Abstract
The present effort was to obtain extracts from various fruit by-products using three extraction systems and to evaluate their polyphenolic content, antioxidant, and α-glucosidase inhibition activity. The fruit by-products were pre-processed by washing, drying, and milling methods to produce the powder. The powder samples were used to obtain extracts using pressurized hot-water (PHWE), enzyme-assisted (EnE) and organic solvent extraction (OSE) systems. The total phenolic content (TPC), total flavonoid content (TFC), antioxidant and α-glucosidase inhibition activity in all samples were assessed by Folin-Ciocalteu, AlCl3 colorimetric, DPPH· & ABST·+ and α-glucosidase inhibitory methods. The results showed that the extracts of peel, seed and other by-products exhibited outstanding TPC, TFC, and strongest antioxidant and α-glucosidase inhibition activity, eventually higher than edible parts of the fruits. For instance, the highest TPC among the peels of various fruits were in mango peel (in all cultivar) followed by litchi peel, banana peel cv. sagor, jackfruit peel, pineapple peel, papaya peel, banana peel cv. malbhog and desi on average in all tested extraction systems. PHWE system yielded significantly (p < 0.05) higher TPC and TFC than other extraction systems. In case of misribhog mango variety, the TPC (mg GAE/g DM) in peels were 180.12 ± 7.33, 73.52 ± 2.91 and 36.10 ± 3.48, and in seeds were 222.62 ± 12.11, 76.18 ± 2.63 and 42.83 ± 12.52 for PHWE, EnE and OSE respectively. This work reported the promising potential of underutilized fruit by-products as new sources to manufacture ingredients and nutraceuticals for foods and pharmaceutical products.
Keywords: Fruit-waste extracts, Total phenolics, Total flavonoids, α-Glucosidase inhibitors, Antioxidant activity
Introduction
Fruit processing industries are generated huge quantities of fruit by-products including peel, seed, shell, hull, husk, stems/stalks, bran, washings, pulp refuse, press cakes which are less or not used at all and create considerable environmental pollution. Fruits, such as mango, jackfruit, pineapple, papaya, litchi, and banana contribute to higher amount of by-products in Bangladesh. The lack of information on the nutritional quality of these by-products excludes their potential use in manufacturing food, feed, pharmaceutical products and their ingredients. The chemical composition of fruit by-products varies according to the type of fruits. The seeds and peels of fruits possess high phytochemical content in terms of antioxidants that can be recovered for secondary use as functional ingredients in many processed foods and feed or drugs (Đilas et al. 2009). Presently, no actions have been taken to valorise these by-products in Bangladesh. They are just treating as food waste by incineration, fermentation, composting, landfill and sometimes using as cattle feed at home. These technologies allow to minimize waste volumes, but fail to turn such wastes into profitable products although having their great potential as important dietary sources of polyphenol compounds with strong antioxidant capacity (Ajila et al. 2007; da Silva et al. 2014). Therefore, the processing of polyphenolic compounds from fruit by-products may lead to the possibility to be used as alternative sources of natural antioxidants to prevent the oxidation of food products and subsequently would result in preserving the nutritional quality and promoting health benefits (Rahman et al. 2017).
Diabetes mellitus is a chronic disease for humans characterized by blood glucose levels. This disease causes an increased production of free radicals or impaired antioxidant defences (Baynes 1991). The excessive production of free radicals damages cellular proteins, membrane lipids and nucleic acids and thus results in destruction of the cells (Giugliano et al. 1996). Diabetes mellitus can be controlled by different means including scavenging of free radicals. Besides, the postprandial glucose levels could be controlled by inhibiting the activity of α-glucosidase that is the key enzyme catalysing the carbohydrate digestion process in small intestine (Kwon et al. 2007). Several synthetic α-glucosidase inhibitors such as acarbose, miglitol, and voglibose have been clinically used to regulate the blood glucose levels of patients suffering from diabetes mellitus (Sh et al. 2011). However, these drugs have been claimed to cause side effects including weight gain, hypersensitivity reaction, abdominal pain, meteorism, diarrhea and hepatotoxicity (Hollander 1992). Consequently, many efforts have been made to search for effective, non-toxic, natural, and inexpensive α-glucosidase inhibitors from natural resources that can prevent the risk of diabetes mellitus and its complications. Plant-based products like fruit by-product are considered as less toxic products having lower side effects than the synthetic ones (WHO 2002).
In addition, fruit by-products are considered as a cheap, natural, and suitable source of functional compounds like phenolic compounds which may be used as biologically active compounds in functional foods and pharmaceuticals as the substitutes of synthetic additives. It is evident that many synthetic additives, being used in food industries, have several side effects like toxicity and carcinogenicity (Zhang et al. 2018). The use of the extracts from fruit by-products as natural food additives or ingredients of food, feed and pharmaceutical products has recently been suggested due to their richness of nutritional and bioactive compounds (Gondi and Prasada Rao 2015).
The extracts from fruit by-products can be obtained by using traditional and innovative extraction methods. However, traditional methods show some drawbacks. For instance, requirement of longer extraction time and high purity solvents, depends on the choice of solvents, loss of a higher amount of solvents through evaporation, low extraction yield. To overcome such drawbacks, two innovative extraction methods such as enzyme-assisted extraction and pressurized hot-water extraction have been introduced in this work.
From the above points of views, the study was aimed to extract the bioactive compounds from fruit by-products through innovative extraction methods and then evaluate total phenolics and total flavonoids content, and their antioxidant and α-glucosidase inhibition activities.
Materials and methods
Samples
Jackfruit (Artocarpus heterophyllus L.), pineapple (Ananas comosus L.), papaya (Carica papaya L.), litchi (Litchi chinensis cv. bombai), three cultivars of banana (Musa acuminata): desi, sagor, malbhog and four cultivars of mango (Mangifera indica L.) such as misribhog, harivangha, langra, and amrapali were collected from local market. Seeds, peels, and other by-products of these fruits were separated from edible part and dried by a cabinet dryer at 60 ± 5 °C. The powder of these by-products was made by a grinder machine (Jaipan, JFM 1300) and was stored at − 18 °C for further analyses.
Reagents
DPPH (2,2-diphenyl-1-picrylhydrazyl), Folin-Ciocalteu reagent (2.0 N), ABTS (2,2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid)), Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), p-Nitrophenyl-α-d-glucopyranoside, α-glucosidase (yeast, EC 3.2.1.20), acarbose, sodium bicarbonate, gallic acid, quercetin, potassium persulphate (K2S2O8), sodium nitrite (NaNO2), and aluminium chloride hexahydrate (AlCl3·6H2O) were of analytical grade (Sigma Aldrich, Germany). Methanol (99.9%) was HPLC grade (Sigma Aldrich). Viscozyme® L was from Sigma Aldrich. Disodium phosphate, monosodium phosphate, hydrochloric acid (HCl), sodium hydroxide (NaOH) were also of analytical grade and purchased from Merck, Germany.
Organic solvent extraction
Organic solvent extraction was carried out according to the procedure described by Zhang et al. (2017) with some modification. Briefly, 2.5 grams of dry powder samples were mixed with 50 mL of 80% methanol (40 ml 99.9% methanol and 10 ml distilled water) in a glass conical flux as solid/liquid ratio of 1:20 (g/mL) and extracted at room temperature for 60 min in a shaking water bath at 100 rpm. Afterward, the sample was centrifuged at 4000 rpm (general centrifuge MF-300, HumanLab Instrument Co., Korea) for 10 min. An aliquot of the supernatant was transferred with a 10 mL plastic syringe and filtered through a Whatman no 1 filter paper before the analysis.
Enzyme-assisted extraction
The enzymatic hydrolysis was carried out by dissolving 2.5 grams of dry powder sample in 50 mL of 0.1 M phosphate buffer solution (pH 4) as per solid/liquid ratio of 1:20 (g/mL) and then the aqueous enzyme solution (Viscozyme® L) was added to the mixture. There were two series of experiments: at first, we searched for optimal condition of enzymatic hydrolysis where the experiments were conducted at 45 °C and pH 4 with different concentrations of enzyme (0–2%) and optimum concentration of the enzyme (1%) was determined. At fixed pH (pH = 4) and optimum enzyme concentration (1%), the incubation temperatures were varied from 35 to 55 °C. Finally, pH value (3.0–7.0) and incubation time (0–12 h) were varied while the concentration of enzyme (1%) and incubation temperature (55 °C) were fixed at optimum conditions.
Using the optimum conditions achieved in the first series of experiments, the second series of experiments were performed to investigate the enzymatic hydrolysis of all samples. All the experimental parameters (enzyme 1%, temperature 55 °C, pH = 4, incubation time 1 h) were the same as in the first series of experiments and the enzymatic hydrolysis was stopped after 60 min. All incubations were performed in a temperature-controlled shaking water bath at 100 rpm. The hydrolysates were centrifuged at 4000 rpm for 10 min. Then, the supernatant was transferred with a 10 mL plastic syringe and filtered through Whatman no 1 filter paper before the analysis. The obtained extracts were analysed for their phenolic & flavonoid content, antioxidant activities and α-glucosidase inhibitory activity.
Pressurized hot-water extraction
The pressurized hot-water extraction of bioactive compounds from fruit by-product was carried out with a laboratory-built pressure cooker system as described by (Plaza and Turner 2015). At first, 10 grams of dry powder samples were mixed with 200 mL distilled water in a 1000 mL pressure cooker as per solid/liquid ratio of 1:20 (g/mL). Then, the pressure cooker was closed and heated by the heater to reach the pressure of 20 psi. Afterward, the pressure cooker was transferred immediately to the shaking water bath. Finally, the extraction was conducted at 100 °C in a temperature-controlled shaking water bath at 100 rpm for 30 min while saturation pressure and temperature were 20 ± 1 psi and 120 ± 5 °C inside the pressure cooker. The resulting slurry was centrifuged at 4000 rpm for 10 min. An aliquot of the supernatant was transferred with a 10 mL plastic syringe and filtered through a Whatman no 1 filter paper before the analysis.
Determination of total phenolic content
The total phenolic content was determined by Folin-Ciocalteu assay according to reference (Hasan et al. 2018) with some modification. A 500 µL of samples extract was mixed with 500 µL Folin–Ciocalteu solution, then 1 mL sodium bicarbonate (7.5% solution) was added to the mixture and finally adjusted to the mark with distilled water to make 10 mL solution. Then, the mixture was vortexed for a few seconds. The solutions were left for 35 min at room temperature in the dark place followed by centrifugation for 10 min at 4000 rpm. Afterward, the absorbance was measured at 750 nm using UV–Vis spectrophotometer (UV-1800, Shimadzu Scientific Instruments Inc., USA). Gallic acid (0–200 µM) was used for calibration of the standard curve. The calibration curve showed the linear regression at R2 = 0.9976 and the results are expressed as mg of Gallic acid equivalent per gram of dry sample (mg GAE/g DM).
Determination of total flavonoid content
Total flavonoid content was measured using the colorimetric method described by Rahman et al. (2017) with some modifications. 1 mL of extracts mixed with 4 mL of distilled water and 0.3 mL of 5% NaNO2 solution in 15 mL falcon tubes. Then, the tubes were allowed to stand for 5 min and subsequently added 0.3 mL of 10% AlCl3 to the mixture and again allowed to stand for further 1 min. Lastly, 2 mL of 1 M NaOH and 2.4 mL of distilled water were added and mixed thoroughly. After centrifugation for 10 min at 4000 rpm, the tubes were kept in the dark place at room temperature for 15 min. The absorbance was read at 510 nm against a blank prepared in a similar manner by replacing the mixture with methanol. The total flavonoid content was calculated from a standard curve of quercetin and the results were expressed as mg of quercetin equivalent per gram of dry sample (mg QE/g DM).
Antioxidant activity
DPPH· scavenging ability
Antioxidant activity of samples was determined by a free radical scavenging assay using DPPH· as the source of the free radicals (Zhang et al. 2016). DPPH· can react directly with most of the antioxidants and be captured by them. The reduction of DPPH· is measured by the decrease in absorbance at a characteristic wavelength and a determined time during the reaction (30 min). A 1.950 mL DPPH· solution was transferred into the cuvette and the absorbance was measured at 515 nm with a UV–Vis spectrophotometer immediately and after 30 min of adding Trolox solution (0, 5, 10, 15, 25, 30 and 50 µM) to produce a calibration curve. Similarly, the absorbance of 50 µL extracts in the DPPH· solution was also measured to evaluate the scavenging capacity of the extract samples. The scavenging ability (%) was calculated as follows:
All the analyses were performed in triplicate. The concentration of samples resulting in 50% inhibition on DPPH· (IC50 value) was calculated by nonlinear curve fitting with OrginPro 8.6 (OriginLab Co., US) and expressed as mg/mL.
ABTS·+ scavenging ability
The total antioxidant capacity was evaluated using the method described by Zhang et al. (2017) with slight modification. The 100 mL ABTS·+ stock solution was prepared with distilled water containing 7 mM ABTS·+ and 2.45 mM potassium persulfate (final concentration), it was then placed in the dark for 12 h at room temperature, and finally, it was diluted with phosphate buffer solution (pH 7) to have an absorbance of 0.7 ± 0.1 at 734 nm before use. Then, the ABTS·+ scavenging ability was measured by mixing 50 µL extracts with 1.950 mL ABTS·+ solution. The absorbance was recorded at 734 nm after 6 min of incubation at room temperature. Trolox was used as a positive control. The percentage ABTS·+ scavenging of the extracts was calculated as:
All the analyses were performed in triplicate. The concentration of samples resulting in 50% inhibition on ABTS·+ (IC50 value) was calculated by nonlinear curve fitting with OrginPro 8.6 (OriginLab Co., US) and expressed as mg/mL.
α-Glucosidase inhibitory activity
The activity of α-glucosidase inhibition of the extracts was determined as previously reported (Zhang et al. 2016). Briefly, 50 µL diluted samples or acarbose at various concentrations were mixed with 50 µL phosphate buffer (0.1 M, pH 6.9) and 100 µL of 0.1 U/mL α-glucosidase solution (in 0.1 M, pH 6.9 phosphate buffer), and allowed to incubate at 25 °C for 10 min. Then, 50 µL of 5 mM of p-Nitrophenyl-α-d-glucopyranoside (pNPG) solution (0.1 M, pH 6.9 phosphate buffer) was added. The mixture was allowed to incubate another 5 min at 25 °C, and then the absorbance was recorded at 405 nm using UV–Vis spectrophotometer. The inhibition percentage was calculated as follows:
where is the absorbance of the control (without sample), is the absorbance of the sample, is the absorbance of the sample blank (without pNPG solution). Acarbose used as a positive reference. The calculation of IC50 value was same as DPPH· and ABTS·+ procedures and expressed as mg/mL.
Statistical analysis
Statistical analyses were carried out using XLSTAT software (Addinsoft). Analysis of variance (ANOVA) and Tukey’s test were used for multiple comparisons of mean. The differences were considered statistically significant at p < 0.05. All the triplicate data were presented as mean ± standard deviation (mean ± SD).
Results and discussion
Effect of extraction methods on the content of total phenolic and total flavonoid
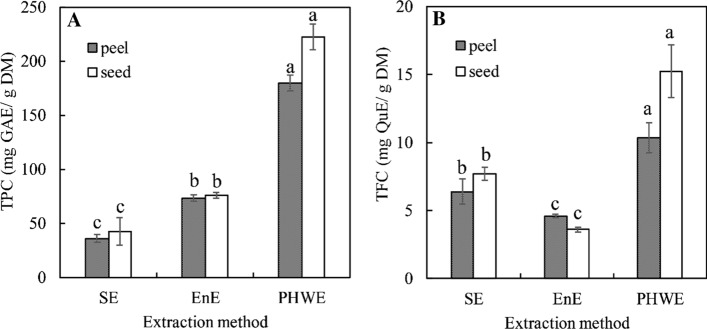
The selection of the extraction methods is very important for the recovery of bioactive compounds. The effect of pressurized hot-water extraction (PHWE), enzyme-assisted extraction (EnE) and organic solvent extraction (OSE) methods on the content of total phenolics and total flavonoids of the extracts from the peel and seed of misribhog variety is shown in Fig. 1 as an example. There were details about the content of total phenolic (TPC) and total flavonoid (TFC) for different fruits by-products in the next section (“Determination of total phenolic content (TPC) and total flavonoid content (TFC)” section). The yield of TPC and TFC were varied according to the extraction methods used. As shown in Fig. 1, the PHWE yielded significantly (p < 0.05) higher amount of TPC and TFC in misribhog mango peel and seed than EnE and OSE methods. The extracts of seeds contained higher TPC and TFC than the extracts of peels on all tested extraction methods that were agreed with Ayala-Zavala et al. (2010). The TPC (mg GAE/g DM) in peels were 180.12 ± 7.33, 73.52 ± 2.91 and 36.10 ± 3.48, and in seeds were 222.62 ± 12.11, 76.18 ± 2.63 and 42.83 ± 12.52 for PHWE, EnE and OSE respectively. The TFC (mg QE/g DM) in peel were 10.36 ± 1.13, 4.60 ± 0.15 and 6.39 ± 0.94, and in seed were 15.25 ± 1.94, 3.60 ± 0.18 and 7.71 ± 0.48 for PHWE, EnE and OSE respectively. The use of elevated temperature and pressure decreases/disrupts the surface tension of the solvent, solutes and matrix interaction, resulting in enhanced solvent permeability into the cells and enhanced mass transfer of the analyte from the sample to the solvent and faster dissolve in the solvent to yield higher amount of total phenolic and total flavonoids (Mustafa and Turner 2011).
Fig. 1.
Effect of extraction methods on the content of total phenolic (a) and total flavonoid (b). OSE organic solvent extraction, EnE enzyme-assisted extraction, PHWE pressurized hot-water extraction; peel and seed from mango cv. misribhog; different letters (a, b, c) on the column mean statistically significant differences (p < 0.05)
With respect to misribhog mango peel and seed extracts, the effect of EnE method on the recovery of TPC was significantly (p < 0.05) higher than OSE method except for the recovery of TFC. This increase in recovery can be attributed to the ability of enzyme (Viscozyme® L) to degrade cell wall and depolymerize plant cell wall polysaccharide, facilitating the release of polyphenols (Hong et al. 2013). All these indicated that both PHWE and EnE are more effective methods to increase polyphenolics yield since they improve the recovery of phenolics from fruit by-products. This can consider that the PHWE and EnE methods are attractive alternatives to traditional solvent maceration for recovery of bioactive compounds from plant matrix.
Determination of total phenolic content (TPC) and total flavonoid content (TFC)
Antioxidant activities from plant sources are mainly derived from phenolic-type components (Bozin et al. 2008), these belongings do not always correlate with the presence of large quantities of polyphenols. Therefore, both sets of data need to be assessed together. Accordingly, the TPC of the extracts from different fruit by-products were determined using Folin-Ciocalteu assay. As shown in Table 1, all tested extraction methods yielded a significant amount (p < 0.05) of total phenolic. In case of mango peel extracts from all cultivars, the TPC (mg/g DM) ranged from 128 to 209, 50 to 73, and 36 to 43, and TPC in mango seed extracts ranged from 205 to 237, 51 to 76 and 36 to 46 using PHWE, EnE, and OSE system respectively. The value of TPC in mango peel and seed extracts using PHWE system was higher than the reported value of Ajila et al. (2007) who noticed 54–100 mg GAE/g DM in Badami and Raspuri mango varieties grown in India and of Sultana et al. (2012) who obtained 63.89–116.80 mg GAE/g DM in langra mango variety while using organic solvent extraction system. A direct comparison with the literature data was not possible due to limited studies on TPC using PHWE from mango peel and seed extracts. Interestingly, Garcia-Mendoza et al. (2015) noticed a positive effect in the TPC, product of the increase of pressure in the extraction process with pressurized liquids. However, the results of TPC in mango peel and seed extracts using EnE and OSE systems were comparable with Ajila et al. (2007) and Sultana et al. (2012). The TPC in jackfruit by-products such as peel, seed, rags and core was also comparable to Zhang et al. (2017) who reported 48.04, 48.04, 11.57 mg GAE/g DM in peel, seed and rags or flake, respectively. The value of TPC in pineapple peel and core, in papaya peel and seed, in litchi peel and seed as well as in banana peel cv. sagor, malbhog and desi in tested extraction methods are agreed with da Silva et al. (2014) for pineapple peel (27.87 mg GAE/g DM) and papaya peel (7.83 mg GAE/g DM), Babbar et al. (2011) for litchi peel (24.6 mg GAE/g DM) & seed (17.9 mg GAE/g DM) and Rebello et al. (2014) for banana peel (29 mg GAE/g DM) with few exceptions. This exception can be explained by the choice of extraction methods, choice of solvent, extraction time and temperature (Zhang et al. 2018). Nevertheless, the highest TPC among the peels of various fruits were in mango peel (in all cultivar) followed by litchi peel, banana peel cv. sagor, jackfruit peel, pineapple peel, papaya peel, banana peel cv. malbhog and desi on average in all tested extraction systems, whereas among the seed and other by-products, the lowest TPC were found in jackfruit seed followed by pineapple core, papaya seed, jackfruit rags, banana seed cv. desi, and litchi seed.
Table 1.
Effect of extraction methods on total phenolic contents of the studied extracts from different fruit by-products
| Fruit | By-product | Total phenolic content (mg GAE/g DM) (mean ± SD)* | ||
|---|---|---|---|---|
| OSEa | EnEb | PHWEc | ||
| Mango cv. Misribhog | Peel | 36.10 ± 3.48c | 73.52 ± 2.91b | 180.12 ± 7.33a |
| Seed | 42.83 ± 12.52c | 76.18 ± 2.63b | 222.62 ± 12.11a | |
| Mango cv. Harivangha | Peel | 40.67 ± 2.59c | 52.49 ± 7.83b | 200.84 ± 12.31a |
| Seed | 38.95 ± 5.49c | 52.22 ± 2.24b | 222.62 ± 9.89a | |
| Mango cv. Langra | Peel | 46.54 ± 5.91c | 52.60 ± 1.44b | 209.32 ± 9.11a |
| Seed | 43.57 ± 8.96c | 49.05 ± 1.31b | 237.22 ± 15.36a | |
| Mango cv. Amrapali | Peel | 43.89 ± 5.16c | 50.94 ± 1.35b | 128.12 ± 8.79a |
| Seed | 36.66 ± 2.74c | 54.92 ± 2.65b | 205.02 ± 11.91a | |
| Jackfruit cv. Local | Peel | 35.13 ± 4.91b | 14.19 ± 0.85c | 47.22 ± 2.31ab |
| Seed | 9.71 ± 1.42ab | 10.54 ± 1.41ab | 7.02 ± 0.39b | |
| Rags | 8.89 ± 0.19c | 19.33 ± 0.16a | 11.90 ± 1.11ab | |
| Core | 15.04 ± 1.41c | 22.25 ± 0.51b | 30.20 ± 4.33a | |
| Pineapple cv. Local | Peel | 15.69 ± 2.43c | 22.23 ± 0.13b | 26.84 ± 1.31ab |
| Core | 8.29 ± 1.10b | 11.75 ± 0.39ab | 8.704 ± 0.31b | |
| Papaya cv. Local | Peel | 12.27 ± 0.41c | 17.44 ± 1.83b | 36.82 ± 0.41a |
| Seed | 6.81 ± 0.08c | 9.79 ± 0.26b | 20.41 ± 0.08a | |
| Litchi cv. Local | Peel | 102.31 ± 2.11a | 40.78 ± 2.89b | 103.57 ± 0.28a |
| Seed | 73.52 ± 1.30a | 5.04 ± 0.15c | 75.64 ± 0.04a | |
| Banana cv. Sagor | Peel | 53.80 ± 2.88ab | 60.44 ± 2.61a | 53.46 ± 2.39ab |
| Banana cv. Malbhog | Peel | 18.17 ± 0.45b | 10.25 ± 1.15c | 34.76 ± 1.45a |
| Banana cv. Local Desi | Peel | 10.17 ± 0.67bc | 6.83 ± 0.28c | 24.58 ± 1.31a |
| Seed | 8.39 ± 0.35c | 17.09 ± 0.23b | 60.16 ± 4.44a | |
*Results are presented as mean values ± SD for triplicate analyses where SD = standard deviation. Different letters (a, b, c) among three extraction systems on the same row indicate significant statistical differences (p < 0.05)
aOrganic solvent extraction
bEnzyme-assisted extraction
cPressurized hot-water extraction
The total flavonoid content (TFC) of the extracts from different fruit by-products was determined by the colorimetric method, where, flavonoid form a pink color complex with AlCl3. Table 2 represents the TFC of the extracts of by-products from jackfruit, pineapple, papaya, litchi, banana and mango fruits. The TFC was higher in litchi peel followed by banana peel cv. sagor, mango peel (in all cultivar), jackfruit peel, banana peel cv. malbhog and desi, pineapple peel, and papaya peel, whereas, among the seed and other by-products, pineapple core showed the lowest TFC followed by jackfruit seed, papaya seed, jackfruit rags and core, banana seed cv. desi, mango seed (all cultivars) and litchi seed on average in three extraction systems. However, the TFC in jackfruit peel, seed, rags, and core was comparable with Zhang et al. (2017), in mango peel and seed was with Ayala-Zavala et al. (2010) and Sultana et al. (2012), in pineapple peel and core, papaya peel and seed with Ayala-Zavala et al. (2010), in litchi peel and seed with Wang et al. (2011) and in banana peel with Aboul-Enein et al. (2016). Nevertheless, the value of the TFC in different fruit by-products in this present investigation was slightly varied with the literature. These could be due to the difference in determination method, variety, growth environment of different fruits as well as in their method of sample preparation.
Table 2.
Effect of extraction methods on total flavonoid contents of the studied extracts from different fruit by-products
| Fruit | By-product | Total flavonoid content (mg QE/g DM) (mean ± SD)* | ||
|---|---|---|---|---|
| OSEa | EnEb | PHWEc | ||
| Mango cv. Misribhog | Peel | 6.39 ± 0.94bc | 4.60 ± 0.15c | 10.36 ± 1.13a |
| Seed | 7.71 ± 0.48b | 3.60 ± 0.18c | 15.25 ± 1.94a | |
| Mango cv. Harivangha | Peel | 6.86 ± 0.97bc | 5.38 ± 0.19b | 7.40 ± 0.64ab |
| Seed | 6.41 ± 0.19b | 3.09 ± 0.09c | 10.21 ± 1.11a | |
| Mango cv. Langra | Peel | 8.61 ± 1.19ab | 6.62 ± 0.12b | 9.30 ± 0.94ab |
| Seed | 6.28 ± 0.31bc | 3.63 ± 0.49c | 9.84 ± 0.84ab | |
| Mango cv. Amrapali | Peel | 8.26 ± 0.71ab | 5.88 ± 0.22b | 9.66 ± 0.85ab |
| Seed | 6.68 ± 0.38a | 2.66 ± 0.07b | 3.95 ± 0.24ab | |
| Jackfruit cv. Local | Peel | 2.96 ± 0.15b | 1.25 ± 0.07c | 11.52 ± 0.81a |
| Seed | 0.31 ± 0.15bc | 0.19 ± 0.03b | 0.48 ± 0.13ab | |
| Rags | 0.49 ± 0.03b | 0.45 ± 0.05b | 1.62 ± 0.11a | |
| Core | 2.56 ± 0.06b | 1.89 ± 0.08c | 9.30 ± 1.03a | |
| Pineapple cv. Local | Peel | 1.56 ± 0.31b | 1.64 ± 0.21b | 2.68 ± 0.36ab |
| Core | 0.16 ± 0.03c | 0.21 ± 0.03b | 0.35 ± 0.03a | |
| Papaya cv. Local | Peel | 0.91 ± 0.09ab | 0.77 ± 0.01bc | 0.60 ± 0.01b |
| Seed | 0.59 ± 0.01c | 0.74 ± 0.01b | 1.05 ± 0.01a | |
| Litchi cv. Local | Peel | 48.67 ± 0.41a | 9.31 ± 0.45c | 13.64 ± 0.04b |
| Seed | 30.57 ± 1.64a | 0.13 ± 0.01c | 8.96 ± 0.02b | |
| Banana cv. Sagor | Peel | 16.44 ± 1.45c | 30.57 ± 1.64a | 21.11 ± 1.87b |
| Banana cv. Malbhog | Peel | 3.63 ± 0.10b | 0.41 ± 0.01c | 7.13 ± 1.11a |
| Banana cv. Local Desi | Peel | 1.17 ± 0.10b | 0.34 ± 0.03c | 4.77 ± 0.45a |
| Seed | 10.79 ± 0.64a | 0.62 ± 0.06c | 7.70 ± 0.85b | |
*Results are presented as mean values ± SD for triplicate analyses where SD = standard deviation. Different letters (a, b, c) among three extraction systems on the same row indicate significant statistical differences (p < 0.05)
aOrganic solvent extraction
bEnzyme-assisted extraction
cPressurized hot-water extraction
In comparison, the extracts of seed, core and other residues like rags of jackfruit exhibited the lower amount of the TPC and TFC than peel (Tables 1, 2) except mango seeds from PHWE. This indicates that the peel of the fruits is the better source of polyphenols than the seed of the fruits. This could be the reason that peel is more prone to undergo abiotic stress due to the external environments such as sunlight, ultraviolet irradiation, temperature, gas and insects which may promote synthesis and accumulation of polyphenols (Zhang et al. 2017). Several researches found higher TPC and TFC in the peel of the fruits. For instance, Someya et al. (2002) found higher phenolic content in the banana peel than pulp. Zhang et al. (2017) found higher phenolic and flavonoid content in jackfruit peel than pulp, flake, and seed. Masci et al. (2016) noticed that the extract of pomegranate peel exhibited much more total phenolic and total flavonoid content than whole fruit extracts.
Antioxidant activities
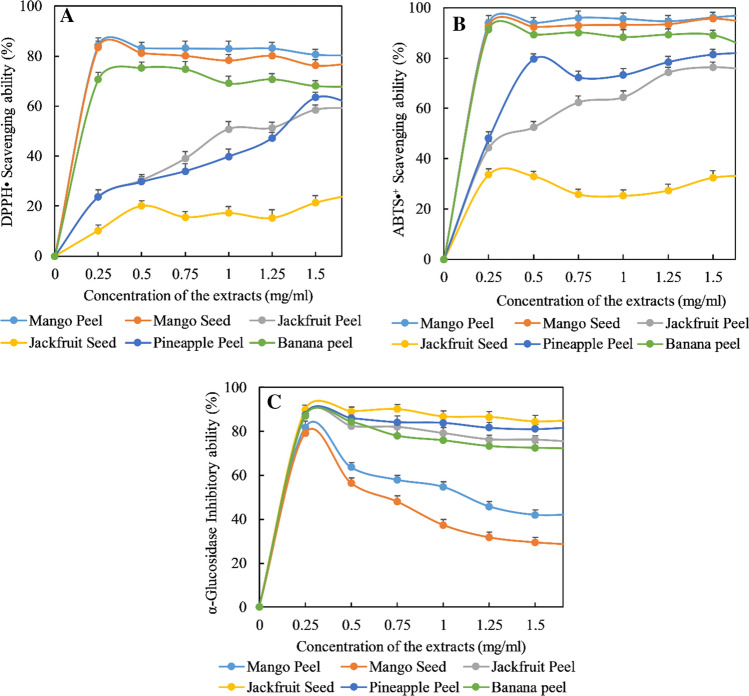
The antioxidant activities of the extracts of peel, seed and other by-products from mango, jackfruit, pineapple, papaya, litchi, and banana were measured using two in vitro assays such as DPPH· and ABTS·+ radical scavenging assay. A single method is not recommended for the evaluation of the antioxidant activity of the plant extracts due to their complex composition (Bozin et al. 2008). Thus, the antioxidant effects of the fruit by-product extracts should be evaluated by combining two different in vitro methods to get the pertinent data. The results are shown in Fig. 2, and the IC50 values of the samples expressed as mg dry matter per milliliter of extracts solution (mg DM/mL) were given in Table 3. All samples exhibited a dose-dependent relationship (Fig. 2) to scavenge free radical, the percentage inhibition was improved with the increasing of sample concentration. But, the extract of jackfruit seed gave ignorable radical scavenging activity with the inhibition from 10 to 25% for DPPH· assay and 25–33% for ABTS·+ assay at the concentration range of 0.25–1.50 mg/mL.
Fig. 2.
DPPH· scavenging ability (a), ABTS·+ scavenging ability (b) and α-glucosidase inhibition activity (c) of the studied extracts from different fruit by-products
Table 3.
Antioxidant activities of the studied extracts from different fruit by-products
| Fruit | By-product | IC50 values (mg DM/mL) (mean ± SD)* | |||||
|---|---|---|---|---|---|---|---|
| DPPH· scavenging ability | ABTS·+ scavenging ability | ||||||
| OSEa | EnEb | PHWEc | OSEa | EnEb | PHWEc | ||
| Mango cv. Misribhog | Peel | 0.15 ± 0.01a | 0.16 ± 0.03a | 0.21 ± 0.05ab | 0.15 ± 0.05a | 0.14 ± 0.02a | 0.13 ± 0.01a |
| Seed | 0.17 ± 0.11a | 0.16 ± 0.09a | 0.35 ± 0.03b | 0.15 ± 0.09a | 0.15 ± 0.06a | 0.21 ± 0.08ab | |
| Mango cv. Harivangha | Peel | 0.16 ± 0.01a | 0.17 ± 0.01a | 0.21 ± 0.06ab | 0.14 ± 0.01a | 0.13 ± 0.01a | 0.13 ± 0.01a |
| Seed | 0.16 ± 0.12ac | 0.15 ± 0.06ac | 0.21 ± 0.06ab | 0.14 ± 0.11ac | 0.14 ± 0.12ac | 0.20 ± 0.13ab | |
| Mango cv. Langra | Peel | 0.15 ± 0.06a | 0.16 ± 0.05a | 0.20 ± 0.03b | 0.14 ± 0.01a | 0.14 ± 0.01a | 0.13 ± 0.01a |
| Seed | 0.17 ± 0.09ac | 0.15 ± 0.02a | 0.21 ± 0.04ab | 0.15 ± 0.10ac | 0.14 ± 0.01a | 0.19 ± 0.12bc | |
| Mango cv. Amrapali | Peel | 0.15 ± 0.01a | 0.16 ± 0.06ab | 0.17 ± 0.01a | 0.15 ± 0.06a | 0.14 ± 0.03a | 0.14 ± 0.01a |
| Seed | 0.17 ± 0.11ac | 0.15 ± 0.03a | 0.23 ± 0.09ab | 0.15 ± 0.08ac | 0.14 ± 0.01a | 0.15 ± 0.07ac | |
| Jackfruit cv. Local | Peel | 0.63 ± 0.09ab | 0.71 ± 0.05b | 0.70 ± 0.02b | 0.53 ± 0.04b | 0.48 ± 0.13ac | 0.53 ± 0.06b |
| Seed | ND | ND | ND | ND | ND | ND | |
| Rags | 1.37 ± 0.14ac | 1.59 ± 0.05b | 1.89 ± 0.09b | 1.20 ± 0.08ab | 1.08 ± 0.05ab | 1.00 ± 0.03a | |
| Core | 1.01 ± 0.07a | 1.36 ± 0.04b | 1.56 ± 0.05c | 0.80 ± 0.16ac | 0.92 ± 0.06c | 0.83 ± 0.02bc | |
| Pineapple cv. Local | Peel | 0.92 ± 0.05c | 0.89 ± 0.06b | 0.79 ± 0.02a | 0.80 ± 0.01c | 0.72 ± 0.09bc | 0.58 ± 0.03a |
| Core | 1.38 ± 0.31ab | 1.63 ± 0.15ab | 1.26 ± 0.09a | 0.95 ± 0.09c | 0.80 ± 0.15b | 0.60 ± 0.14a | |
| Papaya cv. Local | Peel | 0.88 ± 0.15a | 1.49 ± 0.09c | 0.97 ± 0.11ab | 0.45 ± 0.17b | 0.39 ± 0.09ab | 0.39 ± 0.25ab |
| Seed | 0.97 ± 0.05a | 1.10 ± 0.12b | 1.32 ± 0.16c | 1.03 ± 0.11b | 1.01 ± 0.21b | 0.85 ± 0.16a | |
| Litchi cv. Local | Peel | 0.15 ± 0.07a | 0.23 ± 0.04b | 0.16 ± 0.05a | 0.28 ± 0.11c | 0.18 ± 0.07b | 0.14 ± 0.09a |
| Seed | 0.43 ± 0.02b | 0.37 ± 0.11ab | 0.38 ± 0.21ab | 0.40 ± 0.02a | 0.46 ± 0.08b | 0.46 ± 0.12b | |
| Banana cv. Sagor | Peel | 0.23 ± 0.09b | 0.18 ± 0.04a | 0.26 ± 0.13ac | 0.15 ± 0.03a | 0.14 ± 0.03a | 0.15 ± 0.04a |
| Banana cv. Malbhog | Peel | 0.57 ± 0.12bc | 0.48 ± 0.22ab | 0.52 ± 0.08b | 0.90 ± 0.06c | 0.87 ± 0.28bc | 0.60 ± 0.16a |
| Banana cv. Local Desi | Peel | 0.75 ± 0.01b | 0.62 ± 1.24ac | 0.71 ± 0.11bc | 0.53 ± 0.06ab | 0.49 ± 0.11ac | 0.55 ± 0.04ab |
| Seed | 0.65 ± 0.06a | 0.79 ± 0.02b | 0.80 ± 0.06c | 0.78 ± 0.11c | 0.54 ± 0.06a | 0.57 ± 0.04ab | |
*Results are presented as mean values ± SD for triplicate analyses where SD = standard deviation. Different letters (a, b, c) among three extraction systems on the same row indicate significant statistical differences (p < 0.05)
aOrganic solvent extraction
bEnzyme-assisted extraction
cPressurized hot-water extraction
A lower IC50 value reveals that an extract had higher free radical scavenging ability. All the extracts from different fruit by-products showed a significant amount of IC50 value. The results of this study were comparable with the findings by Ajila et al. (2007), Ayala-Zavala et al. (2010), and Sultana et al. (2012) for mango peel and seed, Zhang et al. (2017) for jackfruit peel, Li et al. (2014) for pineapple peel, Ayala-Zavala et al. (2010) for papaya peel and seed, but higher than Babbar et al. (2011) for litchi peel, and Aboul-Enein et al. (2016) for banana peel who were using organic solvents.
The extraction of antioxidant compounds from fruit by-products is influenced mainly by extraction methods and the conditions under the extraction process are carried out. Because each plant material has unique properties in terms of structure and composition when they are coupled with solvents the behaviour of the resulting material-solvent system is unpredictable (Pinelo et al. 2005). The results of this study indicate that the radical scavenging activity of the extracts varies solvent to solvent and by-products to by-products. Notably, organic solvent extracts had higher radical scavenging activity than pressurized hot-water extracts in DPPH· assay, although pressurized hot-water had higher total phenolic and total flavonoid content. This could be as a result of high temperature which may have caused the degradation of polyphenol in the pressurized hot-water system, hence, reducing their radical scavenging potential (Pasrija and Anandharamakrishnan 2015). However, in case of ABTS·+ assay, pressurized hot-water extracts recorded high radical scavenging activity, total phenolic and total flavonoid content in many cases. This indicates that a high total phenolic and total flavonoid content may also amply high radical scavenging activity in some cases. Accordingly, correlation analysis revealed that DPPH· and ABTS·+ scavenging ability was highly correlated with total phenolic content (DPPH & TPC: r = − 0.71 for OSE and EnE and − 0.73 for PHWE; and ABTS &TPC: r = − 0.66 for OSE, − 0.79 EnE and − 0.78 for PHWE). Several studies have shown a high correlation between radical scavenging activity and TPC (Maisuthisakul et al. 2007; Zhang et al. 2017). But correlation coefficient of DPPH· and ABTS·+ radical scavenging activity with total flavonoid content was moderate (r = − 0.45 for OSE, − 0.40 for EnE and − 0.59 for PHWE in case of DPPH and TFC, and r = -0.34 for OSE, − 0.44 for EnE and − 0.56 for PHWE in case of ABTS and TFC). Nevertheless, these indicate that both phenolics and flavonoids are major antioxidant compounds presented in different fruit by-products. The strongest antioxidant activity detected on peel extract of the different fruit by-products that can be explained by its highest content of total phenolic and total flavonoid.
α-Glucosidase inhibitory activity
α-glucosidase breaks down dietary carbohydrates into glucose. The inhibition of α-glucosidase activity is an effective strategy for the control of type 2 diabetes by diminishing the absorption of glucose in the blood (Kalra 2014). Phenolic compounds showed inhibitory activity towards α-glucosidase which is a key enzyme regulating the absorption of glucose in the small intestine (Rahman et al. 2017). In this study, the effect of the extracts from fruit by-products on the inhibition of this enzyme was examined in order to determine its possibility as anti-diabetic effects on the biological system. As shown in Table 4 and Fig. 2, all extracts from peel, seed and other by-products of mango, jackfruit, pineapple, papaya, litchi, and banana fruits were able to inhibit the activity of α-glucosidase and this activity was dose-dependent which was promising for antidiabetic benefits. Zhang et al. (2017) noticed a dose-dependent relationship of the jackfruit peel extracts on the α-glucosidase inhibitory activity.
Table 4.
The α-glucosidase inhibitory activity of the studied extracts from different fruit by-products
| Fruit | By-product | IC50 values (mg DM/mL) (mean ± SD)* | ||
|---|---|---|---|---|
| α-Glucosidase inhibitory activity | ||||
| OSEa | EnEb | PHWEc | ||
| Mango cv. Misribhog | Peel | 0.27 ± 0.01a | 0.27 ± 0.03a | 0.24 ± 0.01b |
| Seed | 0.45 ± 0.06b | 0.30 ± 0.02a | 0.35 ± 0.07a | |
| Manago cv. Harivangha | Peel | ND | 0.40 ± 0.10b | 0.37 ± 0.03a |
| Seed | 0.26 ± 0.02a | 0.32 ± 0.05ab | 0.40 ± 0.09c | |
| Mango cv. Langra | Peel | 0.49 ± 0.11c | 0.35 ± 0.02b | 0.25 ± 0.01a |
| Seed | 0.27 ± 0.03a | 0.39 ± 0.03b | 0.39 ± 0.04b | |
| Mango cv. Amrapali | Peel | 0.23 ± .04a | ND | 0.25 ± 0.02a |
| Seed | 0.26 ± 0.02b | 0.14 ± 0.01a | 0.30 ± 0.03ab | |
| Jackfruit cv. Local | Peel | 0.22 ± 0.01b | 0.18 ± 0.05a | 0.18 ± 0.00a |
| Seed | 0.15 ± 0.01a | 0.15 ± 0.01a | 0.15 ± 0.04a | |
| Rags | 0. 15 ± 0.02a | 0.14 ± 0.02a | 0.19 ± 0.04b | |
| Core | 0. 15 ± 0.01a | 0.14 ± 0.01a | 0.17 ± 0.02ab | |
| Pineapple cv. Local | Peel | 0.16 ± 0.03a | 0.31 ± 0.11b | 0.17 ± 0.01a |
| Core | 0.14 ± 0.03a | 0.14 ± 0.01a | 0.15 ± 0.06ab | |
| Papaya cv. Local | Peel | 0.25 ± 0.02a | 0.94 ± 0.09c | 0.32 ± 0.03b |
| Seed | 22 ± 0.04a | 0.68 ± 0.10c | 0.34 ± 0.03b | |
| Litchi cv. Local | Peel | 0.29 ± 0.01a | 0.26 ± 0.06a | 0.27 ± 0.03a |
| Seed | 0.25 ± 0.05a | 0.28 ± 0.01a | ND | |
| Banana cv. Sagor | Peel | 0.18 ± 0.01a | 0.34 ± 0.02c | 0.29 ± 0.02b |
| Banana cv. Malvhog | Peel | 0.15 ± 0.01a | 0.15 ± 0.01a | 0.33 ± 0.03b |
| Banana cv. Local Desi | Peel | 0.15 ± 0.02a | 0.15 ± 0.01a | 0.20 ± 0.03ab |
| Seed | ND | 0.15 ± 0.01a | 0.17 ± 0.03a | |
*Results are presented as mean values ± SD for triplicate analyses where SD = standard deviation. Different letters (a, b, c) among three extraction systems on the same row indicate significant statistical differences (p < 0.05)
aOrganic solvent extraction
bEnzyme-assisted extraction
cPressurized hot-water extraction
However, the α-glucosidase inhibitory activity of the extracts from different fruit by-products was varied from species to species and cultivar to cultivar. For example, the IC50 values of the α-glucosidase inhibitory activity among different cultivar of mango peel extracts ranged from 0.37 to 0.24 mg/mL in PHWE, 0.40 to 0.39 mg/mL in EnE and 0.49 to 0.23 mg/mL in OSE. These results are higher than Gondi and Prasada Rao (2015) who noticed 3.5 µg/mL in Badami variety mango peel grown in India. On the contrary, the IC50 values of the mango seed extracts in this study exhibited almost similar to Gondi and Prasada Rao (2015) and Irondi et al. (2014) who reported 0.34 to 0.52 mg/mL. The variation of IC50 values in these extracts may be explained with the content of mangiferin and their derivatives, in addition to other phenolic acids, flavonoids, and carotenoids, because these compounds showed an antidiabetic effect in diabetic rats (Gondi and Prasada Rao 2015). In case of jackfruit, the IC50 value of peel was higher than Zhang et al. (2017) but lower in the extracts of seed and flake who reported the IC50 value was 0.05 ± 0.00, 1.79 ± 0.15 and 10.52 ± 0.73 mg/mL in peel, seed and flake respectively. Litchi seed extract showed the lower α-glucosidase inhibitory activity than Sh et al. (2011). The inhibitory potential varies depending on the structural aspects of the phenolic acids, flavonoids, anthocyanins, and the inhibition potential by the compounds also varies depending on the source of an enzyme (Zhang et al. 2016). The IC50 values of the α-glucosidase inhibitory activity for the extracts of peel and seed from papaya and banana in this study were not possible to compare with literature data due to limited information.
The correlation analysis revealed that the α-glucosidase inhibitory activity of the extracts from different fruit by-products was moderately correlated with the antioxidant activities (DPPH· and ABTS·+ scavenging activity) (r = -0.63 for OSE, and r = -0.53 for PHWE) and total phenolic content (r = 0.50 for OSE, and r = 0.65 for PHWE). However, there was a very poor correlation was found on the content of total flavonoid (r = 0.28 for OSE, and r = 0.31 for PHWE) and the extracts from EnE showed no correlation with the antioxidant activities, total phenolic content and total flavonoid content. It could be speculated that phenolics are one of the major α-glucosidase inhibitory compounds presented in the fruits by-products. These findings agree with Gondi and Prasada Rao (2015) and Zhang et al. (2017) who noticed total phenolic content was correlated with DPPH· & ABTS·+ scavenging activity, and α-glucosidase inhibitory activity.
Conclusion
Due to the low cost and easy availability of the fruit by-products, which otherwise would be discarded as wastes, should be treated as novel and potential natural resources of natural antioxidants and α-glucosidase inhibitors to be used as ingredients in foods, feeds, and pharmaceuticals. In this study, the results showed a high correlation between total phenolic content and antioxidant activity and moderate correlation among the content of total phenolic, antioxidant activity and α-glucosidase inhibitory activity. Moreover, the type of solvent used affects the quantity and quality of polyphenol compounds extracted from the fruit by-products. In addition, the pressurized hot-water and enzyme-assisted extraction methods may be recommended to extract polyphenol compounds from fruit by-products since these innovative aqueous solvents are not toxic for human health and could be consumed together with their extracts without further processing. Therefore, the extracts from fruit by-products are promising in the field of food and pharmaceutical industries as rich sources of bioactive compounds or nutraceuticals. Besides, a recognized use of the fruit by-products will also help to mitigate environmental pollution caused by the poor disposal of such by-products. However, more researches are needed to ascertain about the accessibility, bioavailability and overall benefial effects of these extracts before their commercial exploitation considering environmental and economic aspects.
Acknowledgements
The authors would like to thanks to the grant of advanced research in education (GARE), Bangladesh Bureau of Educational Information and Statistics (BANBEIS), Ministry of education, Bangladesh (Grant # SD 2017460).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aboul-Enein AM, Salama ZA, Gaafar AA, Aly HF, Bou-Elella FA, Ahmed HA. Identification of phenolic compounds from banana peel (Musa paradaisica L) as antioxidant and antimicrobial agents. J Chem Pharm Res. 2016;8(4):46–55. [Google Scholar]
- Ajila CM, Naidu KA, Bhat SG, Rao UJSP. Bioactive compounds and antioxidant potential of mango peel extract. Food Chem. 2007;105(3):982–988. doi: 10.1016/j.foodchem.2007.04.052. [DOI] [Google Scholar]
- Ayala-Zavala JF, Rosas-Domínguez C, Vega-Vega V, González-Aguilar GA. Antioxidant enrichment and antimicrobial protection of fresh-cut fruits using their own byproducts: looking for integral exploitation. J Food Sci. 2010;75(8):R175–R181. doi: 10.1111/j.1750-3841.2010.01792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbar N, Oberoi HS, Uppal DS, Patil RT. Total phenolic content and antioxidant capacity of extracts obtained from six important fruit residues. Food Res Int. 2011;44(1):391–396. doi: 10.1016/j.foodres.2010.10.001. [DOI] [Google Scholar]
- Baynes JW. Role of oxidative stress in development of complications in diabetes. Am Diabetes Assoc. 1991;40(4):405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- Bozin B, Mimica-Dukic N, Samojlik I, Goran A, Igic R. Phenolics as antioxidants in garlic (Allium sativum L., Alliaceae) Food Chem. 2008;111(4):925–929. doi: 10.1016/j.foodchem.2008.04.071. [DOI] [Google Scholar]
- da Silva LMR, de Figueiredo EAT, Ricardo NMPS, Vieira IGP, de Figueiredo RW, Brasil IM, Gomes CL. ‘Quantification of bioactive compounds in pulps and by-products of tropical fruits from Brazil. Food Chem. 2014;143:398–404. doi: 10.1016/j.foodchem.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Đilas S, Čanadanović-Brunet J, Ćetković G. By-products of fruits processing as a source of phytochemicals. Chem Ind Chem Eng Q CICEQ. 2009;15(4):191–202. doi: 10.2298/CICEQ0904191D. [DOI] [Google Scholar]
- Garcia-Mendoza MP, Paula JT, Paviani LC, Cabral FA, Martinez-Correa HA. Extracts from mango peel by-product obtained by supercritical CO2 and pressurized solvent processes. LWT Food Sci Technol. 2015;62(1):131–137. doi: 10.1016/j.lwt.2015.01.026. [DOI] [Google Scholar]
- Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19(3):257–267. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- Gondi M, Prasada Rao UJS. Ethanol extract of mango (Mangifera indica L.) peel inhibits α-amylase and α-glucosidase activities, and ameliorates diabetes related biochemical parameters in streptozotocin (STZ)-induced diabetic rats. J Food Sci Technol. 2015;52(12):7883–7893. doi: 10.1007/s13197-015-1963-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan SMK, Asaduzzaman M, Merkyte V, Morozova K, Scampicchio M. Simultaneous kinetic and thermodynamic-based assay to determine the hydrogen peroxide (H2O2) scavenging activity of berry extracts by using reaction calorimetry. Food Anal Methods. 2018;11(2):432–439. doi: 10.1007/s12161-017-1014-z. [DOI] [Google Scholar]
- Hollander P. Safety profile of acarbose, an α-glucosidase inhibitor. Drugs. 1992;44(3):47–53. doi: 10.2165/00003495-199200443-00007. [DOI] [PubMed] [Google Scholar]
- Hong Y-H, Jung EY, Park Y, Shin K-S, Kim TY, Yu K-W, Chang UJ, Suh HJ. ‘Enzymatic improvement in the polyphenol extractability and antioxidant activity of green tea extracts. Biosci Biotechnol Biochem Jpn Soc Biosci Biotechnol Agrochem. 2013;77(1):22–29. doi: 10.1271/bbb.120373. [DOI] [PubMed] [Google Scholar]
- Irondi EA, Oboh G, Akindahunsi AA, Boligon AA, Athayde ML. Phenolic composition and inhibitory activity of Mangifera indica and Mucuna urens seeds extracts against key enzymes linked to the pathology and complications of type 2 diabetes. Asian Pac J Trop Biomed. 2014;4(11):903–910. doi: 10.12980/APJTB.4.201414B364. [DOI] [Google Scholar]
- Kalra S. Alpha glucosidase inhibitors. JPMA J Pak Med Assoc. 2014;64(4):474–476. [PubMed] [Google Scholar]
- Kwon Y-I, Apostolidis E, Kim Y-C, Shetty K. Health benefits of traditional corn, beans, and pumpkin: in vitro studies for hyperglycemia and hypertension management. J Med Food. 2007;10(2):266–275. doi: 10.1089/jmf.2006.234. [DOI] [PubMed] [Google Scholar]
- Li T, Shen P, Liu W, Liu C, Liang R, Yan N, Chen J. Major polyphenolics in pineapple peels and their antioxidant interactions. Int J Food Prop. 2014;17(8):1805–1817. doi: 10.1080/10942912.2012.732168. [DOI] [Google Scholar]
- Maisuthisakul P, Suttajit M, Pongsawatmanit R. Assessment of phenolic content and free radical-scavenging capacity of some Thai indigenous plants. Food Chem. 2007;100(4):1409–1418. doi: 10.1016/j.foodchem.2005.11.032. [DOI] [Google Scholar]
- Masci A, Coccia A, Lendaro E, Mosca L, Paolicelli P, Cesa S. ‘Evaluation of different extraction methods from pomegranate whole fruit or peels and the antioxidant and antiproliferative activity of the polyphenolic fraction. Food Chem. 2016;202:59–69. doi: 10.1016/j.foodchem.2016.01.106. [DOI] [PubMed] [Google Scholar]
- Mustafa A, Turner C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: a review. Anal Chim Acta. 2011;703(1):8–18. doi: 10.1016/j.aca.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Pasrija D, Anandharamakrishnan C. Techniques for extraction of green tea polyphenols: a review. Food Bioprocess Technol. 2015;8(5):935–950. doi: 10.1007/s11947-015-1479-y. [DOI] [Google Scholar]
- Pinelo M, Rubilar M, Jerez M, Sineiro J, Núñez MJ. Effect of solvent, temperature, and solvent-to-solid ratio on the total phenolic content and antiradical activity of extracts from different components of grape pomace. J Agric Food Chem. 2005;53(6):2111–2117. doi: 10.1021/jf0488110. [DOI] [PubMed] [Google Scholar]
- Plaza M, Turner C. Pressurized hot water extraction of bioactives. TrAC Trends Anal Chem. 2015;71:39–54. doi: 10.1016/j.trac.2015.02.022. [DOI] [Google Scholar]
- Rahman MJ, de Camargo AC, Shahidi F. Phenolic and polyphenolic profiles of chia seeds and their in vitro biological activities. J Funct Foods. 2017;35:622–634. doi: 10.1016/j.jff.2017.06.044. [DOI] [Google Scholar]
- Rebello LPG, Ramos AM, Pertuzatti PB, Barcia MT, Castillo-Muñoz N, Hermosín-Gutiérrez I. Flour of banana (Musa AAA) peel as a source of antioxidant phenolic compounds. Food Res Int. 2014;55:397–403. doi: 10.1016/j.foodres.2013.11.039. [DOI] [Google Scholar]
- Sh R, Xu D, Pan Z, Gao Y, Gao Q. Two flavanone compounds from litchi (Litchi chinensis Sonn) seeds, one previously unreported, and appraisal of their a-glucosidase inhibitory activities. Food Chem. 2011;127:1760–1763. doi: 10.1016/j.foodchem.2011.02.054. [DOI] [Google Scholar]
- Someya S, Yoshiki Y, Okubo K. Antioxidant compounds from bananas (Musa Cavendish) Food Chem. 2002;79(3):351–354. doi: 10.1016/S0308-8146(02)00186-3. [DOI] [Google Scholar]
- Sultana B, Hussain Z, Asif M, Munir A. Investigation on the antioxidant activity of leaves, peels, stems bark, and kernel of mango (Mangifera indica L) J Food Sci. 2012;77(8):C849–C852. doi: 10.1111/j.1750-3841.2012.02807.x. [DOI] [PubMed] [Google Scholar]
- Wang H-C, Hu Z-Q, Wang Y, Chen H-B, Huang X-M. Phenolic compounds and the antioxidant activities in litchi pericarp: difference among cultivars. Sci Horticult. 2011;129(4):784–789. doi: 10.1016/j.scienta.2011.05.042. [DOI] [Google Scholar]
- WHO . Traditional medicine strategy 2002–2005. Geneva: World Health Organization; 2002. [Google Scholar]
- Zhang L, Tu Z, Yuan T, Wang H, Xie X, Fu Z. ‘Antioxidants and α-glucosidase inhibitors from Ipomoea batatas leaves identified by bioassay-guided approach and structure-activity relationships. Food Chem. 2016;208:61–67. doi: 10.1016/j.foodchem.2016.03.079. [DOI] [PubMed] [Google Scholar]
- Zhang L, Tu Z, Xie X, Wang H, Wang H, Wang Z, Sha X, Lu Y. Jackfruit (Artocarpus heterophyllus Lam.) peel: a better source of antioxidants and a-glucosidase inhibitors than pulp, flake and seed, and phytochemical profile by HPLC-QTOF-MS/MS. Food Chem. 2017;234:303–313. doi: 10.1016/j.foodchem.2017.05.003. [DOI] [PubMed] [Google Scholar]
- Zhang Q-W, Lin L-G, Ye W-C. Techniques for extraction and isolation of natural products: a comprehensive review. Chin Med. 2018;13(1):20. doi: 10.1186/s13020-018-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]