Abstract
Objective:
To elucidate the clinical values of anti-M3R in Sjögren’s syndrome (SS) in the largest cohort for an anti-M3R study.
Methods:
The plasma of 361 subjects (156 primary-SS[pSS], 62 non-SS-sicca[SICCA], 40 systemic lupus erythematosus [SLE], 50 rheumatoid arthritis[RA], and 53 healthy controls[HC]) was screened using our modified On-Cell-Western assay. Saliva from pSS (n=37) compared to SICCA (n=26) was also analyzed. The sensitivity/specificity of anti-M3R and its association with comprehensive clinical/laboratory features were determined.
Results:
Plasma-anti-M3R was higher in pSS compared to other groups, differentiating pSS with good-to-excellent diagnostic power with a specificity of 85% and a sensitivity between 75% and 98%. pSS plasma-anti-M3R was positively correlated with ocular staining scores, anti-Ro/SSA, IgG, β2-microglobulin, ESR, and ESSDAI. It was negatively correlated with WBC, C4, and salivary scintigraphic indices. Saliva-anti-M3R was 3.59 times higher in pSS than in SICCA. Interestingly, the positivity of plasma-anti-M3R with or without anti-Ro/SSA was higher in those who did not undergo a lip biopsy than in individuals with a biopsy (100% vs. 88.6%, p=0.013, and 90.6% vs. 75%, p=0.023, respectively). The agreement between the 2002 American-European-Consensus-Group criteria and the criteria substituted with plasma-anti-M3R for the lip biopsy reached 92% with a significant kappa of 0.824.
Conclusion:
Anti-M3R enhances sensitivity and specificity for SS diagnosis, correlating with ocular dryness and glandular hypofunction, and the hematological/biological domains of the ESSDAI. Our findings also underscore anti-M3R in SS diagnosis, where clinical assessments by multi-disciplinary specialists, such as lip biopsy, sialometry, or ocular evaluation, are limited.
Keywords: Sjögren’s syndrome, Anti-muscarinic type 3 receptor autoantibodies, Secretory dysfunction, Anti-Ro/SSA, ESSDAI
INTRODUCTION
Sjögren’s syndrome (SS) is an autoimmune disorder characterized by lymphocytic infiltration in the exocrine glands, leading to glandular dysfunction (1). Due to its heterogeneous clinical presentation, SS diagnosis remains a clinical challenge. Novel approaches to improve the specificity and sensitivity of current diagnostic tools are urgently needed (2). To date, autoantibodies against Ro/SSA have been the most used biological measures for SS diagnosis, as defined by the 2002 American-European Consensus Group (AECG) criteria and the 2016 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) criteria (3, 4). Anti-Ro/SSA is known to be associated with systemic extraglandular manifestations, such as vasculitis, Raynaud’s, arthritis, or renal tubular acidosis. However, its role in glandular dysfunction in SS has not been fully understood (5, 6).
Muscarinic-type-3-receptor (M3R), a G-protein-coupled acetylcholine receptor, is known to regulate secretion in salivary acinar cells (7). Out of the five subtypes of MR (M1R to M5R) (8), M3R is highly expressed in the exocrine glands and the M3R knock-out mouse failed to induce saliva secretion (11). Previously, our group and others have reported that autoantibodies against M3R (anti-M3R) can suppress secretion from cells by functioning as an antagonist for the receptor (9–11). Jin et al. reported that incubation of cells with SS IgG significantly decreased M3R membrane localization by inhibiting carbachol-induced intracellular calcium release, which suggests an additional mechanism for secretory dysfunction in SS.
The prevalence of anti-M3R is known to widely vary from 1.92% to 97% in SS, depending on the assay system (i.e. peptide-based ELISA versus cell-based assay) (12). Among studies with cell-based assays, anti-M3R was detected in 60% of SS patients by flow cytometry (13) and 75% of patients tested positive by our modified On-Cell Western (OCW) assay (14). Unlike the conventional ELISA, these techniques allowed binding of autoantibodies to the conformational epitopes of M3R. Our previous study with the assay reported that anti-M3R IgG in plasma was highly prevalent in SS, compared to other disease controls, and that anti-M3R in combination with anti-Ro/SSA outperformed the single analyte in discriminating patients with SS from other groups (14). Moreover, the statistically significant correlation that existed between anti-M3R IgG and the salivary flow rate/focus score in a small set of subjects implied a potential role of these autoantibodies in SS-disease parameters.
In this current study, we applied our in-house, modified OCW assay to screen plasma and saliva samples obtained from the Seoul National University Bundang Hospital (SNUBH) cohort, which is the largest cohort (n=361) for an anti-M3R study, to our knowledge. We aimed to determine the clinical/serological/laboratory characteristics of anti-M3R positive SS patients for its clinical usefulness. More importantly, we explored the potential clinical significance of anti-M3R in diagnosing SS by evaluating the performance of the established SS classification criteria when substituted with anti-M3R for the minor salivary gland lip biopsy (MSGBx).
PATIENTS AND METHODS
Patient enrollment
Participants were recruited at SNUBH from August, 2005 to May, 2016. Primary SS patients (SS, n=156) were diagnosed according to the AECG criteria and patients with rheumatoid arthritis (RA, n=50) fulfilled the 2010 ACR/EULAR criteria (15). The 1997-updated criteria of the 1982-revised ACR criteria were used for systemic lupus erythematosus (SLE, n=40) (16). Non-SS-sicca group (Sicca, n=62) include subjects with dry mouth and/or dry eye, but did not fulfil the AECG criteria. Gender-and age-matched heathy controls (HC, n=53) were enrolled from a routine medical check-up. This study was approved by the Institutional Review Board (B-0506/021–004) and the written informed consent was obtained.
Plasma and saliva collection
Collected blood tubes were centrifuged within 20 to 60 minutes of collection at 2,000 g for 10 min at 4℃. Plasma was separated, aliquoted into cryovials, and stored at −70 °C until analysis. The whole saliva flow rate (WSFR) was determined, as previously described (17), after discontinuation of any xerostomic medication for at least four-fold its half-life. Of 156 SS patients, 141 plasma samples (90.3%) and 36 unstimulated saliva samples (23%) were available for screening.
Clinical parameters
Schirmer’s test was performed in 93 % (146/156) of SS patients and the average value from both eyes was used for analysis. Ocular staining score (OSS) was performed in 64 % (101/156). The ocular surface was stained with a fluorescein strip wetted with buffered saline and OSS was defined as the sum of staining scores of nasal conjunctiva, temporal conjunctiva, and cornea. The staining scores of each area were graded as 0 (no staining), 1 (mild staining limited to <1/3 of the cornea), 2 (moderate staining of <1/2 of the cornea), or 3 (severe staining of > 1/2 of the cornea). EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI) was calculated as described (18) and moderate-to-high activity was defined as ESSDAI ≥ 5 (19).
In SS, the levels of CBC, Westergren erythrocyte sedimentation rate (ESR), C3 and C4, total IgG, and β2-microglobulin (B2M) were determined within 2 weeks of enrollment. MSGBx results were available in 66% (103/156) of SS patients and both the Chisholm-Mason score and focus score were calculated as previously reported (20, 21). Focus scores were categorized into 3 grades (focus score <1, 1≤focus score <2, and 2≤ focus score) for analyses. Salivary gland scintigraphy were performed on 69.8% (109/156) of SS patients. Scintigraphic parameters (parotid uptake ratio PU, submandibular uptake ratio SU, percentage parotid excretion % PE, and percentage submandibular excretion % SE) were calculated by using the region of interests around the frontal skull and salivary glands on anterior images (22). The uptake ratios and percentage excretions were calculated based on the means of right and left parametric values.
The modified OCW assay with the stable cell line expressing human M3R-GFP protein
As described in our previous study (14), stable HEK 293 cells expressing human M3R tagged with GFP were seeded onto the 96-well plates (4 x 10^4 cells/well), followed by plasma (1:400 dilution) or saliva (1:1 dilution) incubation. Goat anti-human IgG (H+L) IRDye800CW secondary antibody (Rockland Immunochemicals, Inc.) at a dilution of 1:800 was used. The plate was screened at 800 nm wavelength on the Odyssey Reader (LI-COR Bioscience) to detect the level of anti-M3R. The mean of at least three values were analyzed. The signal intensities were analyzed by the Odyssey software and normalized by GFP expression levels of the cells in each well, which was measured by a fluorescence microplate reader (BioTeck, 485/20 excitation and 528/20 emission), following our protocol (14).
Statistical analyses
Continuous variables are presented as mean (standard deviation [SD]) or median (interquartile range [IQR]), as appropriate. Group comparisons were performed with analysis of variance (ANOVA) followed by Bonferroni’s post hoc tests or Kruskal-Wallis test followed by Dunn’s post hoc tests. Receiver operating characteristic (ROC) curves were created to explore the ability of anti-M3R to distinguish SS from other groups, and area under the curve (AUC) with 95% confidence intervals (CI) were calculated. Comparison between AUCs was performed using the DeLong’s test (package pROC). Optimum test cut-off values for anti-M3R intensity were based on maximum positive likelihood ratios (+LR) obtained from the ROC curve analysis (HC vs. SS). SS data were categorized into anti-M3R positive and negative, and compared to laboratory features using a Pearson χ² test or the Fisher’s exact test, where appropriate. Spearman rank correlation coefficients were to assess associations between continuous variables. Cohen’s kappa was to determine the level of agreement between the classification criteria. The Prism Software package version 5.0 (GraphPad Software) and R (http://www.r-project.org, version 3.5.1) in RStudio (http://www.rstudio.com, version 1.1.456) were used. The p-value less than 0.05 was considered significant.
RESULTS
P-anti-M3R and S-anti-M3R are significantly upregulated in SS.
The details of the SNUBH cohort are listed in Table 1, including demographics and clinical features. The examples of wells indicate that the signal intensities of P-anti-M3R in SS plasma were markedly higher than in those detected in HC, Sicca, and RA samples (p<0.001) (Fig. 1A). It also reliably distinguished SS from HC (AUC 0.95, 95% CI 0.92 to 0.98), Sicca (AUC 0.95, 95% CI 0.91 to 0.98), or RA (AUC 0.89, 95% CI 0.84 to 0.94) (p<0.0001), while SS from SLE was less discriminatory (AUC 0.52, 95% CI 0.43 to 0.62) (Fig. 1B and 1C). The cut-off of 6.13% yielded a specificity of 85% for the discrimination of SS from HC, Sicca, and RA, with a sensitivity of 98%, 95% and 75%, respectively. Anti-M3R intensity in saliva (S-anti-M3R) was significantly different in SS compared to Sicca (p<0.0001, Fig. 1D), with an AUC of 0.84 (AUC 0.95, 95% CI 0.92 to 0.98) (Fig. 1E). With a cut-off of 14.3% for S-anti-M3R, SS patients were identified with a specificity level of 98% and a sensitivity level of 50%.
Table 1.
Demographic and clinical characteristics of the SNUBH study cohort
| HC (n = 53) | Sicca (n = 62) | SS (n = 156) | SLE (n = 40) | RA (n = 50) | |
|---|---|---|---|---|---|
| Age, years | 50.26 ± 12.81 | 52.42 ± 11.72 | 50.13 ± 12.75 | 32.98 ± 12.26 | 50.38 ± 12.67 |
| Female Sex- | 51/53 (96.2) | 58/62 (93.5) | 152/156 (97.4) | 30/40 (75) | 47/50 (94) |
| WBC (/mm3) | 5654 ± 1356 | 5791 ± 1492 | 4974 ± 1607 | 4130 ± 2651 | 6899 ± 2244 |
| Hb (g/dL) | 13.6 ± 1.0 | 13.3 ± 1.1 | 12.6 ± 1.3 | 11.2 ± 2.3 | 12.5 ± 1.3 |
| Platelet (/mm3) | 260.3 ± 54.7 | 242.5 ± 57.0 | 229.1 ± 65.2 | 182.9 ± 111.4 | 273.8 ± 65.7 |
| ESR (mm/h) | 9.0 ± 7.6 | 11.2 ± 11.2 | 24.5 ± 20.5 | 33.6 ± 24.8 | 19.1 ± 22.5 |
| C3 (mg/dL) | ND | 94.2 ± 19.0 | 105.7 ± 24.0 | 56.2 ± 30.1 | ND |
| C4 (mg/dL) | ND | 21.6± 8.0 | 22.1 ± 10.6 | 11.5 ± 6.8 | ND |
| B2M (mg/L) | ND | 1.9 ± 0.6 | 2.4 ± 1.3 | ND | ND |
| IgG (mg/dL) | ND | 1326.4 ± 278.7 | 1998.0 ± 673.7 | ND | ND |
| Anti-SSA+ | ND | 3/58 (5.2) | 141/156 (90.4) | 25/34 (73.5) | ND |
| Anti-SSB+ | ND | 0/58 (0.0) | 79/156 (50.6) | 10/34 (29.4) | ND |
| Lip Biopsy | ND | 46/62 (74.2) | 103/156 (66.0) | ND | ND |
| Focus Score+ | ND | 1/46 (1.2) | 83/103 (80.6) | ND | ND |
| Chisholm-Mason Scale >1 | ND | 2/46 (4.3) | 87/103 (84.5) | ND | ND |
| Avg Schirmer (mm) | ND | 10.1 ± 7.1 | 7.3 ± 6.9 | ND | ND |
| Unstimulated salivary flow rate (mL/min.) | ND | 0.266 ± 0.0.562 | 0.081 ± 0.102 | ND | ND |
| PU ratio | ND | 5.73 ± 2.69 | 4.10 ± 1.66 | ND | ND |
| SU ratio | ND | 6.44 ± 1.48 | 4.57 ± 1.41 | ND | ND |
| %PE | ND | 38.6 ± 17.3 | 27.5 ± 20.6 | ND | ND |
| %SE | ND | 30.1 ± 14.0 | 16.4 ± 14.4 | ND | ND |
| Lacrimal Dysfunction | ND | 26/55 (47.3) | 125/155 (80.6) | ND | ND |
| Salivary Dysfunction | ND | 38/60 (63.3) | 148/155 (95.5) | ND | ND |
Data are presented as mean ± SD or positive individual/total number (percentage).
ND, not determined.
Figure 1. High prevalence of anti-M3R in SS, detected by the modified OCW assay.
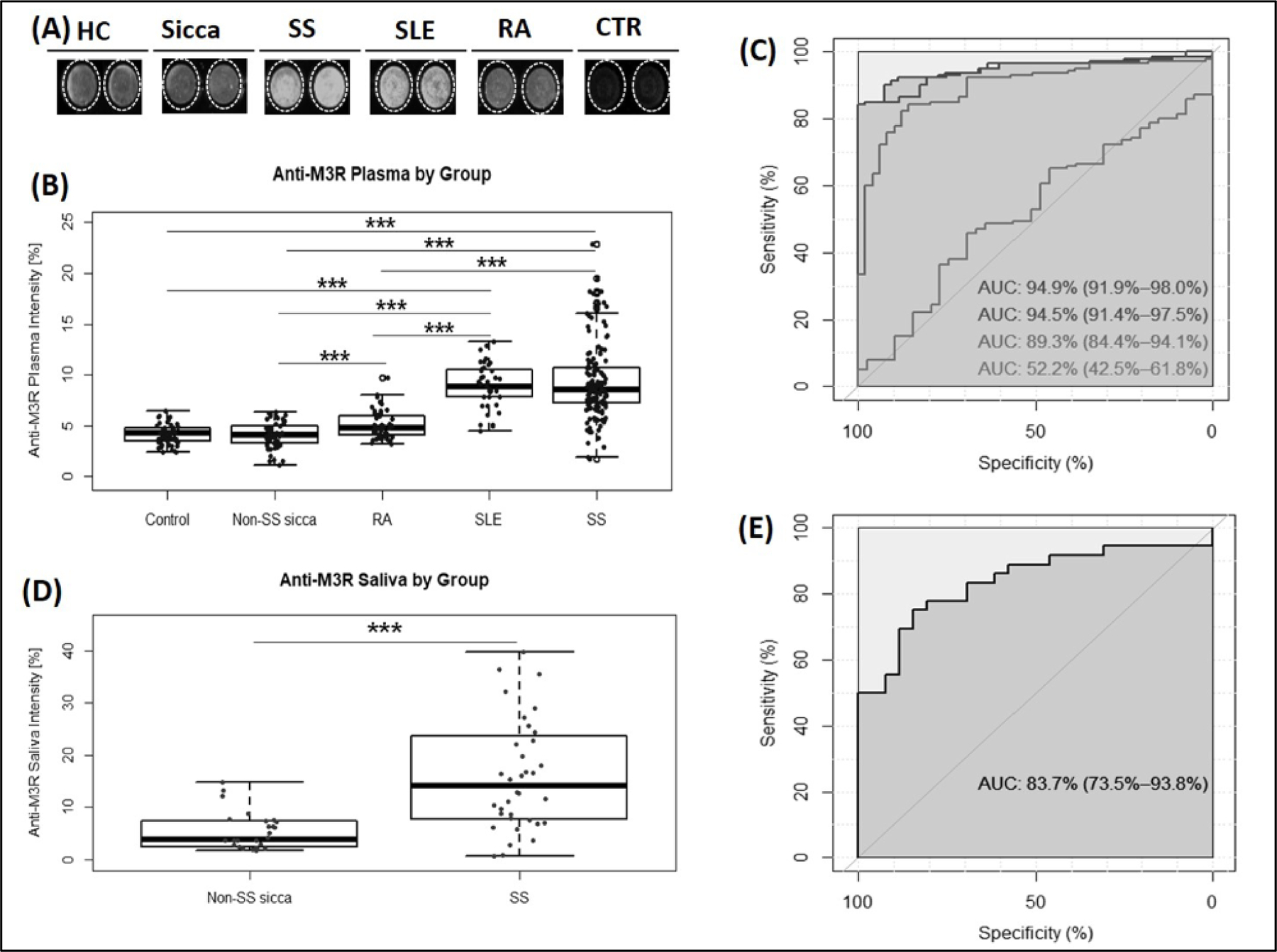
(A) Representative OCW images of P-anti-M3R in HC (n = 53), Sicca (n = 62), SS (n = 156), SLE (n = 40) and RA (n = 50). Control (CTR) is a negative control (2°Ab only). SS and SLE visibly showed higher levels of P-anti-M3R intensity (white shade) as compared to other groups. (B & D) Box-and-whisker plots of anti-M3R intensity in plasma (A) and in saliva (D). The black horizontal line in each box represents the median, with the boxes representing the interquartile range. Whiskers above and below the box indicate 1.5 times the interquartile range from either end of each box and circles represent outliers. Each individual value is plotted as a dot superimposed on the graph. Kruskal-Wallis test followed by Dunn’s posttest (correction for multiple testing) was applied. Significant differences are indicated as *** (p < 0.001). (C) Receiver operating characteristic curves (ROC) of P-anti-M3R for distinguishing patients with SS from HC (the first black line), Sicca (the second line), RA (the third line) and SLE (the fourth line). (E) ROC curve of anti-M3R in saliva for discrimination between SS and Sicca patients.
P-anti-M3R positivity is associated with anti-Ro/SSA and glandular infiltration.
SS patients were categorized into anti-M3R positive and negative groups (Table 2). SS patients with positive P-anti-M3R was significantly associated with anti-Ro/SSA and anti-La/SSB (P<0.001). Additionally, P-anti-M3R positive patients were more likely to have a high grade of mononuclear cell infiltration in the minor salivary glands (p<0.05) and a high level of total IgG (p<0.05). Interestingly, a higher percentage of SS patients with positive P-anti-M3R shows a tendency toward Raynaud’s phenomenon (26.5 % versus 8%, p=0.065) or leukopenia (32.5% versus 12.5%, p=0.052) compared to those with a negative result. As shown in Supplementary Table S1, S-anti-M3R positive SS patients also had a significantly higher prevalence of anti-Ro/SSA (100% versus 76.9%, p=0.040). It is of note that S-anti-M3R was prevalent in those with unstimulated WSFR ≤ 0.1 mL/min (87.0% versus 53.8%, p=0.046).
Table 2.
Comparison between P-anti-M3R positive and negative SS patients.
| Plasma anti-M3R (−), n=24 | Plasma anti-M3R (+), n=117 | P value | |
|---|---|---|---|
| Ocular symptoms* | 20 (83.3%) | 93 (79.5%) | 0.785 |
| Ocular signs* | 17 (70.8%) | 95 (81.9%) | 0.217 |
| Schirmer test ≤ 5 mm | 13/23 (56.5%) | 68/103 (66.0%) | 0.390 |
| OSS ≥ 5 | 5/18 (27.8%) | 24/71 (33.8%) | 0.626 |
| Oral symptoms* | 21 (87.5%) | 101 (86.3%) | 1.000 |
| Salivary gland involvement* | 23 (95.8%) | 111/116 (95.7%) | 1.000 |
| Unstimulated WSFR ≤ 0.1 mL/min | 17/23 (73.9%) | 89/110 (80.9%) | 0.448 |
| Abnormal salivary scintigraphy | 16/18 (88.9%) | 95/107 (88.8%) | 1.000 |
| Anti-Ro/SSA (+) | 14 (58.3%) | 114 (97.4%) | 6.672×10 −7 |
| Anti-La/SSB (+) | 4 (16.7%) | 65 (55.6%) | 0.001 |
| Focus score ≥1 | 13/19 (68.4%) | 59/69 (85.5%) | 0.087 |
| Focus score ≥2 | 4/19 (21.1%) | 33/69 (47.8%) | 0.042 |
| Chisholm–Mason grade = 4 | 5/19 (26.3%) | 41/69 (59.4%) | 0.011 |
| ESSDAI ≥5 | 2 (8.3%) | 20 (17.1%) | 0.368 |
| Extraglandular manifestations | 12 (50.0%) | 65 (55.6%) | 0.619 |
| Raynaud’s phenomenon | 2 (8.3%) | 31 (26.5%) | 0.065 |
| Leukopenia | 3 (12.5%) | 38 (32.5%) | 0.052 |
| Hypocomplementemia C3 or C4 | 1/23 (4.3%) | 12/115 (10.4%) | 0.695 |
| Total IgG ≥ 1700 mg/dL | 10/23 (43.5%) | 79 (67.5%) | 0.028 |
P values were calculated by chi-square or Fischer’s exact test as applicable.
defined according to the 2002 AECG classification criteria.
Anti-M3R is correlated with SS autoantibodies, scintigraphy parameters, and WSFR, analyzed by the bivariate correlation analysis.
Anti-M3R was analyzed for its correlation with hematoimmunological parameters by the bivariate correlation test (Table 3). P-anti-M3R and S-anti-M3R were correlated each other, and both were also correlated with anti-Ro/SSA or anti-La/SSB. Although anti-Ro/SSA and anti-La/SSB levels correlated negatively with age, anti-M3R was not affected by age. P-anti-M3R of SS positively correlated with B2M (R=0.38, p<0.0001) or total IgG (R=0.42, p<0.0001), ESR (R=0.39, p<0.0001), ESSDAI (R=0.24, p=0.004), focus score (R=0.56, p<0.0001), and average OSS (R=0.28, p<0.05). P-anti-M3R correlated negatively with C4 levels (R=−0.20, p<0.05), unstimulated WSFR (R=−0.260, p<0.01), stimulated WSFR (R=−0.286, p<0.01), and WBC (R=−0.34, p<0.0001). This analysis was also strongly supported by our regression analysis, as shown in Supplementary Fig. S1.
Table 3.
Bivariate correlation analysis of SS autoantibodies with clinical parameters
| Plasma anti-M3R (n=141) | Salivary anti-M3R (n=36) | Anti-Ro/SSA (n=156) | Anti-La/SSB (n=156) | |
|---|---|---|---|---|
| Average Schirmer’s | −0.145 (0.055) | −0.237 (0.163) | −0.143 (3.52×10−4) | −0.169 (0.026) |
| Average OSSs | 0.283 (0.02) | 0.519 (0.047) | 0.348 (9.18×10 −5 ) | 0.315 (4.25×10 −4 ) |
| Unstimulated WSFR | −0.260 (3.89×10−4) | −0.512 (0.001) | −0.364 (4.80×10−7) | −0.256 (4.79×10−4) |
| Stimulated WSFR | −0.286 (8.75×10−5) | −0.512 (4.07×10−4) | −4.09 (1.11×10−8) | −0.347 (1.55×10−6) |
| Focus score* | 0.562 (4.02×10 −12 ) | 0.569 (0.001) | 0.549 (1.91×10 −11 ) | 0.444 (1.34×10 −7 ) |
| PU | −0.346 (1.23×10−4) | −0.586 (0.007) | −0.431 (1.22×10−6) | −0.431 (1.23×10−6) |
| SMU | −0.431 (1.08×10−6) | −0.598 (0.005) | −0.517 (2.92×10−9) | −0.429 (1.37×10−6) |
| %PE | −0.386 (1.11×10−5) | −0.400 (0.081) | −0.398 (6.86×10−6) | −0.435 (6.12×10−7) |
| %SE | −0.378 (1.94×10−5) | −0.489 (0.029) | −0.442 (4.71×10−7) | −0.339 (1.52×10−4) |
| Age | −0.136 (0.060) | −0.136 (0.416) | −0.143 (0.049) | −0.254 (3.81×10−4) |
| WBC | −0.341 (1.24×10−6) | −0.330 (0.043) | −0.275 (1.26×10−4) | −0.298 (2.83×10−5) |
| Hb | −0.263 (2.25×10−4) | −0.330 (0.047) | −0.184 (1.26×10−4) | −0.182 (0.012) |
| Platelet | −0.201 (0.005) | 0.125 (0.455) | −0.127 (0.080) | 0.018 (0.805) |
| ESR | 0.389 (2.19×10 −8 ) | 0.545 (4.01×10 −4 ) | 0.361 (2.99×10 −7 ) | 0.459 (2.52×10 −11 ) |
| C4 | −0.204 (0.011) | −0.144 (0.503) | −0.021 (0.799) | −0.052 (0.524) |
| Total IgG | 0.416 (6.70×10 −8 ) | 0.439 (0.036) | 0.407 (1.81×10 −7 ) | 0.488 (1.34×10 −10 ) |
| B2M | 0.379 (1.63×10 −6 ) | 0.542 (0.009) | 0.343 (2.00×10 −5 ) | 0.332 (3.55×10 −5 ) |
| ESSDAI | 0.242 (0.004) | 0.229 (0.319) | 0.320 (1.30×10 −4 ) | 0.308 (2.26×10 −4 ) |
| Plasma anti-M3R | 0.620 (3.27×10 −5 ) | 0.737 (1.00×10 −13 ) | 0.536 (1.02×10 −13 ) | |
| Salivary anti-M3R | 0.620 (3.27×10 −5 ) | 0.781 (1.22×10 −8 ) | 0.608 (5.09×10 −5 ) |
OSS, ocular staining score; WSFR, whole salivary flow rate; PU, parotid gland uptake in the salivary scintigraphy; SMU, submandibular gland uptake in the salivary scintigraphy; %PE, percentage parotid excretion in the salivary scintigraphy; %SE, percentage submandibular excretion in the salivary scintigraphy; WBC, white blood cells; Hb, hemoglobin; ESR, erythrocyte sedimentation rate; B2M, β2 microglobulin; ESSDAI, EULAR Sjögren’s syndrome disease activity index;
stratified into 3 grades (focus score <1, 1≤focus score <2, and 2≤ focus score). Numbers indicate “correlation coefficient (p-value)”.
The relationship between anti-M3R with salivary glandular excretion was determined by analyzing scintigraphic parameters and WSFR (Table 3). P-anti-M3R was found to be inversely proportional to ExSM (the percentage of submandibular excretory function, %SE) and ExP (parotid gland excretory function, %PE) measured by 99mTc-pertechnetate salivary gland scintigraphy (R=−0.386 and R=−0.378, respectively, p<0.0001). It was also negatively correlated with unstimulated and stimulated WSFR (R=−0.260 and R=−0.286, respectively, p<0.0001). We also found that P-anti-M3R was correlated with parotid gland uptake ratio of the radioactive tracer, PU (R=−0.346, p<0.0001) and submandibular gland uptake of the tracer, SMU (R=−0.346 and R=−0.431, respectively, p<0.0001). In addition, S-anti M3R was also found to be associated with these scintigraphic parameters (except for %PE) and WSFR.
P-anti-M3R is associated with scintigraphy parameters and OSS while S-anti-M3R is associated with WSFR, analyzed by the regression analysis.
Supplementary Fig. S2 presents linear relations analyzed by the regression analysis. P-anti-M3R demonstrated a linear relation with ExSM and ExP (Fig. S2A and S2B). S-anti-M3R in our smaller cohort showed phase-one decay association with ExSM and ExP (Fig. S2E and S2F). Stimulated and unstimulated WSFR show a linear relationship with S-anti-M3R, but not with S-anti-M3R (p<0.05) (Fig. S2G and S2H).
We also included ocular test results to analyze their association with levels of anti-M3R. OSS, a diagnostic measure for dry eyes, was directly correlated with P-anti-M3R by the linear regression analysis (p<0.05, Fig. S2I) while S-anti-M3R shows one-phase decay association (Fig. S2J). The average Schirmer’s test showed a tendency of linear relationship with P-anti-M3R (Fig. S3K) or one-phase decay association with S-anti-M3R (Fig S2L).
Most SS patients without MSGBx tested positive for P-anti-M3R and/or anti-Ro/SSA.
When all SS patients were stratified based on the availability of focus score, P-anti-M3R positivity (78.4% versus 90.6%, p=0.063, Table 4) was not statistically different between those underwent MSGBx and those who did not. However, the SS subgroup without MSGBx had significantly higher number of patients who are positive for either P-anti-M3R or anti-Ro/SSA than those who underwent MSGBx (100% versus 90.1%, p=0.016), or for both autoantibodies (90.6% versus 75%, p=0.023).
Table 4.
The prevalence of autoantibodies in SS patients with or without MSGBx.
| Status of positive autoantibodies | MSGBx Performed (n=103) | No MSGBx Performed (n=53) | P values |
|---|---|---|---|
| Anti-Ro/SSA | 88 (85.4%) | 53 (100%) | 0.003 |
| Anti-La/SSB | 44 (42.7%) | 35 (66.0%) | 0.006 |
| P-anti-M3R | 69/88 (78.4%) | 48 (90.6%) | 0.063 |
| Anti-Ro/SSA and anti-La/SSB | 42 (40.8%) | 35 (66.0%) | 0.003 |
| P-anti-M3R and anti-Ro/SSA | 66/88 (75.0%) | 48 (90.6%) | 0.023 |
| P-anti-M3R and anti-La/SSB | 33/88 (36.4%) | 33 (62.3%) | 0.003 |
| Anti-Ro/SSA or anti-La/SSB | 90 (87.4%) | 53 (100%) | 0.005 |
| P-anti-M3R or anti-Ro/SSA | 91/101 (90.1%) | 53 (100%) | 0.016 |
| P-anti-M3R or anti-La/SSB | 81/98 (82.7%) | 50 (94.3%) | 0.047 |
MSGBx, minor salivary gland biopsy
The diagnostic performance of the 2002 AECG criteria for SS improved when substituted with P-anti-M3R for histopathology.
Because SS patients who were positive for both or either autoantibodies were highly prevalent in those who did not undergo a lip biopsy (Table 4), the item of focus score ≥1 in the minor salivary gland in the 2002 AECG criteria system was replaced with P-anti-M3R (Table 5). The data analysis indicates that P-anti-M3R was significantly correlated with the number of the 2002 AECG criteria satisfied (n=125, R=0.540, p=7.847×10−11) and the score of 2016 ACR/EULAR criteria (n=193, R=0.579, p=1.000×10−13). When compared to the original AECG criteria or the 2016 ACR/EULAR criteria, our substituted classification criteria showed a substantial agreement of 92.8% (Cohen’s κ= 0.824) and 90.7% (κ=0.779), respectively. When using the 2002 AECG criteria as the reference, the estimated sensitivity and specificity of the substituted criteria were 92.9% and 92.5%, respectively, with positive likelihood ratio of 12.31. When the performance of the substituted criteria was analyzed based on the 2016 ACR/EULAR criteria, the sensitivity and specificity were 92.7% and 86%, respectively, with the positive likelihood ratio of 6.60.
Table 5.
The diagnostic performance of anti-M3R in place of histopathology in the SS criteria.
| Analyzed criteria | P-anti-M3R substituted criteria | P-anti-M3R substituted criteria | 2016 ACR/EULAR criteria |
|---|---|---|---|
| Reference criteria | 2002 AECG criteria | 2016 ACR/EULAR criteria | 2002 AECG criteria |
| No. of observed agreement | 180 (92.78%) | 179 (90.72%) | 188 (96.91%) |
| Kappa | 0.824 (0.736–0.913)* | 0.779 (0.682–0.876) | 0.924 (0.864–0.984) |
| Sensitivity | 92.91% (87.34–96.55) | 92.70% (86.99–96.44%) | 96.45% (91.92–98.84) |
| Specificity | 92.45% (81.79–97.91%) | 85.96% (74.21–93.74%) | 98.11 (89.93–99.95) |
| Positive likelihood ratio | 12.31 (4.79–31.62) | 6.60 (3.47–12.58) | 51.12 (7.33–356.33) |
| Negative likelihood ratio | 0.08 (0.04–0.14) | 0.08 (0.05–0.16) | 0.04 (0.02–0.09) |
In the substituted classification criteria, the item of histopathology (lip biopsy) in the 2002 AECG criteria was replaced with plasma anti-M3R; n=194;
95% confidential interval.
DISCUSSION
As SS-specific biomarkers are unavailable to date, SS diagnosis requires the measurement of multiple clinical parameters, including less invasive blood tests to invasive MSGBx (23). The pathological role of anti-Ro/SSA in hyposalivation is unclear, although its association with extraglandular manifestations is relatively well accepted (6, 24). Anti-M3R prevalence in SS varies from 1.9% to 97.0%. Most studies on anti-M3R evaluated a small sample size (n<60 in 14 out of 22 studies), mainly utilizing a linear peptide-based ELISA (12). When compared with controls, the prevalence of anti-M3R in SS was significantly higher in 11 studies whereas the other 11 studies showed no significant difference (12). However, by maintaining M3R tertiary structure, our lab and others have consistently reported upregulation of anti-M3R expression in SS patients (13, 25). These studies clearly indicate that anti-M3R requires a detection method designed for conformation-dependent epitopes, which has challenged the establishment and calibration of cell-based assays for anti-M3R screening.
The modified OCW assay has allowed our group to perform a reliable anti-M3R screening method with plasma samples from the UF cohort (14). Anti-M3R levels were significantly elevated in SS plasma in comparison with HC, SLE, or RA (p < 0.01). Furthermore, anti-M3R was associated with anti-Ro/SSA positivity (p=0.035), and indicated positive linear associations with the focus score (p < 0.01) and negative associations with its unstimulated WSFR (p < 0.05) (14). In our current study with the largest SNUBH cohort for an anti-M3R study, we found that P-anti-M3R was significantly elevated in SS, reliably distinguishing SS from other conditions, as shown by the ROC analysis. The cut-off of 6.13 for positive anti-M3R in the unadsorbed, deidentified SNUBH cohort samples was almost identical to the cut-off of 6.24 in our previous study with UF samples (14), indicating the reliability of our cell-based assay.
Interestingly, P-anti-M3R does not substantially distinguish SS from SLE (AUC of 0.56), unlike in the UF study (AUC of 0.72) (14). Potential reasons include: 1) SLE and SS patients demonstrate some common clinical/serological features, which makes SS diagnosis challenging (26, 27). 2) Ethnically heterogeneous (UF) and homogeneous (SNUBH) groups were enrolled for the previous and the current study, respectively. A different prevalence of anti-M3R in different ethnic groups with SS or SLE can be presumed. 3) Notably, the number of anti-Ro/SSA-positive SLE patients at SNUBH is significantly higher than SLE patients at UF (74.3% vs. 38.9%). In addition, almost all SNUBH patients tested positive for anti-nuclear antibody in SS and SLE patients whereas the SS and SLE patients at UF were 80% and 72% positive, respectively (data not shown). It has been reported that anti-Ro/SSA is more commonly detected in Asian SLE patients, including Koreans, than in Caucasians (28, 29). A stringent inclusion and exclusion criteria may minimize differences in subject eligibility among various facilities when multi-center studies are designed.
We also found that S-anti-M3R is significantly higher in SS than in Sicca, reliably distinguishing SS from Sicca (AUC=0.84, p<0.0001). Screening of S-anti-M3R required a 1:1 dilution and the cut-off of 14.3% for positivity while P-anti-M3R required a 1:400 dilution with 6.13% as cut-off. Major antibody classes in saliva are secretory or polymeric IgA (sIgA) and IgG. Unlike sIgA, which is mainly synthesized by plasma cells in the salivary gland and secreted by receptor-mediated transcytosis, most of salivary IgG are derived from serum by passive diffusion though gingival crevices (30). Although salivary glandular/gingival plasma cells may produce salivary IgG, its concentration is much lower than that in serum (31). Therefore, this low sensitivity of detecting S-anti-M3R IgG is unsurprising. Despite its low concentration, salivary IgG levels are known to be correlated with serum IgG levels, reflecting systemic immunity (30). We were unable to determine whether anti-M3R sIgA is elevated in SS due to unavailability of the OCW compatible-IRDye®800CW-anti-IgA secondary antibody on the market. Future analyses of saliva, depending upon the availability of the antibody, may enhance detection sensitivity, will provide a clear insight into the prevalence and usefulness of S-anti-M3R as a non-invasive diagnostic tool for SS.
Our analysis found that P-anti-M3R was significantly associated with important SS-disease parameters, such as anti-Ro/SSA, focus score ≥ 2, grade 4 on the Chisholm-Mason scale, and hypergammaglobulinemia. S-anti-M3R positivity was associated with anti-Ro/SSA and unstimulated WSFR. In addition, our bivariate correlation analysis suggested the potential involvement of P-anti-M3R in extraglandular manifestations, shown as a negative correlation with WBC and platelet. Autoimmune cytopenia is a well-known extraglandular manifestation of SS. A study has shown that anti-M3R enhanced Jurkat T cell death through MHC class I downregulation when co-incubated with NK cells (32). Notably, P-anti-M3R levels were negatively correlated with serum C4 levels in our study, whereas anti-Ro/SSA showed no correlation. In SS, low C4 or C3 is observed in about 15% of SS patients (33) and is considered to be a marker for systemic manifestations and mortality in SS (34). Importantly, low C4 is known to be a risk factor for non-Hodgkin lymphoma in SS (35). Hypergammaglobulinemia was also positively associated with P-anti-M3R. Taken together, P-anti-M3R was significantly correlated with ESSDAI in our SS cohort, especially for the hematological and biological domains. Interestingly, anti-M3R showed a tendency toward a positive association with Raynaud’s phenomenon in our study. No direct evidence is available in the literature for the roles of anti-M3R in Raynaud’s phenomenon. However, a recent study with the M3R knock-out mouse model demonstrating impaired vasodilation (36), suggests a potential role of anti-M3R in impaired vasodilation, which warrants further investigation.
This study also found that P-anti-M3R positivity was similar or more prevalent in SS patients who did not undergo MSGBx than those with MSGBx. The histopathology is a key criterion in the 2002 and 2016 criteria, as focus scores are generally considered to be a robust tool for SS diagnosis. However, focal lymphocytic infiltration was observed in 15% of healthy volunteers without a history of salivary gland dysfunction (37). Furthermore, interobserver and intraobserver agreements in MSGBx were 0.71 and 0.76, respectively, and many pathologists failed to delineate focal lymphocytic sialadenitis from non-specific lymphocytic sialadenitis in a multicenter study (38). In a longitudinal study where MSGBx was repeated after 2 to 3 years, the results changed from focus-negative to focus-positive in 7% of participants and from focus-positive to focus-negative in 11% of subjects (39). Furthermore, this invasive procedure led to lower lip paresthesia in 6% of subjects (40). Due to such limitations, salivary gland ultrasonography has recently been proposed as a substitution for MSGBx. A recent systematic review has shown the sensitivity of ultrasonography ranged from 45.8 to 91.6% and specificity from 73 to 98.1% (41). When compared to the 2002 AECG classification criteria, Mossel et al reported an agreement of 82%, sensitivity of 71%, and specificity of 92% (42), and Takagi et al reported a sensitivity of 81% and specificity of 86% (43). Therefore, higher sensitivity (92.91%) and specificity (92.45%) of P-anti-M3R in the substituted 2002 AECG criteria than those of ultrasonography highlight anti-M3R as a specific and sensitive diagnostic tool for SS, especially in clinics where lip biopsy services may not be immediately available.
In conclusion, P-anti-M3R was highly prevalent in SS patients, which was significantly associated with glandular infiltration and overreactive immune cells. Additionally, the level of P-anti-M3R showed a linear relationship with OSS, salivary excretory function, and parameters in the hematological and biological domains of ESSDAI, especially levels of C4 and IgG. The S-anti-M3R levels were inversely correlated with WSFR. The dissemination of the P-anti-M3R screening assay, which is currently underway, and the development of a non-invasive liquid biopsy utilizing S-anti-M3R is expected to expedite SS diagnosis.
Supplementary Material
Acknowlegements
This study was supported by the National Institute of Dental and Craniofacial Research at the National Institute of Health [DE025726 and DE023838 to S.C., and T90DE021990 to M. M.], and the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education [2015R1D1A1A01060468 to Y.J.L.].
Footnotes
Conflict of Interest
All authors declare no conflicts of interest.
REFERENCES
- 1.Peri Y, Agmon-Levin N, Theodor E, Shoenfeld Y. Sjögren’s syndrome, the old and the new. Best Pract Res Clin Rheumatol 2012;26(1):105–17. [DOI] [PubMed] [Google Scholar]
- 2.Kassan SS, Moutsopoulos HM. Clinical manifestations and early diagnosis of Sjogren syndrome. Archives of internal medicine. 2004;164(12):1275–84. [DOI] [PubMed] [Google Scholar]
- 3.Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: A consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis 2017;76(1):9–16. [DOI] [PubMed] [Google Scholar]
- 4.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 2002;61(6):554–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramos-Casals M, Solans R, Rosas J, Camps MT, Gil A, Del Pino-Montes J, et al. Primary Sjögren syndrome in Spain: clinical and immunologic expression in 1010 patients. Medicine (Baltimore). 2008;87(4):210–9. [DOI] [PubMed] [Google Scholar]
- 6.Baer AN, McAdams DeMarco M, Shiboski SC, Lam MY, Challacombe S, Daniels TE, et al. The SSB-positive/SSA-negative antibody profile is not associated with key phenotypic features of Sjögren’s syndrome. Ann Rheum Dis 2015;74(8):1557–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eglen RM. Muscarinic receptor subtypes in neuronal and non-neuronal cholinergic function. Auton Autacoid Pharmacol 2006;26(3):219–33. [DOI] [PubMed] [Google Scholar]
- 8.Hulme EC, Birdsall NJ, Buckley NJ. Muscarinic receptor subtypes. Annu Rev Pharmacol Toxicol 1990;30:633–73. [DOI] [PubMed] [Google Scholar]
- 9.Cha S, Singson E, Cornelius J, Yagna JP, Knot HJ, Peck AB. Muscarinic acetylcholine type-3 receptor desensitization due to chronic exposure to Sjögren’s syndrome-associated autoantibodies. J Rheumatol 2006;33(2):296–306. [PubMed] [Google Scholar]
- 10.Lee BH, Gauna AE, Perez G, Park YJ, Pauley KM, Kawai T, et al. Autoantibodies against muscarinic type 3 receptor in Sjogren’s syndrome inhibit aquaporin 5 trafficking. PloS one. 2013;8(1):e53113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sumida T, Iizuka M, Asashima H, Tsuboi H, Matsumoto I. Pathogenic role of anti-M3 muscarinic acetylcholine receptor immune response in Sjögren’s syndrome. Presse Med 2012;41(9 Pt 2):e461–6. [DOI] [PubMed] [Google Scholar]
- 12.Yu X, Riemekasten G, Petersen F. Autoantibodies against muscarinic acetylcholine receptor M3 in Sjogren’s syndrome and corresponding mouse models. Front Biosci (Landmark Ed) 2018;23:2053–64. [DOI] [PubMed] [Google Scholar]
- 13.Gao J, Cha S, Jonsson R, Opalko J, Peck AB. Detection of anti-type 3 muscarinic acetylcholine receptor autoantibodies in the sera of Sjogren’s syndrome patients by use of a transfected cell line assay. Arthritis Rheum 2004;50(8):2615–21. [DOI] [PubMed] [Google Scholar]
- 14.Zuo J, Williams AE, Park YJ, Choi K, Chan AL, Reeves WH, et al. Muscarinic type 3 receptor autoantibodies are associated with anti-SSA/Ro autoantibodies in Sjogren’s syndrome. Journal of immunological methods. 2016;437:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69(9):1580–8. [DOI] [PubMed] [Google Scholar]
- 16.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40(9):1725. [DOI] [PubMed] [Google Scholar]
- 17.Lee YJ, Scofield RH, Hyon JY, Yun PY, Lee HJ, Lee EY, et al. Salivary chemokine levels in patients with primary Sjogren’s syndrome. Rheumatology (Oxford). 2010;49(9):1747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seror R, Ravaud P, Bowman SJ, Baron G, Tzioufas A, Theander E, et al. EULAR Sjogren’s syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjogren’s syndrome. Ann Rheum Dis 2010;69(6):1103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seror R, Bootsma H, Saraux A, Bowman SJ, Theander E, Brun JG, et al. Defining disease activity states and clinically meaningful improvement in primary Sjögren’s syndrome with EULAR primary Sjögren’s syndrome disease activity (ESSDAI) and patient-reported indexes (ESSPRI). Ann Rheum Dis 2016;75(2):382–9. [DOI] [PubMed] [Google Scholar]
- 20.Chisholm DM, Mason DK. Labial salivary gland biopsy in Sjogren’s disease. Journal of clinical pathology. 1968;21(5):656–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniels TE, Whitcher JP. Association of patterns of labial salivary gland inflammation with keratoconjunctivitis sicca. Analysis of 618 patients with suspected Sjogren’s syndrome. Arthritis Rheum 1994;37(6):869–77. [DOI] [PubMed] [Google Scholar]
- 22.Kang JY, Jang SJ, Lee WW, Lee YJ, Kim SE. Evaluation of Salivary Gland Dysfunction Using Salivary Gland Scintigraphy in Sjögren’s Syndrome Patients and in Thyroid Cancer Patients after Radioactive Iodine Therapy. Nucl Med Mol Imaging. 2011;45(3):161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niikura AJ, Yamachika S, Yamamoto K, Okamoto MR, Ikeda YF, Nakamura S, et al. Efficient diagnosis of Sjögren’s syndrome to reduce the burden on patients. Mod Rheumatol 2015;25(1):100–4. [DOI] [PubMed] [Google Scholar]
- 24.Yang Z, Liang Y, Zhong R. Is identification of anti-SSA and/or -SSB antibodies necessary in serum samples referred for antinuclear antibodies testing? J Clin Lab Anal 2012;26(6):447–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin M, Hwang SM, Davies AJ, Shin Y, Bae JS, Lee JH, et al. Autoantibodies in primary Sjögren’s syndrome patients induce internalization of muscarinic type 3 receptors. Biochim Biophys Acta 2012;1822(2):161–7. [DOI] [PubMed] [Google Scholar]
- 26.Al Kindi MA, Colella AD, Chataway TK, Jackson MW, Wang JJ, Gordon TP. Secreted autoantibody repertoires in Sjögren’s syndrome and systemic lupus erythematosus: A proteomic approach. Autoimmun Rev 2016;15(4):405–10. [DOI] [PubMed] [Google Scholar]
- 27.Streifler JY, Molad Y. Connective tissue disorders: systemic lupus erythematosus, Sjögren’s syndrome, and scleroderma. Handb Clin Neurol 2014;119:463–73. [DOI] [PubMed] [Google Scholar]
- 28.Cho CS, Park SH, Min JK, Lee SH, Kim HY. Clinical significances of antibodies to Ro/SS-A autoantigens and its subtypes in primary Sjögren’s syndrome. Korean J Intern Med 1997;12(2):176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MOK M-Y. Profile of lupus autoantibodies found in Asia. 2006;9:331–5. [Google Scholar]
- 30.Brandtzaeg P Secretory immunity with special reference to the oral cavity. J Oral Microbiol 2013;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Exum NG, Pisanic N, Granger DA, Schwab KJ, Detrick B, Kosek M, et al. Use of Pathogen-Specific Antibody Biomarkers to Estimate Waterborne Infections in Population-Based Settings. Curr Environ Health Rep 2016;3(3):322–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Namkoong E, Lee SW, Kim N, Choi Y, Park K. Effect of anti-muscarinic autoantibodies on leukocyte function in Sjögren’s syndrome. Mol Immunol 2017;90:136–42. [DOI] [PubMed] [Google Scholar]
- 33.Brito-Zerón P, Acar-Denizli N, Ng WF, Zeher M, Rasmussen A, Mandl T, et al. How immunological profile drives clinical phenotype of primary Sjögren’s syndrome at diagnosis: analysis of 10,500 patients (Sjögren Big Data Project). Clin Exp Rheumatol 2018;36 Suppl 112(3):102–12. [PubMed] [Google Scholar]
- 34.Ramos-Casals M, Brito-Zerón P, Yagüe J, Akasbi M, Bautista R, Ruano M, et al. Hypocomplementaemia as an immunological marker of morbidity and mortality in patients with primary Sjogren’s syndrome. Rheumatology (Oxford). 2005;44(1):89–94. [DOI] [PubMed] [Google Scholar]
- 35.Voulgarelis M, Tzioufas AG. Pathogenetic mechanisms in the initiation and perpetuation of Sjögren’s syndrome. Nat Rev Rheumatol 2010;6(9):529–37. [DOI] [PubMed] [Google Scholar]
- 36.Deng AY, deBlois D, Laporte SA, Gelinas D, Tardif JC, Thorin E, et al. Novel Pathogenesis of Hypertension and Diastolic Dysfunction Caused by M3R (Muscarinic Cholinergic 3 Receptor) Signaling. Hypertension. 2018;72(3):755–64. [DOI] [PubMed] [Google Scholar]
- 37.Radfar L, Kleiner DE, Fox PC, Pillemer SR. Prevalence and clinical significance of lymphocytic foci in minor salivary glands of healthy volunteers. Arthritis Rheum 2002;47(5):520–4. [DOI] [PubMed] [Google Scholar]
- 38.Costa S, Quintin-Roué I, Lesourd A, Jousse-Joulin S, Berthelot JM, Hachulla E, et al. Reliability of histopathological salivary gland biopsy assessment in Sjögren’s syndrome: a multicentre cohort study. Rheumatology (Oxford). 2015;54(6):1056–64. [DOI] [PubMed] [Google Scholar]
- 39.Shiboski CH, Baer AN, Shiboski SC, Lam M, Challacombe S, Lanfranchi HE, et al. Natural History and Predictors of Progression to Sjögren’s Syndrome Among Participants of the Sjögren’s International Collaborative Clinical Alliance Registry. Arthritis Care Res (Hoboken). 2018;70(2):284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pijpe J, Kalk WW, van der Wal JE, Vissink A, Kluin PM, Roodenburg JL, et al. Parotid gland biopsy compared with labial biopsy in the diagnosis of patients with primary Sjogren’s syndrome. Rheumatology (Oxford). 2007;46(2):335–41. [DOI] [PubMed] [Google Scholar]
- 41.Jousse-Joulin S, Milic V, Jonsson MV, Plagou A, Theander E, Luciano N, et al. Is salivary gland ultrasonography a useful tool in Sjögren’s syndrome? A systematic review. Rheumatology (Oxford). 2016;55(5):789–800. [DOI] [PubMed] [Google Scholar]
- 42.Mossel E, Delli K, van Nimwegen JF, Stel AJ, Kroese FGM, Spijkervet FKL, et al. Ultrasonography of major salivary glands compared with parotid and labial gland biopsy and classification criteria in patients with clinically suspected primary Sjögren’s syndrome. Ann Rheum Dis 2017;76(11):1883–9. [DOI] [PubMed] [Google Scholar]
- 43.Takagi Y, Sumi M, Nakamura H, Iwamoto N, Horai Y, Kawakami A, et al. Ultrasonography as an additional item in the American College of Rheumatology classification of Sjögren’s syndrome. Rheumatology (Oxford). 2014;53(11):1977–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


