Abstract
Introduction:
The neurodevelopmental model of psychosis was established over 30 years ago; however, the developmental influence on psychotic symptom expression – how age affects clinical presentation in first-episode psychosis – has not been thoroughly investigated.
Method:
Using generalized additive modeling, which allows for linear and non-linear functional forms of age-related change, we leveraged symptom data from a large sample of antipsychotic-naïve individuals with first episode psychosis (N=340, 12–40 years, 1–12 visits), collected at the University of Pittsburgh from 1990–2017. We examined relationships between age and severity of perceptual and non-perceptual positive symptoms and negative symptoms. We tested for age-associated effects on change in positive or negative symptom severity following baseline assessment, and explored the time-varying relationship between perceptual and non-perceptual positive symptoms across adolescent development.
Results:
Perceptual positive symptom severity significantly decreased with increasing age (F=7.0, p=0.0007; q=0.003) while non-perceptual positive symptom severity increased with age (F=4.1, p=0.01, q=0.02). Anhedonia severity increased with increasing age (F=6.7, p=0.00035; q=0.0003), while flat affect decreased in severity with increased age (F=9.8, p=0.002; q=0.006). Findings remained significant when SES, IQ, and illness duration were included as covariates. There were no developmental effects on change in positive or negative symptom severity (all p>0.25). An association between severity of non-perceptual and perceptual symptoms developed with increasing age starting at age 18.
Conclusion:
These findings suggest that as cognitive maturation proceeds, perceptual symptoms attenuate while non-perceptual symptoms are enhanced, reflecting influences of developmental processes on psychosis expression. Findings underscore how pathological brain-behavior relationships vary as a function of development.
Keywords: schizophrenia, psychotic symptoms, adolescence, antipsychotic-naïve, age effects
Introduction
Over the past 30 years, the neurodevelopmental model of schizophrenia has become a dominant theoretical framework for organizing findings and generating hypotheses related to psychosis pathogenesis. The premise of the model is that an individual’s sensitivity to certain inputs (e.g., teratogens, perinatal complications, adverse childhood experiences) and likelihood of expressing certain clinically significant outputs (e.g., disorganized behavior, hallucinations) are modulated by the individual’s brain maturation, genetic makeup, changes in gene expression across development, and/or epigenetic influences, particularly during adolescence (Weinberger et al., 1986; Murray and Lewis, 1987; Insel, 2010; Owen et al., 2011; Rapoport et al., 2012). Despite the prominence of this model, differences in symptom expression as a result of these maturational changes throughout adolescent and young-adult development have not been examined thoroughly.
Consistent with the proposed model, late adolescence and early adulthood is a time of increased vulnerability for the emergence of symptoms that meet criteria for schizophrenia-spectrum disorders (Amminger et al., 2006; Öngür et al., 2009). However, there is not conclusive evidence of symptomatology changing over the course of development in psychosis. If brain maturation modulates the expression of psychosis (both prevalence and severity of symptoms), it is reasonable to expect, for example, that a 12-year old’s symptom expression differs from a 26-year old’s. Symptom expression of other psychiatric disorders, including depression and anxiety, changes across development, particularly during adolescence. Multiple studies find that the severity and/or prevalence of depression and anxiety symptoms decline in early adolescence, but increase in severity and/or prevalence in mid-late adolescence (Ge et al., 2001; Garber et al., 2002; Van Oort et al., 2009). There is also evidence of changes in specific anxiety symptoms throughout adolescence, with the prevalence of generalized anxiety and social anxiety increasing throughout adolescence (Costello et al., 2003) and panic disorder and separation anxiety symptoms decreasing between early and mid-adolescence (Hale et al., 2008). Similarly, distinct psychotic symptoms could follow unique developmental patterns. Understanding whether and how age varies with psychotic symptom expression could have important implications for creating developmentally-informed assessment and treatment practices, and for understanding the mechanisms underlying specific symptoms.
Previous work suggests that age plays an important role in psychosis symptom development. When positive symptoms are divided into specific sub-groups, there is evidence that perceptual positive symptoms (i.e., illusory sensory experiences such as hallucinations) are present to a greater extent in younger individuals (Mueser et al., 1990), while non-perceptual positive symptoms (e.g., delusions) have greater prevalence in older individuals with psychosis (Häfner et al., 1993). Studies of childhood- or adolescent-onset psychosis find that that these youth endorse higher rates of visual hallucinations than would be expected based on the adult-onset psychosis literature (Green et al., 1992; David et al., 2011). Furthermore, multiple cross-sectional studies of general population cohorts and individuals at high risk for developing psychosis report that younger individuals are more likely to endorse perceptual psychotic experiences in comparison to older individuals (Kelleher et al., 2012b; Brandizzi et al., 2014; Schimmelmann et al., 2015; Schultze-Lutter et al., 2017). However, investigations of age effects on total positive symptoms in chronic and first-episode psychosis fail to find differences between age groups or find significant effects of age on symptom presentation (Haas and Sweeney, 1992; Sharma, 1999; Ballageer et al., 2005; White et al., 2006; Joa et al., 2009). Taken together, these results suggest positive symptoms of psychosis display significant age-related variability, but it is critical to examine developmental patterns within relevant sub-groups of positive symptoms. Age effects have not yet been systematically examined in a longitudinal first-episode psychosis sample, which is less likely to be influenced by disease chronicity and medication effects.
Some investigations of developmental influences on negative symptom severity find that younger people with schizophrenia spectrum disorders showed more prominent negative symptoms (Ballageer et al., 2005; Pencer et al., 2005). However, the majority of studies of participants across the psychosis spectrum fail to find age-associated effects on total negative symptoms (Haas and Sweeney, 1992; White et al., 2006; Joa et al., 2009; Devylder et al., 2013). Late adolescence and early adulthood are times where transitioning to new roles is important (e.g. starting college, beginning full-time work); thus, developmentally-focused explorations of negative symptom severity may be particularly important, as negative symptoms are more closely related to functional impairments than positive symptoms (Ho et al., 1998; Milev et al., 2005).
Table 1 summarizes previous investigations of age effects in symptom presentation across the psychosis spectrum. While some patterns are observed (as described above), there are also inconsistencies. Antipsychotic medication exposure may affect age-symptom associations in psychosis, as antipsychotic medications treat perceptual positive symptoms more quickly or effectively than non-perceptual positive symptoms and negative symptoms (Gunduz-Bruce et al., 2005; Lecrubier et al., 2007; Schneider et al., 2011; Fusar-Poli et al., 2015; Bjarke et al., 2020). All previously published studies in those diagnosed with psychotic disorders include individuals who were currently or previously prescribed antipsychotic medications. Additionally, the majority of previous studies are cross-sectional, precluding the ability to assess within-subject change, and the statistical methods used only assessed linear relationships. Many developmental processes follow a non-linear trajectory and non-linear modeling approaches in developmental neurocognitive science have identified distinct periods of continued refinement of brain structure in typically-developing youth (Simmonds et al., 2014; Calabro et al., 2020). Use of these approaches with longitudinal symptom data may identify distinct periods of change that are obscured in cross-sectional or linear models. Finally, given evidence that neurobiological factors exert differential influences on symptomatology at distinct points in development (Glaser et al., 2011; Jalbrzikowski et al., 2017; Ellwood-Lowe et al., 2018), use of time-varying approaches may prove to be informative.
Table 1.
Summary of previous studies that have examined effects of age on psychotic symptom severity. The table is broken down into A) studies that examine participants across the phase of illness, B) studies that focus on first episode psychosis, C) studies of help-seeking adolescents, and D) population sample studies. Other than Pencer et al. (2005), all studies are cross-sectional in nature. All studies of participants with diagnosed with a psychotic disorder include individuals who are currently or previously have been prescribed antipsychotic medication.
| Author and year | Sample size (N) | Age range or mean & type of analysis (group vs. continuous) | Dependent variables examined | Results |
|---|---|---|---|---|
| A. Studies of psychotic disorder across multiple phases of the illness | ||||
| Mueser et al., (1990) | 117 | Age range: 20–58 yrs; Group: those who endorsed hallucinations vs. those who did not |
Responses to auditory, tactile, visual, and olfactory/gustatory hallucination items on SCID-DSM III (Spitzer & Williams, 1985) | Those who endorsed auditory hallucinations had an earlier age of hospitalization vs. those who did not endorse auditory hallucinations. |
| Haas & Sweeney, (1992) | 71 | 18–55 yrs; Continuous |
Total symptom scores from SAPS and SANS (Andreasen 1984a, 1984b) | No significant effects of age on total positive or negative symptom severity. |
| Sharma et al., (1999) | 160 | Age range not reported. Mean age: 32.3 yrs +/−8 yrs; Continuous |
Hallucinations item score from BPRS (Overall 1962) | No significant effect of age or age of onset on hallucination severity. |
| B. Studies of first episode psychosis | ||||
| Hafner et al., (1992) | 276 | 12–59 yrs; Group: 12–24 yrs (N=90) vs. 25–34 yrs (N=110) vs. 35–59 yrs (N=76) |
Individual positive and negative symptom items measured via a semi-structured interview (Hafner et al., 1992) | Increased delusions of reference in 35–39 yrs vs. 12–24 yrs. Increased delusions of persecution in 25–34 yrs vs. 12–24 yrs. |
| Ballageer et al., (2005) | 201 | 15–30 yrs; Group: 15–18 yrs (N=82) vs. 19–30 yrs (N=119) |
Individual item scores from SAPS and SANS (Andreasen 1984a, 1984b) | Increased affective flattening in the younger (15–18 yrs) vs. older group (19–30 yrs). No significant differences between groups for all other measures. |
| Pencer et al., (2005) | 138 (1–3 visits) |
Group: Adolescents: 15–19 yrs (N=69) Adults: 26–50 yrs (N=69) | Total Positive and Negative symptom scores from the PANSS (Kay et al., 1987) | No significant effect of group at baseline, or 1- or 2-yr follow-up. Younger people showed more prominent negative symptoms at baseline. |
| White et al., (2006) | 188 | Group: 12–19 yrs (N=49) vs. 20–39 yrs (N=139) | Total score for SAPS (Andreasen 1984a, 1984b) | No significant differences in total positive and negative symptom scores between age groups. |
| Joa et al., (2009) | 232 | Age range: 15–65 yrs; Group: ≤18 yrs (N=43) vs. >18 yrs (N=189) | Total Positive and Negative symptom scores from the PANSS (Kay et al., 1987) | No significant differences in total positive and negative symptom scores between age groups. |
| C. Studies of help-seeking adolescents | ||||
| Brandizzi et al., (2014) | 171 | Age range: 11–18 yrs; Group: 11–12 yrs (N=30), 13–14 yrs (N=52), 15–16 yrs (N=49), 17–18 years (N=40) |
Four factor scores from positive scale of the Prodromal Questionnaire (Loewy 2005): Conceptual Disorganization and Suspiciousness, Perceptual Abnormalities, Bizarre Experiences, and Magical Ideation | Increased perceptual positive symptoms in 11–12 yrs vs. 17–18 yrs Increased non-perceptual positive symptoms (bizarre experiences) in 17–18 yrs vs. 15–16 yrs |
| Schultze-Lutter et al., (2017) | 133 | Age range: 8–40 yrs; Group: 8–12 yrs (N=12), 13–15 yrs (N=30), 16–17 yrs (N=33), 18–19 yrs (N=15), 20–24 yrs (N=30) 25–40 yrs (N=13) |
Perceptual (P4) and non-perceptual abnormality scores (P1+P2+P3+P5) from SIPS (McGlashan 2002) | Increased subthreshold perceptual positive symptoms in 8–12 yrs. vs. all other age groups. No significant differences between groups for non-perceptual sub-threshold positive symptoms. |
| DeVylder et al., (2013) | 65 | Age range: 12–30 yrs; Continuous | Ratings from Positive and Negative symptom items from SIPS (McGlashan, 2002) | No significant effects of age for positive or negative symptom scores. |
| D. Population sample studies | ||||
| Kelleher et al., (2012b) | Studies 1 & 2 = 2243 Studies 3 & 4 = 329 | Age range: 11–16 yrs; Studies 1 & 2: Continuous; Studies 3 & 4: Group: 11–12 yrs (N=212), 13–15 yrs (N=117) |
Studies 1 & 2: Auditory hallucination item from Adolescent Psychotic Symptom Screener (Kelleher 2011) Studies 3 & 4: Responses to K-SADS-PL psychosis questions (Kaufman et al. 1996) |
Studies 1 & 2: Decreased auditory hallucination endorsement with increasing age. Studies 3 & 4: 22.6% of 11–12 yrs endorsed psychotic symptoms vs. 7% of 13–15 yrs. |
| Schimmelmann et al., (2015) | 689 | Age range: 8–40 yrs; Group: 8–12 yrs (N=45), 13–15 yrs (N=31), 16–17 yrs (N=78), 18–19 yrs (N=81), 20–24 yrs (N=155), 25–29 yrs (N=144), 30–40 yrs (N=155) |
Perceptual (P4) and non-perceptual abnormality scores (P1+P2+P3+P5) from SIPS (McGlashan 2002) | Increased perceptual positive symptom experiences in 8–12 yrs and 13–15 yrs vs. all other age groups. No significant differences between groups for non-perceptual positive experiences. |
Abbreviations: yrs.: years; SCID-DSMII= Structured Clinical Interview for the DSM-III; SAPS=Scale for the Assessment of Positive Symptoms; SANS=Scale for the Assessment of Negative Symptoms; BPRS=Brief Psychiatric Rating Scale; PANSS=Positive and Negative Syndrome Scale; SIPS=Structured Interview for Prodromal Syndromes; P1=unusual thoughts rating on SIPS; P2=suspiciousness rating on SIPS; P3=grandiosity rating on SIPS; P4=perceptual abnormality rating on SIPS; P5=disorganized communication on SIPS; KSADS-PL=Kiddie Schedule for Affective Disorders and Schizophrenia, Present and Lifetime version.
In this study, we leveraged a longitudinal sample of antipsychotic-naïve (at baseline) first-episode psychosis participants (FEP, N=340, 1–12 visits, 12–40 years) to 1) examine developmental effects on severity of perceptual and non-perceptual positive symptoms, and negative symptoms, 2) investigate developmental effects on change in psychotic symptom severity following first-episode, and 3) explore age-varying relationships between perceptual and non-perceptual positive symptom severity. Based on previous work (Häfner et al., 1992; Kelleher et al., 2012b; Brandizzi et al., 2014; Schimmelmann et al., 2015; Schultze-Lutter et al., 2017), we hypothesized that perceptual positive symptoms would decrease in severity with increasing age and non-perceptual positive symptoms would be stable across adolescent development. Consistent with others (Haas and Sweeney, 1992; White et al., 2006; Joa et al., 2009; Devylder et al., 2013), we hypothesized that negative symptoms would remain stable across adolescent development. All remaining analyses were exploratory.
Materials and Methods
Participants
Participant data was taken from archival and ongoing studies at the University of Pittsburgh (1990–2017). The final sample consisted of 340 individuals with multiple visits (1–12 visits, n = 1068 total). See Figure 1 for demographic information and sample characterization. Study procedures were approved by the University of Pittsburgh Institutional Review Board and performed in accordance with the Declaration of Helsinki. All participants or their legal guardians provided written informed consent after study procedures were fully explained.
Figure 1.
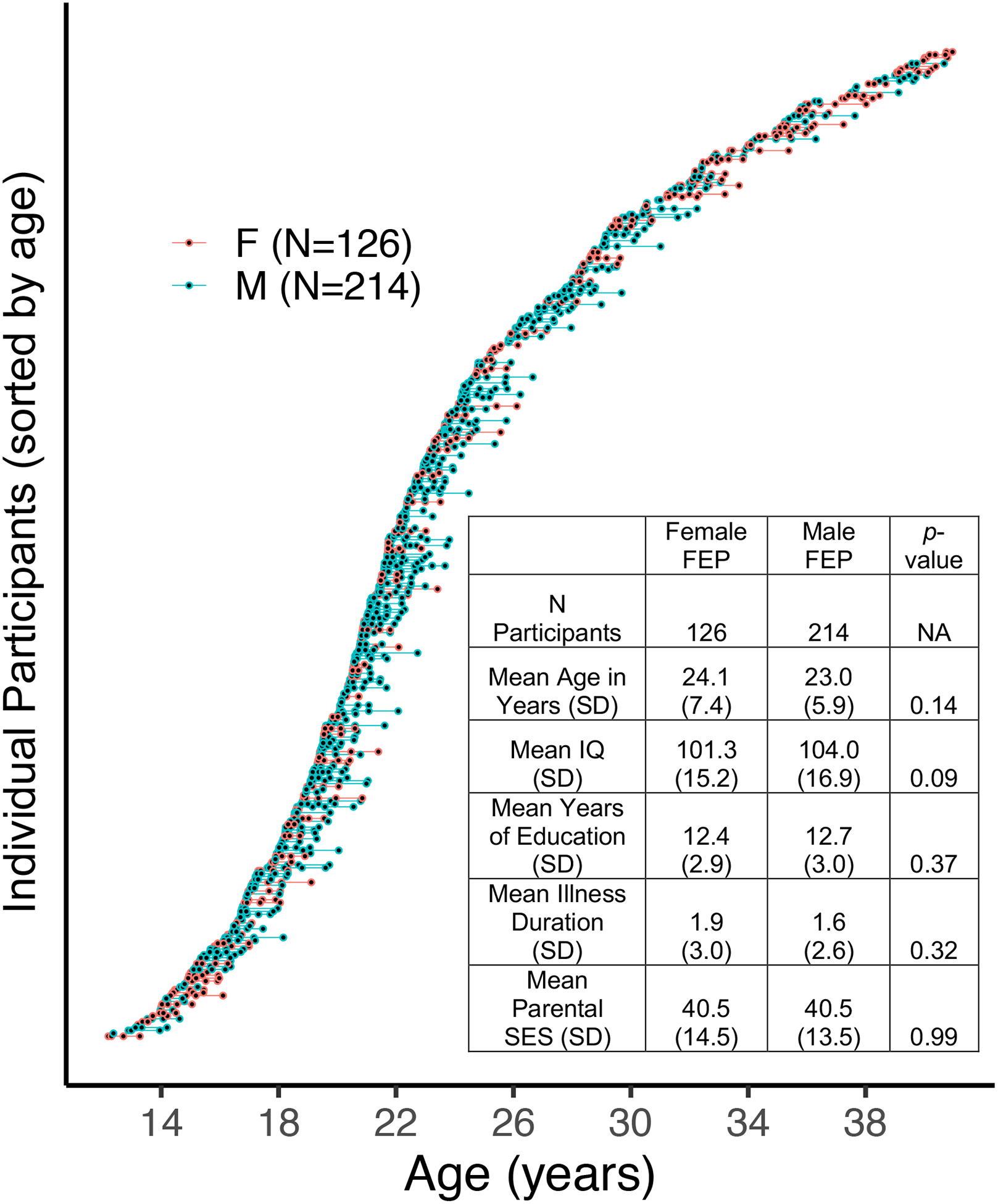
Waterfall plot of all participants and their respective visits (blue circles=male, red circles=female). Each individual circle represents a participant at a particular visit. Lines connecting the circles refer to the time in between visits. A demographic table is in the bottom right of the plot. Two-hundred ninety individuals (85%) had 2 or more visits.
Exclusion criteria for all participants included: medical illness affecting central nervous system function or IQ lower than 75 (determined using the Wechsler Abbreviated Scale of Intelligence, Wechsler, 1999). Inclusion criteria for FEP were as follows: experiencing first psychotic episode, no prior specialized treatment for psychotic symptoms, and antipsychotic-naive. Psychosis diagnoses were determined using available clinical information and data gathered from a Structured Clinical Interview for DSM-IV (SCID, First et al., 2002) conducted by a trained clinician. Senior diagnostician/clinical researchers confirmed diagnoses and illness duration for each client at consensus meetings. See Supplementary Figure S1 for a detailed description of participants removed from final analyses.
Clinical Measures
We assessed positive symptom severity with the Scale for the Assessment of Positive Symptoms (SAPS; Andreasen, 1984b). This scale includes 34 items addressing hallucinations, delusions, bizarre behavior and formal thought disorder on a 0 (absent) to 5 (severe) scale. Consistent with Schimmelmann et al.(2015), we summed individual items from the SAPS (omitting SAPS global rating items) to calculate perceptual (items 1–6, range: 0–30) and non-perceptual (items 8–33, range: 0–120) positive symptom scores.
We assessed negative symptom severity with the Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1984a). The SANS includes 25 items addressing affective flattening, alogia, avolition, anhedonia and attention on a 1 (absent/mild) to 5 (severe) scale (range: 25–125). All 25 items were initially scored for a total negative symptom score (omitting global rating items) and then scored by respective subgroups. A higher score on the SAPS or SANS indicates more severe symptomatology.
Statistical Analyses
Aim 1: Developmental effects of symptomatology in FEP
To assess developmental effects of symptomatology in FEP, data were modeled using penalized splines within a general additive model (GAM; Hastie and Tibshirani, 1986, 1990; Wood, 2017). A GAM is an extension of the general linear model but does not assume a linear relationship between independent and dependent variables, allowing for a more flexible predictor. Smoothed predictor function(s) are automatically derived during model estimation with basis functions (here, thin plate splines: MCGV default). Because incorporating more basis functions incurs greater penalties (using restricted maximum likelihood), GAM addresses many limitations of other non-linear models (e.g., over-fitting, variance/bias trade-offs). The dependent variable was the respective clinical measure being assessed. Fixed effects entered into the model were baseline chronological age (i.e. age at each visit), visit, and sex. To model and account for the non-independence of longitudinal data (multiple visits), subject was included as a random effect (r). Because all clinical symptom data was skewed to the left, we performed a log transformation to normalize distributions.
Due to known sex differences in psychosis age of onset (Häfner et al., 1992; Sharma, 1999; Kirkbride et al., 2006), we first explored smoothed effects for age in sex separately (i.e., moderating effect of sex on age):
We also tested smoothed age effects for both sexes aggregated together:
To determine the best model fit, we used Bayesian information criterion (BIC), a commonly used measure for model selection (Vrieze, 2012).
The broad age range and longitudinal data structure of the study (see Figure 1) allowed us to explore a) developmental effects of symptom expression at first episode and effects of b) illness chronicity. To explore these potentially diverging developmental effects, we first included baseline age and visit as separate predictors in the GAM. However, despite having these entered as separate regressors, by including longitudinal data, we could hypothetically fail to truly measure symptoms at first expression. Thus, we re-ran all analyses using only cross-sectional data (i.e. visit 1). We also examined how socioeconomic status, IQ, length of time between visits, antipsychotic medication exposure, race and illness duration influenced our results. Because psychotic symptoms are temporally associated with cannabis use (Hides et al., 2006; Degenhardt et al., 2007; Corcoran et al., 2008), we re-ran our analyses including cannabis use disorders as a covariate. Using SCID-IV diagnoses, we created three categorical variables: 1) lifetime cannabis use/dependence diagnosis, 2) lifetime cannabis use/dependence diagnosis, excluding those in full remission and 3) current cannabis use/dependence disorders only. We re-ran the analyses including each covariate in the model.
To identify specific developmental periods with significant age-related change in symptom severity, we performed a posterior simulation on the first derivative of GAM fits. Following previous work (Calabro et al., 2020) and established guidelines (Wood, 2017), we used a multivariate normal distribution whose vector of means and covariance were defined by the fitted GAM parameters to simulate 10,000 GAM fits and their first derivatives (generated at 0.1 year age intervals). Significant intervals of age-related change in symptom severity were defined as ages when the confidence intervals (95%) of simulated GAM fits did not include zero (p < .05).
Aim 2: Developmental effects of change in positive and negative symptom severity in FEP
To assess developmental effects of change in symptom severity, we created change scores for between each visit for each symptom measure (e.g., V3-V2, V2-V1). We also calculated a change score with the baseline visit as the reference (V3-V1, V4-V1, etc.). We used GAM and modeled the smoothed effect of age as the predictor and the respective change score as the dependent variable. To account for regression to the mean and initial level of symptom severity, we covaried for symptom severity at V1. We also used BIC to determine the best model fit with respect to sex and then assessed the smoothed effects of age:
vs.
We re-ran these analyses and included the number of days between visits as a covariate.
Aim 3: Interaction between non-perceptual positive symptoms and age on perceptual positive symptom severity
Given evidence that neurobiological factors exert differential influences on symptomatology at distinct points in development (Glaser et al., 2011; Jalbrzikowski et al., 2017; Ellwood-Lowe et al., 2018), we hypothesized there may be developmentally-specific relationships between non-perceptual positive symptoms and perceptual positive symptoms. Thus, we tested how the smoothed effect of age at baseline visit on perceptual positive symptoms varies according the degree of non-perceptual positive symptoms (i.e., the effect of a smoothed interaction between age and non-perceptual positive symptoms):
We used contour plots (mcgv package; Wood, 2011) to visualize the result.
Within each set of analyses, false discovery rate (FDR) was used to correct for multiple comparisons (q<.05)
Results
Severity of distinct positive symptoms changes across adolescent development
Table 2 reports the results of the smoothed effect of age on psychotic symptom severity. Perceptual positive symptoms declined in severity with increasing age, longitudinally (F=7.0, p= 7.0e-04; q=0.003, Figure 2A). Significant periods of age-related change occurred between 14.3–26.8 years old. Effects were driven by auditory and visual hallucinations, while developmental trajectories for somatic and olfactory hallucinations remained stable from 12–40 years (Supplementary Figure S2). Non-perceptual positive symptom severity significantly increased with increasing age (F=4.1, p=0.01, q=0.02, Figure 2B). Significant periods of age-related change occurred between 16.3–22.4 years old. Age-associated increases were driven by delusions and thought disorder (Supplementary Figure S3).
Table 2.
Developmental effects on positive and negative symptoms in first episode psychosis.
| Measure | F | p-value | q-value | Age periods (years) of significant change |
|---|---|---|---|---|
| Positive Symptom Scores | ||||
| Total | 0.3 | 0.6 | 0.6 | NA |
| Perceptual | 7.0 | 7.0e-04** | 0.003** | 14.3–26.8 |
| Non-perceptual | 4.1 | 0.01* | 0.02* | 16.3–22.4 |
| Negative Symptom Scores | ||||
| Total | 1.6 | 0.19 | 0.24 | NA |
| Flat Affect | 9.8 | 0.002** | 0.006** | 12.0–40.0 |
| Anhedonia | 6.7 | 3.5e-05** | 0.0003** | 17.2–23.1 |
| Alogia | 2.5 | 0.07 | 0.12 | NA |
| Apathy | 1.8 | 0.17 | 0.24 | NA |
| Attention | 0.5 | 0.44 | 0.59 | NA |
p < .05
p < .01
Figure 2.
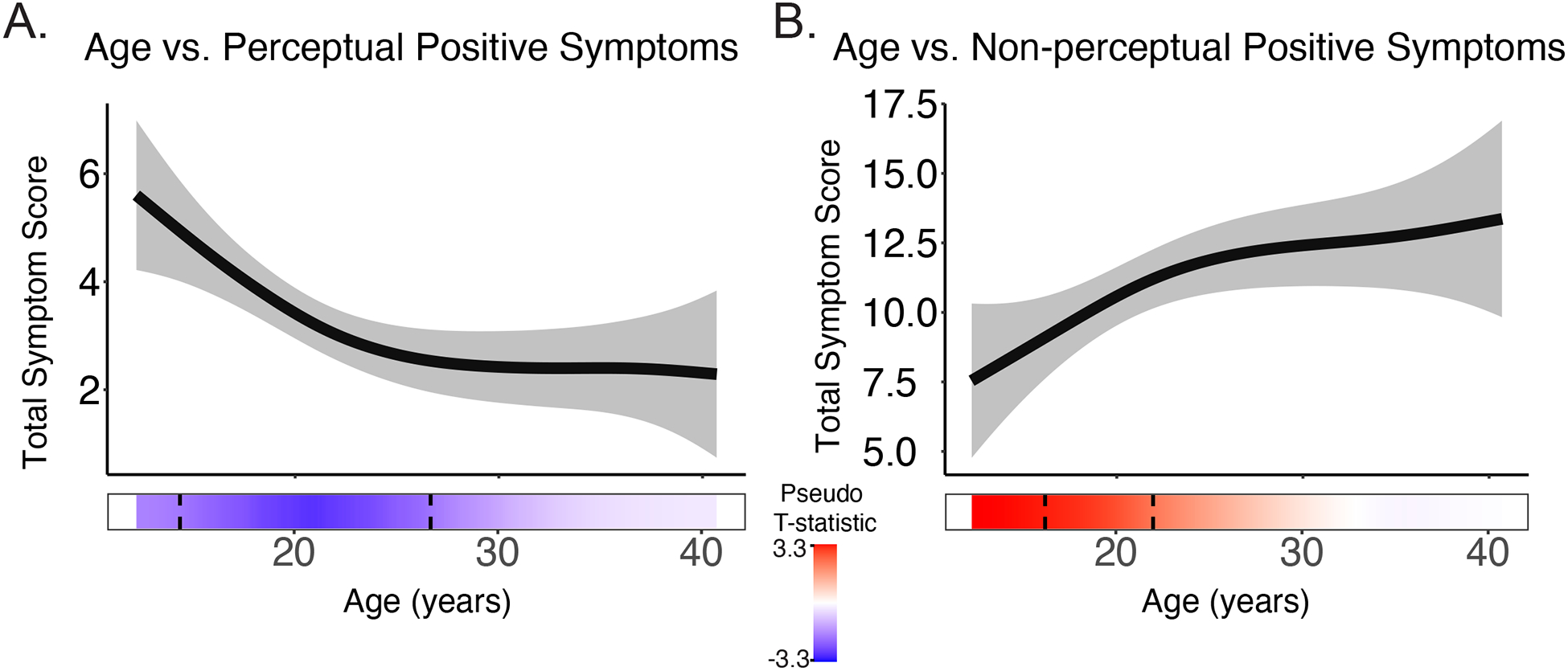
A) Perceptual positive symptoms significantly decreased with increasing age longitudinally, while B) non-perceptual positive symptoms increased with increasing age. The bar underneath the age plot reflects the derivative of the slope, i.e., the rates of change taking place at a particular age, scaled as a pseudo t-statistic, based on the posterior simulation. The dotted lines indicate when significant age associated change is taking place. Brighter red indicates greater age-related increases, while bright blue indicated greater age-related decreases.
There was not a significant effect of age on total positive symptom severity longitudinally (F=0.3, p=0.6, q=0.6). For all models tested, there were no significant main effects of sex (all p>0.5). Furthermore, for all models, BIC estimates showed that including the effect of sex on smoothed age did not significantly improve model fit (Supplementary Table S1).
Nearly all age-related changes remained statistically significant (p<.05) when IQ, parental socioeconomic status, illness duration, antipsychotic medication exposure, race, lifetime and current cannabis use disorders, and length of time between visits were included in the model as covariates (Supplementary Tables S2–S10). When illness chronicity was included as a covariate, the relationship between age and non-perceptual positive symptoms fell to trend level (F=2.3, p=0.07, Supplementary Table S4). Results remained consistent when only cross-sectional data (baseline) or when age at each visit (instead of age at V1) were used in the analyses (Supplementary Tables S11–S12).
Exploratory developmental effects of negative symptoms
Severity of overall negative symptoms did not change across adolescent development (F=1.6, p=0.19, q=0.24). When individual symptoms were examined, anhedonia severity increased with increasing age (F=6.7, p=3.5e-05, q=0.0003), while flat affect severity decreased with increasing age (F=9.8, p=0.002, q=0.006). Symptom severity of alogia, attention and apathy remained stable from ages 12–40 years old. Results are presented in Supplementary Table S13 and Figure S4.
No significant effects of development on symptom change following baseline assessment
There were no significant developmental effects on change in symptom severity when we examined change between all visits (V3-V2, V4-V3, etc.) and change between each visit and the baseline visit (V3-V1, V4-V1, etc.; Figure 3, Supplementary Tables S14 & S15). Mean length of time between visits was 73.3 days (SD: +/−73.5 days, range: 6–700 days, Figure S5). On average, symptom severity was lower in subsequent visits compared to the first visit, regardless of age (all p-values > 0.20). When the number of days between visits was included as a covariate, results remained consistent.
Figure 3.
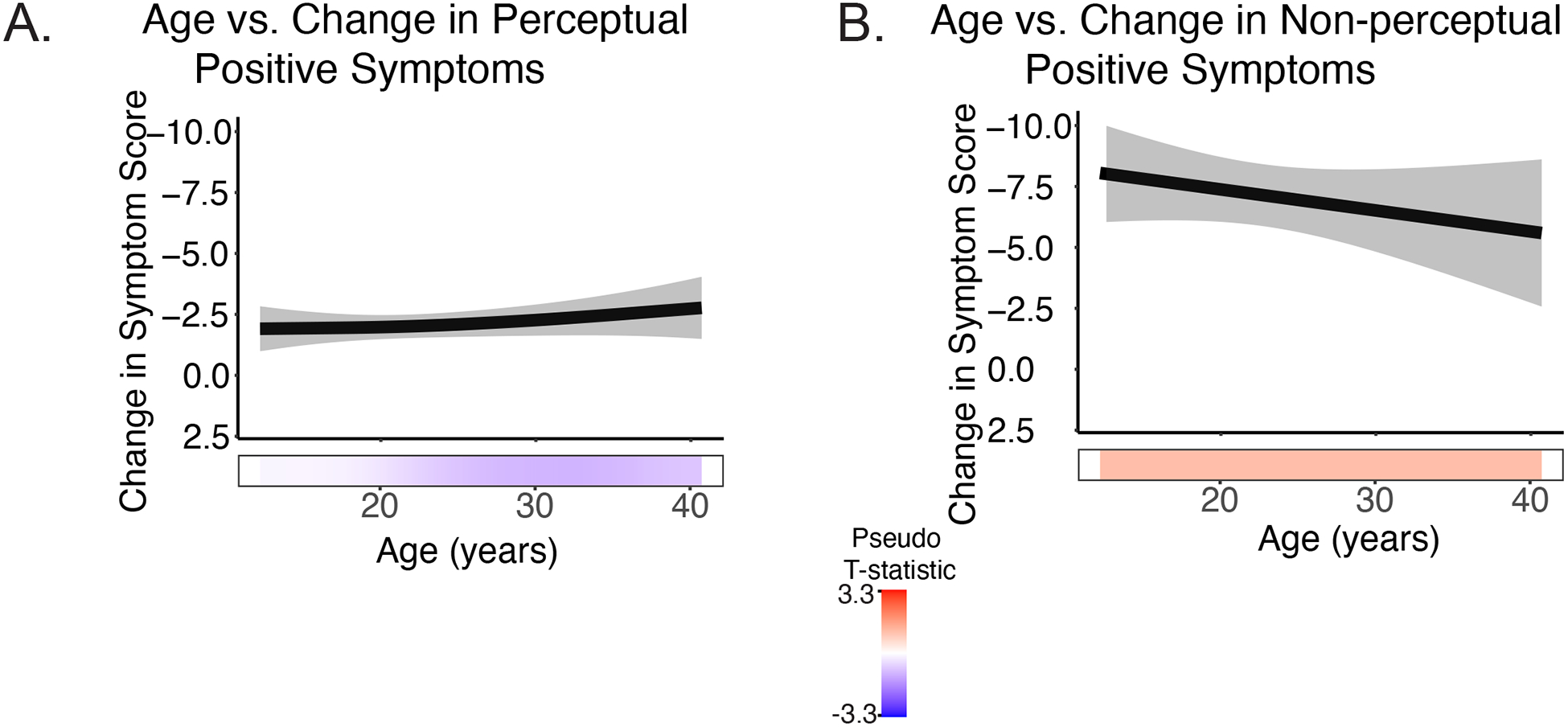
Change in symptom severity remained stable across age for A) perceptual positive symptoms and B) non-perceptual positive symptoms.
Smoothed Interaction Between Age and Non-Perceptual Positive Symptoms on Perceptual Positive Symptoms
There was a statistically significant interaction between the smoothed effect of age and perceptual positive symptoms on non-perceptual positive symptoms (F=13.1, p=2e-16, Figure 4A). In youth (< 18 years), there was not a statistically significant relationship between perceptual symptoms and non-perceptual symptoms (<18 years, b=0.18, p=0.11, Figure 4B). However, in adults (≥ 18 years), there was a statistically significant relationship, as higher levels of perceptual positive symptoms were associated with greater levels of non-perceptual symptoms(18–29 years: b=0.38, p=3.2e-11, Figure 4C; 30–40 years: b=0.60, p=1.2e-8, Figure 4D).
Figure 4.
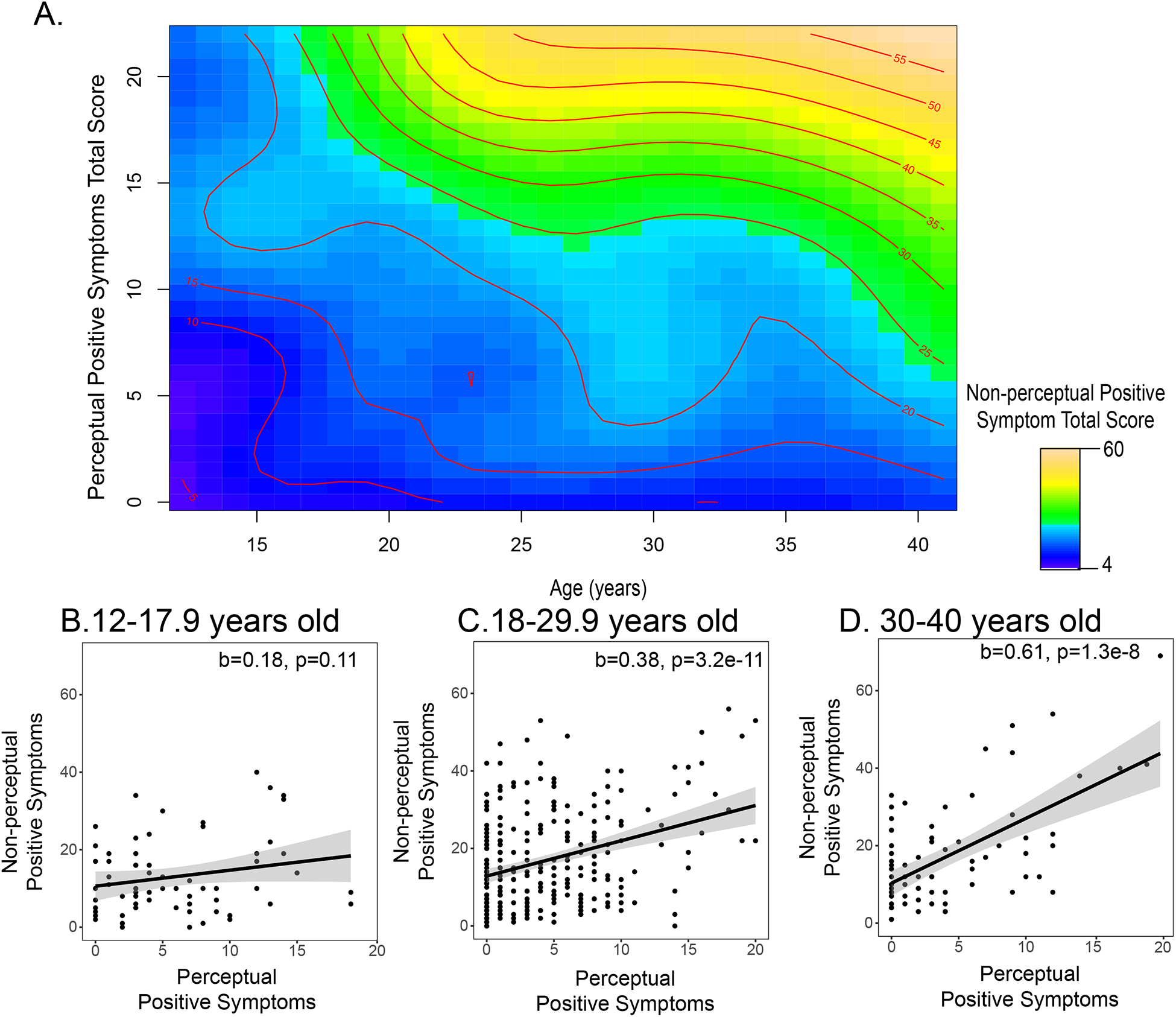
A) A contour plot illustrating how the relationship between perceptual and non-perceptual positive symptoms changes across adolescent development. The color reflects the strength of the severity of non-perceptual positive symptoms, with yellows indicating a higher level of non-perceptual positive symptoms. The severity level of non-perceptual symptoms across different ages is also indicated with red lines and text. To understand this figure, it is helpful to pick a particular age and traverse the height of the graph. At age 15, individuals with greater levels of perceptual positive symptoms (e.g., a score > 10) may have a limited range in the severity of non-perceptual positive symptoms (15–20) and the variables are not strongly associated with one another. At age 35, as individuals’ levels of perceptual positive symptoms increase, their non-perceptual positive symptoms also increase (the change from blue to yellow, and the successive increase in non-perceptual positive symptom severity, observed by the multiple red lines on the right-hand side of the graph). For visualization purposes, we also plot the linear fit between perceptual and non-perceptual positive symptoms in three separate age ranges: B) 12–17.9 years old, C) 18–29.9 years old, and D) 30–40 years old.
Discussion
In a large, antipsychotic-naïve sample of individuals experiencing their first episode of psychosis (12–40 years old), we found distinct patterns of association between development and particular psychotic symptoms. Neither medication exposure at follow-up, IQ, race, cannabis disorder diagnosis, nor socioeconomic status accounted for these associations. We consider these results evidence of selective age-related developmental influences on emerging psychosis. Additionally, the nature of the age-symptom associations may inform our understanding of the pathophysiological processes underlying first-episode psychosis, highlighting the importance of developmentally-informed approaches for both research and treatment in this population.
Distinct developmental trajectories of specific positive symptom severity
We used nonlinear modeling strategies to determine distinct periods of change in positive symptom expression. Between 14–26 years old, perceptual symptom severity decreased significantly, particularly for auditory and visual hallucinations. Somatic and olfactory hallucinations remained stable across adolescent development. Give their low prevalence rate in comparison to visual and auditory hallucinations (Mueser et al., 1990; Lewandowski et al., 2009), it would be difficult to detect significant effects of age on these specific symptoms. Our findings are consistent with reports that hallucinations are more prevalent in cases of childhood- and adolescent-onset psychosis than in adult-onset psychosis (Green et al., 1992; David et al., 2011). These findings dovetail nicely with clinically-ascertained high-risk and population sample findings that younger adolescents are more likely to report perceptual abnormalities than older adolescents and young adults (Kelleher et al., 2012b, 2012a; Brandizzi et al., 2014; Schimmelmann et al., 2015; Schultze-Lutter et al., 2017). Taken together, these findings suggest that across the continuum of psychosis-spectrum severity, perceptual positive experiences decrease with increasing age, possibly reflecting the period of specialization that is indicative of adolescent development.
In contrast, non-perceptual positive symptom severity significantly increased with increasing age from 16–22 years old, an effect driven by delusions and thought disorder. These findings align with those of Hafner et al., (1992), who showed in a chronic schizophrenia-spectrum sample that older participants (>25 years) were more likely to endorse delusions than younger participants (ages 12–24 years). Together, these findings suggest that delusions may be less severe or likely to form in early adolescence, or that they are less impairing or distressing (and therefore less likely to be reported to clinicians).
These developmental differences in perceptual and non-perceptual symptom severity point to potentially distinct treatment needs for individuals diagnosed with psychosis-spectrum disorders in childhood or adolescence versus adulthood. For example, clients in early adolescence may benefit from learning strategies that target effective ways to respond to hallucinations, whereas it may be more effective for older clients to focus on cognitive reappraisal to cope with delusional thoughts. The observed developmental variations could also reflect the fact that symptom expression has different clinical implications at different ages. Types of stressors change across adolescent development (Compas, 1987; Simmons et al., 1987; Eccles et al., 1993; Stroud et al., 2009); perhaps perceptual symptoms are likely to present themselves with stressors that are typical of late childhood/early adolescence, while non-perceptual symptoms are a response to adult stressors. Additionally, the developmental timing of a particular risk factor (e.g., substance use, social adversity) may bring about different types of symptom responses, a phenomenon observed in other psychiatric disorders (see Thapar and Riglin, 2020 for a more thorough discussion).
Distinct developmental trajectories of specific negative symptom severity
Among negative symptoms, affective flattening severity exhibited consistent linear decreases with increasing age. Anhedonia severity increased with increasing age between 17–23 years. There were no significant age-related changes severity of alogia, attention or apathy symptoms. Our findings of decreased affective flattening with increasing age are consistent with previous work (Ballageer et al 2005, Hafner et al. 1992). Worsening anhedonia with increased age may be related to increased feelings of stigma and hopelessness as the psychotic disorder progresses, given that higher levels of internalized stigma and increased feelings of hopelessness are associated with increased negative symptom severity (White et al., 2007; Lysaker et al., 2009; Hill and Startup, 2013). Individuals with psychotic disorders are more likely to endorse feelings of stigma as the disease progresses (Firmin et al., 2019), and, in turn, stigma has been found to predict feelings of hopelessness in individuals with schizophrenia-spectrum disorders (Wood et al., 2017). Future work should explore how the relationships between anhedonia, stigma, and hopelessness change across development.
Most previous publications examined overall negative symptom severity rather than changes in individual negative symptoms; and these studies reported no significant differences in overall negative symptom severity by age of onset (Haas and Sweeney, 1992; White et al., 2006; Joa et al., 2009; Devylder et al., 2013) – we replicate these findings. Further work examining age effects on individual negative symptoms should be done. While increased severity of negative symptoms is associated with greater functional impairment and lower quality of life (Ho et al., 1998; Herbener and Harrow, 2004; Mäkinen et al., 2008; Ventura et al., 2009; Fulford et al., 2013; Santesteban-Echarri et al., 2017), it is unknown if this relationship is stable across adolescent development, and to what extent specific negative symptoms contribute to this association.
No evidence of developmental effects of change in positive and negative symptom severity
There were no significant developmental effects on change in symptom severity across study visits. Across our age range, symptom severity was significantly lower at subsequent visits. An earlier onset of psychosis (<18 years) is often associated with worse long-term outcome (Clemmensen et al., 2012; Immonen et al., 2017) and increased time to symptom remission in first-episode samples (Malla et al., 2006; Veru et al., 2016). However, our results suggest that change in symptom severity is similar across development. Unlike our study, these studies did not quantitatively assess change in symptom presentation (regardless of direction). Furthermore, these studies did not focus on antipsychotic-naïve cases; thus, symptom severity may have been associated, in part, with duration of exposure to medication prior to baseline assessment.
Significant interaction between effect of age and perceptual positive symptoms on non-perceptual positive symptoms
We found that, with increasing age, the relationship between perceptual positive symptom severity and non-perceptual positive symptoms grows significantly stronger. These findings are consistent with the cognitive models of psychosis (Garety et al 2001; Mayer, 1974; Mayer 2006) that propose that abnormal perceptual experiences lead to the formation of delusions. Individuals who experience meaningful and emotionally charged hallucinations will then seek explanations for these experiences, leading to the development of delusions (Garety et al., 2001; Mayer 1974, Mayer 2006). Thus, hallucinations may precede the development of or worsening of delusions as individuals search for a way to explain their unusual perceptions. With increasing age, these explanations (delusions) become more crystallized and/or severe, even if the perceptions lessen. Alternatively, the underlying factor structure of the set of positive symptoms differs between relatively younger adolescents, older adolescents and adults. For example, among younger individuals, perceptual abnormalities load more strongly on a general psychopathology factor (Kelleher et al., 2012b; Lancefield et al., 2016), while among relatively older individuals, the emergence of perceptual abnormalities may reflect a more specific pathology (i.e. psychosis-spectrum disorders).
Possible mechanisms underlying developmental changes in symptom severity
It is important to considerthe physiological underpinnings of developmental influences on symptoms. Aberrant developmental changes in the balance of excitatory and inhibitory neurotransmitters may alter distinct brain circuits, leading to the onset of psychosis (e.g., Mikanmaa et al., 2019). Dysregulated development of dopamine may contribute to this excitatory-inhibitory imbalance, potentially leading to the development of psychotic symptoms (Kapur, 2003; van Nimwegen et al., 2005; Larsen and Luna, 2018). Given that perceptual and non-perceptual symptoms are associated with alterations in particular brain networks (e.g., abnormal perceptual experiences may reflect abnormalities in sensory and temporal regions, while delusions may be due to disrupted connections between frontolimbic areas, Corlett et al., 2010, 2019; Jardri et al., 2013), it is possible that age-associated differences in symptom severity reflect changes in the excitatory-inhibitory balance of distinct areas of the brain. In future work, particular circuits associated with these symptoms should be studied within a developmental framework.
Limitations
This study is not without limitations. As data was retrospectively collected from multiple studies, there was variation in the time between visits (range 6–700 days). Replication in a longitudinal study with uniform follow-up visits is necessary. Furthermore, though all participants were antipsychotic-naïve at baseline, the type of treatment participants engaged in post-baseline varied and was not controlled for in this study. Nonetheless, estimation of age-related developmental effects on the nature and severity of initial presenting symptoms of young people in their first episode of psychosis is an important step in investigating developmental underpinnings of early symptom presentation. Additionally, the age range used in this study (12–40 years) limits the generalizability of these results to individuals >40 years experiencing their first episode of psychosis. Furthermore, while we report our results within context of the neurodevelopmental model of psychosis, we did not associate symptom severity with earlier, pre- and postnatal risk factors (Fusar-Poli et al., 2017; Radua et al., 2018; Ellman et al., 2019). Linking earlier risk factors of psychosis to changes occurring during adolescence is an important next step to further inform the neurodevelopmental model of psychosis. Finally, pubertal development and hormonal changes have been implicated as factors that impact the risk for and sex-related variation in age at onset of psychosis (e.g., Walker and Bollini, 2002; Corcoran et al., 2003; Walker et al., 2008; Markham, 2012); thus, future work should assess how measures of pubertal development relate to positive and negative symptomatology.
Conclusion and Future Directions
We observed distinct age-related developmental effects on psychotic symptoms in an antipsychotic naïve sample with first-episode psychosis. These findings point to the importance of age as an index of developmental effects on specific symptom domains rather than overall symptom severity. Future investigation of specific age-related symptom trajectories may be informative for improving identification of risk factors for psychosis. Furthermore, in the future, approaching psychosis risk characterization and prediction from a developmental perspective may improve identification and prevention efforts. Studies of clinical-high risk cohorts report that higher levels of non-perceptual positive symptoms (e.g., unusual thought content and suspiciousness (Cannon et al., 2008; Cannon et al., 2016)) significantly predict conversion to psychosis, not perceptual positive symptoms, highlighting the important potential for development in future studies of psychosis-risk. Finally, to better understand brain mechanisms underlying developmental effects on symptom severity, it will be useful to conduct a longitudinal neuroimaging study examining the relationship of these developmentally-divergent symptoms and distinct neural regions involved in perceptual and non-perceptual positive symptoms.
Supplementary Material
Acknowledgements
The project described was supported by the National Institutes of Health through grants K01 MH112774 (Maria Jalbrzikowski, PI); R01 MH094328, R01 MH108568P50 (Dean F. Salisbury, PI); MH103204, P50 MH084053, P50 MH045146 (David A. Lewis, MD, Director); UL1 TR001857, UL1 RR024153 (Steven E. Reis, MD, PI); and M01 RR00056 (Arthur Levine, MD, PI).
We thank the faculty and staff of the WPH Psychosis Recruitment and Assessment Core for their assistance in diagnostic and psychopathological assessments. We also thank Leah Vines and Sabrina Catalano for providing feedback to drafts of the manuscript.
References
- Amminger GP, Harris MG, Conus P, Lambert M, Elkins KS, Yuen H-P, McGorry PD (2006) Treated incidence of first-episode psychosis in the catchment area of EPPIC between 1997 and 2000. Acta Psychiatrica Scandinavica 114, 337–345. [DOI] [PubMed] [Google Scholar]
- Andreasen NC (1984a) Scale for the assessment of negative symptoms (SANS). Iowa City: University of Iowa. [Google Scholar]
- Andreasen NC (1984b) Scale for the assessment of positive symptoms (SAPS). Iowa City: University of Iowa. [Google Scholar]
- Ballageer T, Malla A, Manchanda R, Takhar J, Haricharan R (2005) Is Adolescent-Onset First-Episode Psychosis Different From Adult Onset? Journal of the American Academy of Child & Adolescent Psychiatry 44, 782–789. [DOI] [PubMed] [Google Scholar]
- Bjarke J, Sinkeviciute I, Kroken RA, Løberg E-M, Jørgensen HA, Johnsen E, Gjestad R (2020) Different response patterns in hallucinations and delusions to antipsychotic treatment. Nordic Journal of Psychiatry 1–8. [DOI] [PubMed] [Google Scholar]
- Brandizzi M, Schultze-Lutter F, Masillo A, Lanna A, Curto M, Lindau JF, Solfanelli A, Listanti G, Patanè M, Kotzalidis G, Gebhardt E, Meyer N, Di Pietro D, Leccisi D, Girardi P, Fiori Nastro P (2014) Self-reported attenuated psychotic-like experiences in help-seeking adolescents and their association with age, functioning and psychopathology. Schizophrenia Research 160, 110–117. [DOI] [PubMed] [Google Scholar]
- Calabro FJ, Murty VP, Jalbrzikowski M, Tervo-Clemmens B, Luna B (2020) Development of Hippocampal–Prefrontal Cortex Interactions through Adolescence. Oxford Academic; Cerebral Cortex 30, 1548–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmensen L, Vernal DL, Steinhausen H-C (2012) A systematic review of the long-term outcome of early onset schizophrenia. BMC Psychiatry 12, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compas BE (1987) Coping with stress during childhood and adolescence. Psychological Bulletin 101, 393–403. [PubMed] [Google Scholar]
- Corcoran C, Kimhy D, Stanford A, Khan S, Walsh J, Thompson J, Schobel S, Harkavyfriedman J, Goetz R, Colibazzi T (2008) Temporal association of cannabis use with symptoms in individuals at clinical high risk for psychosis. Schizophrenia Research 106, 286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran C, Walker E, Huot R, Mittal V, Tessner K, Kestler L, Malaspina D (2003) The Stress Cascade and Schizophrenia: Etiology and Onset. Schizophrenia Bulletin 29, 671–692. [DOI] [PubMed] [Google Scholar]
- Corlett PR, Horga G, Fletcher PC, Alderson-Day B, Schmack K, Powers AR (2019) Hallucinations and Strong Priors. Trends in Cognitive Sciences 23, 114–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlett PR, Taylor JR, Wang X-J, Fletcher PC, Krystal JH (2010) Toward a neurobiology of delusions. Progress in Neurobiology 92, 345–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A (2003) Prevalence and Development of Psychiatric Disorders in Childhood and Adolescence. American Medical Association; Archives of General Psychiatry 60, 837–844. [DOI] [PubMed] [Google Scholar]
- David CN, Greenstein D, Clasen L, Gochman P, Miller R, Tossell JW, Mattai AA, Gogtay N, Rapoport JL (2011) Childhood Onset Schizophrenia: High Rate of Visual Hallucinations. Adolescent Psychiatry 50, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Tennant C, Gilmour S, Schofield D, Nash L, Hall W, McKAY D (2007) The temporal dynamics of relationships between cannabis, psychosis and depression among young adults with psychotic disorders: findings from a 10-month prospective study. Psychological Medicine 37, 927–934. [DOI] [PubMed] [Google Scholar]
- Devylder JE, Ben-David S, Schobel SA, Kimhy D, Malaspina D, Corcoran CM (2013) Temporal association of stress sensitivity and symptoms in individuals at clinical high risk for psychosis. Psychological Medicine 43, 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JS, Midgley C, Wigfield A, Buchanan CM, Reuman D (1993) Development During Adolescence. American Psychologist 12. [DOI] [PubMed] [Google Scholar]
- Ellman LM, Murphy SK, Maxwell SD, Calvo EM, Cooper T, Schaefer CA, Bresnahan MA, Susser ES, Brown AS (2019) Maternal cortisol during pregnancy and offspring schizophrenia: Influence of fetal sex and timing of exposure. Schizophrenia Research 213, 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwood-Lowe ME, Humphreys KL, Ordaz SJ, Camacho MC, Sacchet MD, Gotlib IH (2018) Time-varying effects of income on hippocampal volume trajectories in adolescent girls. Developmental Cognitive Neuroscience 30, 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firmin RL, Lysaker PH, Luther L, Yanos PT, Leonhardt B, Breier A, Vohs JL (2019) Internalized stigma in adults with early phase versus prolonged psychosis. Early Intervention in Psychiatry 13, 745–751. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, Others (2002) Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition.
- Fulford D, Niendam TA, Floyd EG, Carter CS, Mathalon DH, Vinogradov S, Stuart BK, Loewy RL (2013) Symptom dimensions and functional impairment in early psychosis: More to the story than just negative symptoms. Schizophrenia Research 147, 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Rocchetti M, Sardella A, Avila A, Brandizzi M, Caverzasi E, Politi P, Ruhrmann S, McGuire P (2015) Disorder, not just state of risk: meta-analysis of functioning and quality of life in people at high risk of psychosis. Br. J. Psychiatry 207, 198–206. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Tantardini M, De Simone S, Ramella-Cravaro V, Oliver D, Kingdon J, Kotlicka-Antczak M, Valmaggia L, Lee J, Millan MJ, Galderisi S, Balottin U, Ricca V, McGuire P (2017) Deconstructing vulnerability for psychosis: Meta-analysis of environmental risk factors for psychosis in subjects at ultra high-risk. Eur. Psychiatry 40, 65–75. [DOI] [PubMed] [Google Scholar]
- Garber J, Keiley MK, Martin NC (2002) Developmental trajectories of adolescents’ depressive symptoms: Predictors of change. Journal of Consulting and Clinical Psychology 70, 79–95. [DOI] [PubMed] [Google Scholar]
- Garety PA, Kuipers E, Fowler D, Freeman D, Bebbington PE (2001) A cognitive model of the positive symptoms of psychosis. Psychological Medicine 31, 189–195. [DOI] [PubMed] [Google Scholar]
- Ge X, Conger RD, Elder GH (2001) Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Developmental Psychology 37, 404–417. [DOI] [PubMed] [Google Scholar]
- Glaser B, Gunnell D, Timpson NJ, Joinson C, Zammit S, Smith GD, Lewis G (2011) Age- and puberty-dependent association between IQ score in early childhood and depressive symptoms in adolescence. Cambridge University Press; Psychological Medicine 41, 333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green WH, Padron-Gayol M, Hardesty AS, Bassiri M (1992) Schizophrenia with Childhood Onset: A Phenomenological Study of 38 Cases. Journal of the American Academy of Child & Adolescent Psychiatry 31, 968–976. [DOI] [PubMed] [Google Scholar]
- Gunduz-Bruce H, McMeniman M, Robinson DG, Woerner MG, Kane JM, Schooler NR, Lieberman JA (2005) Duration of Untreated Psychosis and Time to Treatment Response for Delusions and Hallucinations. American Journal of Psychiatry 162, 1966–1969. [DOI] [PubMed] [Google Scholar]
- Haas GL, Sweeney JA (1992) Premorbid and Onset Features of First-Episode Schizophrenia. Schizophrenia Bulletin 18, 373–386. [DOI] [PubMed] [Google Scholar]
- Häfner H, Maurer K, Löffler W, Riecher-Rössler A (1993) The Influence of Age and Sex on the Onset and Early Course of Schizophrenia. British Journal of Psychiatry 162, 80–86. [DOI] [PubMed] [Google Scholar]
- Häfner H, Riecher-Rössler A, Maurer K, Fätkenheuer B, Löffler W (1992) First onset and early symptomatology of schizophrenia: A chapter of epidemiological and neurobiological research into age and sex differences. European Archives of Psychiatry and Clinical Neuroscience 242, 109–118. [DOI] [PubMed] [Google Scholar]
- Hale WW, Raaijmakers Q, Muris P, van HOOF A, Meeus W (2008) Developmental Trajectories of Adolescent Anxiety Disorder Symptoms: A 5-Year Prospective Community Study. Journal of the American Academy of Child & Adolescent Psychiatry 47, 556–564. [DOI] [PubMed] [Google Scholar]
- Hastie T, Tibshirani R (1986) Generalized Additive Models. Institute of Mathematical Statistics; Statistical Science 1, 297–310. [Google Scholar]
- Hastie TJ, Tibshirani RJ (1990) Generalized Additive Models. CRC Press. [DOI] [PubMed] [Google Scholar]
- Herbener ES, Harrow M (2004) Are Negative Symptoms Associated With Functioning Deficits in Both Schizophrenia and Nonschizophrenia Patients? A 10-Year Longitudinal Analysis. Schizophrenia Bulletin 30, 813–825. [DOI] [PubMed] [Google Scholar]
- Hides L, Dawe S, Kavanagh DJ, Young RM (2006) Psychotic symptom and cannabis relapse in recent-onset psychosis: Prospective study. British Journal of Psychiatry 189, 137–143. [DOI] [PubMed] [Google Scholar]
- Hill K, Startup M (2013) The relationship between internalized stigma, negative symptoms and social functioning in schizophrenia: the mediating role of self-efficacy. Psychiatry Research 206, 151–157. [DOI] [PubMed] [Google Scholar]
- Ho B-C, Psych MRC, Nopoulos P, Arndt S (1998) Two-Year Outcome in First-Episode Schizophrenia: Predictive Value of Symptoms for Quality of Life. Am J Psychiatry 6. [DOI] [PubMed] [Google Scholar]
- Immonen J, Jääskeläinen E, Korpela H, Miettunen J (2017) Age at onset and the outcomes of schizophrenia: A systematic review and meta-analysis: Age at onset and the outcomes of schizophrenia. Early Intervention in Psychiatry 11, 453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR (2010) Rethinking schizophrenia. Nature 468, 187–193. [DOI] [PubMed] [Google Scholar]
- Jalbrzikowski M, Larsen B, Hallquist MN, Foran W, Calabro F, Luna B (2017) Development of white matter microstructure and intrinsic functional connectivity between the amygdala and ventromedial prefrontal cortex: associations with anxiety and depression. Biol. Psychiatry 82, 511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardri R, Thomas P, Delmaire C, Delion P, Pins D (2013) The Neurodynamic Organization of Modality-Dependent Hallucinations. Cerebral Cortex 23, 1108–1117. [DOI] [PubMed] [Google Scholar]
- Joa I, Johannessen JO, Langeveld J, Friis S, Melle I, Opjordsmoen S, Simonsen E, Vaglum P, McGlashan T, Larsen TK (2009) Baseline profiles of adolescent vs. adult-onset first-episode psychosis in an early detection program. Acta Psychiatrica Scandinavica 119, 494–500. [DOI] [PubMed] [Google Scholar]
- Kapur S (2003) Psychosis as a State of Aberrant Salience: A Framework Linking Biology, Phenomenology, and Pharmacology in Schizophrenia. American Journal of Psychiatry 160, 13–23. [DOI] [PubMed] [Google Scholar]
- Kelleher I, Connor D, Clarke MC, Devlin N, Harley M, Cannon M (2012a) Prevalence of psychotic symptoms in childhood and adolescence: a systematic review and meta-analysis of population-based studies. Psychological Medicine 42, 1857–1863. [DOI] [PubMed] [Google Scholar]
- Kelleher I, Keeley H, Corcoran P, Lynch F, Fitzpatrick C, Devlin N, Molloy C, Roddy S, Clarke MC, Harley M, Arseneault L, Wasserman C, Carli V, Sarchiapone M, Hoven C, Wasserman D, Cannon M (2012b) Clinicopathological significance of psychotic experiences in non-psychotic young people: evidence from four population-based studies. British Journal of Psychiatry 201, 26–32. [DOI] [PubMed] [Google Scholar]
- Kirkbride JB, Fearon P, Morgan C, Dazzan P, Morgan K, Tarrant J, Lloyd T, Holloway J, Hutchinson G, Leff JP, Mallett RM, Harrison GL, Murray RM, Jones PB (2006) Heterogeneity in Incidence Rates of Schizophrenia and Other Psychotic Syndromes: Findings From the 3-Center ÆSOP Study. Archives of General Psychiatry 63, 250. [DOI] [PubMed] [Google Scholar]
- Lancefield KS, Raudino A, Downs JM, Laurens KR (2016) Trajectories of childhood internalizing and externalizing psychopathology and psychotic-like experiences in adolescence: A prospective population-based cohort study. Cambridge University Press; Development and Psychopathology 28, 527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen B, Luna B (2018) Adolescence as a neurobiological critical period for the development of higher-order cognition. Neuroscience & Biobehavioral Reviews 94, 179–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrubier Y, Perry R, Milligan G, Leeuwenkamp O, Morlock R (2007) Physician observations and perceptions of positive and negative symptoms of schizophrenia: A multinational, cross-sectional survey. European Psychiatry 22, 371–379. [DOI] [PubMed] [Google Scholar]
- Lewandowski KE, DePaola J, Camsari GB, Cohen BM, Öngür D (2009) Tactile, Olfactory, and Gustatory Hallucinations in Psychotic Disorders: A Descriptive Study. 38, 5. [PubMed] [Google Scholar]
- Lysaker PH, Vohs JL, Tsai J (2009) Negative symptoms and concordant impairments in attention in schizophrenia: associations with social functioning, hope, self-esteem and internalized stigma. Schizophrenia Research 110, 165–172. [DOI] [PubMed] [Google Scholar]
- Maher BA (1974) Delusional thinking and perceptual disorder. Journal of Individual Psychology 30, 98–113. [PubMed] [Google Scholar]
- Maher BA (2006) The relationship between delusions and hallucinations. Current Psychiatry Reports 8, 179–183. [DOI] [PubMed] [Google Scholar]
- Mäkinen J, Miettunen J, Isohanni M, Koponen H (2008) Negative symptoms in schizophrenia—A review. Nordic Journal of Psychiatry 62, 334–341. [DOI] [PubMed] [Google Scholar]
- Malla A, Norman R, Schmitz N, Manchanda R, Béchard-Evans L, Takhar J, Haricharan R (2006) Predictors of rate and time to remission in first-episode psychosis: a two-year outcome study. Psychological Medicine 36, 649. [DOI] [PubMed] [Google Scholar]
- Markham JA (2012) Sex steroids and schizophrenia. Reviews in Endocrine and Metabolic Disorders 13, 187–207. [DOI] [PubMed] [Google Scholar]
- Mikanmaa E, Grent-’t-Jong T, Hua L, Recasens M, Thune H, Uhlhaas PJ (2019) Towards a neurodynamical understanding of the prodrome in schizophrenia. NeuroImage 190, 144–153. [DOI] [PubMed] [Google Scholar]
- Milev P, Ho B-C, Arndt S, Andreasen NC (2005) Predictive Values of Neurocognition and Negative Symptoms on Functional Outcome in Schizophrenia: A Longitudinal First-Episode Study With 7-Year Follow-Up. American Journal of Psychiatry 162, 495–506. [DOI] [PubMed] [Google Scholar]
- Mueser KT, Bellack AS, Brady EU (1990) Hallucinations in schizophrenia. Acta Psychiatrica Scandinavica 82, 26–29. [DOI] [PubMed] [Google Scholar]
- Murray RM, Lewis SW (1987) Is schizophrenia a neurodevelopmental disorder? British Medical Journal (Clinical Research Ed.) 295, 681–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nimwegen L, Haan L, Beveren N, Brink W, Linszen D (2005) Adolescence, schizophrenia and drug abuse: a window of vulnerability. Acta Psychiatrica Scandinavica 111, 35–42. [DOI] [PubMed] [Google Scholar]
- Öngür D, Lin L, Cohen BM (2009) Clinical characteristics influencing age at onset in psychotic disorders. Comprehensive Psychiatry 50, 13–19. [DOI] [PubMed] [Google Scholar]
- Owen MJ, O’Donovan MC, Thapar A, Craddock N (2011) Neurodevelopmental hypothesis of schizophrenia. British Journal of Psychiatry 198, 173–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pencer A, Addington J, Addington D (2005) Outcome of a first episode of psychosis in adolescence: a 2-year follow-up. Psychiatry Research 133, 35–43. [DOI] [PubMed] [Google Scholar]
- Radua J, Ramella-Cravaro V, Ioannidis JPA, Reichenberg A, Phiphopthatsanee N, Amir T, Yenn Thoo H, Oliver D, Davies C, Morgan C, McGuire P, Murray RM, Fusar-Poli P (2018) What causes psychosis? An umbrella review of risk and protective factors. World Psychiatry 17, 49–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport JL, Giedd JN, Gogtay N (2012) Neurodevelopmental model of schizophrenia: update 2012. Molecular Psychiatry 17, 1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santesteban-Echarri O, Paino M, Rice S, González-Blanch C, McGorry P, Gleeson J, Alvarez-Jimenez M (2017) Predictors of functional recovery in first-episode psychosis: A systematic review and meta-analysis of longitudinal studies. Clinical Psychology Review 58, 59–75. [DOI] [PubMed] [Google Scholar]
- Schimmelmann BG, Michel C, Martz-Irngartinger A, Linder C, Schultze-Lutter F (2015) Age matters in the prevalence and clinical significance of ultra-high-risk for psychosis symptoms and criteria in the general population: Findings from the BEAR and BEARS-kid studies. World Psychiatry 14, 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider SD, Jelinek L, Lincoln TM, Moritz S (2011) What happened to the voices? A fine-grained analysis of how hallucinations and delusions change under psychiatric treatment. Psychiatry Research 188, 13–17. [DOI] [PubMed] [Google Scholar]
- Schultze-Lutter F, Hubl D, Schimmelmann BG, Michel C (2017) Age effect on prevalence of ultra-high risk for psychosis symptoms: replication in a clinical sample of an early detection of psychosis service. European Child & Adolescent Psychiatry 26, 1401–1405. [DOI] [PubMed] [Google Scholar]
- Sharma R (1999) Hallucinations in the acute schizophrenic-type psychosis: effects of gender and age of illness onset. Schizophrenia Research 37, 91–95. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Hallquist MN, Asato M, Luna B (2014) Developmental stages and sex differences of white matter and behavioral development through adolescence: A longitudinal diffusion tensor imaging (DTI) study. Neuroimage 92, 356–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons RG, Burgeson R, Carlton-Ford S, Blyth DA (1987) The impact of cumulative change in early adolescence. Child Development 58, 1220–1234. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R (2009) Stress response and the adolescent transition: Performance versus peer rejection stressors. Development and Psychopathology 21, 47–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A, Riglin L (2020) The importance of a developmental perspective in Psychiatry: what do recent genetic-epidemiological findings show? Nature Publishing Group; Molecular Psychiatry 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oort FVA, Greaves-Lord K, Verhulst FC, Ormel J, Huizink AC (2009) The developmental course of anxiety symptoms during adolescence: the TRAILS study. Journal of Child Psychology and Psychiatry 50, 1209–1217. [DOI] [PubMed] [Google Scholar]
- Ventura J, Hellemann GS, Thames AD, Koellner V, Nuechterlein KH (2009) Symptoms as mediators of the relationship between neurocognition and functional outcome in schizophrenia: A meta-analysis. Schizophrenia Research 113, 189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veru F, Jordan G, Joober R, Malla A, Iyer S (2016) Adolescent vs. adult onset of a first episode psychosis: Impact on remission of positive and negative symptoms. Schizophrenia Research 174, 183–188. [DOI] [PubMed] [Google Scholar]
- Vrieze SI (2012) Model selection and psychological theory: a discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Psychological Methods 17, 228–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E, Bollini AM (2002) Pubertal neurodevelopment and the emergence of psychotic symptoms. Schizophrenia Research 54, 17–23. [DOI] [PubMed] [Google Scholar]
- Walker E, Mittal V, Tessner K (2008) Stress and the Hypothalamic Pituitary Adrenal Axis in the Developmental Course of Schizophrenia. Annual Review of Clinical Psychology 4, 189–216. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1999) Manual for the Wechsler abbreviated intelligence scale (WASI). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Weinberger DR, Berman KF, Zec RF (1986) Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Archives of General Psychiatry 43, 114–124. [DOI] [PubMed] [Google Scholar]
- White RG, McCleery M, Gumley AI, Mulholland C (2007) Hopelessness in schizophrenia: the impact of symptoms and beliefs about illness. The Journal of Nervous and Mental Disease 195, 968–975. [DOI] [PubMed] [Google Scholar]
- White T, Ho B-C, Ward J, O’Leary D, Andreasen NC (2006) Neuropsychological Performance in First-Episode Adolescents with Schizophrenia: A Comparison with First-Episode Adults and Adolescent Control Subjects. Biological Psychiatry 60, 463–471. [DOI] [PubMed] [Google Scholar]
- Wood L, Byrne R, Burke E, Enache G, Morrison AP (2017) The impact of stigma on emotional distress and recovery from psychosis: The mediatory role of internalised shame and self-esteem. Psychiatry Research 255, 94–100. [DOI] [PubMed] [Google Scholar]
- Wood SN (2011) Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 73, 3–36. [Google Scholar]
- Wood SN (2017) Generalized Additive Models: An Introduction with R, Second Edition. Boca Raton, FL: CRC Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


