Abstract
The aim of the present study was to optimise the extraction conditions of anthocyanins from strawberry fruits and incorporate them in yoghurt to achieve a natural coloration as well as enrich the product with antioxidants. The response surface methodology (RSM) based on Box–Behnken design was studied to assess the influence of the three factors being agitation speed (400–800 rpm), sample to solvent ratio (0.5–2 g/40 mL), and extraction time (1–15 min) on total anthocyanin content and antioxidant activity of strawberries. According to the results, the linear, quadratic and interaction effects of the studied factors on total anthocyanin content and antioxidant activity were determined by the response surface methodology, and the optimal conditions for anthocyanin extraction were 586 rpm for agitation speed, 1.26 g/40 mL for sample to solvent ratio, and 9.36 min for extraction time. Under these extraction conditions, the total anthocyanin content and antioxidant activity recorded by the two validated models were 38.04 mg C3GE/100 g FW and 21.38 mg AAE/100 g FW, respectively. The enriched natural yoghurt contains anthocyanins with a content of 36.50 µg C3GE/100 g and an antioxidant activity of 21.22 µg AAE/100 g. The anthocyanin enriched yoghurt developed in this study may be considered as a functional food with an interesting source of natural antioxidants, and these anthocyanins can substitute synthetic (industrial) colorants.
Keywords: Fragaria ananassa Duch., Modelling optimisation extraction, Response surface methodology, Enriched yoghurt, Natural colorants, Antioxidants
Introduction
Fragaria ananassa known as strawberry is widely grown hybrid species of the genus Fragaria. It is cultivated worldwide for their fruits that are widely appreciated for their characteristic aroma, bright red colour, juicy texture, and sweetness (Manganaris et al. 2014). They are consumed in large quantities, either fresh or in such prepared foods as jam, juice, ice cream, and chocolates. Strawberries are also consumed for their antioxidant quality due to their functional compounds, considered as bioactive healthier molecules, many of which have demonstrated biological activities; among these compounds we can cite vitamins, minerals, polyphenol-proteins, phenolic acids and flavonoids (Karaaslan and Yaman 2017; Hoskin et al. 2019; Kalt et al. 2020). These latter represent the highest amount of bioactive molecules, and anthocyanins are the compounds responsible for the visual appearance of strawberries. Indeed, they provide the red colour for the strawberry fruits and protect them from UV radiations by their antioxidant property (Kalt and Dufour 1997; Nicoué et al. 2007; Zhang et al. 2019; Kalt et al. 2020).
Anthocyanins are the anthocyanidins glycosides, they are frequently found in the nature as glycosylated form that gives them a high solubility in water than their aglycone. They are responsible for fruit and vegetable colour, varying from red to violet. In recent years, there has been increasing interest in anthocyanin pigments by both scientific and industrial communities due to their possible uses in food products, as better industrial natural colorants, supplements and/or health promoting foods, as well as to their disease-preventing benefits (Khoo et al. 2017; Karaaslan and Yaman 2017; Fernandes et al. 2018; Kalt et al. 2020). Indeed, they help to prevent cardiovascular diseases and cancers, sustains normal circulation of the human blood system, and both prevents type II diabetes and regulate blood sugar concentration in diabetic patients, and so on (Huang et al. 2013; Silva et al. 2017; Karaaslan and Yaman 2017). However, there are several challenges related to colour losses that occurred during food processing, storage, and commercialisation related to the low stability of these natural pigments compared to synthetic industrial colorants (Tonutare et al. 2014; Ali et al. 2016; Cortez et al. 2017). Therefore, as objective to extract anthocyanins without losses and maintaining their stability during extraction, their extraction process study from natural sources is necessary and this extraction is influenced by several factors such as type of solvent, extraction time and temperature (Ju and Howard 2003; Türker and Erdoǧdu 2006; Karaaslan and Yaman 2017; Alexandre et al. 2020).
The extraction of anthocyanins from berries by maceration is currently used as a simple economic extraction method without altering the thermo-sensitive anthocyanins, and with maintaining a moderate extraction recovery (da Silva et al. 2007; Cortez et al. 2017; Karaaslan and Yaman 2017).
In addition, among the methodologies used in order to optimise the extraction of anthocyanins, RSM was reported as an interesting tool to determine exactly the influence of several factors on the phenolic extraction, including anthocyanins, and to determine the optimal extraction conditions. This methodology reduces measurements, improves the statistical interpretation, and indicates the quadratic and interaction effects between factors (Saci et al. 2018; Benchikh et al. 2019).
The incorporation of anthocyanins as natural colorants in yoghurt leads to improve the sensory attributes of the final food product including its health benefits are also not neglected. Recently, several studies reported that the enrichment of yoghurt with bioactive compounds from natural sources is the way to improve the dietary intake of consumers with healthier efficiency as well as the physical stability and techno-functional quality of food matrix (Barkallah et al. 2017; Gaglio et al. 2019; Munekata et al. 2020). According to the awareness of consumers, natural colorants are more preferable and appreciated than artificial ones due to their safety and their healthier properties (Ben Mansour and Latrach Tlemcani 2009; Barkallah et al. 2017; Gaglio et al. 2019). Yoghurt is a dairy product, easily digest, that presents a great nutritional value and highly appreciated by the consumers due, especially, to its taste and texture. It is a food ranked among the best consumed product in the world. Thanks to its high consumption rate, the supplementation of yoghurt with natural additives could add an important nutritional and functional value. Therefore, it is necessary to elaborate new healthful and sustainable yoghurt products in the objective to propose a new functional food product enriched with anthocyanins (Bourlioux et al. 2011).
To our knowledge, there are few reports on the extraction conditions of anthocyanins that have been published. Therefore, in the present study, we first proposed an optimised protocol for the extraction of anthocyanins from strawberry fruits and then we performed a preliminary assays aiming to incorporate the extract in yoghurt to propose a functional food product enriched with anthocyanins as natural colorants and antioxidants.
Materials and methods
Chemical reagents
Sodium acetate and potassium chloride were from Biochem, Chemopharma (Cosne-sur-loire, France); 1,1-diphenyl-2-picrylhydrazyl (DPPH) was from Sigma Chemical (Sigma-Aldrich GmbH, Germany); ascorbic acid and hydrochloric acid were from Sigma-Aldrich (St. Louis, USA).
Sample preparation
Strawberry fruits were harvested in April 12th, 2019 from Jijel area (Algeria). They were immediately transported to the laboratory under cold conditions (6 °C). After selection of uniform strawberries, fruits were washed with water and then wiped out. After removal of peduncles, fresh and ripe strawberries were cut in small pieces and then manually ground until obtaining a strawberry puree. This latter was used as a fresh sample for the extraction of strawberry anthocyanins.
Extraction procedure
An aliquot of strawberry fruits (0.5–2 g) was mixed with 40 mL of extraction solvent containing 85% of distilled water and 15% of HCl 0.1 M (pH 1.3). The agitation was carried out by magnetic stirring (AGIMATIC-S, P-SELECTA, Spain) at different speeds (400–800 rpm) and extraction time (1–15 min). After centrifugation (Sigma 2-16 P Centrifuge, Germany) at 2486×g, the extract was subsequently filtered (13 µm, Grade F1001, Chem, Barcelona, Spain).
Determination of total anthocyanin content
The total anthocyanin content (TAC) was determined according to Tonutare et al. (2014). Briefly, an aliquot of 1.5 mL of extract was added to 2.5 mL of 0.025 M potassium chloride (pH 1) and to 2.5 mL of 1 M sodium acetate (pH 4.5). Then, the mixture was incubated in darkness during 30 min, before measuring the absorbance at 510 nm and 700 nm, respectively (Shimadzu, China). TAC was calculated using the derived equation of Beer-Lamber cited as following (Eq. 1). The content was expressed as milligrams equivalents cyanindin-3-glucoside per 100 g of fresh weight (mg C3GE/100 FW).
| 1 |
where TAC is the total anthocyanin content, A is the absorbance of sample, M is the molar mass of cyanindin-3-glucoside that is 449 g/mol, DF is the dilution factor, 1000 is the conversion factor, ε is the molar extinction coefficient of cyanindin-3-glucoside that is 26,900 M−1 cm−1, L is the cuvette optical pathlength (0.5 cm), and m is the weight of the aliquot.
Evaluation of antioxidant activity
Antioxidant activity (AA) was measured by free radical (DPPH) scavenging activity according to the method described by Brand-Williams et al. (1995). Briefly, 100 µL of the extract was added to 1 mL of DPPH solution (60 mM). After 30 min of incubation in the darkness, the decrease in absorbance was determined at 517 nm. Ascorbic acid was used as a standard and the AA was expressed as milligrams ascorbic acid equivalents per 100 g of strawberry fruits fresh weight (mg AAE/100 FW).
Optimisation of anthocyanin extraction by RSM
According to our previous preliminary results (data not published yet), three extraction factors were selected as main parameters influencing independently the extraction of anthocyanins from strawberry fruits. These factors were agitation speed, sample to solvent ratio, and extraction time. In the present work, the same factors were studied by response surface methodology in order to complete the second important step which was the determination of eventual interaction and quadratic effects between these factors. Therefore, Box–Behnken design was adapted as an experimental design to proceed with statistical modelling process to optimise the extraction. As stated above, the independent variables used were agitation speed (x1, rpm), sample to solvent ratio (x2, g/40 mL), and extraction time (x3, min). The coded and real independent variables and the experimental design were mentioned in the Table 1. The lowest, the central and the highest levels of variables were coded as − 1, 0, + 1, respectively.
Table 1.
Factors and levels for response surface methodology, Box–Behnken design matrix (in coded and uncoded level of three variables), experimental data and predicted values for three-level-three-factor response surface analysis
| Run | Variable levelsa | TAC (mg C3GE/100 g FW) | AA (mg AAE/g FW) | ||||
|---|---|---|---|---|---|---|---|
| x1 | x2 | x3 | Observed | Predicted | Observed | Predicted | |
| 1 | 600(0) | 0.5(− 1) | 1(− 1) | 30.26 | 30.05 | 19.29 | 19.52 |
| 2 | 400(− 1) | 1.25(0) | 1(− 1) | 31.98 | 31.85 | 16.82 | 16.70 |
| 3 | 800(+ 1) | 1.25(0) | 1(− 1) | 27.44 | 27.61 | 17.58 | 17.68 |
| 4 | 600(0) | 1.25(0) | 1(− 1) | 24.84 | 25.01 | 16.19 | 15.99 |
| 5 | 400(− 1) | 0.5(− 1) | 8(0) | 30.13 | 30.47 | 15.94 | 15.83 |
| 6 | 800(+ 1) | 0.5(− 1) | 8(0) | 31.31 | 31.36 | 16.76 | 16.43 |
| 7 | 600(0) | 1.25(0) | 8(0) | 37.72 | 37.79 | 21.75 | 21.39 |
| 8 | 600(0) | 1.25(0) | 8(0) | 37.99 | 37.79 | 21.34 | 21.39 |
| 9 | 600(0) | 1.25(0) | 8(0) | 37.66 | 37.79 | 21.08 | 21.39 |
| 10 | 400(− 1) | 2(+ 1) | 8(0) | 33.62 | 33.58 | 15.29 | 15.62 |
| 11 | 800(+ 1) | 2(+ 1) | 8(0) | 31.53 | 31.19 | 12.74 | 12.85 |
| 12 | 600(0) | 0.5(− 1) | 15(+ 1) | 27.80 | 27.63 | 17.67 | 17.88 |
| 13 | 400(− 1) | 1.25(0) | 15(+ 1) | 32.61 | 32.44 | 18.85 | 18.75 |
| 14 | 800(+ 1) | 1.25(0) | 15(+ 1) | 35.06 | 35.19 | 15.48 | 15.60 |
| 15 | 600(0) | 2(+ 1) | 15(+ 1) | 35.39 | 35.60 | 17.85 | 17.62 |
TAC total anthocyanin content, AA antioxidant activity
ax1, agitation speed (rpm); x2, sample to solvent ratio (g/40 mL); x3, extraction time (min)
Data analysis and model validation
The JMP® 14.0.1 software (SAS Institute, Inc., Cary, NC, USA) was used to analyse the experimental data. Accordingly, we fitted the data to a second-order polynomial model and the regression coefficients were obtained and used in the response surface analysis as given in the following equation (Eq. 2):
| 2 |
where a0, ai, aii, and aij are the regression coefficients of intercept, linear, quadratic and interaction terms, respectively, and xi and xj are the independent variables. Fisher's test was used to determine the type of the model equation and Student's t-test was performed to determine the significance of regression coefficients.
In order to validate the obtained theoretical values based on the model accuracy, three experimental extractions were carried out by using optimal conditions obtained by Box–Behnken design, and TPC and AA were determined and compared to the predicted responses.
Incorporation of anthocyanin extract in yoghurt
The extracted colorants using RSM were added immediately in yoghurt formulation in order to improve the food product aspect. The colour of enriched yoghurt must have the same colour of fruits from that the colorant is extracted. Thus, an aliquot of 15 mL of anthocyanin extract was incorporated in 50 g of natural set yoghurt (pH 4.6) without additives (Soummam). The manual homogenisation was performed during few seconds (45 s) until we obtained a homogenous structure of yoghurt with red colour.
Extraction procedure of anthocyanins from enriched yoghurt
As noticed above, the anthocyanin extract was used as colorants enriching yoghurt as well as antioxidants by which the product is protected from oxidation. After 24 h of rest time at 4 °C in glass pots, we would like to assess the remaining of colorants and antioxidants in the product. An aliquot of 2 g of anthocyanin yoghurt was mixed with 20 mL of acidified distilled water containing 85% of water and 15% of HCl 0.1 M. The mixture was agitated at 600 rpm during 15 min, and the mixture was centrifuged at 2486×g during 10 min before filtering the extract as described above (13 µm, Grade F1001, Chem, Barcelona, Spain). The TAC and AA of the obtained anthocyanin yoghurt extract were determined following the same methods described above.
Results and discussion
Analysis of models
The different combinations of the three factors (agitation speed, sample to solvent ratio, and extraction time) and experimental and predicted values of anthocyanin content and AA are given in Table 1. Indeed, the coefficient of determination was very high (R2 = 0.99) denoting that total anthocyanins and AA models have a high level of explanation (Table 2).
Table 2.
Adjustment analysis of the models
| Source | DFa | Sum of squares | F ratio | Prob > F |
|---|---|---|---|---|
| Total anthocyanin content (TAC) | ||||
| Model | 9 | 218.97 | 227.17 | < 0.0001* |
| Error | 5 | 0.54 | ||
| Total model | 14 | 219.51 | ||
| Lack of fit | 3 | 0.47 | 5.11 | 0.168 |
| Pure error | 2 | 0.06 | ||
| Total error | 5 | 0.54 | ||
| R2 | 0.99 | |||
| Adj. R2 | 0.989 | |||
| Antioxidant activity (AA) | ||||
| Model | 9 | 86.58 | 67.81 | < 0.0001* |
| Error | 5 | 0.71 | ||
| Total model | 14 | 87.29 | ||
| Lack of fit | 3 | 0.48 | 1.41 | 0.441 |
| Pure error | 2 | 0.23 | ||
| Total error | 5 | 0.71 | ||
| R2 | 0.99 | |||
| Adj. R2 | 0.996 | |||
aDegrees of freedom
*Value statistically significant at P < 0.05
The analysis of variance of the two models indicates that the squares of means were greater than the squares of means of the residues. Furthermore, the values of Fisher ratio calculated for total anthocyanins (227.17) and AA (67.81) allowed obtaining low probability value of 0.0001 for two models. These results clearly demonstrate that both models have a very high significance regarding experimental responses of total anthocyanins and AA. Otherwise, lack-of-fits calculated from the ratios of square means of lack-of-fits and pure errors (F ratio) were 5.11 for total anthocyanins and 1.41 for AA, corresponding to the probabilities of 0.168 and 0.441, respectively. This denoted that lack-of-fits of studied models were not significant (Table 2). From the analysis of variance as well as lack-of-fits of results, TAC and AA models were judged to be fair and powerful to explain experimental results.
Effect of factors
After checking the overall adjustments, the significance of the two models (TAC and AA) as well as the non-significance of their errors, the individual and combined effects of the factors were analysed. From Table 3, we can see that linear terms of the three factors were significant (P < 0.05) for TAC and AA models, except the effect of extraction time on AA (P > 0.05). The interactions between all factors for TAC and AA were significant (P < 0.05). The three quadratic terms of all factors were statistically significant (P < 0.05) for both responses.
Table 3.
Regression coefficient, standard error, and Student’s t-test results of response surface for total anthocyanin content and antioxidant activity
| Parameter | Estimate | SE | t ratio | Prob. > |t| |
|---|---|---|---|---|
| Total anthocyanin content (TAC) | ||||
| Intercept | 37.79 | 0.19 | 200.01 | < .0001* |
| Agitation speed | − 0.38 | 0.12 | − 3.24 | 0.0229* |
| Sample to solvent ratio | 0.74 | 0.12 | 6.35 | 0.0014* |
| Extraction time | 2.04 | 0.12 | 17.65 | < .0001* |
| Agitation speed * sample to solvent ratio | − 0.82 | 0.16 | − 5.00 | 0.0041* |
| Sample to solvent ratio * extraction time | 1.75 | 0.16 | 10.68 | 0.0001* |
| Agitation speed * extraction time | 3.25 | 0.16 | 19.88 | < .0001* |
| Agitation speed * agitation speed | − 1.97 | 0.17 | − 11.57 | < .0001* |
| (Sample to solvent ratio)2 | − 4.17 | 0.17 | − 24.49 | < .0001* |
| Extraction time * extraction time | − 4.05 | 0.17 | − 23.76 | 0.0229* |
| Antioxidant activity (AA) | ||||
| Intercept | 21.39 | 0.22 | 98.36 | < .0001* |
| Agitation speed | − 0.54 | 0.13 | − 4.07 | 0.0096* |
| Sample to solvent ratio | − 0.95 | 0.13 | − 7.12 | 0.0008* |
| Extraction time | 0.00 | 0.13 | − 0.03 | 0.9786 |
| Agitation speed * sample to solvent ratio | − 0.84 | 0.19 | − 4.47 | 0.0066* |
| Sample to solvent ratio * extraction time | − 1.03 | 0.19 | − 5.48 | 0.0028* |
| Agitation speed * extraction time | 0.82 | 0.19 | 4.35 | 0.0073* |
| Agitation speed * agitation speed | − 3.39 | 0.20 | − 17.28 | < .0001* |
| (Sample to solvent ratio)2 | − 2.82 | 0.20 | − 14.39 | < .0001* |
| Extraction time * extraction time | − 0.82 | 0.20 | − 4.18 | 0.0086* |
SE standard error
*Value statistically significant at P < 0.05
The statistical models of the response surfaces of TACs and AA of strawberry fruits were presented as second-order polynomials. These models include linear, quadratic, and interaction significant effects and were shown in the following equations (Eqs. 3, 4):
| 3 |
| 4 |
Analysis of response surfaces
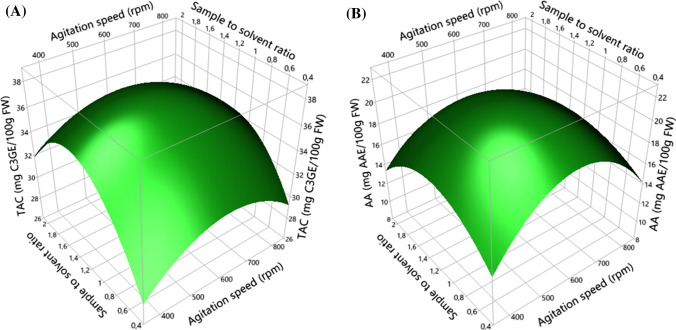
To better illustrate the effects of different factors, three-dimensional graphical representations are generated (Figs. 1, 2, 3). In each surface presentation, two factors vary in the experimental domain and the third is fixed at its central level (level 0). Figure 1 illustrates the three-dimensional spatial representation of agitation speed and sample to solvent ratio effects on anthocyanin recovery and AA. According to the graphs A and B of Fig. 1, it seems that TAC and AA increased with the increasing of agitation speed and sample to solvent ratio until reaching the optimal values of 586 rpm and 1.26 g/40 mL, respectively. At this level, TAC and AA raised to 38.04 mg C3GE/100 g FW and 21.38 mg AAE/100 g FW, respectively. Beyond these optimal values, the TAC and AA decreased significantly (P < 0.05). This is clearly shown by the curvatures of the response surfaces (quadratic effects of factors).
Fig. 1.
Surface response plots showing the effects of agitation speed and sample to solvent ratio on total anthocyanin content (TAC, a) and antioxidant activity (AA, b)
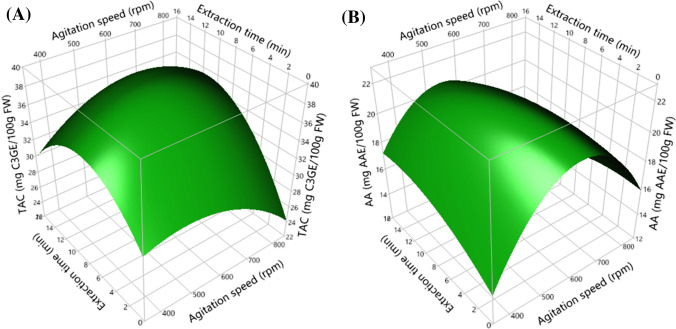
Fig. 2.
Surface response plots showing the effects of agitation speed and extraction time on total anthocyanin content (TAC, a) and antioxidant activity (AA, b)
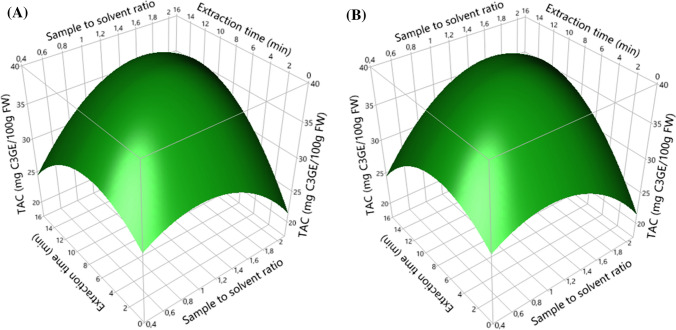
Fig. 3.
Surface response plots showing the effects of sample to solvent ratio and extraction time on total anthocyanin content (TAC, a) and antioxidant activity (AA, b)
The raising of agitation causes the increase in anthocyanin diffusion, but beyond a certain speed, the agitation promotes the solubility of air oxygen in extraction solvent which could induce the oxidation of these bioactive compounds. Shi et al. (2003) mentioned that the oxygen dissolved in the extraction solvent can cause the degradation of antioxidants, especially through the use of large solvent volume, high temperature and long extraction time.
The effect of sample to solvent ratio can be explained by the fact that the extraction efficiency of anthocyanins was decreased under the effect of a weak interaction between the sample and the solvent after optimal sample to solvent ratio, thus the transfer of anthocyanins and their solubility and polarity in the solvent becomes very weak.
Based on results highlighted in Fig. 2, TAC and AA increased until optimal values (586 rpm and 9.36 min, respectively) as a function of the increase in agitation speed and extraction time. However, beyond these optimal conditions, the data showed that the two responses cited above decreased significantly. These results can be explained by the oxidation of anthocyanins due to the simultaneous excess of agitation speed and extraction time beyond optimal values (Shi et al. 2003; Chan et al. 2009). This interpretation is also supported by the high interaction between two cited factors for the TAC and AA obtained by the present results.
Finally, in Fig. 3 we illustrated spatial presentations of response surface models of sample to solvent ratio and extraction time effects on extraction of anthocyanins and AA of strawberry fruits. It seems that TAC and AA significantly increased according to the increase in the two factors up to optimal values of 1.26 g/40 mL and 9.36 min. Beyond these values, both responses gradually decreased (P < 0.05). This diminution was explained by the quadratic effects which pass through the maximums of the AA (curvature of the response surfaces). This can be due to the saturation phenomenon of the solvent and simultaneously to the degradation of anthocyanins already extracted (Shi et al. 2003; Chan et al. 2009), so the results obtained were explained by Fick’s second law of diffusion, which predicts that after a certain time, there will be a final equilibrium between the solute concentration in the solid matrix (plant sample) and in the bulk solution (extraction solvent) (da Silva et al. 2007; Benchikh and Louailèche 2014). Hence, excessive extraction time was not useful to extract more anthocyanins from strawberries.
Determination and validation of optimal conditions
The conditions required maximising total anthocyanin extraction and AA of strawberry fruits as well as TAC and AA were determined. To validate these theoretical values, three extractions are carried out by using optimal conditions obtained by Box–Behnken design. The average experimental values for TAC and AA obtained were 36.50 mg C3GE/100 g FW and 21.22 mg AAE/100 g FW, respectively. These values were very close to those obtained through the two elaborated models which were 38.04 mg C3GE/100 g FW and 21.38 mg AAE/100 g FW for TAC and AA, respectively.
This optimisation study allowed us to set favourable conditions in order to extract anthocyanins with higher compared to those reported by Clifford (2000), da Silva et al. (2007) and Tonutare et al. (2014). This demonstrates the accuracy of the developed models.
Anthocyanins and antioxidant activity of strawberry fruits in the enriched yoghurt
The incorporation of the anthocyanin extract from strawberry fruits in yoghurt was performed in order to substitute the synthetic colorants and antioxidants, hence proposing new functional yoghurt with red natural colorants. These results were 36.50 µg C3GE/100 g of yoghurt for TAC and 21.22 µg AAE/100 g of yoghurt for AA.
The presence of the anthocyanins in the enriched yoghurt at acceptable quantities (10–40 µg/100 g) provides evidence of supplementation. The remaining of the red colour in the final food product can be explained by the fact that the added extract containing anthocyanins are not lost and still present in stable form during and after the incorporation process. This stability maybe related to the adequate environment of food matrix in which these extracted anthocyanins were incorporated occurring good structure stability and suitable functional activity. Anthocyanins are naturally occurring dyes which are characterized by considerable variation in colour intensity and instability during storage. Numerous studies have been carried out in order to set the stability conditions for these compounds. It has been established that anthocyanins are more stable at pH below 5 using low storage temperatures (Wang et al. 2010; Kırca et al. 2007). So in this study, the addition of anthocyanins for a fairly acidic product (yogurt with a pH 4.6) with refrigerated storage (4 °C) gives these antioxidants adequate stability.
Conclusion
As cited above, the present work was focused on optimisation of extraction conditions of anthocyanins from strawberry fruits and their incorporation in yoghurt. From the outcome of our investigation, it is possible to conclude that the use of RSM allowed determining the optimal extraction conditions which were 586 rpm for agitation speed, 1.26 g/40 mL for sample to solvent ratio and 9.36 min for extraction time. Under extraction conditions, the TAC and AA were 38.04 mg C3GE/100 g FW and 21.38 mg AAE/100 g FW, respectively. The extraction conditions that we investigated influenced significantly (P < 0.05) the extraction of anthocyanins from Fragaria ananassa Duch. fruits.
In order to validate the theoretical data, three extractions were performed under the same optimal extraction conditions, and the TAC and AA were determined leading to 36.50 mg C3GE/100 g FW and 21.22 mg AAE/100 g FW, respectively. These results are close to those predicted by our models.
The incorporation of anthocyanin extract in yoghurt was performed to substitute the synthetic (industrial) colorants and antioxidants, to propose new functional yoghurt with red natural colorants. TAC and AA of enriched yoghurt were lower than those obtained by strawberry fruits extract.
Strawberry fruits can be regarded as a good source of anthocyanins which present a good potential for antioxidant capacity that can also be incorporated in yoghurt to obtain new functional foods with benefit for human health.
Acknowledgements
The authors acknowledge the financial support from Directorate-General of Scientific Research and Technological Development (DGRSDT) and Algerian Ministry of Higher Education and Scientific Research. They would also convey special thanks to Dr. Mostapha Bachir bey for his valuable help concerning the scientific opinion and English editing of the manuscript as well as the grammar checking.
Compliance with ethical standards
Conflict of interest
The authors declare that the present work was conducted in the absence of any commercial, financial, personal, or other relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alexandre AMRC, Serra AT, Matias AA, Duarte CMM, Bronze MR. Supercritical fluid extraction of Arbutus unedo distillate residues—impact of process conditions on antiproliferative response of extracts. J CO2 Util. 2020;37:29–38. doi: 10.1016/j.jcou.2019.11.002. [DOI] [Google Scholar]
- Ali O-H, Al-sayed H, Yasin N, Afifi E. Effect of different extraction methods on stablity of anthocyanins extracted from red onion peels (Allium cepa) and its uses as food colorants. Bull Natl Nutr Inst. 2016;47:1–24. doi: 10.21608/bnni.2016.4218. [DOI] [Google Scholar]
- Barkallah M, Dammak M, Louati I, et al. Effect of Spirulina platensis fortification on physicochemical, textural, antioxidant and sensory properties of yogurt during fermentation and storage. LWT Food Sci Technol. 2017;84:323–330. doi: 10.1016/j.lwt.2017.05.071. [DOI] [Google Scholar]
- Ben Mansour H, Latrach Tlemcani L. Are natural food dyes good food additives? Phytothérapie. 2009;7:202–210. doi: 10.1007/s10298-009-0394-7. [DOI] [Google Scholar]
- Benchikh Y, Louailèche H. Effects of extraction conditions on the recovery of phenolic compounds and in vitro antioxidant activity of carob (Ceratonia siliqua L.) pulp. Acta Bot Gallica. 2014;161:175–181. doi: 10.1080/12538078.2014.909325. [DOI] [Google Scholar]
- Benchikh Y, Zaoui A, Derbal R, et al. Optimisation of extraction conditions of phenolic compounds and antioxidant activity of Ruta chalepensis L. using response surface methodology. J Food Meas Charact. 2019;13:883–891. doi: 10.1007/s11694-018-0002-3. [DOI] [Google Scholar]
- Bourlioux P, Braesco V, Mater DDG. Yoghurts and other fermented milks. Cah Nutr Diététique. 2011;46:305–314. doi: 10.1016/j.cnd.2011.07.001. [DOI] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Chan SW, Lee CY, Yap CF, et al. Optimisation of extraction conditions for phenolic compounds from limau purut (Citrus hystrix) peels. Transl Lung Cancer Res. 2009;16:203–213. [Google Scholar]
- Clifford MN. Anthocyanins—nature, occurrence and dietary burden. J Sci Food Agric. 2000;80:1063–1072. doi: 10.1002/(SICI)1097-0010(20000515)80:7<1063::AID-JSFA605>3.0.CO;2-Q. [DOI] [Google Scholar]
- Cortez R, Luna-Vital DA, Margulis D, Gonzalez de Mejia E. Natural pigments: stabilization methods of anthocyanins for food applications. Compr Rev Food Sci Food Saf. 2017;16:180–198. doi: 10.1111/1541-4337.12244. [DOI] [PubMed] [Google Scholar]
- da Silva FL, Escribano-Bailón MT, Pérez Alonso JJ, et al. Anthocyanin pigments in strawberry. LWT Food Sci Technol. 2007;40:374–382. doi: 10.1016/j.lwt.2005.09.018. [DOI] [Google Scholar]
- Fernandes A, Rocha MAA, Santos LMNBF, et al. Blackberry anthocyanins: β-cyclodextrin fortification for thermal and gastrointestinal stabilization. Food Chem. 2018;245:426–431. doi: 10.1016/j.foodchem.2017.10.109. [DOI] [PubMed] [Google Scholar]
- Gaglio R, Gentile C, Bonanno A, et al. Effect of saffron addition on the microbiological, physicochemical, antioxidant and sensory characteristics of yoghurt. Int J Dairy Technol. 2019;72:208–217. doi: 10.1111/1471-0307.12569. [DOI] [Google Scholar]
- Hoskin RT, Xiong J, Esposito DA, Lila MA. Blueberry polyphenol-protein food ingredients: the impact of spray drying on the in vitro antioxidant activity, anti-inflammatory markers, glucose metabolism and fibroblast migration. Food Chem. 2019;280:187–194. doi: 10.1016/j.foodchem.2018.12.046. [DOI] [PubMed] [Google Scholar]
- Huang H-W, Hsu C-P, Yang BB, Wang C-Y. Advances in the extraction of natural ingredients by high pressure extraction technology. Trends Food Sci Technol. 2013;33:54–62. doi: 10.1016/j.tifs.2013.07.001. [DOI] [Google Scholar]
- Ju ZY, Howard LR. Effects of solvent and temperature on pressurized liquid extraction of anthocyanins and total phenolics from dried red grape skin. J Agric Food Chem. 2003;51:5207–5213. doi: 10.1021/jf0302106. [DOI] [PubMed] [Google Scholar]
- Kalt W, Dufour D. Health functionality of blueberries. HortTechnology. 1997;7:216–221. doi: 10.21273/horttech.7.3.216. [DOI] [Google Scholar]
- Kalt W, Cassidy A, Howard LR, et al. Recent research on the health benefits of blueberries and their anthocyanins. Advances in nutrition. Adv Nutr. 2020;11:224–236. doi: 10.1093/advances/nmz065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaaslan NM, Yaman M. Anthocyanin profile of strawberry fruit as affected by extraction conditions. Int J Food Prop. 2017;20:S2313–S2322. doi: 10.1080/10942912.2017.1368548. [DOI] [Google Scholar]
- Khoo HE, Azlan A, Tang ST, Lim SM. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr Res. 2017;61:1361779. doi: 10.1080/16546628.2017.1361779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kırca A, Özkan M, Cemeroğlu B. Effects of temperature, solid content and pH on the stability of black carrot anthocyanins. Food Chem. 2007;101(1):212–218. doi: 10.1016/j.foodchem.2006.01.019. [DOI] [Google Scholar]
- Manganaris GA, Goulas V, Vicente AR, Terry LA. Berry antioxidants: small fruits providing large benefits: berry antioxidants: small fruits providing large benefits. J Sci Food Agric. 2014;94:825–833. doi: 10.1002/jsfa.6432. [DOI] [PubMed] [Google Scholar]
- Munekata PES, Pateiro M, Barba FJ, et al. Development of new food and pharmaceutical products: nutraceuticals and food additives. In: Lorenzo JM, Barba FJ, et al., editors. Advances in food and nutrition research. Cambridge: Academic Press; 2020. pp. 53–96. [DOI] [PubMed] [Google Scholar]
- Nicoué EÉ, Savard S, Belkacemi K. Anthocyanins in wild blueberries of Quebec: extraction and identification. J Agric Food Chem. 2007;55:5626–5635. doi: 10.1021/jf0703304. [DOI] [PubMed] [Google Scholar]
- Saci F, Benchikh Y, Louaileche H, Bachir Bey M. Optimization of ultrasound-assisted extraction of phenolic compounds and antioxidant activity of carob pulp (Ceratonia siliqua L.) using response surface methodology. Ann Univ Dunarea Jos Galati Fascicle VI Food Technol. 2018;42:26–39. [Google Scholar]
- Shi J, Yu J, Pohorly J, et al. Optimization of the extraction of polyphenols from grape seed meal by aqueous ethanol solution. J Food Agric Environ. 2003;1:42–47. doi: 10.1234/4.2003.337. [DOI] [Google Scholar]
- Silva S, Costa EM, Calhau C, et al. Anthocyanin extraction from plant tissues: a review. Crit Rev Food Sci Nutr. 2017;57:3072–3083. doi: 10.1080/10408398.2015.1087963. [DOI] [PubMed] [Google Scholar]
- Tonutare T, Moor U, Szajdak L. Strawberry anthocyanin determination by pH differential spectroscopic method—how to get true results? Acta Sci Pol. 2014;13:35–47. [Google Scholar]
- Türker N, Erdoǧdu F. Effects of pH and temperature of extraction medium on effective diffusion coefficient of anthocynanin pigments of black carrot (Daucus carota var. L.) J Food Eng. 2006;76:579–583. doi: 10.1016/j.jfoodeng.2005.06.005. [DOI] [Google Scholar]
- Wang BC, He R, Liet ZM. Antioxidant activity of blueberry anthocyanins. FTB. 2010;48:42–49. [Google Scholar]
- Zhang J, Singh R, Quek SY. Extraction of anthocyanins from natural sources—methods and commercial considerations. In: Brooks MSL, Celli GB, editors. Food chemistry, function and analysis. Cambridge: Royal Society of Chemistry; 2019. pp. 77–105. [Google Scholar]