Abstract
Context:
Little is known about receipt of specialty-level palliative care by people with hepatocellular carcinoma (HCC), or its impact on healthcare utilization.
Objectives:
Identify patient characteristics associated with receipt of specialty-level palliative care among hospitalized HCC patients and measure association with time to readmission.
Methods:
We used logistic regression to examine relationships between receipt of inpatient palliative care consultation by HCC patients at an academic center (N=811, 2012–2016), and clinical and demographic covariates at index hospitalization. We used a survival analysis model accounting for competing risk of mortality to compare time to readmission among individuals who did or did not receive palliative care during the admission and performed a sensitivity analysis using kernel weights to account for selection bias.
Results:
Overall, 16% received inpatient palliative care consults. Those who received consults had worse laboratory values than those who did not. In a multivariable model, higher MELD-Na, receipt of sorafenib, and higher pain scores were significantly associated with increased odds of palliative care, while liver transplantation and admission to a surgical service were associated with lower odds. For time to readmission (2,076 hospitalizations for 811 individuals with 175 palliative care visits), the sub-hazard ratio for readmission for patients who received consults was 0.26 (95% Confidence Interval [CI] 0.18–0.38) and 0.35 (CI 0.24–0.52) with a kernel-weighted sample.
Conclusion:
Inpatient palliative care consultation was received by individuals with more advanced disease, and was associated with lower readmission hazard. These findings support further research and the development of HCC-specific programs that increase access to specialty-level palliative care.
Keywords: Palliative Care, Hepatocellular Carcinoma, End-Stage Liver Disease, Supportive Oncology, Hospital Readmission
Introduction:
Hepatocellular carcinoma (HCC) has a rising incidence, carries a high mortality rate, and is difficult to treat. HCC accounts for 90% of primary liver tumors and usually develops in the background of underlying cirrhosis (1), or progressive scarring of the liver. The five-year survival of HCC is 18% (2) and curative treatments are available in only a third of cases because of late diagnosis and donor organ shortage (3). Patients may receive non-curative treatments including radio- (4) and chemoembolization (5) chemotherapy (6,7), radiation (8), and immunotherapy (9). The landscape of HCC treatment is developing rapidly (10), with multiple new agents approved for first- and second-line systemic treatment in the last few years.
HCC is unique compared to other solid cancers because of concurrent cirrhosis in the majority of cases, which leads to: (i) a uniquely unpredictable disease trajectory that combines the cancer and end-stage organ failure trajectories (11), (ii) significant symptom burden related to both cancer and cirrhosis, and (iii) a complex treatment algorithm, with many treating clinicians. These factors make patients with HCC and their family caregivers particularly amenable to palliative care consultation, yet little is known about the impact of specialty-level palliative care in this specific patient population.
Palliative care is specialized, interdisciplinary care for people facing serious illnesses. Palliative care can be delivered by specialist palliative care teams, also known as consultative or specialty-level palliative care, or delivered by primary treating clinicians, known as generalist or primary palliative care (12). The focus of this report is the provision of inpatient, specialty-level palliative care to people with HCC. Palliative care teams have been shown to reduce patient (13,14) and family distress (15,16), reduce healthcare utilization (17,18), and improve survival (19,20) largely in the context of cancer. However, cancer represents a heterogeneous group of diseases (21), and there is evidence that the maximal benefits of palliative care differ by type of cancer (14). Evidence suggests that there are unmet palliative care needs among people with HCC and their families (22–24). HCC patients and their caregivers experience distress related to symptoms (23) and uncertainty (22,24) in the face of an increasingly complex series of treatments, yet there is a paucity of research addressing the frequency and impact of palliative care delivery on their care.
The objectives of this study were to: (i) determine HCC patient characteristics associated with receipt of specialty-level palliative care in the hospital setting; and (ii) compare rates of hospital readmission for HCC patients who did and did not receive inpatient palliative care consultation, since this has been examined in oncology patients but not HCC specifically. We hypothesized that HCC patients who received inpatient palliative care consults represent a sicker population as compared to those who did not. Moreover, based on studies of palliative care in other patient populations showing reduced hospital readmissions (25,26), we hypothesized that those who received inpatient palliative care would have a lower hazard of readmission to the hospital. We created a secondary dataset of inpatient hospitalizations of HCC patients at a large academic center, and collected data on patient demographics, health characteristics, and healthcare utilization, to examine patient characteristics associated with palliative care receipt in the hospital and the association between palliative care and hospital readmission.
Methods:
Overview:
This is a retrospective cohort study of patient characteristics and healthcare utilization of hospitalized HCC patients who did and did not receive at least one inpatient palliative care consultation (2012—2016). We identified HCC patients who were seen by palliative care and used logistic regression to determine patient characteristics associated with inpatient palliative care receipt. We measured differences in time to readmission using a competing risks analysis. Stata 16 software was used for the statistical analyses (27).
Source of data:
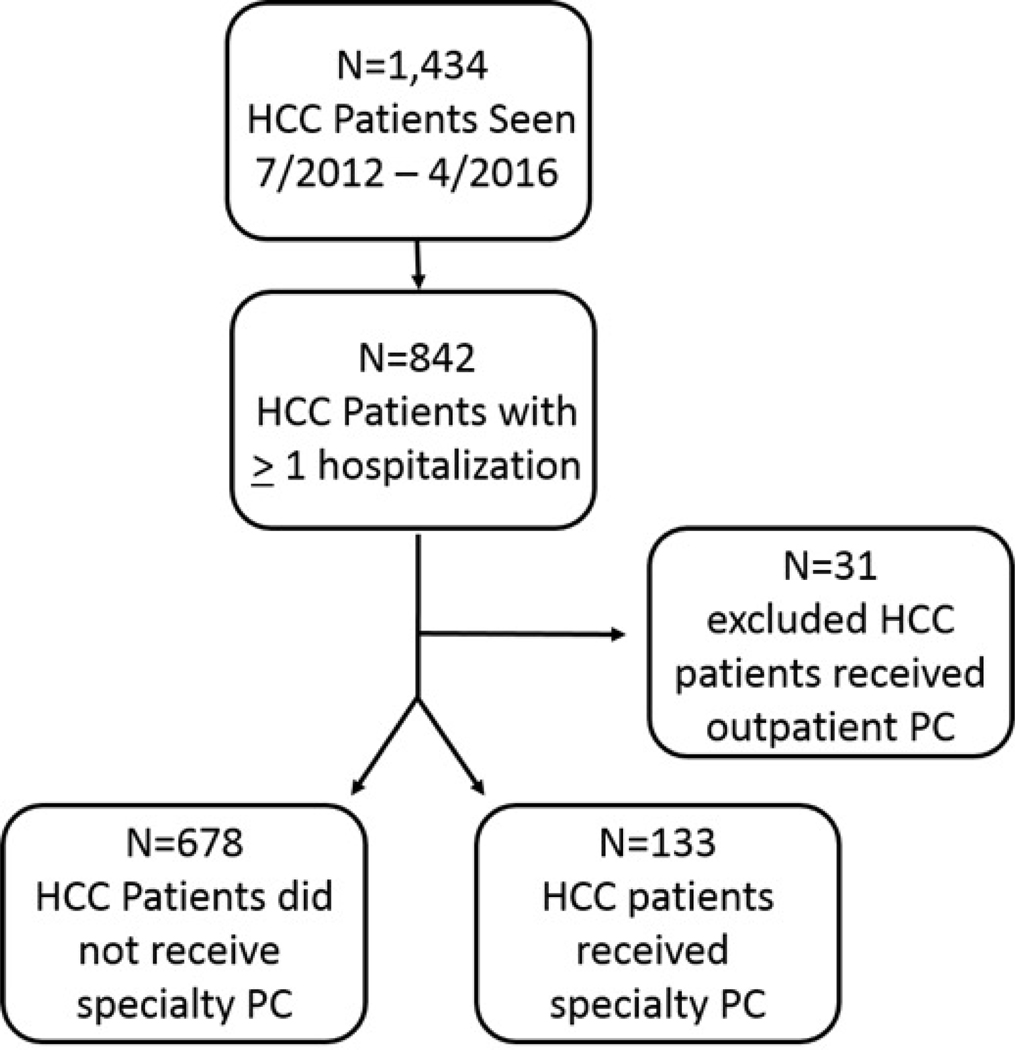
We created a dataset of HCC patients who were seen by palliative care to be used in our analysis (figure 1) by merging data from three sources.
Figure 1.
The dataset was created using a clinical database of HCC patients (top-most box), identifying those who were hospitalized (middle box), and merging with a palliative care clinical database that identifies those seen by palliative care (bottom two boxes).
Liver Cancer Clinical Database:
This dataset contains demographics, disease severity, and treatment data for patients seen at Mount Sinai Hospital (New York, NY) during the study period. It contains details about their disease and treatments. Demographic data are collected at the first visit, and disease and treatment data updated longitudinally.
Palliative Care Clinical Database:
The institution’s palliative care service maintains a clinical database with all patients seen by specialty-level palliative care between 2012 and present that captures dates and setting of the visit. Regular audits ensure completeness and accuracy of data. We used this dataset to identify patients with HCC seen by palliative care.
Clinical and Administrative Data Warehouse:
This institutional database encompasses all inpatient and outpatient encounters within the health system, and was the source of hospitalization data, including admission and discharge dates, primary hospital service, laboratory values, and mortality data.
Study population:
We included adult HCC patients seen July 2012 to April 2016, so that their initial visit to the liver cancer practice occurred after palliative care data are available in 2012. For the first part of the study, we excluded patients who had received outpatient palliative care (N=31) in order to isolate decisions to consult inpatient palliative care.
Outcomes:
We had two outcomes of interest: receipt of an inpatient palliative care consult at any time and number of days to readmission after a hospital discharge.
Other variables of interest:
All variables were collected before the time of the palliative care consultation. Demographic data were collected at the time of presentation to the Liver Cancer Practice, while laboratory and patient-reported pain scores were collected at the time of admission. In the few cases where demographic or disease severity data were missing, we retrieved information through manual chart review.
Variables representing basic demographics were collected from the HCC database, including age, sex, race and ethnicity, distance of home address from the site, marital status, whether they were English-speaking, active alcohol use, primary insurance type, and tobacco-smoking history.
We used additional data from the HCC database and data warehouse to capture HCC and liver disease severity and treatments received, including: source of underlying liver disease, HCC treatments received (embolization, transplant, sorafenib) (1), Model for End-Stage Liver Disease Sodium (MELD-Na) score (28), receipt of rifaximin (for hepatic encephalopathy), and relevant laboratory values at the time of admission, including hemoglobin, prothrombin time, total bilirubin, serum sodium, serum creatinine, and platelet count, which correlates with portal venous hypertension (29).
Additional variables from the data warehouse to capture illness status include patient-reported pain score at admission, receipt of mechanical ventilation, intensive care unit admission, admission on a medical or surgical service, and Charlson Comorbidity Index (30). We collected information about hospitalization dates and mortality from the data warehouse, including both in-hospital death and Social Security Death Index data.
Analysis of Factors Associated with Receipt of Palliative Care Consultation:
Bivariate tests (t-tests, χ2tests) were used to characterize differences among patients who were and were not seen by specialty-level palliative care. We considered p-values of less than 0.05 to be statistically significant. To determine the relationship between these patient characteristics and the receipt of palliative care, we included non-collinear variables (as determined using variance inflation factor) in a multivariable logistic regression model.
Analysis of Time to Readmission:
Our goal was to understand the association between specialty-level palliative care receipt and time to readmission. The unit of analysis was the patient-hospitalization (N=2,076). We used a survival analysis model to measure the difference between median time to readmission among the groups, adjusted for the sex, age, race/ethnicity, primary Medicaid insurance, source of underlying liver disease, history of liver transplantation, receipt of embolization treatments, receipt of sorafenib, receipt of rifaximin, Charlson Co-morbidity Index, pain score at admission, admission to a surgical service, MELD-Na, and platelet count. Because illness severity may be associated with both readmission and mortality risk we used a competing risk approach in which readmission was treated as the “failure” and mortality was treated as a competing risk (26).
Sensitivity analyses:
Our primary readmission analysis accounts for the influence of covariates on time to readmission, but it is likely that a patient’s clinical status influences both the likelihood of palliative care receipt and time to readmission. To isolate the impact of palliative care receipt on time to readmission from the impact of observed factors associated with palliative care receipt and outcomes, we repeated the analysis with propensity score kernel weights. This allowed us to balance our samples of hospitalizations where patients either did or did not receive palliative care to be as similar as possible on observed covariates, other than receipt of palliative care. Propensity score models included variables hypothesized to be associated with both receipt of palliative care and time to readmission (supplementary table S2). Covariate balance across the treatment and comparison group was considered adequate if each covariate had <10% mean standardized difference (31).
Further sensitivity analyses included repeating the analysis including only the index hospital admission for each person, to account for the fact that individuals may have more than one hospitalization represented in the dataset (N=438 individuals); using cluster robust standard errors to account for clustering at the level of the individual; using bootstrapped standard errors, to adjust for uncertainty in the estimation of treatment effect in the propensity score model (32); excluding individuals who had received a liver transplant; and excluding individuals with less than 60 days follow-up time.
Results:
We identified 1,434 individual patients seen in the outpatient liver cancer practice, as determined by their presence in the Liver Cancer clinical database, between July 2012 and April 2016. Of these, 811 individuals had at least one hospitalization at Mount Sinai. Only 133 (16%) received inpatient palliative care (figure 1). In bivariate analyses, receipt of palliative care was more likely among patients who had worse laboratory values (e.g. higher MELD-Na), had mechanical ventilation at any time, had a higher pain score on admission, received rifaximin (treatment of hepatic encephalopathy), received sorafenib, or who were admitted to a medical service in the hospital (table 1). Conversely, those with an unknown cause of liver disease, who had received embolization treatments, underwent a liver transplant, had an admission to an intensive care unit (ICU), or were admitted to a surgical service in the hospital were less likely to have received palliative care.
Table 1.
Sample characteristics of hospitalized HCC patients who either did or did not receive inpatient palliative care in the study sample. Significance was determined using t-tests or χ2-tests as appropriate; significant p-values (p<.05) are denoted with an asterisk.
| Explanatory variable | No Palliative Care (N=678) N (%) or mean (SD) | Palliative Care (N=133) N (%) or mean (SD) | P-value |
|---|---|---|---|
| Age at first HCC visit (years) | 62.5 ± 10.6 | 61.1 ± 9.27 | 0.16 |
| Female sex | 169 (24.9%) | 31 (23.3%) | 0.69 |
| White, non-Hispanic | 195 (28.8%) | 47 (35.3%) | 0.13 |
| Married | 420 (61.9%) | 71 (53.4%) | 0.06 |
| English-speaking | 536 (79.1%) | 115 (86.5%) | 0.05 |
| Home address distance (miles) | 33.4 ± 149 | 25.5 ± 94.6 | 0.56 |
| Medicaid | 217 (32.0%) | 50 (37.6%) | 0.55 |
| Smokes tobacco | 110 (16.2%) | 22 (16.5%) | 0.36 |
| Active alcohol use | 157 (23.2%) | 35 (26.3%) | 0.41 |
| Alcoholic liver disease | 73 (10.8%) | 15 (11.3%) | 0.86 |
| Hepatitis B | 183 (27.0%) | 25 (18.8%) | 0.05 |
| Hepatitis C | 343 (50.6%) | 71 (53.4%) | 0.56 |
| Non-alcoholic steatohepatitis | 53 (7.8%) | 11 (8.3%) | 0.86 |
| Autoimmune liver disease | 16 (2.4%) | 3 (2.3%) | 0.94 |
| Cryptogenic/unknown liver disease | 38 (5.6%) | 14 (10.5%) | 0.04* |
| Charlson co-morbidity index > 2 | 171 (25.2%) | 40 (30.1%) | 0.22 |
| Initial patient-reported pain score (0–10) | 1.1 ± 2.6 | 2.6 ± 3.9 | <0.01* |
| Hemoglobin (mg/dL) | 12.3 ± 2.0 | 11.7 ± 2.3 | <0.01* |
| Platelet count (1000/uL) | 136 ± 82 | 146 ± 96 | 0.23 |
| Serum sodium (mg/dL) | 138 ± 3.9 | 136 ± 4.4 | <0.01* |
| Serum creatinine (mg/dL) | 1.14 ±1.1 | 1.30 ± 1.0 | 0.14 |
| Serum total bilirubin (mg/dL) | 1.82 ± 3.0 | 3.91 ± 6.0 | <0.01* |
| Prothrombin time (s) | 16.4 ± 4.3 | 17.9 ± 4.5 | <0.01* |
| MELD-Na | 13.2 ± 6.5 | 17.8 ± 8.2 | <0.01* |
| Received rifaximin | 79 (11.7%) | 32 (24.1%) | <0.01* |
| Received sorafenib | 108 (15.9%) | 33 (24.8%) | 0.01* |
| Received embolization treatment (TACE/TARE) | 207 (30.5%) | 25 (18.8%) | <0.01* |
| History of liver transplantation | 87 (12.8%) | 7 (5.3%) | 0.02* |
| Received mechanical ventilation | 42 (6.2%) | 16 (12.0%) | 0.02* |
| Admitted to the ICU | 287 (42.3%) | 35 (26.3%) | <0.01* |
| Admission on a surgical service | 463 (68.3%) | 54 (40.6%) | <0.01* |
| Admission on a medical service | 174 (25.7%) | 70 (52.6%) | <0.01* |
In a multi-variable, logistic regression model (Table 2), receipt of sorafenib (odds ratio [OR] 1.71, 95% confidence interval [CI] 1.02–2.85), higher MELD-Na (OR 1.08, CI 1.05–1.11), and higher patient-reported pain score (OR 1.08, CI 1.01–1.16) remained significantly associated with increased odds of palliative care consultation. Receipt of a liver transplant (OR 0.28, CI 0.11–0.72) and admission to a surgical service in the hospital (OR 0.60, CI 0.38–0.94) remained associated with lower odds of palliative care consultation.
Table 2.
Odds ratios of palliative care consultation in a multivariable logistic regression model with inpatient palliative care consultation at any time as the outcome variable; statistically significant findings are shown in bold.
| Explanatory variable | Odds of receiving inpatient palliative
care |
95% confidence interval |
|---|---|---|
| Female sex | 1.04 | 0.60 – 1.68 |
| Age at first visit to HCC practice | 0.99 | 0.97 – 1.01 |
| White, Non-Hispanic | 1.28 | 0.80 – 2.06 |
| Medicaid insurance | 1.49 | 0.91 – 2.43 |
| Hospitalized under a surgical service | 0.60 | 0.38 – 0.94 |
| Alcoholic liver disease | 0.54 | 0.24 – 1.24 |
| Hepatitis B | 0.46 | 0.20 – 1.08 |
| Hepatitis C | 0.66 | 0.33 – 1.32 |
| Non-alcoholic steatohepatitis (NASH) | 0.72 | 0.28 – 1.86 |
| History of liver transplantation | 0.28 | 0.11 – 0.72 |
| Received embolization therapy (TACE/90Y) | 0.74 | 0.45 – 1.24 |
| Received sorafenib | 1.71 | 1.02 – 2.85 |
| Received rifaximin | 1.26 | 0.71 – 2.25 |
| Charlson co-morbidity index | 1.20 | 0.73 – 1.97 |
| Patient-reported pain score (first on admission) | 1.08 | 1.01 – 1.16 |
| MELD-Na score (on admission) | 1.08 | 1.05 – 1.11 |
| Platelet count (on admission) | 1.00 | 1.00 – 1.00 |
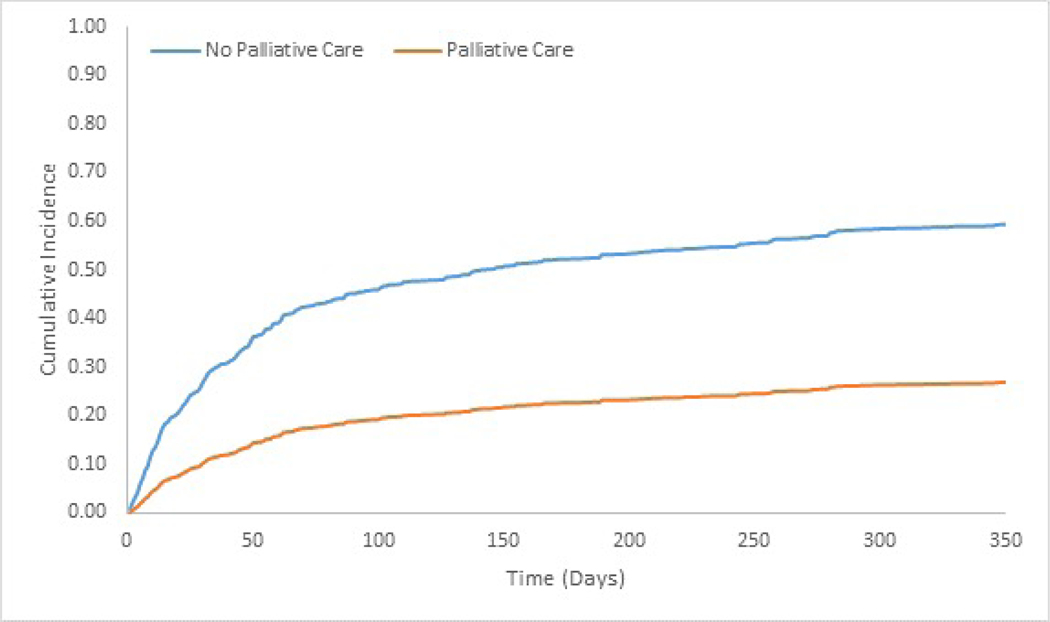
We measured the association between receipt of specialty-level palliative care with the subhazard of hospital readmission using a survival analysis model accounting for competing risk of mortality, with readmission as the “failure” and mortality as a competing risk (Figure 2, Supplementary Table S1). The sub-hazard ratio (SHR) for hospital readmission for those who received inpatient palliative care consultation was 0.26 (CI 0.18–0.38). Results were substantively similar, though modestly more conservative, in sensitivity analyses accounting for observed selection bias (SHR 0.35 (CI 0.24–0.52), kernel weighted sample). Additionally, there was no change in the treatment effect of lowering the hazard of readmission when using models that use only index hospitalizations in the analysis, cluster robust standard errors, bootstrapped standard errors, individuals who did not undergo liver transplant, and only those with 60 days or greater follow-up period.
Figure 2.
Cumulative incidence functions showing time to readmission after hospital discharge for a cohort of HCC patient hospitalizations at a single academic center accounting for competing risk of mortality; the height of the curve represents with a readmission at a given time in days.
Discussion:
This is the first report that we are aware of that investigates factors associated with receipt of palliative care by people with HCC, and the impact of palliative care consultations on their time to readmission. While there are reports of rates of HCC patients’ referral rates to hospice at the end of life (33,34), there is very little reported about patterns of referral to palliative care across the trajectory of HCC.
In the bivariate and multivariable analyses, the laboratory values of the palliative care group were consistent with a “sicker” population, with worse liver function: a higher MELD-Na score is associated with higher odds of receiving palliative care. This is consistent with literature showing that palliative care consultation for people with liver disease occurs infrequently and late in the course of their illness (35,36). One exception was that ICU admission was associated with lower frequency of palliative care consultation in the sample, possibly as a result of those receiving curative treatments for early stage HCC. We did not have the necessary data to stratify the sample by reason for ICU admission.
We found that palliative care receipt was more likely among patients receiving treatments for advanced HCC. While there have been major advancements in HCC therapies over the last few years, during the study period (2012–2016) sorafenib was the only systemic treatment available for HCC and prognosis was limited (37). In our study, people who received sorafenib were about 70% more likely to receive palliative care as those who did not, when controlling for potential confounding variables. Those who have a history of liver transplantation, which is available only to those with early stage disease, were about 70% less likely to receive a palliative care consultation when hospitalized than those who had not had a transplant. Patients who were admitted to a surgical service in the hospital were 40% less likely to receive palliative care than those who were not. Admission to a surgical service may indicate earlier stage disease since therapies for early stage HCC are surgical in nature (resection or transplantation) and those for advanced HCC are typically medical (systemic treatment).
These results suggest that either those receiving potentially curative treatment like transplant have less need, or less perceived need by treating clinicians, for palliative care services. While little is known of current unmet HCC patient palliative care needs, there is evidence that advanced and terminal stage HCC patients have significant unmet symptomatic and advance care planning needs (23,24). Gastroenterologists and hepatologists also report significant concern about patients having a negative perception of palliative care (38); which may serve as a barrier to earlier referral.
Our study of hospital readmission rates is consistent with published studies showing that palliative care consultations result in lower readmission rates (25,26). This is the first such study in patients with HCC. In a recent randomized trial of early palliative care for people with end-stage liver disease, Shinall and colleagues report a readmission hazard ratio of 0.36 (CI 0.16–0.83) (39). Additional research is needed to understand the mechanism of our finding. As suggested by May and colleagues (40), we hypothesize that in a population with heavy healthcare utilization patterns, even at the end of life (41), discussions of treatment preferences have potential to reduce rates of healthcare use by aligning treatment plans with patient goals and preferences.
This study has several limitations. First, palliative care referral in sicker HCC patients means that the referral may coincide with higher risk of mortality. We have therefore matched the sample as described, in order to make the two groups of subjects as similar as possible on observed markers of illness status. We used a time to readmission model that accounted for the competing risk of mortality and augmented mortality data in the electronic health record with data from the Social Security Death Index, which includes additional data on out-of-hospital deaths. While there may still be missing death data, we have taken measures to balance the sample as well as possible to account for possible differential between the groups.
Second, there may be unmeasured factors that may impact a person’s likelihood of receiving a palliative care consultation. These include the care preferences and attitudes toward palliative care held by patients, family caregivers, and primary treating clinicians. This creates opportunity for collaboration between palliative care and HCC-treating clinicians, to address concerns or misconceptions about the role of palliative care and reduce patient suffering.
Third, care received outside of the study institution is not included in the study. However, we do not anticipate that one group is more likely to receive care elsewhere, so healthcare usage data from different health systems should not significantly affect estimates of the palliative care treatment effect.
Finally, the use of data from a single center is a potential threat to the generalizability of the results. However, many HCC patients receive care at similar large academic medical centers and larger studies suggest that palliative care delivery to HCC patients remains limited and late in the course of disease. We believe our single-center results within the largest liver cancer and hospital-based palliative care programs in the U.S. provide information that justifies further, prospective studies to develop and test early palliative care interventions for HCC patients and families.
In conclusion, our data suggest that hospitalized patients with HCC receive palliative care consultations late in the course of their illness, and that those that do receive inpatient palliative care may have lower hazard of readmission. Given our findings, and existing clinical guidelines calling for the inclusion of palliative care in routine oncologic care (42), we need more information about the nature of “upstream” needs of HCC patients across the spectrum of HCC. Future work should address specific unmet needs of HCC patients and their families, as well as modifiable barriers to palliative care referrals, like clinician knowledge and attitudes about palliative care and lack of availability of palliative care services. Ideally, clinical programs will increase palliative care availability and be tailored to the specific needs of this growing population.
Supplementary Material
Key Message:
Little is known about the timing and impact of specialty-level palliative care received by people with hepatocellular carcinoma. This retrospective cohort study shows that HCC patients with more advanced disease are more likely to receive inpatient palliative care, and that inpatient palliative care consultation is associated with lower readmission hazard.
Acknowledgements
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government.
Funding: Funding has been provided by a National Palliative Care Research Center Junior Faculty Career Development Award (no grant number, CDW), an American Cancer Society Mentored Research Scholar Grant (MRSG-19-040-01-PCSM, CDW), the Claude D. Pepper Older Americans Independence Center (P30 AG028741, CDW, NEG, JRM), and a Veterans Affairs Health Services Research and Development Service Investigator Initiated Research Grant (VA HSRD IIR 16–140, MMG).
Footnotes
Disclosures : None of the authors have any conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2016;2:16018. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Ward EM, Johnson CJ, et al. Annual Report to the Nation on the Status of Cancer, 1975–2014, Featuring Survival. J Natl Cancer Inst 2017;109(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Journal Hepatol 2012;56(4):908–943. [DOI] [PubMed] [Google Scholar]
- 4.Edeline J, Gilabert M, Garin E, Boucher E, Raoul JL. Yttrium-90 microsphere radioembolization for hepatocellular carcinoma. Liver Cancer 2015;4(1):16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qu XD, Chen CS, Wang JH, et al. The efficacy of TACE combined sorafenib in advanced stages hepatocellullar carcinoma. BMC Cancer 2012;12:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359(4):378–390. [DOI] [PubMed] [Google Scholar]
- 7.Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389(10064):56–66. [DOI] [PubMed] [Google Scholar]
- 8.Kalogeridi MA, Zygogianni A, Kyrgias G, et al. Role of radiotherapy in the management of hepatocellular carcinoma: A systematic review. World J Hepatol 2015;7(1):101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389(10088):2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol 2018;15(10):599–616. [DOI] [PubMed] [Google Scholar]
- 11.Murray SA, Kendall M, Boyd K, Sheikh A. Illness trajectories and palliative care. BMJ (Clinical Research Ed) 2005;330(7498):1007–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quill TE, Abernathy AP. Generalist plus specialist palliative care—creating a more sustainable model. N Engl J Med 2013;368(13):1173–1175. [DOI] [PubMed] [Google Scholar]
- 13.Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA 2009;302(7):741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Temel JS, Greer JA, El-Jawahri A, et al. Effects of Early Integrated Palliative Care in Patients With Lung and GI Cancer: A Randomized Clinical Trial. J Clin Oncol 2017;35(8):834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teno JM, Clarridge BR, Casey V, et al. Family perspectives on end-of-life care at the last place of care. JAMA 2004;291(1):88–93. [DOI] [PubMed] [Google Scholar]
- 16.Gelfman LP, Meier DE, Morrison RS. Does palliative care improve quality? A survey of bereaved family members. J Pain Symptom Manage 2008;36(1):22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.May P, Normand C, Morrison RS. Economic impact of hospital inpatient palliative care consultation: review of current evidence and directions for future research. J Palliat Med 2014;17(9):1054–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.May P, Garrido MM, Cassel JB, et al. Prospective Cohort Study of Hospital Palliative Care Teams for Inpatients With Advanced Cancer: Earlier Consultation Is Associated With Larger Cost-Saving Effect. J Clin Oncol 2015;33(25):2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. New Engl J Med 2010;363(8):733–742. [DOI] [PubMed] [Google Scholar]
- 20.Bakitas MA, Tosteson TD, Li Z, et al. Early Versus Delayed Initiation of Concurrent Palliative Oncology Care: Patient Outcomes in the ENABLE III Randomized Controlled Trial. J Clin Oncol 2015;33(13):1438–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaertner J, Wolf J, Hallek M, Glossmann JP, Voltz R. Standardizing integration of palliative care into comprehensive cancer therapy--a disease specific approach. Support Care Cancer 2011;19(7):1037–1043. [DOI] [PubMed] [Google Scholar]
- 22.Hansen L, Rosenkranz SJ, Vaccaro GM, Chang MF. Patients With Hepatocellular Carcinoma Near the End of Life: A Longitudinal Qualitative Study of Their Illness Experiences. Cancer Nursing 2015;38(4):E19–27. [DOI] [PubMed] [Google Scholar]
- 23.Hansen L, Dieckmann NF, Kolbeck KJ, Naugler WE, Chang MF. Symptom Distress in Patients With Hepatocellular Carcinoma Toward the End of Life. Oncol Nurs Forum 2017;44(6):665–673. [DOI] [PubMed] [Google Scholar]
- 24.Hansen L, Rosenkranz SJ, Wherity K, Sasaki A. Living With Hepatocellular Carcinoma Near the End of Life: Family Caregivers’ Perspectives. Oncol Nurs Forum 2017;44(5):562–570. [DOI] [PubMed] [Google Scholar]
- 25.O’Connor NR, Moyer ME, Behta M, Casarett DJ. The Impact of Inpatient Palliative Care Consultations on 30-Day Hospital Readmissions. J Palliat Med 2015;18(11):956–961. [DOI] [PubMed] [Google Scholar]
- 26.May P, Garrido MM, Del Fabbro E, et al. Evaluating Hospital Readmissions for Persons With Serious and Complex Illness: A Competing Risks Approach. Med Care Res Rev 2019:1077558718823919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.StataCorp. Stata Statistical Software: Release 15. College Station, TX: StataCorp LP. 2017. [Google Scholar]
- 28.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000;31(4):864–871. [DOI] [PubMed] [Google Scholar]
- 29.Afdhal N, McHutchison J, Brown R, et al. Thrombocytopenia associated with chronic liver disease. J Hepatol 2008;48(6):1000–1007. [DOI] [PubMed] [Google Scholar]
- 30.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 31.Garrido MM, Kelley AS, Paris J, et al. Methods for constructing and assessing propensity scores. Health Serv Res 2014;49(5):1701–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Austin PC, Small DS. The use of bootstrapping when using propensity-score matching without replacement: a simulation study. Stat Med 2014;33(24):4306–4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanoff HK, Chang Y, Reimers M, Lund JL. Hospice Utilization and Its Effect on Acute Care Needs at the End of Life in Medicare Beneficiaries With Hepatocellular Carcinoma. J Oncol Pract 2016:JOP2016017814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou WY, El-Serag HB, Sada YH, et al. Determinants and Outcomes of Hospice Utilization Among Patients with Advance-Staged Hepatocellular Carcinoma in a Veteran Affairs Population. Digest Dis Sci 2018;63(5):1173–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kathpalia P, Smith A, Lai JC. Underutilization of palliative care services in the liver transplant population. World J Transplant 2016;6(3):594–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poonja Z, Brisebois A, van Zanten SV, Tandon P, Meeberg G, Karvellas CJ. Patients with cirrhosis and denied liver transplants rarely receive adequate palliative care or appropriate management. Clin Gastroenterol Hepatol 2014;12(4):692–698. [DOI] [PubMed] [Google Scholar]
- 37.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. New Engl J Med. 2008;359(4):378–390. [DOI] [PubMed] [Google Scholar]
- 38.Ufere NN, Donlan J, Waldman L, et al. Physicians’ Perspectives on Palliative Care for Patients With End-Stage Liver Disease: A National Survey Study. Liver Transpl 2019;25(6):859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shinall MC Jr., Karlekar M, Martin S, et al. COMPASS: A Pilot Trial of an Early Palliative Care Intervention for Patients With End-Stage Liver Disease. J Pain Symptom Manage 2019;58(4):614–622 e613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.May P, Normand C, Del Fabbro E, et al. Economic Analysis of Hospital Palliative Care: Investigating Heterogeneity by Noncancer Diagnoses. MDM Policy Pract 2019;4(2):2381468319866451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ufere NN, Halford JL, Caldwell J, et al. Health Care Utilization and End-of-Life Care Outcomes for Patients With Decompensated Cirrhosis Based on Transplant Candidacy. J Pain Symptom Manage 2019; 59(3):590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferrell BR, Temel JS, Temin S, Alesi ER, Balboni TA, Basch EM, Firn JI, Paice JA, Peppercorn JM, Phillips T, Stovall EL, Zimmermann C, and Smith TJ. Integration of Palliative Care Into Standard Oncology Care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2016:1–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.