Abstract
Objective.
To characterize polysomnographic sleep architecture in children with Down syndrome and compare findings in those with and without obstructive sleep apnea.
Study Design.
Case series with retrospective review.
Setting.
Single tertiary pediatric hospital (2005–2018).
Methods.
We reviewed the electronic health records of patients undergoing polysomnography who were referred from a specialized center for children with Down syndrome (age, ≥12 months). Continuous positive airway pressure titration, oxygen titration, and split-night studies were excluded.
Results.
A total of 397 children were included (52.4% male, 81.6% Caucasian). Mean age at the time of polysomnography was 4.7 years (range, 1.4–14.7); 79.4% had obstructive sleep apnea. Sleep variables were reported as mean (SD) values: sleep efficiency, 85% (11%); sleep latency, 29.8 minutes (35.6); total sleep time, 426 minutes (74.6); rapid eye movement (REM) latency, 126.8 minutes (66.3); time spent in REM sleep, 22% (7%); arousal index, 13.3 (5); and time spent supine, 44% (28%). There were no significant differences between those with obstructive sleep apnea and those without. Sleep efficiency <80% was seen in 32.5%; 34.3% had a sleep latency >30 minutes; 15.9% had total sleep time <360 minutes; and 75.6% had an arousal index >10/h. Overall, 69.2% had ≥2 metrics of poor sleep architecture. REM sleep time <20% was seen in 35.3%. REM sleep time decreased with age.
Conclusion.
In children with Down syndrome, 32.5% had sleep efficiency <80%; 75.6% had an elevated arousal index; and 15.9% had total sleep time <360 minutes. More than a third of the patients had ≥3 markers of poor sleep architecture. There was no difference in children with or without obstructive sleep apnea.
Keywords: Down syndrome, obstructive sleep apnea, pediatrics, sleep architecture
Obstructive sleep apnea (OSA) is seen in 1.2% to 5.7% of children in the United States and occurs at much higher rates in children with Down syndrome (DS), with estimated rates of 30% to 60%.1–6 Children with DS are thought to be predisposed to OSA due to numerous anatomic and physiologic factors, including midface hypoplasia, relative macroglossia, adenotonsillar hypertrophy, glossoptosis, and global hypotonia. The long-term sequelae of untreated OSA have been well studied and include growth failure, impairment in neurocognitive development, pulmonary hypertension, and cor pulmonale.1–6
The high rate of sleep abnormalities in children with DS has largely been attributed to OSA and/or sleep-disordered breathing. There has been little reported on other sleep disorders in children with DS beyond OSA. Additionally, behavioral interventions for children with DS can be challenging, particularly in those with disrupted sleep. Poor daytime behavior may often be attributed to, or felt to be exacerbated by, presumed OSA. There are limited data suggesting that sleep fragmentation in children with DS is attributed to factors other than OSA, and it has been proposed that children with DS have altered sleep architecture as compared with children without DS.7,8 Whereas there are normative data on sleep architecture in typically developing children, large-scale data on sleep architecture in children with DS have yet to be established.9–12 Additionally, very little attention has been given to differences across age categories in children with DS.
The objective of this study was to characterize polysomnographic sleep architecture in children with DS and compare findings in those with and without OSA, as well as to identify differences by age. Additionally, we aimed to determine how many metrics of poor sleep architecture were present in this cohort of children with DS, with and without OSA.
Materials and Methods
Patient Population
We performed a retrospective review of consecutive patients undergoing polysomnography (PSG) between 2005 and 2018 in a specialized center for children with DS at Cincinnati Children’s Hospital Medical Center. Children aged ≥12 months were included. The data collected included demographic information, sleep study diagnosis, and PSG parameters. Patients with tracheostomy tubes and those undergoing titration of oxygen or positive pressure were excluded. Given the large number of patients, age categories were devised on the basis of differences seen clinically and in sleep parameters by age. This study was approved by the Cincinnati Children’s Hospital Medical Center’s Institutional Review Board.
Overnight PSG
All patients underwent overnight PSG (up to 12 hours) in our pediatric sleep laboratory. Patients went to bed at a time of their preference, and studies were terminated when they awoke spontaneously, in accordance with their home wake times. The following parameters were recorded simultaneously: body position, bilateral electrooculogram, >3-channel electroencephalogram, chin electromyogram, anterior tibialis electromyogram, tracheal microphone, electrocardiogram, pulse oximetry, thoracic and abdominal inductance plethysmography, nasal pressure transduction, and end tidal CO2. A certified sleep technician performed, and a board-certified sleep specialist interpreted, scoring of the PSG based on standard criteria as defined by the American Academy of Sleep Medicine.13,14 Patients were considered to have OSA if their PSG showed an obstructive apnea-hypopnea index ≥1 event/h (1 to <5, mild; ≥5 to <10, moderate; ≥10, severe). Patients were considered to have elevated periodic limb movements of sleep if their PSG showed >5 leg movements/ h.
Definition of Sleep Architecture Variables
The American Academy of Sleep Medicine standard definitions were used for the following variables of sleep architecture.14 Sleep efficiency was defined as a percentage of total sleep time (TST) divided by time in bed. Sleep latency was time to sleep onset after hookup was complete. Rapid eye movement (REM) latency was time in minutes to first REM sleep period after sleep onset. All sleep-stage percentages were calculated as the time in each stage (in minutes) divided by the TST: non-REM (NREM) stages 1 to 3 and REM. The number of REM cycles was reported. The arousal index was the number of arousals per hour of sleep. Body position was reported as a percentage of time in each position (supine, prone, side) divided by TST.
Definition of Poor Sleep Architecture
Based on clinical experience and limited normative data in the literature on sleep architecture in typically developing children, we defined metrics indicative of poor sleep architecture as the following (Table 1): sleep efficiency <80%, sleep latency ≥30 minutes, TST <360 minutes, REM sleep latency <90 minutes, REM cycles <3, arousal index >10/h, and time spent in REM sleep <20%.9–12 In regard to NREM sleep stages, we reported those who were outside the following percentages: NREM stage 1, >5%; NREM stage 2, <40% or >55%; NREM stage 3, <15%. We reported the percentage of children who had each metric of poor sleep architecture, and we summed the number of metrics that each child had as an indicator of severity of poor sleep architecture.
Table 1.
Metrics of Poor Sleep Architecture.
| Metric | Cutoff value |
|---|---|
| Sleep | |
| Efficiency | <80% |
| Latency | ≥30 min |
| Total sleep time | <360 min |
| Arousal index | >10/h |
| REM | |
| Sleep | <20% |
| Sleep latency | <90 min |
| Cycles | <3 |
Abbreviations: REM, rapid eye movement.
Statistical Analysis
Statistical analyses were conducted with SAS version 9.4 (SAS Institute Inc). The distributions of sleep architecture variables were evaluated with means with standard deviations and medians with interquartile ranges. The relationships of sleep architecture characteristics with age (categorized as ≥12 months to 2 years, ≥3 to 5 years, ≥6 to 9 years, ≥10 years) were tested with analysis of variance or the Kruskal-Wallis test. Differences in the percentage of children with poor sleep metrics by age category were tested with Pearson’s chi-square. Differences in sleep architecture characteristics and the percentage of children with poor sleep metrics by OSA diagnosis were tested with t tests and Pearson’s chi-square, respectively.
Results
Demographics and Clinical Characteristics
A total of 544 children were included; 66 studies were excluded because they were not diagnostic. Of the remaining 478 studies, 397 were in children ≥12 months of age and were included in the final analysis. The average age of the patient at time of PSG was 4.7 years (SD, 2.6).
Among these 397, 208 (52.4%) were male; 324 (81.6%), Caucasian; 33 (8.3%), African American; 9 (2.3%), Asian; 27 (6.8%), other; and 4 (1%), unknown. All children had a diagnosis of DS. OSA was diagnosed on the PSG in 315 children (79.4%; median obstructive apnea-hypopnea index, 3.3; interquartile range, 2–7.4) and periodic limb movements of sleep in 8 (2%).
PSG Parameters
For the entire population, the mean sleep efficiency was 85% (SD, 11%); sleep latency, 29.8 minutes (35.6); TST, 426 minutes (74.6); and time in bed, 521.7 minutes (60). Sleep variables were reported as mean values: REM latency, 126.8 minutes (66.3); time spent in REM sleep, 22% (7%); number of REM cycles, 5 (1.8); arousal index, 13.3 (5); and time spent in each body position—supine, 44% (28%); prone, 30% (25%); and side, 35% (21%). Mean NREM stages 1 to 3 were 3% (2%), 47% (7%), and 28% (7%), respectively. Table 2 shows sleep architecture variables.
Table 2.
Distribution of Sleep Architecture Variables for 397 Children With Down Syndrome as Assessed With Polysomnography.
| Variable | Mean (SD) |
|---|---|
| Sleep | |
| Efficiency, % | 82 (11) |
| Latency, min | 29.8 (35.6) |
| Total time, min | |
| Sleep | 426.0 (74.6) |
| In bed | 521.7 (60) |
| REM latency, min | 126.8 (66.3) |
| NREM stage, % | |
| 1 | 3 (2) |
| 2 | 47 (7) |
| 3 | 28 (7) |
| REM | |
| Sleep, % | 22 (7) |
| Cycles | 5.0 (1.8) |
| Arousal index, No./h | 13.3 (5.0) |
| Time spent, % | |
| Supine | 44 (28) |
| Prone | 30 (25) |
| Side | 35 (21) |
Abbreviations: NREM, non–rapid eye movement; REM, rapid eye movement.
Table 3 presents a breakdown of sleep architecture variables by age group. The number of REM cycles decreased with increasing age, and REM latency increased with age. In evaluating the relationship between sleep architecture and age, NREM stage 2 significantly increased with age (P = .0004), and REM significantly decreased with age (P < .0001; Figure 1). Additionally, the arousal index decreased through age 9 years and then increased for children >10 years old (P = .0001; Figure 2). Outlier values for the arousal index were seen in each age group, particularly in years 3 to 5, with a maximum value of 39.4/h. No other variables showed change with age.
Table 3.
Distribution of Sleep Architecture Variables for Children With Down Syndrome by Age.a
| Variables | 1–2 y (n = 47) | 3–5 y (n = 274) | 6–9 y (n = 52) | ≥10 y (n = 24) | P valueb |
|---|---|---|---|---|---|
| Sleep | |||||
| Efficiency, % | 81 (9) | 83 (11) | 83 (9) | 74 (17) | .002 |
| Latency, min | 27.5 (35.3) | 29.3 (33.6) | 27 (32) | 46.9 (57.5) | .37c |
| Median [IQR] | 14.0 [4.5–36.5] | 19.4 [5–39.9] | 16.5 [4.8–38.5] | 24.8 [12.6–55.5] | |
| Total time, min | |||||
| Sleep | 426.0 (62.5) | 430.1 (73.3) | 426.6 (61.2) | 378.2 (115.6) | .01d |
| In bed | 525.9 (61) | 523.0 (59.6) | 518.8 (50) | 505.5 (80.8) | .53 |
| REM | |||||
| Cycles | 6.8 (1.9) | 5 (1.6) | 4.4 (1.4) | 3.3 (1.5) | <.0001 |
| Latency, min | 67.8 (33) | 130.0 (60.3) | 143.8 (69) | 171.2 (101.1) | <.0001 |
| Time spent, % | |||||
| Supine | 48 (33) | 43 (27) | 40 (25) | 65 (30) | .0009d |
| Prone | 36 (31) | 30 (25) | 32 (26) | 21 (24) | .40 |
| Side | 34 (21) | 35 (20) | 39 (21) | 31 (23) | .46 |
Abbreviations: IQR, interquartile range; REM, rapid eye movement.
Values are presented as mean (SD) unless noted otherwise.
P value reported from analysis of variance comparing 4 age groups.
Kruskal-Wallis test reported.
Significant differences between ≥ 10 years and other age groups. No differences across 3 younger groups.
Figure 1.
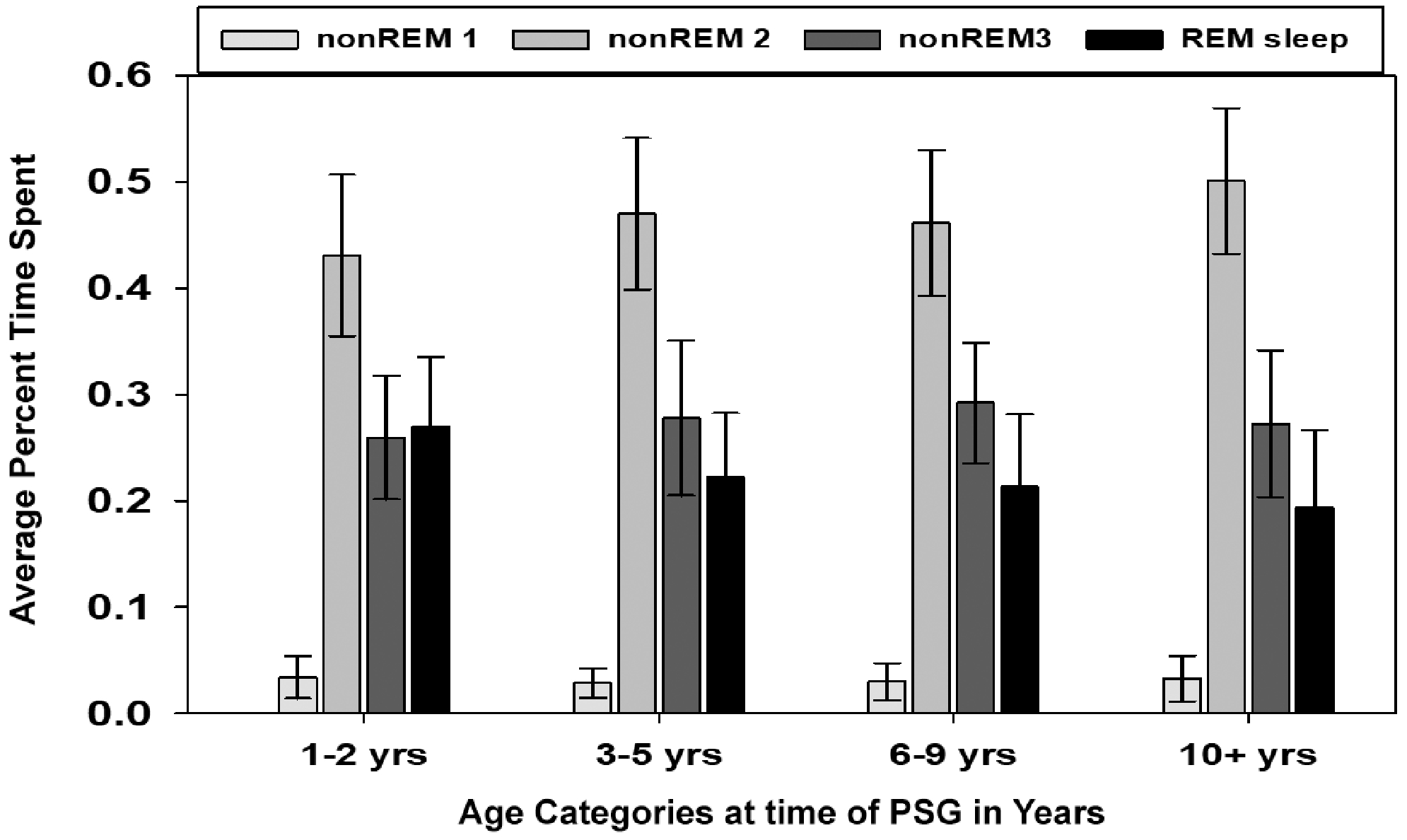
Average percentage time spent in sleep stages for children with Down syndrome undergoing polysomnography (PSG) by age category. Error bars represent SD. Comparisons across age: non-REM stage 1 (P = .11), non-REM stage 2 (P = .0004), non-REM stage 3 (P = .15), and REM (P <.0001). REM, rapid eye movement.
Figure 2.
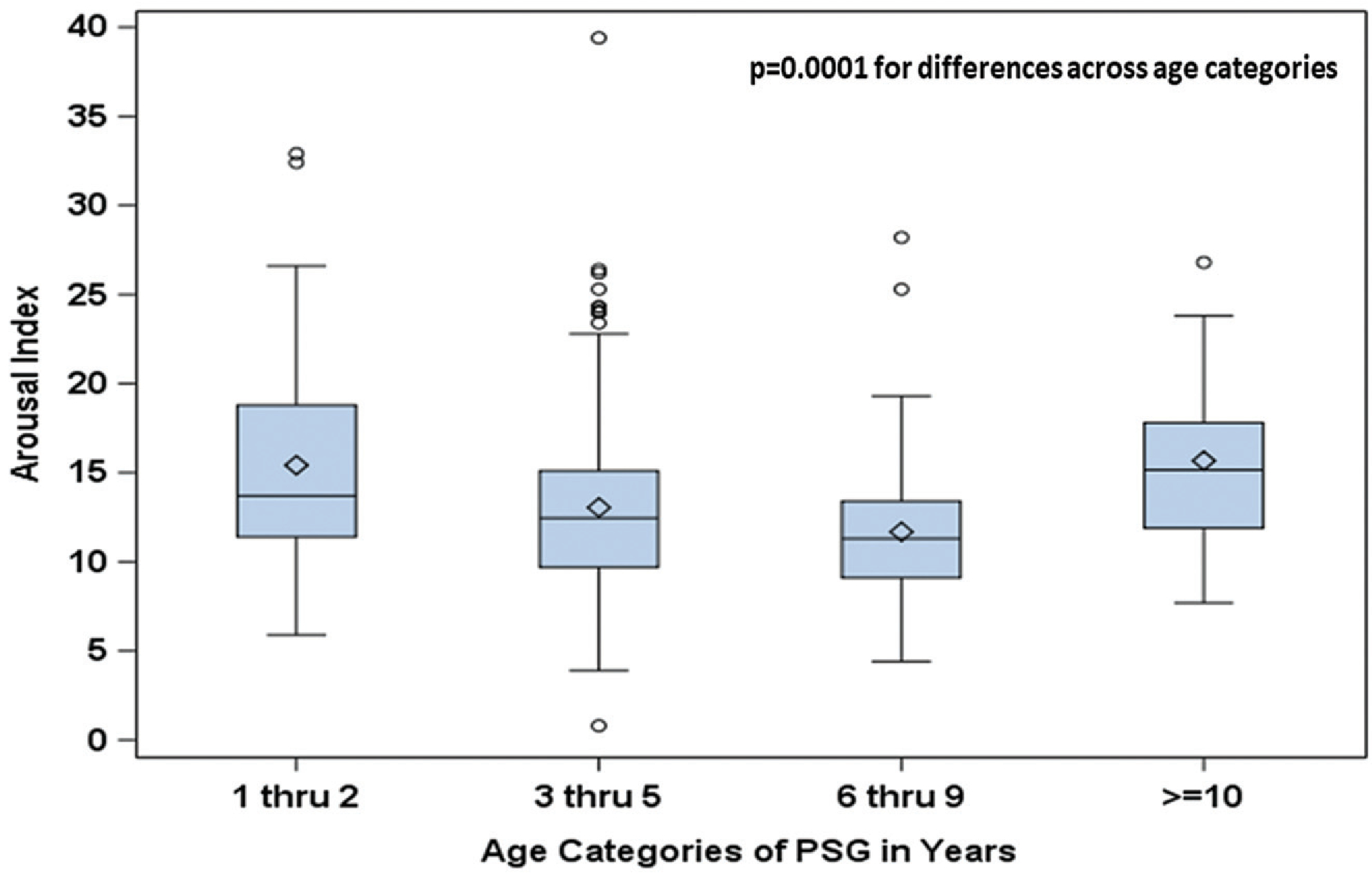
Box plots of arousal index for children with Down syndrome by age category. Diamond marker, mean; line inside box, median (50th percentile); top/bottom of box, 25th/75th percentiles, respectively; whiskers (error bars) are 1.5 × interquartile range above/below the 75th/25th percentile.
Poor Sleep Architecture
Table 4 illustrates the proportion of children with poor sleep architecture metrics, as defined in the Materials and Methods section. Additionally, the percentage of patients with poor sleep architecture is depicted by age category. When these metrics were evaluated as a function of age, the percentage of patients with REM latency <90 minutes decreased with increasing age (P < .0001). In addition, the percentage of patients with REM sleep time <20% increased with age, occurring in 11.1% for those 1 to 2 years old and in 62.5% for children with DS who were ≥10 years of age (P = .001). Similarly, the percentage of patients with <3 REM cycles increased with age (P < .0001). The arousal index decreased from 1 to ≤10 years of age, at which point an increase was seen (P = .009). Figure 3 shows the percentage of patients with ≥1 metric of poor sleep architecture for the entire population and by age categories. For the total population, 69.2% had ≥2 metrics of poor sleep architecture, and 39% had ≥3. Overall, 5% of children had none.
Table 4.
Children With Down Syndrome With Poor Sleep Architecture Metrics.a
| Metric | Total (N = 397) | 1–2 y (n = 47) | 3–5 y (n = 274) | 6–9 y (n = 52) | ≥10 y (n = 24) | P valueb |
|---|---|---|---|---|---|---|
| Sleep | ||||||
| Efficiency, <80% | 128 (32.5) | 19 (40.4) | 81 (29.8) | 16 (30.8) | 12 (52.2) | .09 |
| Efficiency, <90% | 297 (75.4) | 40 (85.1) | 197 (72.4) | 41 (78.9) | 19 (82.6) | .20 |
| Latency, ≥30 min | 134 (34.3) | 15 (31.9) | 92 (34.3) | 18 (34.6) | 9 (37.5) | .97 |
| Total sleep time, <360 min | 63 (15.9) | 6 (12.8) | 39 (14.2) | 8 (15.7) | 10 (41.7) | .005 |
| REM latency, <90 min | 135 (34.5) | 37 (78.7) | 82 (30.5) | 13 (25) | 3 (13) | <.0001 |
| NREM stage | ||||||
| 1 | 31 (7.9) | 5 (10.9) | 15 (5.5) | 6 (11.5) | 5 (20.8) | .02c |
| 2 | 102 (26) | 16 (34.8) | 69 (25.4) | 12 (23.5) | 5 (20.8) | .49 |
| 3 | 6 (1.5) | 2 (4.4) | 3 (1.1) | 1 (1.9) | 0 | .21c |
| REM | ||||||
| Sleep, <20% | 136 (35.3) | 5 (11.1) | 94 (35.5) | 22 (43.1) | 15 (62.5) | .001 |
| Sleep, <10% | 11 (2.9) | 0 | 7 (2.6) | 2 (3.9) | 2 (8.3) | .18c |
| Cycles, <3 | 19 (5.2) | 0 | 9 (3.6) | 2 (3.9) | 8 (34.8) | <.0001c |
| Arousal index, >10/h | 300 (75.6) | 43 (89.4) | 202 (73.7) | 34 (65.4) | 22 (91.7) | .009 |
Abbreviations: NREM, non–rapid eye movement; REM, rapid eye movement.
Values are presented as No. (%).
P value comparing all 4 age groups.
Fisher’s exact test.
Figure 3.
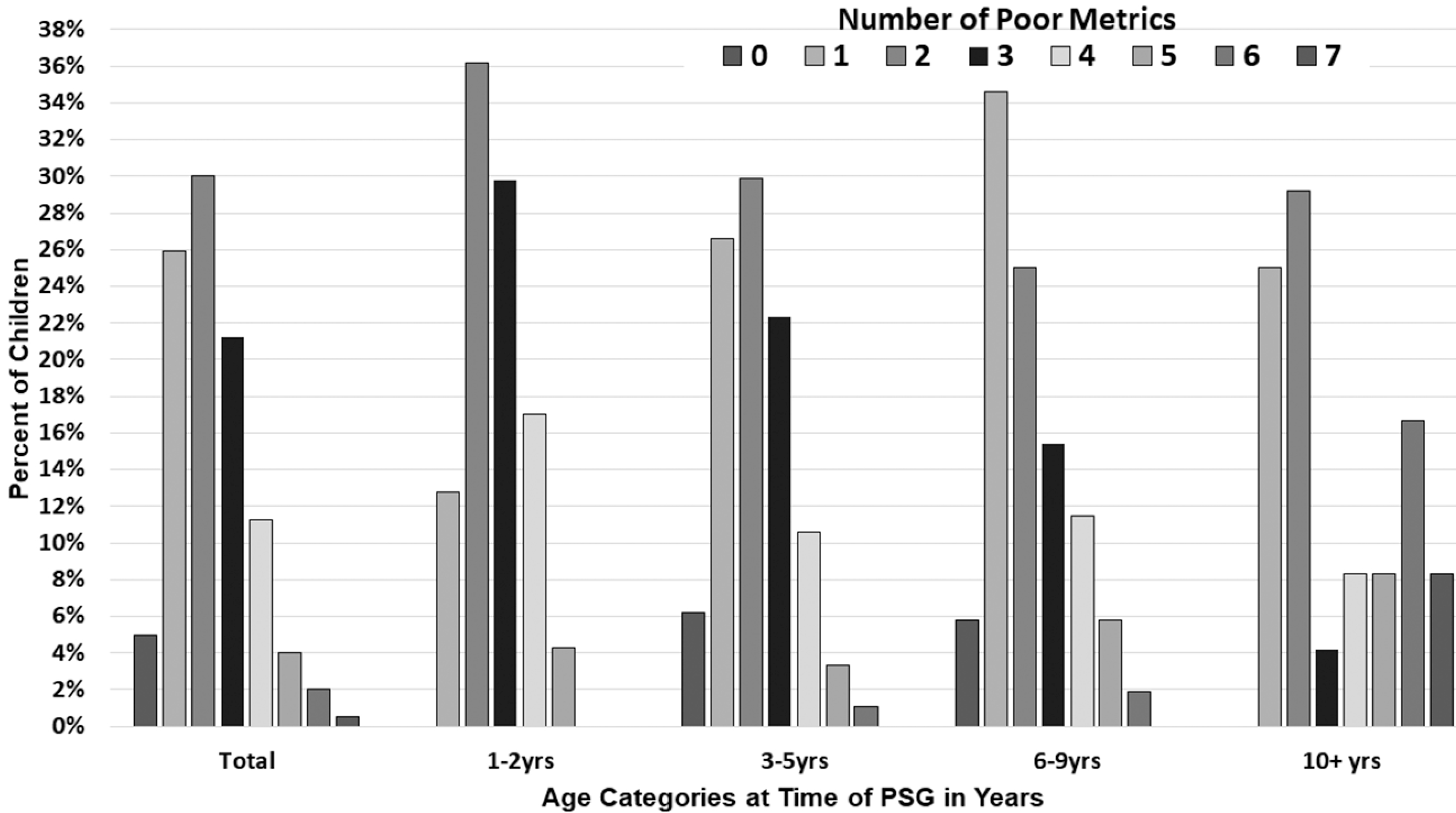
Percentage of children with Down syndrome with poor sleep architecture metrics, for total population and by age category. Poor sleep architecture metrics as defined in the Materials and Methods section. PS, polysomnography.
OSA Comparison
Sleep architecture variables were then compared between those with and without OSA. As shown in Table 5, there were no significant differences between the OSA and non-OSA groups except for the arousal index. The mean arousal index was 13.9 (5) in those with OSA and 11.3 (4.2; P < .001) in without OSA. This trend continued with increasing OSA severity (Supplementary Table S1, available online). The percentage of patients with each metric of poor sleep architecture was also similar between the groups (Table 6), again without change with increasing severity of OSA (Supplemental Table S2). Additionally, the number of patients with ≥1 metrics of poor sleep was similar between the groups (Figure 4).
Table 5.
Sleep Architecture Variables for Children With Down Syndrome for Those With and Without a Diagnosis of OSA.a
| Variable | OSA (n = 315) | No OSA (n = 82) | P value |
|---|---|---|---|
| Sleep | |||
| Efficiency, % | 82.3 (11) | 80.6 (12) | .21 |
| Latency, min | 29.5 (36) | 31 (34.4) | .60b |
| Median [IQR] | 17.5 [0–202] | 20.6 [0–150.8] | |
| Total time, min | |||
| Sleep | 430.4 (71.3) | 409.1 (84.7) | .03 |
| In bed | 524.6 (54.3) | 510.8 (77.5) | .13 |
| REM latency, min | 124.1 (64.9) | 137.2 (71.1) | .12 |
| NREM stage, % | |||
| 1 | 3 (1) | 3 (2) | .90 |
| 2 | 47 (7) | 47 (7) | .59 |
| 3 | 28 (7) | 29 (8) | .30 |
| REM | |||
| Sleep, % | 22.8 (6) | 21 (7) | .03 |
| Cycles | 5.1 (1.8) | 4.6 (1.4) | .01 |
| Arousal index per hour | 13.9 (5) | 11.2 (4.1) | <.0001 |
Abbreviations: IQR, interquartile range; NREM, non–rapid eye movement; OSA, obstructive sleep apnea; REM, rapid eye movement.
Values are presented as mean (SD).
Wilcoxon rank sum test.
Table 6.
Children With Down Syndrome and Poor Sleep Architecture Metrics: Those With and Without a Diagnosis of OSA.a
| Metric | OSA (n = 315) | No OSA (n = 82) | P value |
|---|---|---|---|
| Sleep | |||
| Efficiency, <80% | 101 (32.4) | 27 (32.9) | .92 |
| Efficiency, <90% | 228 (73.1) | 69 (84.2) | .04 |
| Latency, ≥30 min | 104 (33.7) | 30 (35.6) | .62 |
| Total sleep time, <360 min | 46 (14.6) | 17 (21) | .16 |
| REM latency, <90 min | 112 (36) | 23 (28.8) | .22 |
| NREM | |||
| Stage 1 | 20 (6.4) | 11 (13.8) | .03 |
| Stage 2 | 81 (25.9) | 21 (26.3) | .95 |
| Stage 3 | 6 (1.9) | 0 | .61b |
| REM | |||
| Sleep, <20% | 103 (33.9) | 33 (40.7) | .25 |
| Sleep, <10% | 6 (2) | 5 (6.2) | .06b |
| Cycles, <3 | 15 (5.1) | 4 (5.4) | >.99b |
| Arousal index, >10 | 249 (79.1) | 51 (62.2) | .002 |
Abbreviations: NREM, non–rapid eye movement; OSA, obstructive sleep apnea; REM, rapid eye movement.
Values are presented as No. (%).
Fisher’s exact test.
Figure 4.
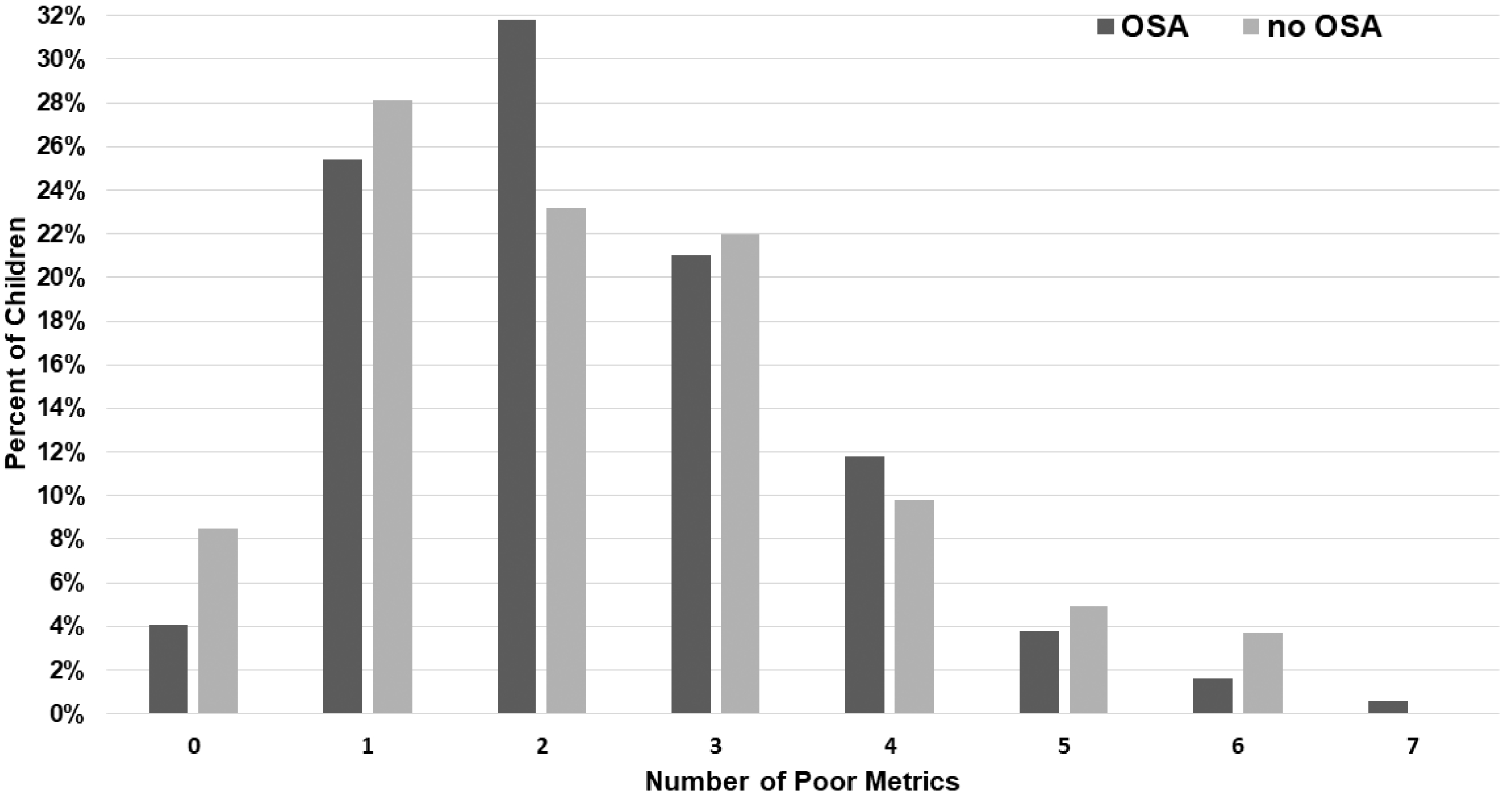
Percentage of poor sleep architecture metrics for children with Down syndrome, with and without obstructive sleep apnea (OSA).
Discussion
In this study, we evaluated polysomnographic variables in 397 children with DS, with and without OSA. Overall, the PSG parameters of mean sleep efficiency, TST, time spent in REM sleep, and arousal index were all reassuring in regard to feasibility and tolerability of overnight PSG in children with DS. However, many children exhibited poor sleep architecture. Using metrics of poor sleep architecture based on clinical experience and normative data in typically developing children,9–12 we found that one-third of children with DS exhibited reduced sleep efficiency (<80%), three-quarters had an elevated arousal index (>10/h), one-third demonstrated prolonged sleep latency (>30 minutes), and about 16% had reduced TST (<360 minutes). Additionally, more than one-third of children with DS had reduced REM sleep time (<20%), and time spent in REM sleep decreased with increasing age. There was no difference in children with or without OSA. It should be noted that the rate of OSA in this group was 79.3%; 82 children did not have OSA. This is one of the largest samples reported on children with DS without OSA. More research is needed in this area; however, our data indicate that the 2 groups are similar across the majority of sleep architecture parameters.
Sleep disruption in children with DS has largely been attributed to OSA due to the high rate seen in this population. Very few studies have examined sleep architecture in children with DS. Levanon et al reported on 23 children with DS and a control group of 13 children with primary snoring. They found that sleep fragmentation, specifically frequent arousals and awakenings, was not solely attributed to OSA.7 In their series, 11 children with DS underwent PSG; 12 had a partial sleep evaluation. Upon comparison with the control group, there was no significant difference in time spent in each sleep stage, but there were significantly more arousals and awakenings in the group with DS, attributable to limb jerks >50% of the time. Additionally, the mean number of limb movements in the group of children with DS was 8.3/h (6.5), which was a markedly higher rate than in their children without DS. Our data of periodic limb movement of sleep in 2% of children with DS do not support this abnormality, and it certainly warrants further study.
In a study of 130 children with DS aged 0 to 17.8 years, Nisbet et al hypothesized a sleep phenotype in children with DS, using matched controls grouped by age.8 All but 6 children with DS had OSA, which was significantly different from children without DS and slightly higher than that reported in the literature. When compared with controls, children with DS demonstrated lower sleep efficiency at ages >2 years, decreased TST in ages 12 to 17.9 years, but no difference in sleep latency, REM latency, or sleep fragmentation index, defined by the authors as sleep-stage transitions or awakenings per hour. In regard to sleep stages, there was decreased NREM stage 2 sleep in all ages, higher NREM stage 3 sleep, and decreased REM sleep in the older age groups. Although the number of children without OSA was small, there was no difference in sleep architecture findings between those with OSA and those without. Nisbet et al proposed a sleep phenotype unique to DS of increased slow-wave sleep and decreased REM sleep.
Sleep disruption in children with DS is likely multifactorial. In an effort to better understand this, we aimed to define metrics of poor sleep. We used relatively well-established normative data of PSG variables in typically developing children to establish metrics of poor sleep, which is a novel paradigm. Traeger et al published a review of the literature of normative data on sleep architecture, as well as their results in 66 children aged 2 to 9 years, which they thought to be in line with previous publications.9 The majority of studies noted by Traeger et al were of smaller numbers, and many included home sleep studies. In terms of their patients’ sleep architecture, mean sleep efficiency was 89%; TST, 461 minutes; percentage REM sleep time, 21%; and arousal index, 8.8/h (sleep latency was not included in their analysis). Eight percent of patients had a periodic limb movement index of >5/h. These data are in line with those of Beck and Marcus on sleep architecture values for typically developing children aged 1 to 18 years, who reported the usual value for the following variables: sleep efficiency, 89%; sleep latency, 23 minutes; arousal index, 9 to 16/h; NREM stage 1, 4% to 5%; NREM stage 2, 44% to 56%; NREM stage 3, 29% to 32% (<10 years) and 20% (>10 years); REM, 17% to 21%; and periodic limb movement index, ≤4.3/h.10 Based on the aforementioned values, our data indicated that overall sleep efficiency is slightly lower than normative data, as one-third of our population have sleep efficiency <80%. Similarly, in the children with DS included in our study, the sleep latency is shorter; the arousal index is higher; and time spent in REM sleep is slightly higher. In regard to sleep stages, overall the medians are consistent with normative data; however, the percentage of time in REM sleep decreased by age in our group, which is consistent with normal aging but likely represents an abnormality in DS, as also seen in the work by Nisbet et al, who proposed a sleep phenotype in DS.8 Though, we did not see an increase in slow-wave sleep, also called NREM stage 3, which they described.
Although a single abnormal polysomnographic variable such as low sleep efficiency may be indicative of sleep disruption, based on clinical practice, it is more likely that the combination of one or more poor metric will cause significant disruption. In our population, more than half had ≥2 metrics of poor sleep architecture; 39% had ≥3; and 7% had ≥5. Five percent of patients had a sleep study in line with normative data, without any abnormalities in the 7 defined metrics. Furthermore, these findings occurred in children with and without OSA. In other words, if you remove OSA, the findings appear to be the same. Only the arousal index differed between the groups, which may represent respiratory events scored with an arousal in the children with OSA. When compared with normative data from children without DS, these findings suggest 2 possibilities: sleep architecture in children with DS is different, or children with DS demonstrate poor sleep architecture. Furthermore, our results suggest that OSA is not the only driver of poor sleep architecture in these children. As it is difficult to determine what the impact of poor sleep architecture is on behavioral issues and/or daytime sleepiness, particularly in children with DS, further studies are needed.
The biggest strength of our study is the large number of patients included (N = 397). Additionally, as this was a population identified for PSG from a multidisciplinary clinic that cares for children with DS within a division of developmental and behavioral pediatrics, we think that there was less bias than in a population referred for sleep-disordered breathing. A high percentage of OSA was seen (78.9%), which is in keeping with what we see at our institution, where we have a high percentage of screening PSGs based on the American Academy of Pediatrics’ guidelines for health maintenance in children with DS.15 Additionally, our large number of PSGs—with a relatively high median TST, in line with normative data for typically developing children—reflects the strength of our pediatric sleep laboratory, where we often have 2 sleep respiratory therapists available for hookup, with child life on hand as needed. Of our study group, 15.9% had a TST <360 minutes, which is noteworthy in children with developmental delay. A limitation in the study is the retrospective nature, as well as the lack of additional clinical data that may affect sleep architecture, namely body mass index. Additionally, we did not exclude patients who had undergone surgical intervention for OSA, as we wanted a large mixed cohort. Future studies will include these variables and interventions to improve sleep architecture and will investigate the association between behavioral issues and poor sleep architecture.
Conclusion
In children with DS, 32.5% had a sleep efficiency <80%; 75.6% had an elevated arousal index >10/h; and 15.9% had a TST <360 minutes. In terms of sleep stage, there was decreased time spent in REM sleep with increasing age. More than a third of the patients had ≥3 metrics of poor sleep architecture, with 69.2% having ≥2. There was no difference in PSG parameters among children with or without OSA. Our findings indicate that children with DS have poor sleep architecture that is independent of OSA; the majority of the population had ≥1 metrics of poor sleep architecture. Our data support the notion of a sleep phenotype in children with DS that merits further study.
Supplementary Material
Footnotes
Disclosures
Competing interests: Stacey L. Ishman has done consulting for Inspire Medical.
Sponsorships: None.
Funding source: None.
Supplemental Material
Additional supporting information is available in the online version of the article.
References
- 1.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:576–84. [DOI] [PubMed] [Google Scholar]
- 2.Marcus CL, Keens TG, Bautista DB, von Pechmann WS, Ward SL. Obstructive sleep apnea in children with Down syndrome. Pediatrics. 1991;88:132–139. [PubMed] [Google Scholar]
- 3.de Miguel-Diez J, Villa-Asensi JR, Alvarez-Sala J. Prevalence of sleep-disordered breathing in children with Down syndrome: polygraphic findings in 108 children. Sleep. 2003;26(8):1006–1009. [DOI] [PubMed] [Google Scholar]
- 4.Shott SR, Amin R, Chini B, Heubi C, Hotze S, Akers R. Obstructive sleep apnea: should all children with Down syndrome be tested? Arch Otolaryngol Head Neck Surg. 2006;132: 432–436. [DOI] [PubMed] [Google Scholar]
- 5.Maris M, Verhulst S, Wojciechowski M, Van de Heyning P, Boudewyns A. Prevalence of obstructive sleep apnea in children with Down syndrome. Pediatrics. 2016;39(3):699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzgerald DA, Paul A, Richmond C. Severity of obstrucive sleep apnoea in children with Down syndrome who snore. Arch Dis Child. 2007;92:423–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levanon A, Tarasiuk A, Tal A. Sleep characteristics in children with Down syndrome. J Pediatr. 1999;134;755–760. [DOI] [PubMed] [Google Scholar]
- 8.Nisbet LC, Phillips NN, Hoban TF, O’Brien LM. Characterization of a sleep architectural phenotype in children with Down syndrome. Sleep Breath 2015;16:1065–1071. [DOI] [PubMed] [Google Scholar]
- 9.Traeger N, Schultz B, Pollock AN, Mason T, Marcus CL, Arens R. Polysomnographic values in children 2–9 years old: additional data and review of literature. Pediatric Pulmonol. 2005;40(1):22–30. [DOI] [PubMed] [Google Scholar]
- 10.Beck SE, Marcus CL. Pediatric polysomnography. Sleep Med Clin. 2009;4(3):393–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mason TBA, Teoh L, Calabro K, et al. Rapid eye movement latency in children and adolescents. Pediatr Neurol. 2008; 39(3):162–169. [DOI] [PubMed] [Google Scholar]
- 12.Domany KA, Nahman-Averbuch H, King CD, et al. Clinical presentation, diagnosis and polysomnographic findings in children with migraine referred to sleeps clinic. Sleep Med. 2019; 63:57–63. [DOI] [PubMed] [Google Scholar]
- 13.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events. American Academy of Sleep Medicine; 2007. [Google Scholar]
- 14.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knollman PD, Heubi CH, Meinzen-Derr J, et al. Adherence to guidelines for screening polysomnogaphy in children with Down syndrome. Otolaryngol Head Neck Surg. 2019;16(1): 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


