Abstract
The main focus of this study was to develop and calibrate a computerized image analysis system in order to measure the color of banana (Musa Cavendish) under microwave treatment. Bananas were cut into 2 mm slice thickness and dried at two different microwave power level; 540 W and 180 W. An algorithmic was developed which converted RGB color value from a color image into CIE L*a*b* values very well (ErrorL* = 2.163%, Errora* = 4.458%, Errorb* = 5.224%). Once the calibration is completed, it was applied to measure the color change in the banana slice during drying. The value of L* decreased from 89.01 to 71.17 and from 82.60 to 72.53 for both microwave treated samples suggesting browning is taking place during the drying operation. The value of a* increased from − 0.80 to 11.50 and from − 3.90 to 5.18 for 540 and 180 W microwave treated banana slices respectively suggesting tendency of redness increased. The same type of increment was observed for b* value. It changed from 36.46 to 60.51 and 34.02 to 72.82 for 540 and 180 W microwave treated banana slices, respectively. Artificial Neural Network (ANN) modeling was used for prediction of the developed CVS’s values.
Keywords: Banana, Microwave drying, CIE L*a*b*, Image analysis, Color measurement, Artificial Neural Network (ANN)
Introduction
Banana is one of the most consumed fruit all over the world. It derived its name from the Arabic word ‘banan’ which means finger. Banana is a general term grasping various species or crossbreeds in the variety Musa of the family Musaceae. As per the latest data available from the United Nations Food and Agriculture Organization, the world production of banana for the year 2017 is 113.918 million tonnes from 5.637 ha area (FAO Stat 2019). Bananas are predominantly cultivated in Asia, Latin America, and Africa. India is the largest producer of banana, which produced 26.75% alone in total world production, followed by China 10.02%, Indonesia 6.28%, Brazil 5.85%, and Ecuador 5.51%. Bananas contain significant amount of several vitamins, antioxidant (Someya et al. 2002), carbohydrates (Bello-Pérez et al. 1998) and minerals especially potassium (D’Elia et al. 2011). Once the banana is harvested in ripe green stage, its ripening begins, which results in the irreversible chemical changes, that lead to change of its texture (Quevedo et al. 2008). As the ripening of banana continues, it changes the peel color to yellow from green. Mendoza and Aguilera (2006) reported that change in peel color occurs due to the formation of pigments (such as carotenoids). Among the various physical properties of any agricultural commodities and food products like their shape, size, color, freshness and, the absence of visual defects, color is the most dominating deciding attribute. It plays a major role in acceptance or non-acceptance of the product (Costa et al. 2011). The very first attribute of any product encountered by consumers is its color. They tend to associate color with other attributes like flavor, safety, level of satisfaction, and nutrition. It is often necessary in food engineering research to analyze the food surface color, both quantitatively and qualitatively. The study of color distribution comes under quantitative analysis. It can be correlated with other data and assist in the study of temperature profile or study of moisture content distribution. Color can be analyzed and objectively measured by a novel computerized image analysis technique known as Computer Vision System (CVS). It works on theoretical and algorithmic basis; it acquires an image or image sequence and, extracts and analyzes useful information from it. CVS has been used to measure color of different food products such as apple (Unay et al. 2011), dates (Al Ohali 2011), mango (Nandi et al. 2014), and banana (Mendoza and Aguilera 2004). CVS is more versatile and less expensive than the traditional instruments to measure the color (Yam and Papadakis 2004; Nandi et al. 2014; Saldaña et al. 2014). It is advantageous in many ways such as it is easy and quick, it generates precise descriptive data (Brosnan and Sun 2004), and it is non-destructive and consistent (Du and Sun 2004) as well. CVS also holds permanent record which allows further analysis later (Gümüş et al. 2011), reduces manpower requirement, and best of all it is not only efficient in operation, but it is cost-effective as well (Lu et al. 2000).
Non-destructive techniques of quality evaluation are gaining the attention of researchers nowadays. Still, most of them are non-feasible in the agricultural industry as they are more expensive and sometimes rather impractical to use outside the laboratory. A low-cost method that can be applied on-line for monitoring and quality inspection of the product would be the ideal one. Many studied have been conducted on quality and maturity indices of agricultural products such as papaya (Ali et al. 2014), mango (Elsayed et al. 2016), tomato (Rungpichayapichet et al. 2017), etc.; but the problem of expensive laboratory equipment is associated with them. There are a lot of instruments available in the market to measure the color, but most of them are contact type. They need to keep in touch with the sample to perform the measurements. The main drawback with this color-measuring instrument is the requirement of homogenous representative sample and limitations in physical characteristics (size, shape, roundness, and geometry of the inspected area). These commercial instruments will give the best results when the geometry of the product is fixed, and the area under observation is small, but this kind of measurement will be quite unrepresentative for most of the food products that are heterogeneous in nature (Antonelli et al. 2004). Hence, to increase the accuracy of measurement, repetitive observations will be required, but this will result in very tedious and time-consuming operations. As some of the food products need to process within a time-bounded period, repetitive observation is not a viable approach. Instead, an approach such as Computer Vision System (CVS) must be taken into account that uses overall surface to obtain relevant stats about the color, which is also important in industry for quality evaluation where color homogeneity is a matter of concern. In these situations, contact-type color measuring instruments are not appropriate to fulfill the requirement. One of the important techniques for pattern recognition in CVS is texture image analysis. It is used to estimate heterogeneous gray color from images and to characterize the order of components on the surface of the product. This method works on the principle that texture of the image reflects the variation in pixel or surface intensity which possess not only the information about color parameters (lightness, hue, saturation) but geometric structure of the product as well (Gonzales-Barron and Butler 2008).
Artificial Neural Networks (ANNs) is a very promising tool when it comes to predict the values for a complex non-linear system (Zuñiga et al. 2014). Various authors (Zapotoczny 2011; Zaborowicz et al. 2017) has shown that ANN can perform better in pattern recognition and classification than the classical regression model. ANNs perform these tasks by training of neural network repeatedly with known inputs and the corresponding output. Many fields such as medical science, food processing, as well as automobile and many other horizons are focusing on the ANNs application. The status of current process control along product quality control inspection systems holds great potential for the design of intricate and robust algorithms which can be used in real-time inspection lines and will provide fruitful result.
The objective of this study was the development of a CVS to measure color in L*a*b* color units from RGB color images. Artificial Neural Network (ANN) modeling was used for prediction of the developed CVS’s values. Banana slices were given microwave drying treatment, and the effect of this drying method on color parameter was examined by developed CVS system.
Materials and methods
CVS design
Images were captured using an image acquisition system. Illumination was achieved by four fluorescent lamps (Philips, Natural Daylight, 18 W). Specification of the illumination system is given in Table 1. Canon digital SLR camera (Canon EOS 1500D, Singapore) with 24.1 megapixels of the resolution was used to capture the images and placed vertically 22.5 cm from the samples such that the angle between the axis of the lens and sources of illumination is close to 45°. The images were taken transferred through USB port to the Intel(R) Core(TM) I5-550 CPU@3.20 GHz. In order to calibrate the CVS, seven color charts were taken; each color chart was divided into four regions. The color value of each region was measured with Konika Minolta CR400 Colorimeter (with three replicates).
Table 1.
Image acquisition system specification
| Lightening system specification | Camera specification | ||
|---|---|---|---|
| Variable | Value | Variable | Value |
| Lighting | Fluorescent lamp | Focal distance | 20.7 mm |
| Number of the lamp | 4 | Zoom | 9 |
| Length of the lamp | 60 cm | Flash | Off |
| Color temperature | 6500 K | Iso velocity | 100 |
| Lamp power | 18 W | White balance | Fluorescence H |
| Color rendering index | 95% | Operation mode | Manual |
| Angle between camera and sample | 45° | Aperture | f/80 |
CVS implementation
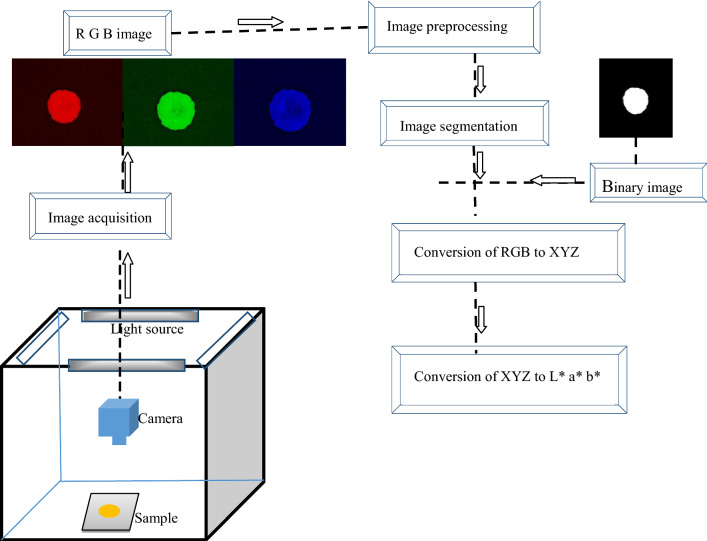
Image acquisition of each chart was taken by a Charge-Coupled Device (CCD) camera (RGB digital image). MATLAB software (version 9.3.0, release 2017b, The MathWorks Inc.) was used for the measurement of R, G, and B color values of the corresponding regions. The extraction of the color information from images was performed on a Graphical User Interface (GUI), which was created in MATLAB. GUI was started after switching on the lighting system and waited for 10 min, assuring light is uniformly distributed, and banana slices were conditioned in the CVS. Image acquisition and segmentation follow the preceding operation to obtain the color parameters from the RGB color image using equations. In the first step, RGB is converted to XYZ (Eqs. 1–3) (Poynton 1996).
| 1 |
| 2 |
| 3 |
Subsequently, these obtained R″, G″ and B″ values were converted XYZ using CIE standard for illuminant D65 correlated with color temperature of 6500 K and 2° observer (Eq. 4) (Blasco et al. 2007)
| 4 |
In the second step XYZ is converted to CIE Lab color space (Eq. 5)
| 5 |
where 95.047, 100 and 108.883 are tri-stimulus values which were obtained by the weighted-ordinate method (Δʎ = 1 nm) (CIE 1995).
Once x, y and z were calculated VarX, VarY and VarZ were obtained using Eqs. 6–8
| 6 |
| 7 |
| 8 |
And at last L*, a* and b* were obtained using Eqs. 9–11
| 9 |
| 10 |
| 11 |
The deviation or error of measured R, G, and B color values from CVS and that of colorimeter were measured. The Mean Normalized Error (MNE) for color parameters was obtained by comparing with model estimates (León et al. 2006) (Eq. 12).
| 12 |
where J: parameter were obtained with colorimeter; J*: parameter were obtained with Computer Vision System; ∆J: ∆L* = 100, ∆a* = 240, ∆b* = 240, N = 28. To measure the overall error of the model Eq. 13 can be used.
| 13 |
Image segmentation
It is an essential step in CVS and can be defined as extracting the information from the right Region of Interest (ROI) in an image. If it is not done promptly by excluding the background or keeping a significant portion of food, meaningful information cannot be obtained. Many algorithms have been developed in the past for image segmentation (Du and Sun 2004). One of the most common methods proposed by Otsu (1979), color image is converted into binary image by thresholding the grey-level of the image data. In a binary image, each pixel can take only two values; ‘0’ or ‘1’ where ‘0’ represents the ROI or foreground in an image, and ‘1’ represents background as well. Nevertheless, clearly distinct threshold is very complex to obtain by histograms of the grey-level image, which can be overcome by using morphological operation before further processing (Riquelme et al. 2008). The process flow diagram of color conversion process from RGB images to L*, a*, and b* is shown in Fig. 1. In this research work algorithms for image segmentation was developed in MATLAB software.
Fig. 1.
Process flow diagram of color conversion process from RGB images to L*a*b*
Raw material and microwave treatment
Bananas of maturity stage second were procured from the Precision Farming Development Centre (PFDC) of IIT Kharagpur for the experiment. Bananas were selected based on uniformity and color and stored at the temperature of 18 °C and relative humidity 50%. Bananas were cut into 2 mm slice and dried using a domestic microwave oven at 180 W, and 540 W. Images were taken for every 7 min interval using the developed image acquisition system.
Artificial Neural Network (ANN) model
Prior to the training of the neural network, data were normalized within the range 0 to 1 so that all values come under a specified range (Mery and Pedreschi 2005). This was performed according to Eq. 14.
| 14 |
where αi, αmin, αmax are original, minimum and maximum values respectively of the input variable can have and corresponding output values were also obtained. The feed-forward neural network with back propagation and one hidden layer, network model was used in this study. These data were divided into three sets of data randomly. 70% of the data were used for training purposes, 15% were used for testing, and the remaining 15% of data were used for validation purpose. Color parameters obtained from the colorimeter were used as the output layer while parameters obtained from CVS were used as input layer. The optimum number of hidden neurons must be selected very precisely for ANN construction. If there are less number of neurons than optimum, it will lead to the weak prediction while excess numbers will result in overtraining of the ANN (Rafiq et al. 2016). In this regard, training of ANN was started with three neurons and went increasing until optimum number is found.
Results and discussions
Calibration of CVS
Images were taken from the developed image acquisition system, and L*, a*, and b* values were calculated using the developed algorithm. There was a small difference observed between CVS data and colorimeter data (Fig. 2).
Fig. 2.
Comparison among colorimetric parameters obtained by CVS and colorimetric parameters obtained by colorimeter a a*, b b*, c L*
By observing the figure, it is clear that the model taken to convert RGB values into L*a*b* is giving acceptable results. Relationship error for all the three attributes was relatively low and within 10%. It was 2.163% for L*, 4.458% for a*, and 5.224% for b*, and overall error was 3.948%. Gutiérrez-Pulido and Vara-Salazar (2008) reported that if the error is below 10%, the model taken to predict the data is acceptable. A strong correlation coefficient was also observed and shown in Fig. 2.
Image texture analysis
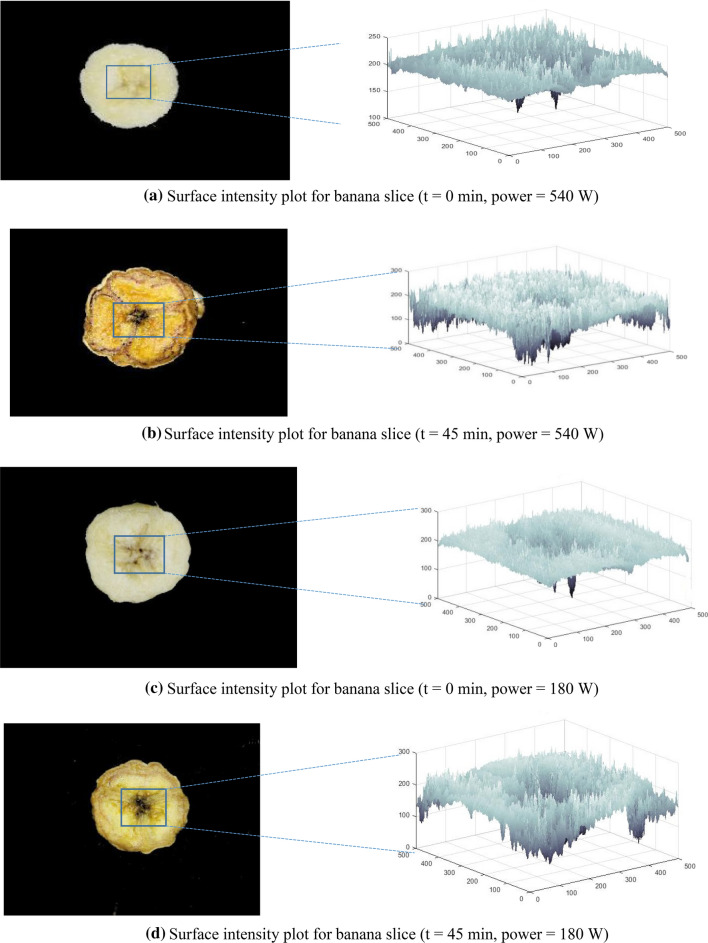
Figure 3 shows the images of banana slices before drying (t = 0 min) and after drying (t = 45 min) along with their respective gray-level intensity or surface intensity plot. These were obtained by plotting the pixel coordinated (x, y) against the gray level of each pixel (z coordinate). By observing the plots, it becomes clear that browning in banana slices is taking place predominantly during drying operation (Fig. 3b, d). SI plot of the treated sample seems more-jagged after drying than the SI plot of the sample before drying. It is because of color change that took place during the drying operation. Figure 3 showed that as the drying continue from zero minute to 45 min the region of interest of banana slice got darkened from light yellow. Surface intensity plots are the distribution of pixels values of ROI. In other words it can be said that it is the product of the reflection of the light by the sample under conditions used (Quevedo et al. 2009). The darkened banana slice (at 45 min) does not reflect the light as the fresh banana slice (at 0 min) which result in the heterogeneous distribution of surface intensity plots.
Fig. 3.
Surface intensity plot for banana slice: a t = 0 min, power = 540 W, b t = 45 min, power = 540 W, c t = 0 min, power = 180 W, d t = 45 min, power = 180 W
Application of developed CVS on banana
Color of banana has been measured in L*a*b*. The L*a*b* or CIELab color space is an international standard for color measurement adopted by Commission International de l’Elcairage (CIE). The value of L* represents the luminance or lightness within the range from 0 to 100, while the other chromatic component a* indicates green to red, and b* indicates blue to yellow color which ranges from − 120 to + 120. There is a strong correlation between color parameter and chemical composition. Changes in the color are the indication of change in the quality of food product (Saldaña et al. 2014). Color distortion in banana takes place due to the formation of brown or black pigments caused by phenol oxidation which is catalyzed by polyphenol oxidase (PPO) and peroxidase (POD) (Ko et al. 2009). As the L* represents the luminance or lightness, a decrease in the L* value indicates that the sample is getting darker. It decreased from 89.01 to 71.17 and 82.60 to 72.53 for 540 W and 180 W microwave treated banana slices respectively (Fig. 4).
Fig. 4.

Change in color parameters with time for a 540 W treated banana slice b 180 W treated banana slice
The value of a* appears in the range − 120 to + 120. Values near the negative region indicate greenish in the sample; when it turns to positive, it indicates redness. Same is observed in the banana sample. The value of a* changed from − 0.80 to 11.50 and − 3.90 to 5.18 for 540 and 180 W microwave treated banana slices respectively (Fig. 4). Same as color parameter a*, b* value also ranges from − 120 to 120. The negative value of b* describes the blueness in the sample, and it turns yellow when the value reaches to positive region. The value of b* changed from 36.46 to 60.51 for 540 W microwave treated banana slices and 34.02 to 72.82 for another sample (Fig. 4).
ANN modeling
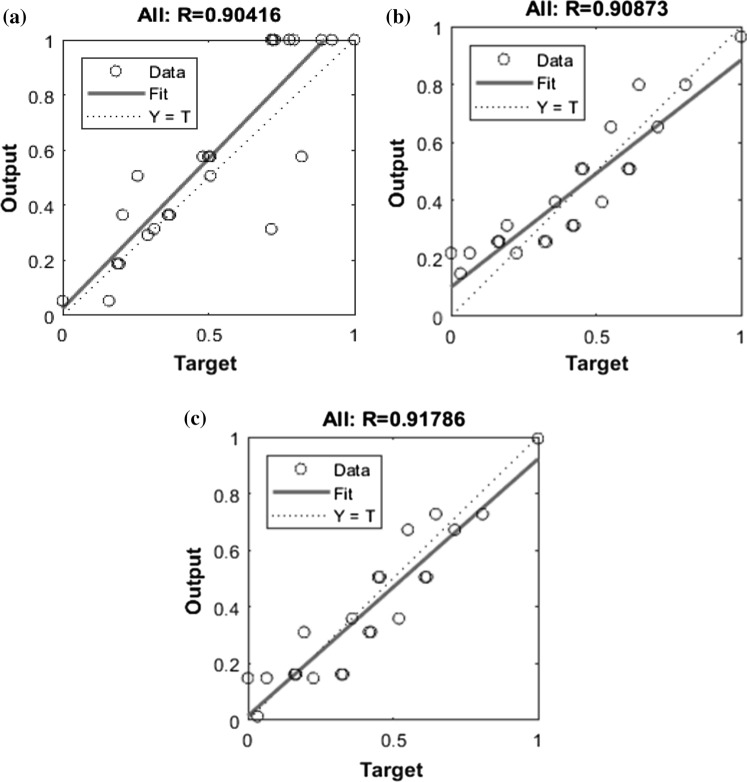
The experimental data were tested further and validated by a feed-forward neural network with back propagation. The input is fed to hidden layer with tanh activation function. While modeling the responses in ANN, various number of iterations were performed to find out the optimum number of neurons in the hidden layer. The number of neurons varied between 2 and 20 for each hidden layer. Optimum number of neurons were decided based on minimum MSE and maximum R-value. The network containing 14, 8 and 18 neurons in the hidden layer was found to predict the value of L*, a* and b*, respectively, with minimum MSE and maximum R-value. The value of MSE and R-value were 7.35 × 10−4 and 0.9041, respectively, for L* prediction, while 5.45 × 10−4 and 0.90873 for a* prediction and 4.23 × 10−3 and 0.91786 for b* prediction. The regression plots for the ANN model are presented in Fig. 5.
Fig. 5.
Estimate of a L*, b a*, and c b* for ANN model for actual vs predicted value
Conclusion
Color has been analyzed and objectively measured by a novel computerized image analysis technique in this study and applied to banana slices. An algorithmic was developed which converted RGB value from a color image into CIELab values very well. A GUI was also developed which acquires images, extracts them and analyzes useful information from it. This research proves that the application of CVS is feasible in the agricultural industry as this is less expensive and very practical to use outside the laboratory. The value of L* decreased, suggesting browning is taking place during the drying operation. The value of a* and b* increased during drying. It is concluded that the designed system can be very useful in explaining the color changes of minimally processed fruits. Homogenous representative sample requirement, which is the limitation of using colorimeter, has been eliminated in the developed CVS.
Acknowledgements
We acknowledge technical staffs of Thermal Property Laboratory of Agricultural and Food Engineering Department, Indian Institute of Technology, Kharagpur-721302, India, in conducting this research.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Al Ohali Y. Computer vision based date fruit grading system: design and implementation. J King Saud Univ Comput Inf Sci. 2011;23:29–36. doi: 10.1016/j.jksuci.2010.03.003. [DOI] [Google Scholar]
- Ali A, Ong MK, Forney CF. Effect of ozone pre-conditioning on quality and antioxidant capacity of papaya fruit during ambient storage. Food Chem. 2014;142:19–26. doi: 10.1016/j.foodchem.2013.07.039. [DOI] [PubMed] [Google Scholar]
- Antonelli A, Cocchi M, Fava P, et al. Automated evaluation of food colour by means of multivariate image analysis coupled to a wavelet-based classification algorithm. Anal Chim Acta. 2004;515:3–13. doi: 10.1016/j.aca.2004.01.005. [DOI] [Google Scholar]
- Bello-Pérez LA, Pano de Léon Y, Agama-Acevedo E, Paredes-López O. Isolation and partial characterization of amaranth and banana starches. Starch Stärke. 1998;50:409–413. doi: 10.1002/(SICI)1521-379X(199810)50:10<409::AID-STAR409>3.0.CO;2-W. [DOI] [Google Scholar]
- Blasco J, Aleixos N, Moltó E. Computer vision detection of peel defects in citrus by means of a region oriented segmentation algorithm. J Food Eng. 2007;81:535–543. doi: 10.1016/j.jfoodeng.2006.12.007. [DOI] [Google Scholar]
- Brosnan T, Sun DW. Improving quality inspection of food products by computer vision—a review. J Food Eng. 2004;61:3–16. doi: 10.1016/S0260-8774(03)00183-3. [DOI] [Google Scholar]
- CIE 1995 (1995) Industrial colour-difference evaluation (Technical Report) CIE. Cent Bur Comm Int L’Eclairage 116
- Costa C, Antonucci F, Pallottino F, et al. Shape analysis of agricultural products: a review of recent research advances and potential application to computer vision. Food Bioprocess Technol. 2011;4:673–692. doi: 10.1007/s11947-011-0556-0. [DOI] [Google Scholar]
- D’Elia L, Barba G, Cappuccio FP, Strazzullo P. Potassium intake, stroke, and cardiovascular disease: a meta-analysis of prospective studies. J Am Coll Cardiol. 2011;57:1210–1219. doi: 10.1016/j.jacc.2010.09.070. [DOI] [PubMed] [Google Scholar]
- Du CJ, Sun DW. Recent developments in the applications of image processing techniques for food quality evaluation. Trends Food Sci Technol. 2004;15:230–249. doi: 10.1016/j.tifs.2003.10.006. [DOI] [Google Scholar]
- Elsayed S, Galal H, Allam A, Schmidhalter U. Passive reflectance sensing and digital image analysis for assessing quality parameters of mango fruits. Sci Hortic (Amsterdam) 2016;212:136–147. doi: 10.1016/j.scienta.2016.09.046. [DOI] [Google Scholar]
- FAO Stat (2019) Retrieved May 10, 2019. http://www.fao.org/faostat/en/#home
- Gonzales-Barron U, Butler F. Fractal texture analysis of bread crumb digital images. Eur Food Res Technol. 2008;226:721–729. doi: 10.1007/s00217-007-0582-3. [DOI] [Google Scholar]
- Gümüş B, Balaban M, And MÜ-TJ of F, 2011 U (2011) Machine vision applications to aquatic foods: a review. Turkish J Fish Aquat Sci 11
- Gutiérrez-Pulido H, Vara-Salazar RD la (2008) Factorial designs 2 k. Analysis and design of experiments
- Ko WH, Su CC, Chen CL, Chao CP. Control of lethal browning of tissue culture plantlets of Cavendish banana cv. Formosana with ascorbic acid. Plant Cell Tissue Organ Cult. 2009;96:137–141. doi: 10.1007/s11240-008-9469-7. [DOI] [Google Scholar]
- León K, Mery D, Pedreschi F, León J. Color measurement in L*a*b*units from RGB digital images. Food Res Int. 2006;39:1084–1091. doi: 10.1016/j.foodres.2006.03.006. [DOI] [Google Scholar]
- Lu J, Tan J, Shatadal P, Gerrard DE. Evaluation of pork color by using computer vision. Meat Sci. 2000;56:57–60. doi: 10.1016/S0309-1740(00)00020-6. [DOI] [PubMed] [Google Scholar]
- Mendoza F, Aguilera JM. Application of image analysis for classification of ripening bananas. J Food Sci. 2004;69:E471–E477. doi: 10.1111/j.1365-2621.2004.tb09932.x. [DOI] [Google Scholar]
- Mendoza F, Dejmek P, Aguilera JM. Calibrated color measurements of agricultural foods using image analysis. Postharvest Biol Technol. 2006;41:285–295. doi: 10.1016/j.postharvbio.2006.04.004. [DOI] [Google Scholar]
- Mery D, Pedreschi F. Segmentation of colour food images using a robust algorithm. J Food Eng. 2005;66:353–360. doi: 10.1016/j.jfoodeng.2004.04.001. [DOI] [Google Scholar]
- Nandi CS, Tudu B, Koley C. Machine vision based techniques for automatic mango fruit sorting and grading based on maturity level and size. Cham: Springer; 2014. pp. 27–46. [Google Scholar]
- Otsu N. Threshold selection method from gray-level histograms. Czas Stomatol. 1979;26:855–860. doi: 10.1109/TSMC.1979.4310076. [DOI] [Google Scholar]
- Poynton C (1996) A technical introduction to digital video. Retrieved May 10, 2019
- Quevedo R, Mendoza F, Aguilera JM, et al. Determination of senescent spotting in banana (Musa cavendish) using fractal texture Fourier image. J Food Eng. 2008;84:509–515. doi: 10.1016/j.jfoodeng.2007.06.013. [DOI] [Google Scholar]
- Quevedo R, Díaz O, Caqueo A, et al. Quantification of enzymatic browning kinetics in pear slices using non-homogenous L*color information from digital images. LWT Food Sci Technol. 2009;42:1367–1373. doi: 10.1016/j.lwt.2009.03.011. [DOI] [Google Scholar]
- Rafiq A, Makroo HA, Hazarika MK. Artificial Neural Network-based image analysis for evaluation of quality attributes of agricultural produce. J Food Process Preserv. 2016;40:1010–1019. doi: 10.1111/jfpp.12681. [DOI] [Google Scholar]
- Riquelme MT, Barreiro P, Ruiz-Altisent M, Valero C. Olive classification according to external damage using image analysis. J Food Eng. 2008;87:371–379. doi: 10.1016/J.JFOODENG.2007.12.018. [DOI] [Google Scholar]
- Rungpichayapichet P, Nagle M, Yuwanbun P, et al. Prediction mapping of physicochemical properties in mango by hyperspectral imaging. Biosyst Eng. 2017;159:109–120. doi: 10.1016/j.biosystemseng.2017.04.006. [DOI] [Google Scholar]
- Saldaña E, Siche R, Castro W, et al. Measurement parameter of color on yacon (Smallanthus sonchifolius) slices using a computer vision system. LWT Food Sci Technol. 2014;59:1220–1226. doi: 10.1016/j.lwt.2014.06.037. [DOI] [Google Scholar]
- Someya S, Yoshiki Y, Okubo K. Antioxidant compounds from bananas (Musa Cavendish) Food Chem. 2002;79:351–354. doi: 10.1016/S0308-8146(02)00186-3. [DOI] [Google Scholar]
- Unay D, Gosselin B, Kleynen O, et al. Automatic grading of Bi-colored apples by multispectral machine vision. Comput Electron Agric. 2011;75:204–212. doi: 10.1016/j.compag.2010.11.006. [DOI] [Google Scholar]
- Yam KL, Papadakis SE. A simple digital imaging method for measuring and analyzing color of food surfaces. J Food Eng. 2004;61:137–142. doi: 10.1016/S0260-8774(03)00195-X. [DOI] [Google Scholar]
- Zaborowicz M, Boniecki P, Koszela K, et al. Application of neural image analysis in evaluating the quality of greenhouse tomatoes. Sci Hortic (Amsterdam) 2017;218:222–229. doi: 10.1016/J.SCIENTA.2017.02.001. [DOI] [Google Scholar]
- Zapotoczny P. Discrimination of wheat grain varieties using image analysis and neural networks. Part I. Single kernel texture. J Cereal Sci. 2011;54:60–68. doi: 10.1016/J.JCS.2011.02.012. [DOI] [Google Scholar]
- Zuñiga A, Mora M, Oyarce M, Fredes C. Grape maturity estimation based on seed images and neural networks. Eng Appl Artif Intell. 2014;35:95–104. doi: 10.1016/j.engappai.2014.06.007. [DOI] [Google Scholar]