Abstract
As clinical guidelines for cancer prevention refer individuals to primary care physicians (PCPs) for risk assessment and clinical management, PCPs may be expected to play an increasing role in cancer prevention. It is crucial that PCPs are adequately supported to assess an individual’s cancer risk and make appropriate recommendations. The objective of this study is to assess use, familiarity, attitude and behaviors of PCPs regarding breast and ovarian cancer risk and prevention, to better understand the factors that influence their prescribing behaviors. We conducted a cross-sectional, web-based survey of PCPs in the United States, recruited from an opt-in healthcare provider panel. Invitations were sent in batches until the target sample size of 750 respondents (250 each for OB/GYN, internal medicine and family medicine) was met. Self-reported use of breast/ovarian cancer risk assessments was low (34.7%-59.2%) compared with discussion of cancer family history (96.9%), breast exams (87.1%) and mammograms (92.8%). While most respondents (48.0-66.8%) were familiar with cancer prevention interventions, respondents who reported to be less familiar were more likely to report cautious attitudes. When presented with hypothetical cases depicting patients at different breast/ovarian cancer risks, up to 34.0% of respondents did not select any of the clinically recommended course(s) of action. This survey suggests that PCP use of breast/ovarian cancer risk assessment tools and ability to translate the perceived risks to clinical actions is variable. Improving implementation of cancer risk assessment and clinical management guidelines within primary care may be necessary to improve the appropriate prescribing of cancer prevention interventions.
Keywords: cancer prevention, risk assessment, primary care physicians
Introduction
Risk assessment for breast and ovarian cancer include assessment of hereditary and demographic factors including age, family history, genetic testing results (self/relatives) and ancestry (1, 2) (National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®), https://www.nccn.org/professionals/physician_gls/default.aspx). Breast cancer screening recommendations for women at average risk include mammography, although guidelines are inconsistent in terms of starting age and frequency (3-5) (Susan G. Komen Breast Cancer Screening for Women at Average Risk, https://ww5.komen.org/BreastCancer/BreastCancerScreeningforWomenatAverageRisk.html). For women deemed at high risk for breast cancer (e.g. 5-year risk ≥1.67% or lifetime risk of ≥20% (6)), screening recommendations begin at a younger age (Susan G. Komen Breast Cancer Screening for Women at Higher Risk, https://ww5.komen.org/BreastCancer/BreastCancerScreeningForWomenAtHigherRisk.html), and suggested risk reduction interventions include lifestyle behaviors, increased mammography screening, chemopreventive agents and/or mastectomy (1, 7, 8). Selective estrogen receptor modulators (SERMs) and aromatase inhibitors (AIs) have shown to provide a 50-65% relative risk reduction in women at high risk of breast cancer (6, 9-16).
For ovarian cancer, screening is not recommended for women who are not known to have a high-risk hereditary cancer syndrome (17). The strongest ovarian cancer risk factor is family history and/or the presence of a BRCA1 or BRCA2 germline mutation, with a lifetime risk of 39% (95% CI 18-54%) and 11% (95% CI 2.4-19%) risk, respectively (18). Risk reduction interventions for ovarian cancer are mainly surgical (i.e. salpingo-oophorectomy) but may also include use of oral contraceptives (19, 20). Large epidemiologic studies have demonstrated that women who have reported use of oral contraceptives have a reduced lifetime ovarian cancer risk ranging from 35-80%, with longer use being associated with increased risk reduction (21, 22). The American College of Obstetricians and Gynecologists (ACOG) and the Society of Gynecologic Oncology (SGO) have published recommendations that women with increased risk for ovarian cancer consider use of oral contraceptives (19, 23). The most effective intervention for ovarian cancer is risk-reducing bilateral salpingo-oophorectomy, which reduces the risk of ovarian and associated cancers by 80% in women with BRCA1/2 mutations (24).
Primary Care Physicians (PCPs) play an increasing role in cancer risk assessment and prescribing of preventive interventions (25). Patients rely on their PCPs to identify their cancer risk and provide recommendations for risk reduction (26-28). The willingness of a PCP to recommend a cancer preventive intervention is associated with their familiarity with the intervention (29, 30). However, PCPs face barriers of evolving guidelines and lack of recognition of evolving risk factors (31), while high-risk women are reluctant or skeptical of cancer risk reduction, all of which contribute to limited uptake of most cancer preventive interventions (32-35).
As PCPs are the first referral for individuals concerned with cancer risk and prevention, it is imperative that they receive the necessary support to adequately assess an individual’s cancer risk and make appropriate recommendations. We undertook a large survey-based study to explore the knowledge, use, familiarity and behaviors of PCPs with respect to breast/ovarian cancer risk and prevention for high-risk women (31). We previously reported a set of results from this survey, which evaluated recognition of risk factors, recommendation and prescribing behaviors. These results demonstrated that perception of importance and recommendations of cancer screening and prevention significantly differed by provider type, reflecting the demographics of the populations seen by the respective PCPs. In addition, survey responses showed that PCPs were less likely to recognize clinical or epidemiologic cancer risk factors, and prescribing behaviors of PCPs were related to familiarity, with physicians more likely to reinforce a specialist’s recommendation for breast/ovarian cancer prevention rather than prescribe an intervention (31).
This current study expands on our previous results, evaluating use of risk assessment tools, familiarity and attitudes towards cancer prevention interventions and behaviors of PCPs in response to patient scenarios, to better understand the factors that influence their prescribing behaviors.
Methods
Study Design
Survey design was previously published (31). We recruited a cross-sectional sample of PCPs from an online healthcare provider panel hosted by a healthcare market research firm, M3 Global Research (https://www.m3global.com/). M3 validates their panel members’ registration information against the American Medical Association’s (AMA) database. M3 Global Research follows the rules, regulations and standards of Market Research industry and is ISO certified. The survey was reviewed and approved by the ICF Institutional Review Board (IRB).
Survey invitations were emailed to random samples of panel members between June 8-14, 2018. To be eligible, respondents were required to: (1) be a physician specializing in family medicine, internal medicine, or obstetrics/gynecology (OB/GYN); (2) interact with patients on a weekly or daily basis; (3) reside and practice medicine in the United States; and (4) be able to read and understand English to provide informed consent and complete the survey. Participants meeting all inclusion criteria reviewed and electronically provided written (digital) informed consent before completing the online survey. Multiple survey completions were avoided by use of a unique URL for each survey. Invitations were sent daily in batches until the target sample size of 750 respondents [250 for each physician type—family medicine, internist and obstetrics/gynecology (OB/GYN)] was met.
A total of 6,148 panel members were invited, of whom 953 responded, and 750 completed the survey within one week. Respondents who completed the survey were provided with a link to a National Cancer Institute (NCI) webpage with information and resources about breast and gynecologic cancer prevention, and received remuneration for their participation.
Survey Instrument
The survey was developed to evaluate self-reported cancer prevention perceptions, cancer risk assessment and prescribing behaviors of PCPs. We collected physician demographics, use of cancer risk assessment tools/resources, attitude towards cancer prevention interventions, and familiarity with breast and ovarian cancer prevention interventions. The survey required a response to all items. The entire survey, respondent demographics and preliminary analyses were previously published (31).
The survey included four hypothetical clinical case vignettes describing female patients presenting with varying demographic, family history and clinical characteristics associated with breast and/or ovarian cancer risk. Using a set of preselected responses, the survey asked PCPs to select recommended course(s) of action for each hypothetical patient.
Statistical Analysis
All analyses were conducted using SPSS version 22 (SPSS Inc., Chicago, IL). Descriptive statistics [frequency counts and percentages, means with standard deviations (SDs)] were calculated for all variables. Chi-squared tests were used for comparisons between all the physician types and, for those comparisons that were found to differ, t-tests were performed to compare frequencies of responses between the different physician type pair combinations.
Logistic regression models assessed the associations between physician demographics and familiarity and attitude toward cancer prevention interventions. Independent variables were selected according to their a priori importance and from bivariate analyses. The potential independent variables were first examined for multicollinearity. To summarize the respondents’ familiarity and attitude with a single variable, we developed post hoc scales for each of the options. The scales had good internal consistency (Cronbach α 0.92 for familiarity and Cronbach α 0.80 for attitude). The variables were then categorized by visual binning of equal percentiles on scanned cases (less and more familiar or less and more cautious attitude, for those attitudes that leaned towards risk-averse).
Because of the structure of some of the questions, some responses were categorized. To explore differences between physician specialties, we categorized physician use of breast and/or ovarian cancer risk assessment and screening tools into “used” (including “often” and “occasionally”), “not used” (including “rarely” and “never”) and “not familiar with this assessment”; dichotomized self-reported ratings of familiarity with cancer preventive interventions into “familiar” (including “extremely familiar and “moderately familiar”) and “not as familiar” (including “somewhat familiar”, “slightly familiar” and “not at all familiar”). To explore physician attitudes regarding cancer prevention interventions, we categorized agreement levels into “agree” (including “strongly agree” and “somewhat agree”) and “disagree” (including “strongly disagree” and “somewhat disagree”) as well as “no opinion” and “don’t know/unsure”. A p-value<0.05 was considered to be statistically significant.
Results
Use of Breast and Ovarian Cancer Risk Assessment Tools and Resources
Our previous findings showed that PCPs recognized genetic factors but were less likely to recognize epidemiologic risk factors (31). Respondents were asked about their use of various breast and ovarian cancer assessments in asymptomatic patients to determine cancer risk (Table 1). Most respondents reported discussing cancer family history (96.9%), performing breast exams (87.1%) and ordering mammograms (92.8%) to assess breast/ovarian cancer risk, with no significant difference between provider types. Other risk assessments were less likely to be utilized, such as breast cancer risk assessment tools (e.g., Gail Model, BOADICEA, Claus, BRCAPRO, Cuzick-Tyrer) (50.9%), genetic testing for BRCA1 or BRCA2 (59.2%) and multi-gene testing (34.7%). Self-reported use of these risk assessments significantly differed by provider type: OB/GYN physicians, compared to both family medicine physicians and internists, were significantly more likely to report using breast cancer risk assessment tools (p-value<0.001), genetic testing for BRCA1 or BRCA2 (p-value<0.001), and multi-gene testing (p-value<0.001). For example, 80.8% of OB/GYN physicians but only 45.6% and 51.2% of family medicine physicians and internists, respectively, reported using BRCA1/2 genetic testing. No significant differences were found between family medicine physicians and internists for utilization of any of the risk assessment tools.
Table 1.
Physician use of breast and/or ovarian cancer risk assessment and screening tools.
| Family Medicine n (%) |
Internist n (%) |
OB/GYN n (%) |
Total n (%) |
|
|---|---|---|---|---|
| Discussion of Cancer Family History | ||||
| Used | 246 (98.4%) | 235 (94.0%) | 246 (98.4%) | 727 (96.9%) |
| Not used | 4 (1.6%) | 13 (5.2%) | 3 (1.2%) | 20 (2.7%) |
| Not familiar with this assessment | 0 (0%) | 2 (0.8%) | 1 (0.4%) | 3 (0.4%) |
| Breast Exam | ||||
| Used | 212 (84.8%) | 200 (80.0%) | 241 (96.4%) | 653 (87.1%) |
| Not used | 38 (15.2%) | 48 (19.2%) | 7 (2.8%) | 93 (12.4%) |
| Not familiar with this assessment | 0 (0%) | 2 (0.8%) | 2 (0.8%) | 4 (0.5%) |
| Mammogram | ||||
| Used | 248 (99.2%) | 240 (96.0%) | 238 (95.2%) | 696 (92.8%) |
| Not used | 2 (0.8%) | 9 (3.6%) | 7 (2.8%) | 18 (2.4%) |
| Not familiar with this assessment | 0 (0%) | 1 (0.4%) | 1 (0.4%) | 2 (0.3%) |
| Breast Cancer Risk Assessment Tool (e.g., Gail Model, BOADICEA, Claus, BRCAPRO, Cuzick-Tyrer)* | ||||
| Used | 110 (44.0%) | 104 (41.7%) | 168 (67.2%) | 382 (50.9%) |
| Not used | 88 (35.2%) | 97 (38.8%) | 59 (23.6%) | 244 (32.5%) |
| Not familiar with this assessment | 52 (20.8%) | 49 (19.6%) | 23 (9.2%) | 124 (16.5%) |
| Genetic Testing for BRCA1 or BRCA2* | ||||
| Used | 114 (45.6%) | 128 (51.2%) | 202 (80.8%) | 444 (59.2%) |
| Not used | 133 (53.2%) | 119 (47.6%) | 48 (19.2%) | 300 (40.0%) |
| Not familiar with this assessment | 3 (1.2%) | 3 (1.2%) | 0 (0%) | 6 (0.8%) |
| Multi-Gene Testing* | ||||
| Used | 62 (24.8%) | 68 (27.2%) | 130 (52.0%) | 260 (34.7%) |
| Not used | 146 (58.4%) | 153 (61.2%) | 101 (40.4%) | 400 (53.3%) |
| Not familiar with this assessment | 42 (16.8%) | 29 (11.6%) | 19 (7.6%) | 90 (12.0%) |
Indicates significant difference between provider types (p≤ 0.05)
Familiarity with Cancer Prevention Interventions
Because our and other studies have demonstrated that familiarity is associated with prescribing behaviors, respondents were asked about their familiarity with breast and ovarian cancer preventive intervention options (Table 2). Overall, most respondents were familiar with cancer prevention intervention options (51.5-66.8%), with less familiarity with aromatase inhibitors (48.0%). Familiarity significantly differed by provider type. OB/GYN physicians, compared to family medicine physicians, were significantly more likely to report being familiar with tamoxifen (p-value<0.001), raloxifene (p-value<0.001), aromatase inhibitors (p-value<0.001), prophylactic mastectomy (p-value=0.001), oophorectomy (p-value<0.001), and risk-reducing salpingo-oophorectomy(p-value<0.001). OB/GYN physicians, compared to internists, were significantly more likely to report being familiar with tamoxifen (p-value<0.05;), oophorectomy (p-value<0.001), and risk-reducing salpingo-oophorectomy (p-value<0.001). For example, 93.6% of OB/GYN physicians but only 42.0% and 47.6% of family medicine physicians and internists, respectively, reported being familiar with risk-reducing salpingo-oophorectomy. Internists compared to family medicine physicians, were significantly more likely to report being familiar with tamoxifen (p-value<0.05), raloxifene (p-value<0.05), and aromatase inhibitors (p-value<0.001). For example, 57.6% of OB/GYN physicians and 51.2% of internists but only 35.2% of family medicine physicians reported being familiar with aromatase inhibitors.
Table 2.
Physician familiarity with breast and/or ovarian cancer preventive intervention options.
| Family Medicine n (%) |
Internist n (%) |
OB/GYN n (%) |
Total n (%) |
|
|---|---|---|---|---|
| Tamoxifen* | ||||
| Familiar | 130 (52.0%) | 153 (61.2%) | 182 (72.8%) | 465 (62.0%) |
| Not as familiar | 120 (48.0%) | 97 (38.8%) | 68 (27.2%) | 285 (38.0%) |
| Raloxifene* | ||||
| Familiar | 103 (41.2%) | 133 (53.2%) | 150 (60.0%) | 386 (51.5%) |
| Not as familiar | 147 (58.8%) | 117 (46.8%) | 100 (40.0%) | 364 (48.5%) |
| Aromatase inhibitors* | ||||
| Familiar | 88 (35.2%) | 128 (51.2%) | 144 (57.6%) | 360 (48.0%) |
| Not as familiar | 162 (64.8%) | 122 (48.8%) | 106 (42.4%) | 390 (52.0%) |
| Prophylactic mastectomy* | ||||
| Familiar | 146 (58.4%) | 161 (64.4%) | 180 (72.0%) | 487 (64.9%) |
| Not as familiar | 104 (41.6%) | 89 (35.6%) | 70 (28.0%) | 263 (35.1%) |
| Oophorectomy* | ||||
| Familiar | 128 (51.2%) | 143 (57.2%) | 230 (92.0%) | 501 (66.8%) |
| Not as familiar | 122 (48.8%) | 107 (42.8%) | 20 (8.0%) | 249 (33.2%) |
| Risk-reducing salpingo-oophorectomy* | ||||
| Familiar | 105 (42.0%) | 119 (47.6%) | 234 (93.6%) | 458 (61.1%) |
| Not as familiar | 145 (58.0%) | 131 (52.4%) | 16 (6.4%) | 292 (38.9%) |
Indicates significant difference between provider types (p≤ 0.05)
Attitudes Regarding Cancer Prevention Interventions
Respondents were asked to respond with their level of agreement regarding various statements related to breast or ovarian cancer prevention (Table 3). Most respondents agreed that benefits of cancer prevention outweigh the risks; however, attitudes significantly differed by provider type. OB/GYN physicians, compared to family medicine physicians and internists, were significantly more likely to agree that the benefits of preventive agents in breast cancer outweigh the risks (p-value<0.05 and p-value<0.001, respectively), and were less likely to agree that the evidence that they significantly reduce breast cancer risk is controversial (p-value<0.001 and p-value<0.05, respectively), the risk of endometrial cancer is too great to prescribe tamoxifen for breast cancer reduction (both p-value<0.05), risk of thromboembolic disease is too great to prescribe preventive agents for breast cancer reduction (both p-value<0.001), and evidence that preventive surgery significantly reduces breast cancer risk is controversial (both p-value<0.05). For example, 74.4% of OB/GYN physicians but only 40.0% and 38.8% of family medicine physicians and internists, respectively, reported disagreeing that the risk of endometrial cancer is too great to prescribe tamoxifen for breast cancer reduction. OB/GYN physicians, compared to family medicine physicians and internists, were also more likely to agree that it is easy for them to determine who is eligible to take preventive agents for breast cancer reduction (p-value<0.001 and p-value<0.05, respectively).
Table 3.
Physician attitudes regarding breast or ovarian cancer prevention interventions.
| Family Medicine n (%) |
Internist n (%) |
OB/GYN n (%) |
Total n (%) |
|
|---|---|---|---|---|
| The benefits of preventive agents in breast cancer outweigh the risks.* | ||||
| Agree | 148 (59.2%) | 143 (57.2%) | 180 (72.0%) | 471 (62.8%) |
| Disagree | 24 (9.6%) | 25 (10.0%) | 19 (7.6%) | 68 (9.1%) |
| No Opinion | 60 (24.0%) | 57 (22.8%) | 45 (18.0%) | 162 (21.6%) |
| Don’t know/Unsure | 18 (7.2%0 | 25 (10.0%) | 6 (2.4%) | 49 (6.5%) |
| The evidence that preventive agents significantly reduces breast cancer risk is controversial.* | ||||
| Agree | 94 (37.6%) | 85 (34.0%) | 57 (22.8%) | 236 (31.5%) |
| Disagree | 65 (26.0%) | 85 (34.0%) | 137 (54.8%) | 287 (38.3%) |
| No Opinion | 72 (28.8%) | 56 (22.4%) | 47 (18.8%) | 175 (23.3%) |
| Don’t know/Unsure | 19 (7.6%) | 24 (9.6%) | 9 (3.6%) | 52 (6.9%) |
| The risk of endometrial cancer is too great to prescribe tamoxifen for breast cancer reduction.* | ||||
| Agree | 46 (18.4%) | 47 (18.8%) | 37 (14.8%) | 130 (17.3%) |
| Disagree | 100 (40.0%) | 97 (38.8%) | 186 (74.4%) | 383 (51.1%) |
| No Opinion | 75 (30.0%) | 76 (30.4%) | 27 (10.8%) | 178 (23.7%) |
| Don’t know/Unsure | 29 (11.6%) | 30 (12.0%) | 0 (0%) | 59 (7.9%) |
| The risk of thromboembolic disease is too great to prescribe preventive agents for breast cancer reduction.* | ||||
| Agree | 65 (26.0%) | 62 (24.8%) | 27 (10.8%) | 154 (20.5%) |
| Disagree | 104 (41.6%) | 112 (44.8%) | 181 (72.4%) | 397 (52.9%) |
| No Opinion | 62 (24.8%) | 58 (23.2%) | 42 (16.8%) | 162 (21.6%) |
| Don’t know/Unsure | 19 (7.6%) | 18 (7.2%) | 0 (0%) | 37 (4.9%) |
| It is easy for me to determine who is eligible to take preventive agents for breast cancer reduction. | ||||
| Agree | 56 (22.4%) | 70 (28.0%) | 100 (40.0%) | 226 (30.1%) |
| Disagree | 138 (55.2%) | 126 (50.4%) | 111 (44.4%) | 375 (50.0%) |
| No Opinion | 46 (18.4%) | 41 (16.4%) | 37 (14.8%) | 124 (16.5%) |
| Don’t know/Unsure | 10 (4.0%) | 13 (5.2%) | 2 (0.8%) | 25 (3.3%) |
| The benefits of preventive surgery in breast cancer outweigh the risks. | ||||
| Agree | 146 (58.4%) | 142 (56.8%) | 163 (65.2%) | 451 (60.1%) |
| Disagree | 33 (13.2%) | 40 (16.0%) | 36 (14.4%) | 109 (14.5%) |
| No Opinion | 61 (24.4%) | 51 (20.4%) | 47 (18.8%) | 159 (21.2%) |
| Don’t know/Unsure | 10 (4.0%) | 17 (6.8%) | 4 (1.6%) | 31 (4.1%) |
| The evidence that preventive surgery significantly reduces breast cancer risk is controversial.* | ||||
| Agree | 92 (36.8%) | 89 (35.6%) | 60 (24.0%) | 241 (31.1%) |
| Disagree | 88 (35.2%) | 98 (39.2%) | 144 (57.6%) | 330 (44.0%) |
| No Opinion | 51 (20.4%) | 51 (20.4%) | 20 (16.0%) | 142 (18.9%) |
| Don’t know/Unsure | 19 (7.6%) | 12 (4.8%) | 6 (2.4%) | 37 (4.9%) |
| The benefits of preventive surgery in ovarian cancer outweigh the risks.* | ||||
| Agree | 139 (55.6%) | 142 (56.8%) | 212 (84.8%) | 493 (65.7%) |
| Disagree | 29 (11.6%) | 28 (11.2%) | 27 (10.8%) | 84 (11.2%) |
| No Opinion | 61 (24.4%) | 58 (23.2%) | 10 (4.0%) | 129 (17.2%) |
| Don’t know/Unsure | 21 (8.4%) | 22 (8.8%) | 1 (0.4%) | 44 (5.9%) |
| The evidence that preventive surgery significantly reduces ovarian cancer risk is controversial.* | ||||
| Agree | 84 (33.6%) | 79 (31.6%) | 56 (22.4%) | 219 (29.2%) |
| Disagree | 71 (28.4%) | 82 (32.8%) | 171 (68.4%) | 324 (43.2%) |
| No Opinion | 70 (28.0%) | 65 (26.0%) | 21 (8.4%) | 156 (20.8%) |
| Don’t know/Unsure | 25 (10.0%) | 24 (9.6%) | 2 (0.8%) | 51 (6.8%) |
Indicates significant difference between provider types (p≤ 0.05)
Similar differences in attitudes were seen regarding preventive surgery in ovarian cancer, as OB/GYN physicians were significantly more likely than family medicine physicians or internists to agree the benefits of preventive surgery in ovarian cancer outweigh the risks (both p-value<0.001), and less likely to agree that the evidence that preventive surgery significantly reduces ovarian cancer risk is controversial (both p-value<0.001). For example, 84.8% of OB/GYN physicians but only 55.6% and 56.8% of family medicine physicians and internists, respectively, reported agreeing that benefits of preventive surgery in ovarian cancer outweigh the risks.
Association Between Familiarity and Attitudes
Among all participants, there was a significant association between familiarity with cancer prevention interventions and attitude toward cancer prevention interventions (p-value<.0001). Logistic regression models explored the familiarity predictor variables with cancer prevention interventions and attitude toward cancer prevention interventions (Table 4). Family medicine respondents and internal medicine physicians were less likely to be familiar with cancer prevention interventions than OB/GYN physicians (odds ratio [OR] 0.20, 95% CI 0.13-0.31; 95% CI 0.23-0.57 respectively), and almost 4 times more likely to have cautious attitudes towards cancer prevention interventions than OB/GYN physicians (OR 4.43, 95% CI 2.86-6.85; OR 3.57, 95% 2.29-5.56 respectively).
Table 4.
Logistic regression models of physician demographics, familiarity and attitudes toward chemoprevention interventions.
| Familiar with Cancer Prevention Interventions |
Attitude toward Cancer Prevention Interventions |
|||
|---|---|---|---|---|
| Odds Ratio (OR) |
95% CI | Odds Ratio (OR) |
95% CI | |
| Gender | ||||
| Female | 1 | [Reference] | 1 | [Reference] |
| Male | 0.97 | [.67, 1.42] | 1.41 | [.97, 2.03] |
| Age | 1.00 | [.96, 1.05] | 1.00 | [.90, 1.05] |
| Medical Specialty | ||||
| OB/GYN | 1 | [Reference] | 1 | [Reference] |
| Family medicine | 0.20* | [.13, .31] | 4.43* | [2.86, 6.85] |
| Internist | 0.36* | [.23, .57] | 3.57* | [2.29, 5.56] |
| Geographic location | ||||
| Urban | 1 | [Reference] | 1 | [Reference] |
| Suburban | 1.87* | [1.25, 2.79] | 0.72 | [.49, 1.08] |
| Rural/remote | 0.97 | [.53, 1.77] | 0.63 | [.35, 1.12] |
| Years practicing specialty | 1.01 | [.96, 1.07] | 1.00 | [.95, 1.05] |
| Type of practice | ||||
| Solo practice | 1 | [Reference] | 1 | [Reference] |
| Single Specialty Group* | 0.54* | [.31, .94] | 1.30 | [.75, 2.25] |
| Multi-Specialty Group | 0.68 | [.38, 1.21] | 1.29 | [.73, 2.28] |
| Direct hospital employee | 0.54 | [.27, 1.11] | 1.40 | [.70, 2.82] |
| Faculty practice plan | 0.51 | [.18, 1.41] | 0.74 | [.27, 2.07] |
| Average number of patients | ||||
| 1-15 | 1 | [Reference] | 1 | [Reference] |
| 16-25 | 1.53 | [.96, 2.43] | 0.82 | [.52, 1.29] |
| 26 or more | 2.34* | [1.32, 4.13] | 0.73 | [.43, 1.27] |
Indicates significant difference between provider types (p≤ 0.05)
In addition, as shown in Table 4, self-reporting of being more familiar with cancer prevention interventions was associated with practicing in a suburban setting compared to an urban location (OR 1.87, 95% CI 1.25-2.79), and seeing 26 or more patients on average per day compared to seeing 1-15 an average daily (OR 2.34, 95% CI 1.32-4.13). Being less familiar with cancer prevention interventions was associated with practicing in a single specialty group compared to a solo practice (OR 0.54, 95% CI 0.31-0.94). Age, gender, and years in a specialty practice were not significantly associated with familiarity or attitude toward cancer prevention interventions.
Course of Action for Patients at Risk
To determine PCPs’ understanding and ability to recognize women at various degrees of breast/ovarian cancer risk (average risk, family history, genetic predisposition, or presence of precursor), and apply the appropriate recommendation guidelines (1-5, 7) (National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) https://www.nccn.org/professionals/physician_gls/default.aspx; Susan G. Komen Breast Cancer Screening for Women at Higher Risk; https://ww5.komen.org/BreastCancer/BreastCancerScreeningForWomenAtHigherRisk.html; Susan G. Komen Breast Cancer Screening for Women at Average Risk, https://ww5.komen.org/BreastCancer/BreastCancerScreeningforWomenatAverageRisk.html), respondents were presented with hypothetical cases depicting women with breast/ovarian cancer risk factors, and asked to select course(s) of action (Table 5). Figure 1 depicts the breakdown of responses for the clinically recommended course(s) of action for each case. A more detailed text description of the results presented in Table 5 and Figure 1 can be found in Supplementary Data.
Table 5.
Recommended course of action for vignettes featuring patients at various levels of risk for breast and/or ovarian cancer.†
| Family Medicine n (%) |
Internist n (%) |
OB/GYN n (%) |
Total n (%) |
|
|---|---|---|---|---|
| Patient 1 is 40 years old and has: No personal history of breast cancer; No personal history of DCIS; No personal history of LCIS; and no first-degree relative with breast cancer. | ||||
| Continue routine care. | 202 (80.8%) | 188 (75.2%) | 197 (78.8%) | 587 (78.3%) |
| Order a screening mammogram for this patient.* | 149 (59.6%) | 136 (54.4%) | 196 (78.4%) | 481 (64.1%) |
| Talk with the patient about her lifestyle behaviors. | 110 (44.0%) | 112 (44.8%) | 113 (45.2%) | 335 (44.7%) |
| Discuss the pros and cons of getting genetic testing with this patient.* | 19 (7.6%) | 16 (6.4%) | 5 (2.0%) | 40 (5.3%) |
| Discuss the pros and cons of using a preventive agent with this patient. | 10 (4.0%) | 16 (6.4%) | 7 (2.8%) | 33 (4.4%) |
| Discuss the pros and cons of getting prophylactic surgery with this patient.* | 10 (4.0%) | 14 (5.6%) | 1 (0.4%) | 25 (3.3%) |
| Refer the patient to a specialist. | 14 (5.6%) | 17 (6.8%) | 6 (2.4%) | 37 (4.9%) |
| Patient 2 is 35 years old and has: No personal history of breast cancer; No personal history of DCIS; No personal history of LCIS; One first-degree relative with breast cancer, diagnosed before age 50; and one first-degree relative with ovarian cancer. | ||||
| Continue routine care.* | 116 (46.4%) | 90 (36.0%) | 77 (30.8%) | 283 (37.7%) |
| Order a screening mammogram for this patient. | 146 (58.4%) | 139 (55.6%) | 132 (52.8%) | 417 (55.6%) |
| Talk with the patient about her lifestyle behaviors. | 139 (55.6%) | 124 (49.6%) | 120 (48.0%) | 383 (51.1%) |
| Discuss the pros and cons of getting genetic testing with this patient.* | 168 (67.2%) | 161 (64.4%) | 211 (84.4%) | 540 (72.0%) |
| Discuss the pros and cons of using a preventive agent with this patient. | 44 (17.6%) | 57 (22.8%) | 56 (22.4%) | 157 (20.9%) |
| Discuss the pros and cons of getting prophylactic surgery with this patient. | 33 (13.2%) | 34 (13.6%) | 45 (18.0%) | 112 (14.9%) |
| Refer the patient to a specialist. | 143 (57.2%) | 139 (55.6%) | 126 (50.4%) | 408 (54.4%) |
| Patient 3 is 35 years old and has: No personal history of breast cancer; No personal history of DCIS; No personal history of LCIS; Two first-degree relatives with breast cancer; and was found to carry a pathogenic BRCA1 mutation. | ||||
| Continue routine care.* | 71 (28.4%) | 71 (28.4%) | 45 (18.0%) | 187 (24.9%) |
| Order a screening mammogram for this patient. | 148 (59.2%) | 158 (63.2%) | 165 (66.0%) | 471 (62.8%) |
| Talk with the patient about her lifestyle behaviors. | 117 (46.8%) | 122 (48.8%) | 114 (45.6%) | 353 (47.1%) |
| Discuss the pros and cons of using a preventive agent with this patient.* | 73 (29.2%) | 102 (40.8%) | 132 (52.8%) | 307 (40.9%) |
| Discuss the pros and cons of getting prophylactic surgery with this patient.* | 112 (44.8%) | 122 (48.8%) | 169 (67.2%) | 402 (53.6%) |
| Refer the patient to a specialist. | 207 (82.8%) | 203 (81.2%) | 205 (82.0%) | 615 (82.0%) |
| Patient 4 is 65 years old and has: No personal history of breast cancer; No personal history of DCIS; No personal history of LCIS; No first-degree relatives with breast cancer; and one breast biopsy showing atypical hyperplasia. | ||||
| Continue routine care.* | 127 (50.8%) | 113 (45.2%) | 85 (34.0%) | 325 (43.3%) |
| Order a screening mammogram for this patient. | 181 (72.4%) | 170 (68.0%) | 161 (64.4%) | 512 (68.3%) |
| Talk with the patient about her lifestyle behaviors. | 105 (42.0%) | 109 (43.6%) | 110 (44.0%) | 324 (43.2%) |
| Discuss the pros and cons of getting genetic testing with this patient. | 36 (14.4%) | 46 (18.4%) | 40 (16.0%) | 122 (16.3%) |
| Discuss the pros and cons of using a preventive agent with this patient.* | 36 (14.4%) | 50 (20.0%) | 69 (27.6%) | 155 (20.7%) |
| Discuss the pros and cons of getting prophylactic surgery with this patient. | 21 (8.4%) | 34 (13.6%) | 31 (12.4%) | 86 (11.5%) |
| Refer the patient to a specialist.* | 105 (42.0%) | 121 (48.4%) | 142 (56.8%) | 368 (49.1%) |
Responses are not mutually exclusive; respondents could select one or more course of action.
Indicates significant difference between provider types (p≤ 0.05)
Grey shading indicates the clinically recommended courses of action.
Figure 1.
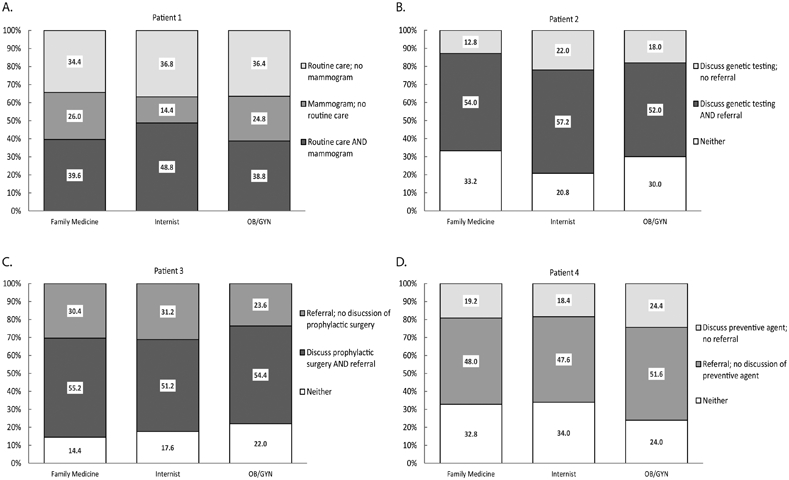
Breakdown of physician responses for clinically recommended course of action for case vignettes presented in Table 5. A) Patient 1 (40 years old; no personal history of breast cancer; no personal history of DCIS; no personal history of LCIS; no first-degree relative with breast cancer); B) Patient 2 (35 years old; no personal history of breast cancer; no personal history of DCIS; no personal history of LCIS; one first-degree relative with breast cancer, diagnosed before age 50; one first-degree relative with ovarian cancer); C) Patient 3 (35 years old; no personal history of breast cancer; no personal history of DCIS; no personal history of LCIS; two first-degree relatives with breast cancer; found to carry a pathogenic BRCA1 mutation); D) Patient 4 (65 years old; no personal history of breast cancer; no personal history of DCIS; no personal history of LCIS; no first-degree relatives with breast cancer; one breast biopsy showing atypical hyperplasia). Light gray bars: percentage of physicians who selected the specified clinically recommended course of action; dark gray bars: percentage of physicians who selected both clinically recommended courses of actions; white bars: percentage of physicians who selected neither of the clinically recommended specified courses of action.
Discussion
We assessed PCP understanding of breast and ovarian cancer risk management by querying use of risk assessment tools, familiarity with cancer preventive interventions, and knowledge/understanding of clinical guidelines via hypothetical case studies.
Our results show that while PCPs are familiar with and reported use of common cancer risk assessments (i.e. family history and breast exam), the specialized tools aimed at identifying high risk populations (e.g. breast cancer risk assessment tools, genetic testing) are less utilized. Physicians’ familiarity with cancer prevention interventions was significantly associated with their attitudes towards risks/benefits, such that respondents who reported to be less familiar were more likely to report cautious attitudes towards cancer prevention interventions. In general, OB/GYN physicians were more likely to report using and/or being familiar with the breast/ovarian cancer risk assessment tools and cancer preventive intervention agents, which likely reflects the female patient population managed by OB/GYN physicians, in which breast and cancer risk assessment is more commonly practiced.
The American Society for Clinical Oncology (ASCO) (36), National Comprehensive Cancer Network (NCCN) (37), and the National Institute for Health and Care Excellence (NICE) (38), have released recommendations that PCPs consider and discuss the use of chemopreventive agents with women at increased risk of breast cancer, including asymptomatic women age ≥35 years, with previous diagnoses of benign breast lesions (e.g. atypical ductal or lobular hyperplasia and lobular carcinoma in situ). However, these professional societies represent oncologists and therefore these recommendations and guidelines may not be effectively reaching the primary care setting. The United States Preventive Services Task Force (USPSTF), which includes primary care, released a statement recommending (B rating) that clinicians offer to prescribe tamoxifen, raloxifene, AIs for prevention in this increased risk population (7). However, uptake of these recommendations remains low, in part due to reluctance of PCPs, and reluctance by women to use these medications due to perceived side effects, cost and/or skepticism of efficacy (6, 26).
The hypothetical cases represent the types of patients who may seek risk management from their PCPs, and are meant to determine their knowledge/understanding of clinical management guidelines. For Patient 1, who is not necessarily at increased risk of breast or ovarian cancer, 100% of the responses selected at least one of the clinically recommended courses of action. The difference between responses from OB/GYN physicians regarding ordering a screening mammogram likely reflects inconsistent mammography guidelines related to patient age, with the OB/GYN professional society ACOG recommending mammograms starting at age 40 whereas other guidelines recommend mammograms starting at later ages (3-5, 39). As expected risk in the vignettes increased due to family history (Patients 2 and 3), genetic mutation (Patient 3) or a precancerous lesion (Patient 4), such that their clinical management goes beyond routine care, up to 34.0% of the total responses did not select any of the clinically recommended courses of action (Figure 1).
As clinical management guidelines recommend at-risk individuals to seek guidance from their PCPs, it is clear that PCPs may be expected to take on a greater role in cancer risk assessment and management. For example, the USPSTF recently released a recommendation (A rating) that PCPs apply familial risk assessment tools to women with a personal or family history of breast or ovarian/tubal/peritoneal cancers, or who have an ancestry indicative of BRCA1/2 gene mutations (2). This reliance on PCPs could provide a challenge considering that cancer risk models continuously evolving to include additional etiologic and genetic risk factors (1, 40) and these updated models may not be effectively incorporated into primary care practices. Previous studies have shown that guideline dissemination is not necessarily adequate to change practice behavior (41, 42). Integration of cancer risk assessment and clinical management guidelines within routine primary care workflow, including reminder systems (chart-based or computerized) (43, 44), cancer risk and prevention-related decision support tools (30, 45, 46) (https://clinicaltrials.gov/ct2/show/NCT02986230), and provider assessment and feedback (which have been successful for improving screening rates for breast, cervical and colon cancer) (47), is imperative for widespread implementation and to make an impact on prescribing behaviors of PCPs (42, 48).
The survey study design, while assessing the understanding and knowledge of a large sample size, carries some limitations. The identification of respondents via a database of physicians who have previously agreed to participate in scientific research studies and the limited response rate pose the risk of sample bias, such that findings may not be applicable to the overall PCP population. To overcome this limitation, we aimed for a large sample size representing three specialties. Also, the survey study design limits further exploration and/or clarification of responses. Based on the results of this survey, we plan to conduct working group discussions with relevant stakeholders to not only further evaluate the responses, but also to explore potential opportunities to provide more robust and useful resources to the primary care community.
Clinical management guidelines reinforce patients’ reliance on their PCPs to provide risk assessment and management recommendations. In order to ensure that both physicians and patients receive updated comprehensive care, cancer prevention resources must be more readily integrated into primary care, such that PCPs become more familiar and therefore comfortable applying cancer risk assessments and prescribing cancer preventive interventions to their patients.
Supplementary Material
Acknowledgements
This work was supported by National Cancer Institute contract HHSN261201400002B, task order no. HHSN26100011 to ICF (B. Tennant, B. Bloodgood Sheppard, K.I. Coa, S.S. Kay).
The authors wish to thank Dr. Deborah M. Winn for reviewing the manuscript and providing valuable feedback.
Footnotes
The authors declare no potential conflicts of interest.
References
- 1.Daly MB, Pilarski R, Yurgelun MB, Berry MP, Buys SS, Dickson P, et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 1.2020. J Natl Compr Canc Netw. 2020;18:380–91. [DOI] [PubMed] [Google Scholar]
- 2.Force USPST, Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, et al. Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer: US Preventive Services Task Force Recommendation Statement. JAMA : the journal of the American Medical Association. 2019;322:652–65. [DOI] [PubMed] [Google Scholar]
- 3.Bevers TB, Helvie M, Bonaccio E, Calhoun KE, Daly MB, Farrar WB, et al. Breast Cancer Screening and Diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1362–89. [DOI] [PubMed] [Google Scholar]
- 4.Oeffinger KC, Fontham ET, Etzioni R, Herzig A, Michaelson JS, Shih YC, et al. Breast Cancer Screening for Women at Average Risk: 2015 Guideline Update From the American Cancer Society. JAMA : the journal of the American Medical Association. 2015;314:1599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siu AL, Force USPST. Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164:279–96. [DOI] [PubMed] [Google Scholar]
- 6.Crew KD, Albain KS, Hershman DL, Unger JM, Lo SS. How do we increase uptake of tamoxifen and other anti-estrogens for breast cancer prevention? NPJ Breast Cancer. 2017;3:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Force USPST Owens DK, Davidson KW Krist AH, Barry MJ, Cabana M, et al. Medication Use to Reduce Risk of Breast Cancer: US Preventive Services Task Force Recommendation Statement. JAMA : the journal of the American Medical Association. 2019;322:857–67. [DOI] [PubMed] [Google Scholar]
- 8.Sauter ER. Breast Cancer Prevention: Current Approaches and Future Directions. Eur J Breast Health. 2018;14:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer Prev Res (Phila). 2010;3:696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goss PE, Ingle JN, Ales-Martinez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381–91. [DOI] [PubMed] [Google Scholar]
- 11.Powles TJ, Ashley S, Tidy A, Smith IE, Dowsett M. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst. 2007;99:283–90. [DOI] [PubMed] [Google Scholar]
- 12.Cuzick J, Forbes JF, Sestak I, Cawthorn S, Hamed H, Holli K, et al. Long-term results of tamoxifen prophylaxis for breast cancer−-96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. 2007;99:272–82. [DOI] [PubMed] [Google Scholar]
- 13.Veronesi U, Maisonneuve P, Rotmensz N, Bonanni B, Boyle P, Viale G, et al. Tamoxifen for the prevention of breast cancer: late results of the Italian Randomized Tamoxifen Prevention Trial among women with hysterectomy. J Natl Cancer Inst. 2007;99:727–37. [DOI] [PubMed] [Google Scholar]
- 14.Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA : the journal of the American Medical Association. 1999;281:2189–97. [DOI] [PubMed] [Google Scholar]
- 15.Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Kornitzer M, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125–37. [DOI] [PubMed] [Google Scholar]
- 16.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA : the journal of the American Medical Association. 2006;295:2727–41. [DOI] [PubMed] [Google Scholar]
- 17.Force USPST Grossman DC, Curry SJ Owens DK, Barry MJ Davidson KW, et al. Screening for Ovarian Cancer: US Preventive Services Task Force Recommendation Statement. JAMA : the journal of the American Medical Association. 2018;319:588–94. [DOI] [PubMed] [Google Scholar]
- 18.Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker JL, Powell CB, Chen LM, Carter J, Bae Jump VL, Parker LP, et al. Society of Gynecologic Oncology recommendations for the prevention of ovarian cancer. Cancer. 2015;121:2108–20. [DOI] [PubMed] [Google Scholar]
- 20.Temkin SM, Bergstrom J, Samimi G, Minasian L. Ovarian Cancer Prevention in High-risk Women. Clin Obstet Gynecol. 2017;60:738–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beral V, Doll R, Hermon C, Peto R, Reeves G. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008;371:303–14. [DOI] [PubMed] [Google Scholar]
- 22.Friebel TM, Domchek SM, Rebbeck TR. Modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ACOG Committee on Practice Bulletins-Gynecology, Committee on Genetics, Society of Gynecologic Oncology. Practice Bulletin No 182: Hereditary Breast and Ovarian Cancer Syndrome. Obstet Gynecol. 2017;130:e110–e26. [DOI] [PubMed] [Google Scholar]
- 24.Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101:80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harry ML, Truitt AR, Saman DM, Henzler-Buckingham HA, Allen CI, Walton KM, et al. Barriers and facilitators to implementing cancer prevention clinical decision support in primary care: a qualitative study. BMC Health Serv Res. 2019;19:534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samimi G, Heckman-Stoddard BM, Kay SS, Bloodgood B, Coa KI, Robinson JL, et al. Acceptability of localized cancer risk reduction interventions among individuals at average or high risk for cancer. Cancer Prev Res (Phila). 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol. 2010;28:3090–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravdin PM. The lack, need, and opportunities for decision-making and informational tools to educate primary-care physicians and women about breast cancer chemoprevention. Cancer Prev Res (Phila). 2010;3:686–8. [DOI] [PubMed] [Google Scholar]
- 29.Blakeslee SB, McCaskill-Stevens W, Parker PA, Gunn CM, Bandos H, Bevers TB, et al. Deciding on breast cancer risk reduction: The role of counseling in individual decision-making - A qualitative study. Patient Educ Couns. 2017;100:2346–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kukafka R, Fang J, Vanegas A, Silverman T, Crew KD. Pilot study of decision support tools on breast cancer chemoprevention for high-risk women and healthcare providers in the primary care setting. BMC Med Inform Decis Mak. 2018;18:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samimi G, Heckman-Stoddard BM, Holmberg C, Tennant B, Sheppard BB, Coa KI, et al. Cancer Prevention in Primary Care: Perception of Importance, Recognition of Risk Factors and Prescribing Behaviors. Am J Med. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith SG, Sestak I, Forster A, Partridge A, Side L, Wolf MS, et al. Factors affecting uptake and adherence to breast cancer chemoprevention: a systematic review and meta-analysis. Ann Oncol. 2016;27:575–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pruthi S, Heisey RE, Bevers TB. Chemoprevention for Breast Cancer. Ann Surg Oncol. 2015;22:3230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bambhroliya A, Chavez-MacGregor M, Brewster AM. Barriers to the Use of Breast Cancer Risk Reduction Therapies. J Natl Compr Canc Netw. 2015;13:927–35. [DOI] [PubMed] [Google Scholar]
- 35.Liede A, Cai M, Crouter TF, Niepel D, Callaghan F, Evans DG. Risk-reducing mastectomy rates in the US: a closer examination of the Angelina Jolie effect. Breast Cancer Res Treat. 2018;171:435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Visvanathan K, Fabian CJ, Bantug E, Brewster AM, Davidson NE, DeCensi A, et al. Use of Endocrine Therapy for Breast Cancer Risk Reduction: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2019;37:3152–65. [DOI] [PubMed] [Google Scholar]
- 37.Bevers T US Preventive Services Task Force (USPSTF), Breast Cancer Risk Reduction Version 1.2020. [Google Scholar]
- 38.National Institute for Health and Care Excellence. Familial breast cancer: classification, care and managing breast cancer and related risks in people with a family history of breast cancer. Clinical guideline [CG164]; 2013. [Google Scholar]
- 39.ACOG Committee on Practice Bulletins-Gynecology, Practice Bulletin Number 179: Breast Cancer Risk Assessment and Screening in Average-Risk Women. Obstet Gynecol. 2017;130:e1–e16. [DOI] [PubMed] [Google Scholar]
- 40.Konstantinopoulos PA, Norquist B, Lacchetti C, Armstrong D, Grisham RN, Goodfellow PJ, et al. Germline and Somatic Tumor Testing in Epithelial Ovarian Cancer: ASCO Guideline. J Clin Oncol. 2020:JCO1902960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bero LA, Grilli R, Grimshaw JM, Harvey E, Oxman AD, Thomson MA. Closing the gap between research and practice: an overview of systematic reviews of interventions to promote the implementation of research findings. The Cochrane Effective Practice and Organization of Care Review Group. BMJ. 1998;317:465–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zapka JG, Lemon SC. Interventions for patients, providers, and health care organizations. Cancer. 2004;101:1165–87. [DOI] [PubMed] [Google Scholar]
- 43.Davis DA, Thomson MA, Oxman AD, Haynes RB. Changing physician performance. A systematic review of the effect of continuing medical education strategies. JAMA : the journal of the American Medical Association. 1995;274:700–5. [DOI] [PubMed] [Google Scholar]
- 44.Baron RC, Melillo S, Rimer BK, Coates RJ, Kerner J, Habarta N, et al. Intervention to increase recommendation and delivery of screening for breast, cervical, and colorectal cancers by healthcare providers a systematic review of provider reminders. Am J Prev Med. 2010;38:110–7. [DOI] [PubMed] [Google Scholar]
- 45.Silverman TB, Vanegas A, Marte A, Mata J, Sin M, Ramirez JCR, et al. Study protocol: a cluster randomized controlled trial of web-based decision support tools for increasing BRCA1/2 genetic counseling referral in primary care. BMC Health Serv Res. 2018;18:633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ozanne EM, Howe R, Omer Z, Esserman LJ. Development of a personalized decision aid for breast cancer risk reduction and management. BMC Med Inform Decis Mak. 2014;14:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sabatino SA, Habarta N, Baron RC, Coates RJ, Rimer BK, Kerner J, et al. Interventions to increase recommendation and delivery of screening for breast, cervical, and colorectal cancers by healthcare providers systematic reviews of provider assessment and feedback and provider incentives. Am J Prev Med. 2008;35:S67–74. [DOI] [PubMed] [Google Scholar]
- 48.Curry SJ, Byers T, Hewitt ME, National Cancer Policy Board (U.S.). Fulfilling the potential of cancer prevention and early detection. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


