Abstract
Cocoa (Theobroma cacao L.), one of the most important agricultural commodity products, is the key raw material for chocolate manufacturing. It is a source naturally occurring polyphenolic compounds and have been widely studied for their beneficial effects to human health. The objective of this study was to evaluate the fermentation time required to obtain more bioactive compounds and higher antioxidant activity in order to propose a mixture of unfermented and fermented cocoa beans in varying concentrations. Samples were collected every 12 h over a fermentation period of 144 h and evaluated according to their physico-chemical characteristics, as well as the content of bioactive compounds. It was verified that after 48 h of fermentation occurred a significant reduction in slate seeds, the appearance of partially fermented beans and the elevation of acidity and temperature. Until this period, a higher content of bioactive compounds with antioxidant activity was also observed. Thus, it is possible to propose a blend of cocoa beans fermented for 48 h and completely fermented beans to elaborate functional chocolates.
Keywords: Methylxantines, Phenolic compounds, Antioxidant activity, Theobroma cacao L.
Introduction
Many studies have evaluated fruits, vegetables and tea as major sources of dietary antioxidative phenolics, but Lee et al. (2003) demonstrated the importance of cocoa with respect to the content of these compounds. Cocoa (Theobroma cacao L.), the key raw material for chocolate manufacturing, is a cash crop of huge economic significance worldwide (Krähmer et al. 2015), since its products have greater antioxidant capacity and a higher amounts of flavonoids per serving than either tea or red wine (Lee et al. 2003).
Naturally occurring polyphenolic compounds have been widely studied for their beneficial effects to human health, as they can combat free radicals, which are harmful to the body and to food systems (Othman et al. 2007). In addition, they are responsible for the cardiovascular protective, antitumor, anti-inflammatory, antineurodegenerative, antibacterial and anticariogenic properties of functional foods (Aprotosoaie et al. 2016).
Cocoa beans have a high phenolic content of approximately 12–18% (dry weight), and 95% are flavanol monomers (epicatechin and catechin) and procyanidin oligomers (dimmer to decamer). Epicatechin has been reported as the main monomeric flavanol in cocoa beans. Depending on the variety, fresh cacao beans contain approximately 12.8 to 43.2 mg g−1 of (−)-epicatechin and 20 to 30-times less (+)-catechin (Payne et al. 2010).
In addition to polyphenols, cocoa is also rich in methylxanthines, bioactive compounds that are associated with some physiological effects to various body systems, including the central nervous, gastrointestinal, respiratory and renal systems. The major methylxanthines in cocoa are theobromine (3.7%) and caffeine (0.2%) (Belšcak et al. 2009).
However, despite their health benefits, phenolics and methylxanthines have a negative influence on taste, conferring astringency and bitterness, and also affect the stability and digestibility of products with high levels of these compounds. As a result, they require subsequent treatments, including fermentation, drying and roasting, to obtain their unique sensory characteristics (Wood and Lass 2001).
Microbial fermentation triggers many chemical reactions that promote promising biochemical characteristics in the cocoa beans. The pulp sugar convertes into ethanol, lactic acid and acetic acid and generates heat that causes the death of the beans. Several enzymatic reactions also contribute to the formation of desirable flavor and color in the beans. In addition, these spontaneous biochemical changes within the beans also reduce their bitterness and astringency. Thus, unfermented cocoa beans will not produce a distinctive chocolate aroma after roasting because the cocoa flavor precursors have not fully formed. In this case, the acidity of the beans is also high (Lagunes-galvez et al. 2007).
During the transformation of fresh cocoa beans to chocolate, the concentration of bioactive compounds can be affected by a variety of biological and processing conditions including fermentation, drying, and roasting (Afoakwa et al. 2012). It is estimated that the phenolic content decreases approximately 70%, and (−)-epicatechin is reduced by 90% of its initial concentration. At the same time, catechins form complex tannins, and anthocyanins are hydrolyzed to anthocyanidins, which polymerize themselves. This can be catalyzed by the enzyme polyphenoloxidase, epicatechin epimerization caused by pH changes during fermentation, and the polymerization of quinones during drying, among other reasons (Oracz et al. 2015).
Therefore, the objective of this study was to evaluate the fermentation time required to obtain more bioactive compounds and higher antioxidant activity in order to propose a mixture of unfermented and fermented cocoa beans in varying concentrations to propose mixtures of cocoa beans as a raw material for the production of functional chocolates.
Materials and methods
Materials
Cocoa seeds were obtained from a farm located in the south of Bahia, on the Ilhéus-Uruçuca highway, in January 2016. Fermentation and drying were performed on the farm following the standards of the producer. The cocoa pods were manually opened with a machete after 48 h of harvest, and the seeds with pulp were separated and immediately submitted to fermentation in three 40-kg wooden boxes.
Approximately 300 g of cocoa beans were removed at random from each fermentation box after each 12 h interval until the end of the fermentation (144 h), and temperature measurements were taken at heights of 6 and 24 cm. The average temperature was 45 °C, not exceeding 50 °C. The cocoa was turned and moved to another box. Samples were taken from different parts of the box after turning to ensure sampling uniformity. The pulp was removed from the grains by rubbing with saw dust. After cleaning, the beans were dried in the sun on stainless steel barges (12 days). The samples were stored in hermetically sealed bags, sent to the laboratory of the Faculty of Pharmacy at UFBA and kept in a refrigerator at 8 °C before further analysis. After the cutting test, the dried cocoa beans were peeled to separate the tips of the shells for analysis. All evaluations were performed in triplicate.
Cut test
After fermentation and drying, 300 beans were randomly collected from each trial batch and submitted to the cut test. This test was performed using a longitudinal section to evaluate the quality of beans according to cotyledon staining and degree of fermentation. The results of the cut test are expressed in percentage (Brasil 2008). Normative Instruction 38/2008 of MAPA (Brasil 2008) was used to define the official cocoa bean standard, considering its identity and quality requirements, sampling, presentation, marking and labeling as aspects of the classification of the product.
The cut test involves counting of 300 beans. These 300 beans are then cut lengthwise through the middle and examined. Separate counts are made of the number of beans which are defective in that they are mouldy, slaty, insect damaged, germinated or flat. The results for each kind of defect are expressed as a percentage of the 300 beans examined. The amount of defective beans revealed in the cut test gives manufacturers an indication of the flavour characteristics of the beans.
Physicalchemical characterization
Moisture (Method 931.04), pH (Method 931.04), titratable total acidity (Method 942.15) were conducted according with the official methods of the AOAC (1995).
Fermentation index (FI)
Fermentation index (FI) determination method is based on the degradation of anthocyanins in products such as cyanidin-3-β-D-galactoside and cyanidin-3-α-L-arabinoside during the fermentation process. Anthocyanin pigments under acidic conditions give a red to purple color, with a maximum absorbance of 500–550 nm prior to fermentation. The oxidation of these products is suspected to contribute to the development of brown pigments with absorbance values below 500 nm (Misnawi et al. 2003). Therefore, the fermentation index is defined as the absorbance ratio measured between 460 nm and 530 nm and was determined according to the method described by Gourieva and Tserevinov (1979).
In this procedure, 20 mg of ground and defatted cocoa bean was extracted with 10 mL of a methanol:HCl (97:3) solution. The homogenate was allowed to stand at 8 °Cfor 16–19 h and then filtered. Measurements were performed individually in a spectrophotometer UV–Vis (BEL PHOTONICS UV-M51) at wavelengths of 460 and 530 nm, and the sample fermentation index was obtained by calculating the ratio of absorbances.
Sample preparation for analysis
Methanolic extracts were obtained according to Oliveira et al. (2011). Two grams of cocoa beans was weighed and degreased with 6 mL of petroleum ether, vortexed (Phoemix, model AP-56) for 5 min and centrifuged (Mikro 220R, Lettich zenthifugen) at 6000 rpm for 15 min. The supernatant was discarded, and an additional 6 mL of petroleum ether was added, repeating the procedure five times. Ten milliliters of aqueous methanol (80%) was poured into tubes containing 100 mg of defatted sample, vortexed for 5 min and centrifuged for 25 min. After filtration with filter paper (Qualy 11,0 J.Prolab), the methanolic extracts were stored in a dark bottle at − 6 °C.
Total polyphenol content (TPC)
Quantification of the phenolic compounds was performed using the Folin-Ciocalteu method described by Carrillo et al. (2014). Briefly, 100 μL of the previously prepared extract, 2.5 mL of 10% Folin–Ciocalteu’s reagent and 2 mL of 7.5% Na2CO3 were mixed. After 2 h, the absorbance of the blue color was measured on a UV–VIS spectrophotometer (BEL PHOTONICS UV-M51) at 760 nm against a blank sample. A calibration curve with epicatechin as a standard was produced (0.06–1.00 mg mL−1). Total polyphenol content (TPC) was expressed as milligram equivalents of epicatechin per gram of sample (mg ECE g−1 sample).
Monomeric phenols and methylxanthines
Qualitative and quantitative determination of monomeric phenolic compounds (catechin, epicatechin and galic acid) and methylxantines (caffeine and theobromine) was performed according to the method described by Maciel et al. (2017), with some adaptations. Twenty microliters of each sample was analyzed using the HPLC system (Perkin Elmer Model Flexar coupled to a UV/VIS detector) and a C-18 column (4.6 × 250 mm, 5 μm). The column was maintained at 30 °C for all analyses, and the wavelength used for detection was 280 nm, with the total running time of 24 min. For HPLC analysis, the compounds were identified by comparing the retention time to those of pure standards (Sigma-Aldrich). The mobile phase used was (A): water acidified with 0.05% phosphoric acid and (B): methanol: acetonitrile (2:4 v/v) in an isocratic ratio (86:14 v/v), with a flow rate of 0.4 mL min−1.
Total flavonoids content
Total flavonoids content (TFC) was quantified according to Lee et al. (2003). Three hundred microliters of the phenolic extract was transferred to a 10-mL volumetric flask containing 4 mL of deionized water. Then, 0.3 mL of 5% sodium nitrite (NaNO2) solution was added. After 5 min, 0.3 mL of 10% aluminum chloride (AlCl3) solution was added. After 1 min, 2 mL of 1 M sodium hydroxide (NaOH) was added, and the flask was filled with distilled water. The absorbance was measured at 510 nm with UV–VIS spectrophotometer (BEL PHOTONICS UV-M51), using white as reference. The TFC in each extract was measured using a standard curve prepared with epicatechin (0.06–1.20 mg mL−1), and the result was expressed as milligram equivalents of epicatechin per gram of sample (mg ECE g−1 sample).
Total anthocyanins content
Total anthocyanins (TA) were determined according to Fuleki and Francis (1968). Briefly, 0.1 g of sample was added to 4 mL of 95% methanol:1.5 N HCl (85:15, v/v) and manually stirred for 2 min before standing overnight at room temperature (30 °C) while protected from light exposure. The extracts were filtered through filter paper (Qualy 11.0 J.Prolab), and the residue was submitted to exhaustive extraction with the same methanolic solution until colorless. The filtered solution was transferred to a 10-mL volumetric flask and left to stand for 90 min at room temperature, protected from light exposure. The total anthocyanins were quantified by UV–VIS spectrophotometry (BEL PHOTONICS UV-M51). To calculate the concentration, we use Eq. 1:
| 1 |
where: A = absorbance at 535 nm; fd = dilution factor; and ε = molar absorptivity (98.2) at 535 nm.
Antioxidant activity
Analysis of radical scavenging activity
The antioxidant activity was evaluated using the free radical sequestration method with DPPH (2,2-diphenyl-1-picrylhydrazyl), as described by Vinson et al. (2006). A volume of 0.1 mL of the 2.5 mg mL−1 methanolic extracts was submitted to reaction with 4 mL of 0.004% (w/v) DPPH solution. After 20 min in the absence of light, absorbance readings at 517 nm were performed in a UV–Vis spectrophotometer (BEL PHOTONICS UV-M51). The ability to sequester free radicals was expressed as the per cent inhibition of radical oxidation and calculated according to Eq. 2:
| 2 |
where ADPPH is the absorbance of the DPPH solution and AExtr is the absorbance of the sample in solution. AExtr was calculated based on the difference in the absorbance of the test sample solution and the blank. The antioxidant activity of each sample (IC50) is defined as the final concentration (in µg mL−1) of the dry extract present in the cuvette that was required to decrease the initial DPPH concentration by 50%.
Ferric reducing antioxidant power (FRAP)
Reduction power according to the ferric reducing antioxidant power (FRAP) method was evaluated according to the methodology described by Pulido et al. (2000), with some adaptations. The FRAP assay was determined based on the reduction of Fe3+-TPTZ to a blue Fe2+-TPTZ complex. The FRAP reagent was prepared by mixing 300 mM of acetate buffer (pH 3.6), 10 mM of TPTZ in 40 mM of HCl and 20 mM of FeCl3·6H2O in a ratio of 10:1:1. A total of 90 μL of methanolic extract and 270 μL of distilled water was then added to the test tubes. FRAP reagent (2.7 mL) was pipetted into the same test tubes and incubated at 37 °C for 30 min. Absorbance was read using a spectrophotometer (BEL PHOTONICS UV-M51) at 595 nm. In the FRAP assay, the antioxidant potential of the sample was determined based on a standard curve (0.50–2.50 mM mL−1), which was plotted using FeSO4·7H2O. The results were expressed as μMFe2+ g−1 samples.
Cupric reducing antioxidant capacity (CUPRAC)
Determination of antioxidant activity by copper reduction in the methanolic extracts was performed according to Apak et al. (2008), with some adaptations. To a test tube, 1 mL of 10−2 M copper (II) chloride (CuCL2) (aqueous solution), 1 mL of neocuproin (Nc) (methanolic solution) at a concentration of 7.5 × 10−3 M and 1 mL of ammonium acetate (NH4Ac) 1 M at pH 7.0 were added. This mixture was vortexed, and 100 mL of the phenolic extract and 1 mL of distilled water were added to yield a final volume of 4.1 mL. The tubes were capped, and after 30 min, the absorbance at 450 nm was recorded in a UV–Vis spectrophotometer (BEL PHOTONICS UV-M51). The analytical curve was prepared with epicatechin (3.46–26.67 μM L−1), and the result was expressed as µmol of epicatechin equivalent per g of sample (µMECE g−1).
Statistical analysis
Data are presented as the mean ± SD. Statistical significance of the differences between groups was evaluated by one-way ANOVA using Minitab® 17.3.1, using the Tukey test at 5% significance for mean separation. Pearson’s correlation (r-value) was also performed using the same statistical program.
Results and discussion
Cut test and fermentation index
During the fermentation of cocoa beans, acetic acid is produced by bacteria and yeast metabolism. Changes in the color of the beans, ranging from purple to brown, were reported during cocoa fermentation (Efraim et al. 2010; Sulaiman 2014). The cut test is a widely used visual method, due to its simplicity and low cost, to evaluate the quality of a random sample of cocoa beans through analysis of its color and compartmentalization and is frequently used as FI (Misnawi et al. 2003). On the other hand, several more accurate chemical methods are available. Among these, a quantitative fermentation index has been proposed for cocoa beans. In this study, both techniques were used because unlike the fermentation index, the cut test has no pattern for over-fermented cocoa beans and is additionally a very subjective technique.
Table 1 shows the results of the cut test for the fermented and dried cocoa beans and the mean FI data after the various evaluated fermentation periods.According to the data, the greatest variation occurred after 48 h of fermentation, when the number of slaty beans decreased from 78% to 1%, and purple beans increased from 5 to 70%. There was also an increase in the percentage of Partially brown beans in this period, from 15 to 28%. In total, brown beans appeared only after 72 h of fermentation, reaching 53% in 144 h. Similar data were found by Efraim et al. (2010) and Afoakwa et al. (2012) by investigating the effect of fermentation time, the type of drying and the method of storage before fermentation. Partially brown beans are not defective, as they change to brown upon storage and the trade accepts up to 30–40%; however, samples containing over 50% partially brown beans are unacceptable (Wood and Lass 2001). Based on the results of cut test, the beans were adequately fermented after 120 h of fermentation, when the percentage of partially brown beans was below 50%.
Table 1.
Results from the Cut Test and FI of cocoa beans throughout fermentation
| Percentage of bean color and defects | Fermentation time (h) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 12 | 24 | 36 | 48 | 60 | 72 | 84 | 96 | 108 | 120 | 132 | 144 | ||
| Cut test | Purple (%) | – | 2 | 1 | 6 | 5 | 70 | 49 | 30 | 20 | 24 | 18 | 20 | 17 |
| Purple/Brown (%) | 1 | 1 | 4 | 6 | 15 | 28 | 34 | 49 | 54 | 49 | 52 | 38 | 29 | |
| Fully brown (%) | – | – | – | – | – | – | 16 | 21 | 25 | 25 | 27 | 40 | 53 | |
| White (%) | 1 | 1 | – | 1 | 1 | – | – | – | – | – | – | – | – | |
| Slaty (%) | 98 | 96 | 94 | 87 | 78 | 1 | – | – | – | – | – | – | – | |
| Broken (%) | – | – | – | – | – | – | – | – | – | 2 | 2 | 1 | – | |
| Flat (%) | – | – | – | – | 1 | – | 1 | – | 1 | – | 1 | – | – | |
| No. of cocoa beans in 100 g | 77,8 | 79,3 | 78,6 | 78,6 | 76,5 | 75,9 | 78,3 | 81,0 | 80,6 | 80,7 | 80,5 | 81,3 | 81,4 | |
| FI | Fraction I (530 nm) | 1.240 | 1.201 | 1.139 | 1.029 | 0.626 | 0.433 | 0.354 | 0.275 | 0.293 | 0.327 | 0.375 | 0.271 | 0.417 |
| Fraction II (460 nm) | 0.488 | 0.478 | 0.530 | 0.495 | 0.330 | 0.274 | 0.308 | 0.259 | 0.336 | 0.366 | 0.413 | 0.301 | 0.441 | |
| FI (Fraction II/Fraction I) | 0.394ª | 0.398ª | 0.467ab | 0.481b | 0.526b | 0.633c | 0.870d | 0.941d | 1.157e | 1.120ef | 1.100ef | 1.109ef | 1.058f | |
*Means with the same letter are not significantly different by ANOVA with the Tukey test (p < 0.05). According to the methodology used, the extract is divided into fractions and the reading is performed on the spectrophotometer in different wave lengths to obtain the result
In all evaluated periods, the absence of moldy, insect-attacked or germinated beans was observed. The amount of flat, broken and white beans was less than 2%, and the number of beans in 100 g category was less than 110. Therefore, according to the cut test, from 60 h of fermentation, the beans were classified as Type I, with superior quality (Brasil 2008).
The fermentation index in cocoa beans is due to the diffusion of polyphenols during fermentation, followed by oxidation and reduction with other cellular compounds, which then turn brown in the cocoa beans (Kresnowati and Febriami 2015; Hernandez et al. 2016). The polyphenol oxidase involved in the oxidation reaction catalyzes o-diphenol into o-quinone, leading to the formation of brown color in cocoa beans (Hernandez et al. 2016). Polyphenol oxidase works best between 42 and 45 °C, which usually occurs on the third day of fermentation and optimal pH conditions (Caligiani et al. 2007).
Results of the spectral measurements of the fermentation index showed that the increase in fermentation time led to drastic decrease in absorbance, falling from 1.240 to 0.417 in 6 days, when reading at 530 nm. These high initial values can be attributed to the presence of high amount of anthocyanin pigments in unfermented beans. On the other hand, the absorbance at 460 nm showed marginal decrease of 0.488 to 0.441. These resulted in an increased fermentation index of the grains from 0.394 to 1.058 at 0 to 144 h of fermentation. It can be observed that there is significant difference between the data up to 48 h of fermentation. Anthocyanin pigments in acid conditions impart a red to purple color with a maximum absorbance at 500–550 nm before fermentation; compounds with absorbance values below 500 nm increase through fermentation, as observed by spectral measurements (Misnawi et al. 2003).
A similar finding was reported by Afoakwa et al. (2012), who observed rapid changes in FI during the first 4 days of fermentation and an increase from 0.774 to 1.050 on the sixth day, likely because the condensation product became less soluble with increasing fermentation. Sulaiman (2014) reported mean FI values above 1.000 from 72 h of fermentation.
According to Sulaiman (2006), for fermentation index less than 1.000, cocoa beans are considered under-fermented, values between 1.000 and 1.599 correspond to completely fermented beans and values over 1.600 correspond to over-fermented beans.
Chemically, the disappearance of purple color can be explained by anthocyanin hydrolysis, which occurs mainly between the first and third day of fermentation (Afoakwa et al. 2012; Misnawi et al. 2003). Thus, anthocyanin content is considered a good index for determining the degree of fermentation of cocoa beans.
Thus, using only the FI parameter as a reference, fermented cocoa beans up to 84 h are still considered unfermented due to their high anthocyanin content. This pigment impart a red-to-purple color, with a maximum absorbance at 500–550 nm under acidic conditions.
According to Efraim et al. (2010), the low productivity and high demand for fermented and dried beans in the processing industries have led to the reduction in fermentation time from 6–7 days to 2–3 days, causing a decrease in the quality of cocoa products as well as technological problems for processing in these industries.
Physicochemical changes of cocoa beans during fermentation
Table 2 presents the physico-chemical analysis (temperature, moisture, pH and titratable total acidity) of dried and ground beans for fermentation times studied.
Table 2.
Physical chemistry characterization of cocoa beans
| Fermentation time (h) | Parameters | |||
|---|---|---|---|---|
| Temperature (°C) | pH | Titratable Acidity1 | Moisture2 | |
| 0 | 27.4 ± 0.00 | 6.64 ± 0.03a | 5.57 ± 0.26d | 5.42 ± 0.14c |
| 12 | 30.0 ± 0.13 | 6.61 ± 0.02ab | 5.65 ± 0.82d | 6.11 ± 0.08abc |
| 24 | 32.0 ± 0.12 | 6.56 ± 0.02b | 6.84 ± 0.52d | 6.04 ± 0.12abc |
| 36 | 31.4 ± 0.06 | 6.44 ± 0.01c | 6.65 ± 0.41d | 5.72 ± 0.29bc |
| 48 | 32.2 ± 0.42 | 6.16 ± 0.02d | 8.48 ± 1.11cd | 5.54 ± 0.20bc |
| 60 | 34.6 ± 1.75 | 5.60 ± 0.00e | 12.83 ± 0.91c | 5.64 ± 0.27bc |
| 72 | 46.6 ± 0.63 | 4.98 ± 0.01f | 23.23 ± 1.22b | 6.81 ± 0.37ª |
| 84 | 42.5 ± 0.48 | 4.83 ± 0.01g | 26.59 ± 2.29ab | 6.31 ± 0.06ab |
| 96 | 45.1 ± 0.41 | 4.75 ± 0.01h | 30.11 ± 1.32ª | 6.71 ± 0.17ª |
| 108 | 42.2 ± 0.27 | 4.70 ± 0.00hi | 30.71 ± 0.86ª | 6.11 ± 0.05abc |
| 120 | 42.7 ± 0.74 | 4.67 ± 0.01i | 30.73 ± 0.42ª | 6.29 ± 0.06ab |
| 132 | 44.2 ± 0.95 | 4.68 ± 0.01i | 26.53 ± 2.18ab | 5.56 ± 0.40bc |
| 144 | 44.7 ± 1.18 | 4.72 ± 0.01hi | 26.52 ± 2.52ab | 5.82 ± 0.16bc |
*Means with the same letter in the columns are not significantly different by ANOVA with the Tukey test (p < 0.05)
1(mEqNaOH/100 g); 2%
The initial fermentation temperature was 27.4 °C, reaching a maximum of 46.6 °C after 72 h, and accompanied by a reduction to 44.78 °C at the end of 144 h. The constant increase in temperature may be associated with the release of heat throughout the process, due to the conversion of the available fermentable substrate (sugar) into the desired by-products of the Metabolite. In good commercial fermentations, the temperature of the seed mass should reach 45 to 48 °C in approximately 72 h, similar to that measured in this study.
The fermentation index has been associated with other parameters, such as pH, reducing sugars, free amino acids and cocoa color pigments but, as mentioned above, only on dried cocoa beans (Ilangantileke et al. 1991). In this study, the pH of fresh cocoa fell from an initial value of 6.64 to 4.72 at the end of the fermentation. A similar trend was reported by other researchers (Sulaiman 2014, Krähmer et al. 2015). Based on these observations, the final pH was slightly low. The acidity of the cocoa beans increased from 5.57 mEqNaOH 100 g−1 at the beginning of fermentation to 26.52 mEqNaOH 100 g−1 after 144 h. According to Krähmer et al. (2015), the acidity of cocoa is not inherent in the seeds but is acquired during fermentation. During the first stage, acidity and pH were constant and temperatures was below 40 °C. In the second phase of fermentation, the transformation of ethanol into organic acids (acetic and lactic acid) occurs through the action of microorganisms (Misnawi et al. 2003). In addition, an increase in titratable acidity and a consequent decrease in pH after 48 h of fermentation can be observed.
Moisture of the analyzed beans ranged from 5.42% to 5.82%. In a study by Efraim et al. (2010), the moisture decreased from 6.38% to 6.29% from the third day of fermentation and showed no significant difference. The moisture values found for all the fermentation periods were close to the allowed range (between 6% and 8%), thus avoiding mold formation and insect attack (Brasil 2008).
Thus, from 48 h of fermentation, the environmental conditions (temperature, pH and acidity) become extremely unfavorable to the seed embryo, which dies and loses its germination capacity, becoming a bean. These factors also lead to cellular decomposition and the formation of aroma precursors.
Profile of bioactive compounds during fermentation
Total phenol content (TPC), flavonoids (TFC) and anthocyanins (TA) in defatted cocoa extracts are presented in Table 3.
Table 3.
Profile of bioactive compounds during fermentation
| Fermentation time (h) | TPC (mgECE g−1) | TFC (mgECE g−1) | TA (mg 100 g−1) |
|---|---|---|---|
| 0 | 395.15 ± 0.89ª | 116.99 ± 8.80a | 352.81 ± 7.36a |
| 12 | 270.06 ± 38.54b | 97.99 ± 14.21abc | 307.93 ± 2.06ab |
| 24 | 398.00 ± 8.74ª | 108.54 ± 7.49ab | 275.36 ± 31.11b |
| 36 | 373.02 ± 12.67ª | 87.67 ± 8.63bc | 325.57 ± 10.66ª |
| 48 | 260.06 ± 15.35bc | 80.04 ± 2.61 cd | 207.54 ± 20.93c |
| 60 | 208.85 ± 8.39bcde | 58.64 ± 6.59de | 110.29 ± 2.49d |
| 72 | 190.83 ± 8.57cde | 38.95 ± 5.98ef | 94.50 ± 14.36d |
| 84 | 209.92 ± 4.82bcde | 50.54 ± 1.75ef | 88.98 ± 4.66d |
| 96 | 136.22 ± 0.71e | 43.78 ± 4.78ef | 71.94 ± 2.13d |
| 108 | 254.89 ± 13.38bcd | 47.86 ± 4.18ef | 80.88 ± 3.92d |
| 120 | 178.87 ± 3.03de | 46.19 ± 2.11ef | 76.60 ± 3.31d |
| 132 | 135.33 ± 8.74e | 30.59 ± 3.01f | 65.40 ± 2.01d |
| 144 | 154.96 ± 3.03e | 42.34 ± 6.04ef | 70.86 ± 4.56d |
*Means with the same letter in the columns are not significantly different by ANOVA with the Tukey test (p < 0.05)
TPC in fresh cocoa beans is comparatively higher than in fermented beans, due to degradation during fermentation. Thus, the average total content ranged from 395.15 to 154.96 mgECE g−1 for unfermented seeds and after 144 h of fermentation, respectively, representing a reduction of 60%. However, after up to 48 h of fermentation, the reduction was only 34%. This observation agrees with previous reports by Dare et al. (2013), for aqueous extracts of unfermented T. cacao seeds, where the values presented were also high (382.61 mgTAE g−1). Afoakwa et al. (2012) and Onomo et al. (2015) studied fermented cocoa beans for the same period of time and found a mean concentration of these substances of 140.34 mgCE g−1 and 142.51 mgGAE g−1, respectively. In contrast, Brito et al. (2017) found lower concentrations of total phonolics, ranging from 77.31 mgCE g−1 (unfermented) to 53.03 mgCE g−1 (fermented).
According to Azizah et al. (1999), this dissimilarity among the obtained results can be explained by the difference in the solvent extractors and the standards used in the quantification. In addition, it is known that TPC can vary according to the variety of cocoa beans, geographic origin, degree of maturity (harvest season) and post-harvest conditions, such as fermentation, drying, roasting, processing and storage.
Flavonoids in cocoa are mainly flavan-3-ols, which occur both as monomers of epicatechin and catechin and as polymerized flavanols or procyanidins with appreciable amounts of anthocyanins (especially cyanidin glycosides) and flavonols (Payne et al. 2010).
Total flavonoids in the samples of unfermented cocoa beans were higher than the fermented beans, from 116.99 to 42.34 mgECE g−1. Similar to that observed for phenolic compounds, the flavonoid content fell by up to 31% after 48 h of fermentation.
Similar results were reported by Onomo et al. (2015) in a study with beans derived from four cocoa clones and their offspring, with values between 60 and 165 mgGAE g−1. In a study by Genovese and da Lannes (2009) using different extracts for the analysis of flavonoids in cocoa powder, much lower values were reported, ranging from 0.90 to 1.20 mgRE g−1.
In our study, the content of anthocyanins decreased by 41% after 48 h and 80% after 144 h (Table 4). The major anthocyanins in cacao beans are cyanidin-3-α-L-arabinoside and cyanidin-3-β-D-galactoside, and their reduction can be explained by the fact that during fermentation, these compounds are hydrolyzed to anthocyanidins by glycosidases, resulting in the brightening of cotyledons (Oracz et al. 2015).
Table 4.
Contents of monomeric phenols [(−)-epicatechin, (+)-catechin and gallic acid] and methylxanthines (caffeine and theobromine) in cocoa bean extracts determined by HPLC analysis
| Fermentation time (h) | Phenolic Compound | Methylxanthines | |||
|---|---|---|---|---|---|
| (−)-Epicatechin (mg g−1) | (+)-Catechin (mg g−1) | Gallic acid (mg g−1) | Caffeine (mg g−1) | Theobromine (mg g−1) | |
| 0 | 41.73 ± 3.40a | 4.11 ± 0.49a | 24.57 ± 1,87ab | 15.63 ± 2.39a | 17.29 ± 0.33ª |
| 12 | 30.35 ± 0.85b | 2.94 ± 0.38ab | 31.04 ± 0,73ª | 8.51 ± 0.35bc | 14.82 ± 0.62ab |
| 24 | 30.14 ± 1.70b | 3.98 ± 0.70ª | 23.15 ± 1,80ªb | 11.13 ± 1.12ab | 16.98 ± 0.46ª |
| 36 | 19.98 ± 3.80 cd | 2.21 ± 0.22bc | 19.06 ± 3,74b | 8.19 ± 1.64bc | 12.34 ± 1.80abc |
| 48 | 22.09 ± 0.27bc | 2.25 ± 0.06bc | 25.72 ± 1,22ªb | 10.14 ± 0.60abc | 14.69 ± 0.67abc |
| 60 | 6.99 ± 2.21de | 1.41 ± 0.21bc | 26.95 ± 3,12ªb | 7.13 ± 0.73bc | 12.23 ± 1.05abc |
| 72 | 8.92 ± 1.68e | 1.38 ± 0.21bc | 30.24 ± 0.77ªb | 6.46 ± 1.25bc | 10.89 ± 0.85bc |
| 84 | 4.56 ± 1.41e | 0.78 ± 0.05c | 25.79 ± 2.07ªb | 4.66 ± 0.66c | 8.89 ± 1.56c |
| 96 | 6.94 ± 1.46e | 1.05 ± 0.12c | 24.08 ± 1.68ªb | 6.04 ± 0.93bc | 10.88 ± 1.65bc |
| 108 | 7.70 ± 1.09e | 1.20 ± 0.12c | 24.77 ± 3.41ªb | 9.05 ± 0.23bc | 13.54 ± 0.91abc |
| 120 | 7.46 ± 1.33e | 0.99 ± 0.05c | 27.06 ± 0.09ªb | 5.83 ± 0.54bc | 11.93 ± 1.09abc |
| 132 | 5.43 ± 0.49e | 0.89 ± 0.29c | 27.79 ± 2.01ªb | 5.84 ± 0.63bc | 9.89 ± 0.73bc |
| 144 | 6.71 ± 0.10e | 1.36 ± 0.07bc | 29.25 ± 0.51ªb | 5.88 ± 0.53bc | 9.79 ± 0.22bc |
*Means with the same letter in the columns are not significantly different by ANOVA with the Tukey test (p < 0.05)
Brito et al. (2017) reported a similar reduction for anthocyanins, with results varying from 301 mg 100 g−1 for fresh cocoa beans to 63 mg 100 g−1 after 7 days of fermentation. Niemenak et al. (2006) identified variations in the content of the majority anthocyanins in the cocoa bean from 46.6 to 455.2 mg 100 g−1 (cyanidin-3-α-L-arabinoside) and from 29.4 to 281.7 mg 100 g−1 (cyanidin-3-β-D-galactoside) in freshly harvested seeds of different cocoa pods from different clones.
Based on the classification of unfermented beans using the cut test (time less than 132 h) and the FI (time less than 96 h), the highest content of phenolic compounds was at 48 h fermentation, with a great reduction after this period. Considering temperature, pH and acidity, the second day of fermentation is when the major transformations in the beans begin to occur, leading to the formation of aroma and flavor precursors.
Monomeric phenols and methylxanthines by HPLC
In this study, two flavan-3-ols, a phenolic acid and two methylxanthines were identified by their retention times, HPLC–UV (280 nm) spectra and chromatographic comparisons to primary patterns, using methanol, acetonitrile and phosphoric acid diluted as solvents.
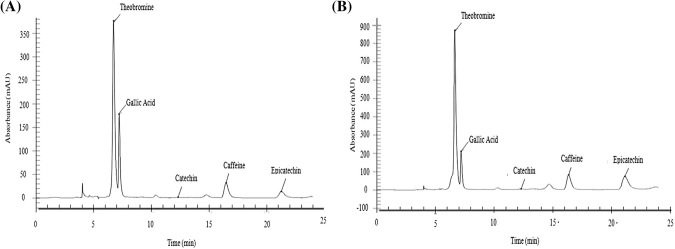
There are clear differences in the chromatograms of the unfermented (A) and fermented (B) cotyledon samples (Fig. 1), with the unfermented sample having a richer profile.All values, except for that of gallic acid, diminished following the fermentation of cotyledon samples (Table 4). The reduction of these compounds in the cotyledon following fermentation has been reported previously (Cruz et al. 2015, Krähmer et al. 2015) and directly depends on reaction time.
Fig. 1.
Representative chromatograms of unfermented (a) and fermented (b) cocoa samples at λ = 280 nm (mAU Absorption Units)
Among the bioactive compounds, catechin had the lowest content (from 4.11 to 1.36 mg g−1), representing a reduction of 66% after 144 h of fermentation. Epicatechin showed high concentrations at the beginning of fermentation (41.73 mg g−1), with a reduction of 47% in the first 48 h and 84% by the end of the fermentation. Gallic acid content was high throughout fermentation (from 24.57 to 29.25 mg g−1), with no significant differences between the times (p < 0.05).
Niemenak et al. (2006) determined the content of monomeric phenols in freshly harvested seeds of various clones and found higher catechin indexes (1.25–14.42 mg g−1) and epicatechin (14.43–43.90 mg g−1), in line with our results. However, using samples of fermented beans, Onomo et al. (2015) reported higher epicatechin content (29.80 mg g−1 on average) and lower catechin content (mean of 0.31 mg g−1) relative to our results.
Hernández-Hernández et al. (2018) showed that the fermentation had a different influence on the epicatechin and catechin contents of the beans. Both had a reduction in the epicatechin concentration above 70% of the initial value. On the other hand, catechin concentrations did not change during fermentation.
For gallic acid, Leite et al. (2013) reported considerably lower values compounds (0.12 to 0.13 mg g−1) in the cocoa mass of different cultivars, which were resistant and not resistant to “witch’s broom disease”. Similarly, Cruz et al. (2015) evaluated the content of phenolic compounds at various fermentation times and reported average values for gallic acid of 0.12 mg g−1 in fresh beans at 0.09 mg g−1 after 120 h of fermentation. This acid is the basic constituent of the hydrolysable tannins and is responsible for the astringent sensation in the mouth (Leite et al. 2013).
Like phenolic compounds, the content of methylxanthines has reduced during fermentation. Theobromine concentration decreased from 17.29 mg g−1 in fresh seeds to 9.79 mg g−1 after 144 h of fermentation. Some studies highlight theobromine as the predominant compound in the extracts (Belšcak et al. 2009; Carrillo et al. 2014; Hernández-hernández et al. 2018).
Similar results were described by Batista et al. (2016) and Hernández-Hernández et al. (2018) for the theobromine content in fermented and unfermented cocoa samples, corroborating the present work.
In much of the research conducted, the caffeine content was much lower than that presented in this study (< 3.5 mg g−1 on average) (Belšcak et al. 2009; Carrillo et al. 2014; Cruz et al. 2015; Batista et al. 2016; Hernández-hernández et al. 2018). According to Aneja and Gianfagna (2001), the highest content of methylxanthines and phenolic compounds may be related to the biochemical mechanisms of plant tissue defense.
As in the total phenolic compounds content, the content of monomeric polyphenols and methylxanthines presents a reduction pattern after 48 h of fermentation, and thus, this period is most suitable for use in the elaboration of chocolates with functional characteristics.
Antioxidant activity
The antioxidant activity against the DPPH radical was determined by IC50, which is defined as the amount of antioxidant needed to decrease the initial concentration of the DPPH radical by 50% (Table 5). A lower IC50 value indicates the strongest ability of extracts to act as DPPH scavengers (Othman et al. 2007).
Table 5.
Antioxidant capacity of cocoa product extracts determined by DPPH, FRAP and CUPRAC assays
| Fermentation time (h) | Antioxidant Activity | ||
|---|---|---|---|
| DPPH (IC50) | FRAP (μmolFe2+ 100 g−1) | CUPRAC (mgECE g−1) | |
| 0 | 13.30 ± 0.06e | 329.71 ± 36.56abc | 38.84 ± 30.29a |
| 12 | 13.80 ± 0.47de | 354.32 ± 23.89ab | 31.95 ± 2.23b |
| 24 | 13.47 ± 0.05e | 389.04 ± 15.90ª | 40.04 ± 2.02ª |
| 36 | 13.83 ± 0.31de | 333.71 ± 46.33abcd | 30.25 ± 3.13bc |
| 48 | 14.32 ± 0.05de | 255.71 ± 9.75bcdef | 28.30 ± 0.99bc |
| 60 | 17.96 ± 2.01cde | 177.08 ± 7.12efg | 23.51 ± 2.40 cd |
| 72 | 20.30 ± 2.23c | 263.88 ± 1.08bcdef | 18.54 ± 0.06de |
| 84 | 22.22 ± 0.22c | 280.33 ± 6.79bcde | 17.33 ± 1.14de |
| 96 | 21.16 ± 1.35c | 257.85 ± 26.03cde | 18.70 ± 1.79de |
| 108 | 19.38 ± 2.24cd | 281.57 ± 33.80bcde | 18.82 ± 1.26de |
| 120 | 21.11 ± 1.60c | 219.42 ± 1.96defg | 18.10 ± 1.14de |
| 132 | 28.81 ± 2.87b | 163.71 ± 20.24 fg | 9.71 ± 0.92f |
| 144 | 43.02 ± 2.28a | 141.46 ± 18.97 g | 14.32 ± 0.92ef |
*Means with the same letter in the columns are not significantly different by ANOVA with the Tukey test (p < 0.05)
The IC50 for the cocoa extracts increased from 13.30 to 43.02 µg mL−1 until the end of the fermentation. However, there was no significant difference (p < 0.05) between the initial values for up to 48 h of fermentation.
In a study with 26 cacao genotypes, Hernández-Hernández et al. (2018) verified that the genotypes previously identified as having the highest concentrations of theobromine, epicatechin, and catechin had some of the lowest IC50 values, and consequently, the antioxidant activities were higher.
According to Oliveira et al. (2011), there appears to be significant relationship between the antioxidant activity of cocoa extracts and the total concentration of phenolic compounds, since extracts with higher concentrations of monomeric phenols also have higher antioxidant activity.
A low correlation coefficient was found between TPC and DPPH in the methanolic extracts (r = −0.680), indicating that only small amounts of phenolic antioxidants in cocoa products are responsible for the activity through the elimination of free DPPH radicals. A slightly higher coefficient was found for theobromine (r = −0.691). These results are in full agreement with those reported by Othman et al. (2007) and Belšcak et al. (2009), suggesting that the high elimination capacity of cocoa extract can be attributed to other methanol-soluble compounds such as methylxanthines.
The data presented in Table 5 show a reduction in the FRAP value of 329.71 μmol Fe2+ 100 g−1 in fresh seeds to 141.46 μmol Fe2+ 100 g−1 in fermented beans. Unlike the DPPH assay, the FRAP assay showed a high, positive correlation coefficient with the total polyphenol content (r = 0.809), and flavonoids (r = 0.779), catechin (r = 0.708) and epicatechin (r = 0.711). A similar result was reported by Othman et al. (2010) in the ethanolic extracts of fermented cocoa beans, ranging from 77.47 to 143.37 μmol Fe2 + 100 g−1, showing a positive and high correlation based on the FRAP assay between antioxidant potential and epicatechin content. In the same way, Belšcak et al. (2009) demonstrated a good correlation between total phenolics and antioxidant capacity in various cocoa products.
The values obtained for the Cuprac assay varied from 38.84 to 14.32 mgECE g−1 of the sample. No studies were found in the literature evaluating the antioxidant activity of cocoa beans using this method. This method should be advantageous over the FRAP and DPPH assays because, in addition to the faster kinetics of copper(II) redox chemistry as opposed to ferric ion, it also has a higher sensitivity for measuring both hydrophilic and lipophilic antioxidants (Apak et al. 2008).
The Cuprac assay had the highest correlation coefficient based on total phenolics (r = 0.914), flavonoids (r = 0.977), monomeric phenols (r = 0.932 (epicatechin) and r = 0.936 (catechin)) and methylxanthines (r = 0.831 (caffeine) and r = 0.901 (theobromine)).
According to Apak et al. (2008), higher pH is correlated with a greater dissociation tendency of acids and phenols, with an increase in negative charge and facilitation of electron output (oxidation), evidencing the antioxidant characteristics. In this case, it is possible to emphasize that until 48 h of fermentation, there is a greater antioxidant activity in the cacao seeds.
Conclusion
The fermentation index showed that there was a difference between the samples during the fermentation, even at the initial times (0 to 48 h), when the concentration of the slaty beans was still high. It was observed that up to 48 h, there was high antioxidant activity due to the lower acidity found. As fermentation further progresses, there is a significant reduction in these compounds, mainly epicatechin. Thus, due to the high concentration of bioactive compounds and antioxidant activity the time of 48 h this would be the most adequate time to interrupt fermentation and use beans in the mixture to obtain raw material for the production of functional chocolates.
Acknowledgements
To Capes (Coordination and Improvement of Higher Level or Education Personnel) for the scholarship and the CNPq (National Council for Scientific and Technological Development) for financial support.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Afoakwa EO, Quao J, Budu AS, Takrama J, Saalia FK. Changes in total polyphenols, o-diphenols and anthocyanin concentrations during fermentation of pulp pre-conditioned cocoa (Theobroma cacao) beans. Int Food Res J. 2012;19(3):1071–1077. [Google Scholar]
- Aneja M, Gianfagna T. Induction and accumulation of caffeine in young actively growing leaves of cocoa (Theobroma cacao, L.) by wounding or infection with Crinipellis perniciosa. Physiol Mol Plant Pathol. 2001;59(1):13–16. doi: 10.1006/pmpp.2001.0337. [DOI] [Google Scholar]
- AOAC . Official methods of association of official analytical chemists. 16. Arlington: Association of Official Analytical Chemists; 1995. [Google Scholar]
- Apak R, Guçlu K, Ozyurek M, Celik SE. Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. Microchim Acta. 2008;160(4):413–419. doi: 10.1007/s00604-007-0777-0. [DOI] [Google Scholar]
- Aprotosoaie AC, Luca SV, Miron A. Flavor chemistry of cocoa and cocoa products: an overview. Compreh Rev Food Sci Food Safety. 2016;15:73–91. doi: 10.1111/1541-4337.12180. [DOI] [PubMed] [Google Scholar]
- Azizah AH, Ruslawati NMN, Tee TS. Extraction and characterization of antioxidant from cocoa by-products. Food Chem. 1999;64:199–202. doi: 10.1016/S0308-8146(98)00121-6. [DOI] [Google Scholar]
- Batista NN, Andrade DP, Ramos CL, Dias DR, Schwan RF. Antioxidant capacity of cocoa beans and chocolate assessed by FTIR. Food Res Int. 2016;90:313–319. doi: 10.1016/j.foodres.2016.10.028. [DOI] [PubMed] [Google Scholar]
- Belšcak A, Komes D, Horzic D, Ganic KK, Karlovic D. Comparative study of commercially available cocoa products in terms of their bioactive composition. Food Res Int. 2009;42(5–6):707–716. doi: 10.1016/j.foodres.2009.02.018. [DOI] [Google Scholar]
- BRASIL (2008) Ministério da Agricultura, Pecuária e Abastecimento - Instrução Normativa nº 38 de 23 de junho de 2008. Regulamento Técnico da Amêndoa de Cacau. Diário Oficial da União
- Brito BNC, Chisté RC, DA Pena R, Gloria MBA, Lopes AS. Bioactive amines and phenolic compounds in cocoa beans are affected by fermentation. Food Chem. 2017;228(1):484–490. doi: 10.1016/j.foodchem.2017.02.004. [DOI] [PubMed] [Google Scholar]
- Caligiani A, Cirlini M, Palla G, Ravaglia R, Arlorio M. GC-MS detection of chiral markers in cocoa beans of different quality and geographical origin. Chirality. 2007;19:329–334. doi: 10.1002/chir.20380. [DOI] [PubMed] [Google Scholar]
- Carrillo LC, Londoño-Londoño J, Gil A. Comparison of polyphenol, methylxanthines and antioxidant activity in Theobroma cacao beans from different cocoa-growing areas in Colombia. Food Res Int. 2014;60:273–280. doi: 10.1016/j.foodres.2013.06.019. [DOI] [Google Scholar]
- Cruz JFM, Leite PB, Soares SE, Bispo ES. Bioactive compounds in different cocoa (Theobroma cacao L.) cultivars during fermentation. Food Sci Technol. 2015;35(2):279–284. doi: 10.1590/1678-457X.6541. [DOI] [Google Scholar]
- Dare CA, Onwumelu RN, Oyedapo OO. Biochemical studies on the effects of polyphenols from fermented and unfermented acetone extracts of Theobroma Cacao L (cocoa) seeds on antioxidant enzymes of streptozotocin-induced diabetic rats. Niger J Biochem Mol Biol. 2013;28(1–2):44–58. [Google Scholar]
- Efraim P, Pezoa-García NH, Jardim DCP, Nishikawa A, Haddad R, Eberlin MN. Influence of cocoa beans fermentation and drying on the polyphenol content and sensory acceptance. Ciência e Tecnologia em Alimentos. 2010;30(l1):142–150. doi: 10.1590/S0101-20612010000500022. [DOI] [Google Scholar]
- Fuleki T, Francis FJ. Quantitative methods for anthocyanins: 1. Extraction and determination of total anthocyanin in cranberries. J Food Sci. 1968;33(1):72–77. doi: 10.1111/j.1365-2621.1968.tb00887.x. [DOI] [Google Scholar]
- Genovese MI, da Lannes SC. Comparison of total phenolic content and antiradical capacity of powders and “chocolates” from cocoa and cupuassu. Ciência Tecnologia de Alimentos. 2009;29(4):810–814. doi: 10.1590/S0101-20612009000400017. [DOI] [Google Scholar]
- Gourieva KB, Tserevinov OB (1979) Methods of evaluating the degree of fermentation of cocoa beans. USSR Patent 64654, 1979
- Hernandez CH, Lopez-Andrade PA, Ramirez-Guillermo MA, Ramirez DG, Perez JFC. Evaluation of different fermentation processes for use by small cocoa growers in Mexico. Food Sci Nutr. 2016;4(5):690–695. doi: 10.1002/fsn3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Hernández C, Viera-Alcaide I, Sillero AMM, Fernández-Bolaños J, Rodríguez-Gutiérrez G. Bioactive compounds in Mexican genotypes of cocoa cotyledon and husk. Food Chem. 2018;240:831–839. doi: 10.1016/j.foodchem.2017.08.018. [DOI] [PubMed] [Google Scholar]
- Ilangantileke SG, Wahyudi T, Bailon G. Assessment methodology to predict quality of cocoa beans for export. J Food Qual. 1991;14:481–496. doi: 10.1111/j.1745-4557.1991.tb00088.x. [DOI] [Google Scholar]
- Krähmer A, Engel A, Kadow D, Ali N, Umaharan P, Kroh LW, Schulz H. Fast and neat:determination of biochemical quality parameters in cocoa using near infrared spectroscopy. Food Chem. 2015;181:152–159. doi: 10.1016/j.foodchem.2015.02.084. [DOI] [PubMed] [Google Scholar]
- Kresnowati PMTA, Febriami H. Mapping the effects of starter culture addition on cocoa bean fermentation. ASEAN Eng J Part B. 2015;5(1):25–37. [Google Scholar]
- Lagunes-Galvez S, Loiseau G, Paredes JL, Barel M, Giraud JP. Study on the microflora and biochemistry of cocoa fermentation in the Dominican Republic. Int J Food Microbiol. 2007;114:124–130. doi: 10.1016/j.ijfoodmicro.2006.10.041. [DOI] [PubMed] [Google Scholar]
- Lee KW, Kim YJ, Lee HJ, Lee CY. Cocoa has more phenolic phytochemicals and a higher antioxidant capacity than teas and red wine. J Agric Food Chem. 2003;51(25):7292–7295. doi: 10.1021/jf0344385. [DOI] [PubMed] [Google Scholar]
- Leite PB, Maciel LF, Opretzka LCF, Soares SE, Bispo ES. Phenolic compounds, methylxanthines and antioxidant activity in cocoa mass and chocolates produced from “witch broom disease” resistant and non resistant cocoa cultivars. Ciência e Agrotecnologia. 2013;37(3):244–250. doi: 10.1590/S1413-70542013000300007. [DOI] [Google Scholar]
- Maciel LF, Felício ALSM, Hirooka EY. Bioactive compounds by UPLC-PDA in different cocoa clones (Theobroma cacao L.) developed in the Southern region of Bahia, Brazil. Br Food J. 2017;119(9):2117–2127. doi: 10.1108/BFJ-09-2016-0423. [DOI] [PubMed] [Google Scholar]
- Misnawi S, Jamilah B, Nazamid S. Effects of incubation and polyphenol oxidase enrichment on colour, fermentation index, procyanidins and astringency of unfermented and partly fermented cocoa beans. Int J Food Sci Technol. 2003;38:285–295. doi: 10.1046/j.1365-2621.2003.00674.x. [DOI] [Google Scholar]
- Niemenak N, Rohsius C, Elwers S, Ndoumou DO, Lieberei R. Comparative study of different cocoa (Theobroma cacao L.) clones in terms of their phenolics and anthocyanins contents. Journal of Food Composition and Analysis. 2006;19:612–619. doi: 10.1016/j.jfca.2005.02.006. [DOI] [Google Scholar]
- Oliveira CS, Maciel LF, Miranda MPS, Bispo ES. Phenolic compounds, flavonoids and antioxidant activity in different cocoa samples from organic and conventional cultivation. Br Food J. 2011;113(9):1094–1102. doi: 10.1108/00070701111174550. [DOI] [Google Scholar]
- Onomo PE, Niemenak N, Djocgoue PF, Ondobo ML, Ndoumou DO. Heritability of polyphenols, anthocyanins and antioxidant capacity of Cameroonian cocoa (Theobroma cacao L.) beans. Afr J Biotechnol. 2015;14(36):2672–2682. doi: 10.5897/AJB2015.14715. [DOI] [Google Scholar]
- Oracz J, Zyzelewicz D, Nebesny E. Changes in the flavan-3-ols, anthocyanins, and flavanols composition of cocoa beans of different Theobroma cacao L. groups affected by roasting conditions. Eur Food Res Technol. 2015;24(5):663–681. doi: 10.1007/s00217-015-2494-y. [DOI] [Google Scholar]
- Othman A, Ismail A, Abdul ghani N, Adenan I. Antioxidant capacity and phenolic content of cocoa beans. Food Chem. 2007;100:1523–1530. doi: 10.1016/j.foodchem.2005.12.021. [DOI] [Google Scholar]
- Othman A, Jalil AMM, Weng KK, Ismail A, Abdghani N, Adenan I. Epicatechin content and antioxidant capacity of cocoa beans from four different countries. Afr J Biotechnol. 2010;9(7):1052–1059. doi: 10.5897/AJB09.1219. [DOI] [Google Scholar]
- Payne MJ, Hurst WJ, Miller K, Rank C, Stuart DA. Impact of fermentation, drying, roasting, and Dutch processing on epicatechin and Catechin content of cacao beans and cocoa ingredients. J Agric Food Chem. 2010;58(19):10518–10527. doi: 10.1021/jf102391q. [DOI] [PubMed] [Google Scholar]
- Pulido R, Bravo L, Saura-Calixo F. Antioxidant activity of dietary polyphenols as determinedby a modified ferric reducing/antioxidante power assay. J Agric Food Chem. 2000;48(8):3396–3402. doi: 10.1021/jf9913458. [DOI] [PubMed] [Google Scholar]
- Sulaiman KB (2006) Determination of fermentation index and ph. Kertas Kerja VII, Kursus Penggredan Biji Koko Kering (Lanjutan); Tawau, Sabah, Malaysia; Malay
- Sulaiman KB. Impact of fermentation duration on the quality of malaysian cocoa beans using shallow box. Asia Pac J Sci Technol. 2014;19:74–80. [Google Scholar]
- Vinson JA, Proch J, Bose P, Muchler S, Taffera P, Shuta D, Samman N, Agbor GA. Chocolate is a powerful ex vivo and in vivo antioxidant, an anti-atherosclerotic agent in an animal model, and significant contributor to antioxidants in European and American diets. J Agric Food Chem. 2006;54(21):8071–8076. doi: 10.1021/jf062175j. [DOI] [PubMed] [Google Scholar]
- Wood GAR, Lass RA. Cocoa, 4ed. Oxford: Blackwell Science; 2001. p. 620. [Google Scholar]