Abstract
Biogenic amines (BAs) are organic nitrogenous compounds, formed mostly by decarboxylation of corresponding amino acids. BAs are responsible for several biological events. However, if the concentration of BAs reached the threshold level, it causes mild to serious health problems in human. The objective of this manuscript was to summarize the prevalence and prevention of Bas formation, detection methods and factors affecting the BAs formation in fermented fish and meat products. Meat sausages are the fermented meat product that contains high BAs. Fish sauces are reported to have high BAs compared to other fish products. Several chemosensors and chromatography methods are available to screen and detect BAs in foods. The prevention measures are vital to avoid toxic outbreaks. The use of starter culture, application of physical factors, control of environmental factors, and use of polyphenols could prevent or diminish the formation of BAs in fermented foods. The literature survey warrants that the development of potent starter with desirable characters, maintenance of hygienic food production and regular monitoring of commercial products are necessary to ensure the quality and safety of fermented fish and meat product.
Keywords: Biogenic amine, Sausages, Fish sauces, Fermentation, Fermented foods, Starter culture
Introduction
Food becomes poisonous, due to the microbial contaminations and its toxic metabolites, which is a serious public health issue. Food poisoning may occur at any stage of food preparations like harvesting of raw materials, handling, processing, cooking, storage, and transport, etc. Though pathogenic microbial contamination and infection are a major cause of foodborne diseases, toxic substances formed in the food materials are critical in terms of food safety (Özogul and Hamed 2018). Foodborne diseases are one of the hindrances of economic growth, which affects a nation’s productivity and increases medical expenses (Hussain and Dawson 2013).
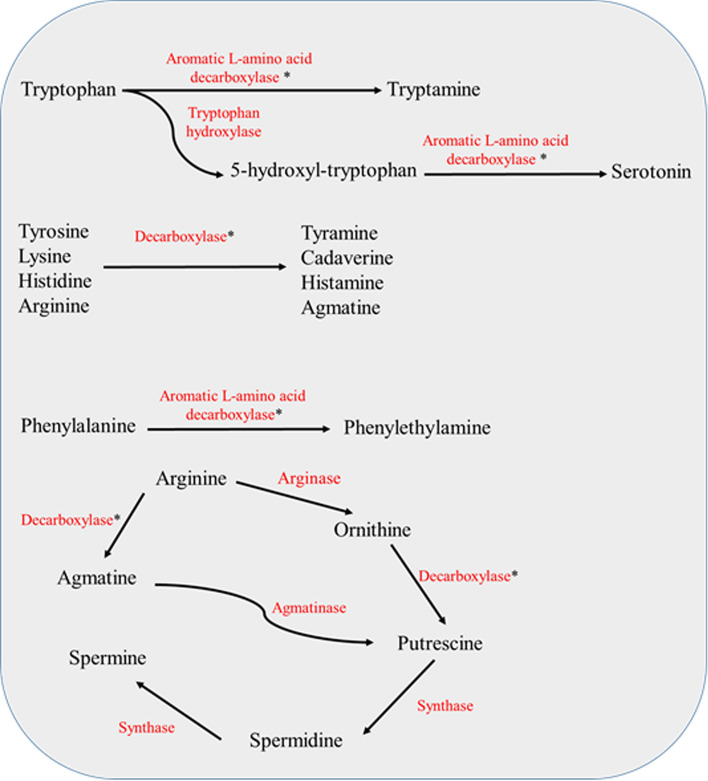
Biogenic amines (BAs) are low molecular weight organic compounds. Decarboxylation of amino acids, amination, and transamination of ketones and aldehydes resulting in the formation of BAs (Fig. 1), which is a normal metabolic event that occurs in all the living cells. Thus, BAs are omnipresent in plants, animals, microorganisms, and humans. BAs are categorized as aromatic and heterocyclic, di-, tri-, and polyamines, aliphatic amines, and aliphatic volatile amines based on the chemical structure. Moreover, BAs are classified as mono, di, and polyamines based on their amine group (Ferreira and Pinho 2006).
Fig. 1.
Formation of biogenic amines. *Decarboxylation reaction release CO2 as by product
BAs are associated with several biological functions that includes the regulation of blood pressure, growth and nerve conditions, immune development, and metabolic activities. Besides, the high concentration of BAs (1000 mg of total BAs/kg; < 8 mg of histamine) in the system cause a minor allergic reaction to some serious health problems in respiratory, cardiovascular and nervous systems to the susceptible individuals (Erdag et al. 2018) (Fig. 2).
Fig. 2.
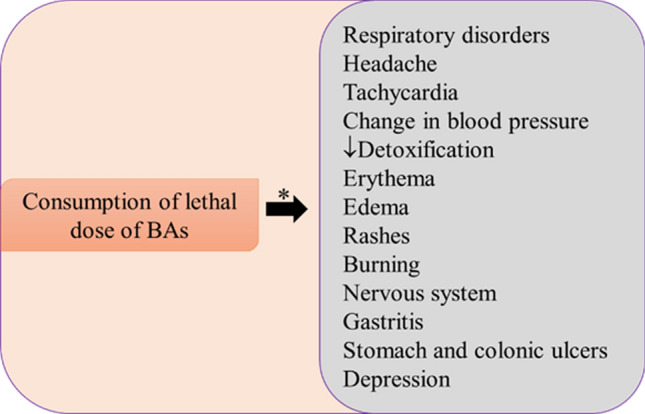
The lethal effects of biogenic amines toxicity in humans. *The severity of harmfulness of BAs overdose are associated with the individual’s health status
The production of fermented fish and meat-based food products include controlled-starter based method, and spontaneous natural process. Even though the availability of free amino acids is the major factor, which affects the quality and quantity of BAs in foods (Pinho et al. 2001), the microbiological quality of the raw material is a major concern regarding BAs. Lactobacillus, Leuconostoc, Lactococcus, Enterobacter, Escherichia, Enterococcus, Streptococcus, Staphylococcus, Shigella, Salmonella, Pseudomonas, and Bacillus species are the common microbes that produce BAs through amino acid decarboxylase activity (Suzzi and Gardini 2003). Among them, Lactobacillus spp. are majorly responsible for histamine, tyramine, and putrescine load in foods while Enterobacteriaceae and Enterococcus spp. contributing the putrescine and cadaverine, and tyramine load in foods (Bover-Cid and Holzapfel 1999).
The objective of this manuscript was to narrate the prevalence and prevention of BAs in fermented fish and meat products. The detection methods and factors affecting the BAs formation have also been discussed.
The relevant literature were collected from Web of Science, PubMed, Scopus, and Google Scholar using the keywords “biogenic amine” and “fermented food”. The papers published about BAs in fermented fish and meat products were selected and used for the manuscript preparations.
Prevalence of BAs in fermented seafood and meat products
Sausages and meat products
Maria group assessed the BAs, polycyclic aromatic hydrocarbons (PAHs), and heavy elements content of fermented dry sausages of Portuguese and Serbian using reversed phase-HPLC method. Cadaverine, putrescine, and tyramine were found in both Portuguese and Serbian sausages while histamine was not observed in a significant amount. Though tested sausage samples meet the quality standard of the European Union, lead was detected in all the samples. Moreover, a low amount of heavy elements was also observed in the samples. The study suggested that the selection of starter culture and optimized smoking phase are the critical steps in fermented sausage production (Alves et al. 2017).
Erkmen and Bozkurt (2004) evaluated the microbiological and chemical safety of the factory and butcher made sucuks (Turkish traditional dry fermented sausage). Both factory and butcher made sucuks samples showed positive for BAs, but the concentration of BAs was higher in butcher’s sucuks when compared to the factory made sucuks. Histamine, putrescine, spermine, and cadaverine were found in the sucuks samples. The concentration of them varied from not-detectable to toxic level. The amount of microbial load was also found to be varied among the samples, and butcher’s sucuks showed slightly high pH value than that of the factory sucuks. The increased BAs in butcher’s sucuks samples was attributed to the hygiene issues during the production and ripening process (Erkmen and Bozkurt 2004). Recently, Ciçek and Tokatli (2018) estimated the BAs content of Turkish traditional fermented sausage (Bez Sucuk) made with different ratio of meat and fat. Bez Sucuk containing more meat exhibited high content of tyramine, histamine, cadaverine, putrescine 2-phenylethylamine, and tryptamine when compared to other samples after 14 days of the maturation period. The spermine and spermidine were not detected in any of the samples. High meat content might provide more amino acids that are converted as BAs. The results suggested that the Bez Sucuk prepared with 70:30 ratio of meat: fat exhibited low BAs during preparation and storage, and ripening period also greatly influences the BAs load in the samples (Ciçek and Tokatli 2018).
Eerola group investigated the level of BAs in Finnish sausages. A significant amount, and even toxic level of BAs such as tyramine, histamine, phenylethylamine, putrescine, and cadaverine were detected in Finnish sausages. The study suggested that the unhygienic production and ripening process are the major source of BAs contamination, and BAs could be an indicator of the quality of the fermented meat sausages (Eerola et al. 1998).
González-Tenorio et al. (2013) examined the changes of BAs content in the soft and dry-ripped sausages [with different activity water (aw) content] stored at 5 °C for 42 days or at 12 °C for 240 days, respectively. All the samples were detected with a notable amount of tyramine. The soft sausages showed an overall higher amount of BAs. The long storage period, aw value at packaging, lactic acid bacteria, and Enterobacteriaceae load are greatly associated with the BAs level in soft sausages. The study proposed that drying and storage temperature, usage of decarboxylase negative-starters, and maintaining the low aw value might effectively reduce the formation of BAs in sausages (González-Tenorio et al. 2013).
Ikonic et al. (2013) analyzed the formation of BAs and proteolysis in Petrovská klobása (dry-fermented sausage of Northern Serbia; made at low ambient temperature without starter and additives) during 120 days of storage period. The results of proteolysis analysis suggested that the sausage samples endure slow fermentation. Tryptamine, phenylethylamine, and spermine were detected in all the detection points while tyramine was detected after 30 days of storage. Similarly, putrescine was not spotted in all the samples, but after 30 days, the content of putrescine was at a noticeable level. Histamine, spermidine, and serotonin were not detected in any of the samples. The level of BAs in Petrovská klobása has not reached the lethal level during the storage period (Ikonic et al. 2013).
Kameník et al. (2012a) examined the content of BAs and polyamines in mold-fermented sausages (Fuet, and Hungarian-type sausage) in comparison with smoked-traditional fermented sausages (Nitran). Putrescine and tyramine, histamine and tyramine, and histamine were predominantly found in fuet, Hungarian-type sausages, and Nitran sausages, respectively. Overall, the BAs content in mold-fermented sausages did not differ from smoked sausages (Kameník et al. 2012a).
The physiochemical analysis showed that fermented wild boar and deer meat sausages made in Croatia contains a notable amount of tyramine (47.3 to 219.0 mg/kg), and a low level of histamine. The microbiological analysis displayed the sausage specific lactic acid bacterial species, and the microbial communities exhibited intraspecies diversity. More than 30% of the sausages were found to be contaminated with pathogenic microbes (Maksimovic et al. 2018).
Parente et al. (2001) studied the rate of BAs content in Italian dry sausages (Salsiccia and Soppressata) made in different food production plants (two of the production process involved commercial starter) were assessed during 40 days of the ripening process. The content of tyramine, cadaverine, and putrescine were found to be increased in sausages while histamine and 2-phenylethylamine were detected occasionally during the ripening process. The microbiological studies suggested that the microbial load and use of starter culture were not significantly correlated with the BAs content (Parente et al. 2001).
Malcata group investigated the changes in amino acids and BAs content in dry ripped sausages (made from horse, beef and turkey meats) during 28 days of refrigerated (4 °C) storage. About 285, 263, and 6 mg of putrescine, histamine, and cadaverine were found in per kg of turkey sausages, respectively. Followed by beef and horse sausages that showed maximum BAs load. The amino acid content of beef and horse sausages were found to be increased while turkey sausages showed a significant reduction of amino acid content. Overall, the turkey sausages were noted with a lethal level of BAs during storage. Therefore, special concern should be raised among the consumer of turkey sausages regarding the health safety (Rabie et al. 2014).
Wójciak and Solska (2016) examined the formation of toxic chemical compounds in marinated fermented beef samples during storage (up to 3 years), and results showed that the beef samples marinated with acid whey and salt exhibited high total BAs content (1.159 g/kg). Putrescine, cadaverine, and tyramine were found in beef samples while histamine, spermidine, spermine, agmantine and N-nitrosamines were not detected in any of the samples at any point of analysis (Wójciak and Solska 2016).
Santiyanont et al. (2019) analyzed the BAs content and microbial load in Thai fermented pork meat product, nham, during the fermentation process. Three different batches of nham were prepared with a different batch of raw materials but using same method of preparation and in the same manufacturing facility. The samples were collected at different time point to assess the microbial load and BAs. The results showed that nham prepared with high-quality raw materials exhibited an acceptable level of BAs content while other batches showed high total BAs and tyramine content. The study also suggested that Weissella hellenica exhited a negative association with BAs formation in nham, and further studies on the mechanism of BAs suppression by W. hellenica could aid to produce high-quality nham (Santiyanont et al. 2019).
Fish products
Malcata group investigated the occurrence of BAs in Egyptian fermented fish, meat and dairy foods. The fermented sausages, mesh and blue cheese showed high BAs content (2.4, 2.1, and 2 g/kg, respectively) while smoked cooked sausages showed low BAs content (0.1 g/kg) when compared to the other samples. Histamine, tyramine, putrescine, and cadaverine are predominant BAs found in the samples. The high level of histamine, and tyramine were found in Feseekh (fermented salted fish), and blue cheese, respectively. There results showed that some Egyptian fermented foods contain hazards materials in terms of BAs (Rabie et al. 2011).
Ghazali group examined the BAs content in the commercial Ikan pekasam (Fermented fish product) samples (Ezzat et al. 2015). Histamine, tyramine, putrescine, cadaverine, spermidine, spermine, tryptamine, and phenylethylamine were found at various concentrations in both traditionally prepared and acid-assisted Ikan pekasam samples made from Javanese carp and black tilapia, but the concentration of BAs was in acceptable range (> 1000 ppm; Nout (1994) reported that approximately 1000 ppm is considered as the hazardous level of total BAs). The results suggested that the commercially available Malaysian Ikan pekasam might be safe for human consumption regarding BAs load (Ezzat et al. 2015).
Jiang et al. (2014) studied the rate of BAs content in thirty-five commercial Yulu (Chinese fermented fish sauce) samples. Putrescine, cadaverine, histamine, tyramine, tryptamine, spermidine and spermine were found in Yulu samples. Several samples showed more than tolerable level of total BAs, histamine, and tyramine. The study suggested that the commercially a viable Yulu product should undergo a routine quality check to ensure the safety of the product (Jiang et al. 2014).
Köse et al. (2012) investigated for the BAs content in smoked, fermented and marinated fish products originated from Turkey and Europe. To the maximum of 1.862 and 1.544 g of cadaverine and histamine were observed in per kg of smoked bonito, and fish paste, respectively, which may cause serious health problems. The fermented foods were accounted for the high content of putrescine. Tyramine, tryptamine and phenylethylamine were also found in significant levels. The study suggested that some of the tested fish products are lethal to human health, and European food samples were relatively safe in terms of histamine content (Köse et al. 2012).
Moon et al. (2010) examined the BAs content in Korean fermented fish products (anchovy and sand lance sauces, shrimp, clam, and squid pastes). Sand lance (538.2 and 212.8 mg/kg) and anchovy (810.5 and 202.5 mg/kg) sauces accounts for high content of tyramine and histamine. Particularly, aekjeot and jeotgal samples exhibited high BAs content (specifically high histamine load) than that of the other samples. The study warrant that routine cautious screening of BAs in the fermented fish product is necessary to ensure the safety of the consumers (Moon et al. 2010).
Yongsawatdigul et al. (2007) studied the influence of specific starters (Virgibacillus sp. SK33 and SK37, and Staphylococcus sp. SK1-1-5) on total amino acids and BAs content in fermented Thai fish sauces. The fish sauces made with SK33 and SK37 were comparable with traditional fermented fish sauces in terms of amino acid content, and the use of SK33 effectively reduced the histamine formation during fermentation. However, strain SK1-1-5 has the potential to quicken the fermentation process and the quality of the sauce regarding the sensory features. The results suggested that the use of a specific starter culture could reduce the formation of BAs during the production of fish sauces (Yongsawatdigul et al. 2007) (Table 1).
Table 1.
Prevalence of biogenic amines (BAs) in fermented seafood and meat products
| Food products | BAs found | Detection methods | Remarks | References |
|---|---|---|---|---|
| Fermented meat sausages | Cadaverine, putrescine, tyramine | RP-HPLC | Histamine content was not at a significant level | Alves et al. (2017) |
| Sucuks (Turkish- fermented sausage) | Histamine, putrescine, spermine, cadaverine | HPLC | Butcher’s sucuks contain more BAs | Erkmen and Bozkurt (2004) |
| Bez Sucuks (Turkish- fermented dry sausage) | Tyramine, histamine, cadaverine, putrescine, 2-phenylethylamine, and tryptamine | HPLC | The sausages made with high meat ratio exhibited high BAs content | Ciçek and Tokatli (2018) |
| Soft-fresh Mexican chorizo, and dry-ripened Spanish chorizo sausages | Tyramine | HPLC | Soft sausages exhibited high BAs content | González-Tenorio et al. (2013) |
| Finnish dry sausages | Tyramine, histamine, phenylethylamine, putrescine, and cadaverine | HPLC | BAs were found in many samples and are sourced from the unhygienic production process. | Eerola et al. (1998) |
| Petrovská klobása (dry-fermented sausage of Northern Serbia) | Tryptamine, tyramine phenylethylamine, putrescine, spermine, and cadaverine, | HPLC | Histamine, spermidine, and serotonin were not detected | Ikonic et al. (2013) |
| Dry fermented Sausages (Fuet, Nitran, Hungarian-type sausage) | Tyramine, putrescine, histamine, and cadaverine | RP-HPLC | Mold-fermented sausages do not differ from smoked sausages regarding BAs content | Kameník et al. (2012a, b) |
| Fermented wild boar and deer meat sausages | Tyramine, and histamine | ELISA and HPLC | Presumptive pathogens and high level of tyramine was detected in > 30% of the samples | Maksimovic et al. (2018) |
| Salsiccia and Soppressata (Italian dry sausages) | Tyramine, putrescine, and cadaverine | HPLC | 2-Phenylethylamine and histamine were observed rarely | Parente et al. (2001) |
| Dry sausages made from horse, beef and turkey meats | Putrescine, cadaverine, and histamine | Amino acid analyzer | Turkey meats sausages were dangerously contaminated with BAs | Rabie et al. (2014) |
| Egyptian fermented foods | Histamine, tyramine, putrescine, and cadaverine | Ion-exchange chromatography | Salted fermented fish and blue cheese have major health risks | Rabie et al. (2011) |
| Fermented beef | Putrescine, cadaverine, and tyramine | Amino acid analyzer | Histamine was not detected | Wójciak and Solska (2016) |
| Nham (Fermented pork product) | Tyramine | HPLC | Quality of the raw materials and starter culture influence the BAs content of nham samples | Santiyanont et al. (2019) |
| Ikan pekasam (Fermented fish product) | Histamine, tyramine, putrescine, cadaverine, spermidine, spermine, tryptamine, and phenylethylamine. | RP-HPLC | BAs content was at an acceptable level | Ezzat et al. (2015) |
| Yulu (Chinese fermented fish sauce) | Putrescine, cadaverine, histamine, and tyramine | HPLC | More than acceptable level of histamine was found in some of the samples | Jiang et al. 2014 |
| Fish products | Histamine, phenylethylamine, tyramine, and tryptamine | HPLC | Relatively low BAs content was observed in European fish products when compared to other tested samples | Kose et al. (2012) |
| Fermented fish products | Histamine, and tyramine | HPLC | Study warrant careful examination of BAs in fish products | Moon et al. (2010) |
| Fermented fish sauce | Cadaverine, histamine, and tyramine | HPLC | Use of starter culture reduced the formation of histamine in fish sauce | Yongsawatdigul et al. (2007) |
HPLC high-performance liquid chromatography, RP-HPLC reversed phase high performance liquid chromatography
Factors affecting BAs formation
Several factors such as raw material quality, temperature, moisture, fermentation duration, storage conditions, starter culture, and size of the product (in case of sausages) influences the formation and accumulation of BAs in meat and fish products. The use of decarboxylase-negative starter culture may decrease the formation of BAs during the production and storage of sausages (Bover-Cid et al. 2000).
Generally, in industry, high temperature and high relative humidity conditions are applied to warrant the quicker acidification of sausages, which may reduce the production time but it may not reduce the BAs formation. The high temperature, high relative humidity, and diameter of sausages (llonganissa is larger sized sausage) are the major technical parameters, which are greatly associated with the formation and accumulation of BAs during the spontaneous fermentation process of sausage preparation (Latorre-Moratalla et al. 2010). The study confirmed that maintaining the low temperature and relative humanity could reduce the accumulation of BAs during the production of dry fermented sausages (Latorre-Moratalla et al. 2010). The influence of high temperature, high relative humidity and diameter of sausage on BAs formation in sausages prepared with starter Lactobacillus curvatus CTC273 (BAs producer strain) has been studied. BAs content (especially tyramine, cadaverine, and phenylethylamine) was found to be increased in llonganissa type sausages. The fermentation condition endorsed the decarboxylase activity and facilitated the accumulation of BAs. The results suggested that the accumulation of BAs is chiefly depends on the proteolytic and decarboxylase activity of the residing microbes (Latorre-Moratalla et al. 2012).
Kuley group examined the effect of brine solutions (10 and 20% NaCl) and LAB strains (Lactococcus lactic subsp. cremoris, L. lactic subsp. lactic, L. plantarum, and S. thermophilus) in fermented trout fillets production. The pathogenic strains were introduced in the production process and various production conditions with or without LAB and brine solution were performed. The results showed that the addition of LAB did not influence the quality of the product in terms of BAs content, while brine solution showed inhibitory effects on some of the BAs, especially histamine content (Kuley et al. 2011).
Tabanelli group reviewed the influence of genetic, metabolic factors, residing microbes, decarboxylase activity, environmental factors, and technological factors like use of starter, additives, antimicrobial substances, and storage conditions on BAs formation in fermented foods in detail (Gardini et al. 2016). Temperature, salt concentration, and pH are the main environmental factors, which affect the BAs formation in fermented foods especially in sausages. These factors can stimulate the formation BAs via providing the optimal condition for the growth of aminogenic microbes and activity of decarboxylase enzyme (Bargossi et al. 2015), and these conditions should support the enzyme activity rather than microbial growth for the formation of BAs (Marcobal et al. 2006). Moreover, in some cases, the decarboxylase activity does not require an intact cellular system (Moreno-Arribas and Lonvaud-Funel 2001; Kanki et al. 2007; Tabanelli et al. 2012).
The cooking methods significantly affected the BAs formation in foods. Grilling and frying process accelerates the histamine formation in fish, meat, and vegetables whereas boiling prevent or reduce the histamine content compared to that of the raw and processed foods (Chung et al. 2017).
The studies concluded that the quality of the raw material, environmental factors (temperature, pH, and humidity), technological factors (starter culture, additives, antimicrobial substances, and storage conditions), and cooking methods are the major elements that affect the BAs content in fermented fish and meat products (Fig. 3).
Fig. 3.
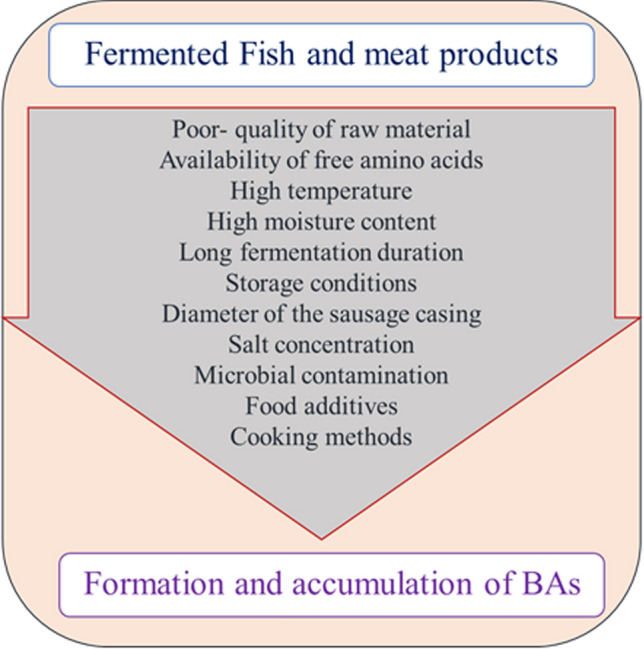
Factors influencing the formation and accumulation of biogenic amines in fermented fish and meat products
Detection of BAs
Several chemosensors (aggregation-induced emission (AIE) based, ligand exchange (LE) based, photoinduced electron transfer (PET)/internal charge transfer (ICT) based, chromogenic, ratiometric fluorescent, fluorescence resonance energy transfer (FRET) based, metal–ligand charge transfer (MLCT) based, fluorescent quantum dots, chemodosimeters, etc.,) are used to detect the BAs in the different matrix, especially in foods. Kaur et al. (2018) have detailed the complete mechanism of chemosensors for BAs detection recently. Nakamura et al. (2011) demonstrated the detection and identification of BAs using the fluorometric sensor array of AIE-active tetraphenylethenes (TPEs) containing different carboxylic acid groups. Ten different amines (including five BAs such as histamine, phenethylamine, spermine, spermidine, and tryptamine) were identified using AIE-active TPE receptors containing different carboxylic acid group (recognition unit; denoted as R) such as TPE 1 (R = COOH), TPE 2 (R = OC4H8COOH), and TPE 3 (R = OC2H4OC2H4COOH). AIE-active TPE displayed blue emission (at 480 nm) only when interaction (electrostatic interactions or/and hydroden bonding) occurs between the amines and carboxylic acid present in TPE. The intensity of fluorescence depends on the amines, which could be used to distinguish between the amines. The pattern of the different fluorescence responses of the TPEs (TPE 1, TPE 2, TPE 3) against the amines were classified by analyzing all the measured (480 nm) fluorescence intensity using linear discriminant analysis (LDA). Therefore, LDA analysis differentiated each amine (10 amines) qualitatively. The classification accuracy of LDA model was 98% based on the leave-one-out classification. Nakamura et al. (2011) also examined the histamine concentration in a tuna fish matrix (extract obtained from dicholoromethane/hexane (1:1 v/v) extraction of canned tuna fish) to demonstrate a test applying their findings for checking the quality and freshness of the food. Even at a 50 ppm concentration of histamine, the fluorescence was clearly visible to the naked eye and the fluorescence intensity measured at 480 nm increased linearly with the concentration (0-100 ppm) of histamine (Nakamura et al. 2011). Kim and Kim (2016) demonstrated the detection of polyamines (spermidine and spermine) using the sulfonated probe (2-SO3−) based on the AIE approach. The sulfonated probe shows a a weak background emission (at 541 nm) and maximum absorption (at 458 nm) during the non-aggregation state. Anionic probe (2-SO3−) displays a red-shifted emission (at 600 nm) upon electrostatic interaction with multicationic analyte (polyamines). A shift in the maximum absorption (from 458 to 462 nm) occurs as a result of the interaction between the spermine (tetracationic) and sulfonated probe. Interaction of spermidine (tricationic) with the sulfonated probe also displayed shifted emission but the fluorescence was less compared to that of the spermine. Since spermidine is tricationic, it interacted with less equivalents of sulfonated probe compared to that of the spermine, which was confirmed by binding studies. Presence of other BAs showed no significant hindrance during the detection of spermine and spermidine in artificial urine samples (Kim and Kim 2016).
Seto et al. (2010a) demonstrated the detection and imaging of histamines in living RAW 264 cells using a novel fluorescent probe composed of 2 moieties (an iminodiacetic acid-Ni2+ complex and Nile red). The mechanism is based on the LE reaction, the fluorescence is turned on only when Ni2+ dissociates from the probe and interact with histamine. The fluorescence intensity measured at 670 nm increased linearly with the concentration (0-1000 µM) of histamine. The detection limit of this approach was reported to be 54 µM histamine. Same group also reported the LE based detection and monitoring of histamines in living RAW 264 cells using a fluorescent probe composed of calcein-Ni2+ complex (Seto et al. 2010b). The probe displays fluorescence (at 515 nm) only when Ni2+ dissociates from the probe and interact with histamine. Biomonitoring of histamine in RAW 264 cells was successfully reported in both studies (Seto et al. 2010a, b) using the LE mechanism based chemosensors. Oshikawa et al. (2016) demonstrated the detection of histamines released during the degranulation of mast cell by developing fluorescent probes. Fluorescent coumarin derivatives complex were synthesized and screened the fluorescence responses upon addition of excess histamine in Hank’s balanced salt solution (HBSS). The fluorescent complex composed of an iminodiacetic acid displayed significant fluorescence response upon addition of histamine. Therefore, fluorescent probe composed of iminodiacetic acid (two sets)-metal ion (Co2+ or Ni2+) complex and cyanine (Cy5) dye was developed. The probe with metal ion complex displays fluorescence only when the metal ion dissociates from the probe and interact with histamine i.e., histamine induced coordination displacement mechanism based fluorescence emission. The HBSS solution containing probe with Co2+ complex displays significant and rapid fluorescence response when compared to that of the Ni2+ complex upon addition of histamine. The probe (Co2+ complex of Cy5 dye) was further modified (with lipophilic biocompatible anchor for membrane) to attach itself to the cell membrane, which enabled effective detection and real-time monitoring of histamines released during the degranulation of mast cell (Oshikawa et al. 2016). Singh et al. (2014) reported the detection of BAs (histamine and spermine) using a novel fluorescent organosilicon based chemosensors. 1,2,3-triazole based silatrane-scaffolds (TBSs) chemosensors such as 1-(3-(silatranyl)propyl)-1H-(1,2,3-triazol-4-yl)benzene (TBSs 1), N-((1-(3-(silatranyl)propyl)-1H-1,2,3-triazol-4-yl)methyl)phthalimide (TBSs 2), 2-((1-(3-(silatranyl)propyl)-1H-1,2,3-triazol-4-yl)methoxy)benzaldehyde (TBSs 3), N-((1-(3-(silatranyl)propyl)-1H-1,2,3-triazol-4-yl)methyl)morpholine (TBSs 4), and (1-(3-(silatranyl)propyl)-1H-1,2,3-triazol-4-yl)methanol (TBSs 5) were synthesized, characterized and then the affinity of the TBSs (1-5) towards BAs (1,4-diaminobutane, 1,5-diaminopentane, 1,2-diaminopropane, histamine, 2-phenylethylamine, spermidine, spermine, and tyramine) were assessed in CH3-CN: H2O (98: 2; v/v). TBSs 3 displayed fluorescent enhancement (at 393 nm) upon addition of spermine (70 µM) to the solution of TBSs 3 (10 µM). The fluorescence intensity (measured at 393 nm) of TBSs 3 (10 µM) increased linearly with the concentration (2-50 µM) of spermine. Fluorescence intensity enhancement occurs as a result of inhibition of PET channel due to the interaction of TBSs 3 and spermine. The interaction is hypothesized as interaction occurred between 2 molecules of TBSs 3 and spermine molecules. TBSs 3 displayed detection limit of about 7 µM concentration of spermine and this approach was utilized for the detection of spermine in the urine of a tumor patient. TBSs 4 displayed distinct fluorescence response (at 376 nm) upon addition of histamine (100 µM) to the solution of TBSs 4 (10 µM). The fluorescence intensity (at 376 nm) of TBSs 4 (10 µM) increased linearly with the concentration (5-64 µM) of histamine with a detection limit of 5 µM. Distinct fluorescence response occurred as a result of charge transfer within the molecule upon the interaction of TBSs 4 and histamine. The interaction is hypothesized that electronegative groups of TBSs 4 forms hydrogen bonds with the histamine molecules. TBSs 3 and 4 chemosensors effectively recognized the spermine and histamine, respectively (Singh et al. 2014).
Singh and Kaur group demonstrated the detection of BAs using a novel chemosensor based on the metal complex of fluorescent organic nanoparticles (FONs) of receptor (denoted as 1; naphthyl unit present in the receptor is the fluorophore) with ferric ions (Fe3+) (Chopra et al. 2015a). The prepared receptor 1 subjected to water soluble FONs (FONs1) and its complex with metal (FONs1.Fe3+) using re-precipitation technique. The probe FONs1.Fe3+ complex (in H2O: DMF solution; 99: 1, v/v) was used to check the recognition ability of probe towards BAs (100 µM) such as 1,4-diaminobutane, 1,3-diaminopropane, 1,5-diaminopentane, histamine, 2-phenylethylamine, spermidine, spermine, and tyramine hydrochloride. The probe FONs1.Fe3+ displayed enhancement (sevenfold) in the fluorescence intensity upon addition of tyramine indicating that tyramine displaces ferric ions from the probe. The fluorescence intensity (at 445 nm) of the probe increased linearly with the concentration (0-90 µM) of tyramine with a detection limit of 4.95 µM (Chopra et al. 2015a). Similarly, FONs of another new receptor (denoted as 2) complexed with Fe3+ ions (FONs2.Fe3+) was used for the detection of spermidine. The probe FONs2.Fe3+ complex (in H2O: DMF solution; 99: 1, v/v) displayed enhancement in the fluorescence intensity upon addition of spermidine. The fluorescence intensity of the probe increased linearly with the concentration (0–125 µM) of spermidine with a detection limit of 3.68 µM (Chopra et al. 2015b). Kaur et al. (2016) reported a Biginelli-based organic nanoprobe (FONs receptor. Ag+ complex) for the simultaneous detection of tyramine and 1,2-diaminopropane. The probe displayed fluorescence (at 446 nm) upon addition of tyramine and the fluorescence intensity (at 446 nm) increased linearly with the concentration (0-60 µM) of tyramine with a detection limit of 3.91 µM. This turn on fluorescence is due to cation displacement upon addition of tyramine. The probe displayed a distinct blue shift (at 404 nm) upon addition of 1,2-diaminopropane and the fluorescence intensity (at 446 nm) increased linearly with the concentration (0-65 µM) of 1,2-diaminopropane with a detection limit of 4.21 µM. This new fluorescence response occurred due to the modulation of ICT upon addition of 1,2-diaminopropane. This approach was utilized for the real-time analysis of BAs in milk and white wines (Kaur et al. 2016).
Yin and Ye group reported the detection of BAs using a novel colorimetric method based on arylalkylamine N-acetyltransferase (Leng et al. 2015). The enzyme arylalkylamine N-acetyltransferase was isolated from mosquitoes (Aedes aegypti) and then genetically modified. Histamine is converted as acetyl-histamine by arylalkylamine N-acetyltransferase through transferring an acetyl group from acetyl coenzyme A to histamine. Chromogenic 2-nitro-5-thiobenzoate anion (yellow product, strong absorbance at 412 nm) is formed by the reaction of free thiol group of coenzyme A with the aromatic disulphide (5,5´-dithiobis-(2-nitrobenzoic acid)). This approach was used to detect the histamine with the detection limit of 5 µM and also used to detect tyramine and phenylethylamine. This approach was applied for the real sample analysis of BAs in white wine and milk (Leng et al. 2015). Chopra et al. (2017) have reported the chromogenic chemosensor based simultaneous detection of spermidine and spermine in vapors and aqueous phase using the Cu2+ complex of organic nanoparticles (ONPs) receptor. They designed the chemosensor (ONPs receptor.Cu2+ complex) receptor containing imine and amide linkages, possessing sp2 nitrogen binging sites from pyridine and imine linkages, oxygen donor sites from carbonyl and OH groups, which may provide binding of multiple cations. The binding sites of the receptor are well placed that the coordination sphere of each metal ion shouldn’t get saturated with the binding sites of the receptor. The cation must complete the coordination sphere by utilizing the solvent or monodenate anion. BAs (2 µM) such as 1,4-diaminobutane, 1,2-diaminopropane, histamine, 2-phenylethylamine, spermidine, spermine, and tyramine were used to check the detection ability of ONPs receptor.Cu2+ complex, while the chromogenic chemosensor displayed visible color change upon addition of spermidine and spermine. Reduction of absorbance (at 400 nm) was observed in the host solution and new distinct peaks were displayed upon addition of spermidine (at 602 nm) and spermine (at 555 nm). The absorbance of the new peak increased linearly with the increased concentration of each BAs with a detection limit of 35 nM spermidine and 36.2 nM spermine. This approach was applied for the real sample analysis of BAs in mushroom and meat and the chromogenic chemosensor displayed visible color change due to the vapors of spermidine emitted from mushroom and vapors of spermine emitted from meat (Chopra et al. 2017).
Tu et al. (2016) demonstrated the ratiometric fluorescence chemosensor based detection of spermine using a novel self-assembled platform, where squaraine (SQ) dye act as the nucleus and micelles of pyrene derivative act as the shell. The spherical self-assembled micelles changed its sphere form to rod-like formation, as a result of reduced interfacial curvature by electrostatic interactions upon addition of spermine. Parallel oriented array of H-aggregated SQ dye surrounded by micelles displayed absorption peaks at 467, 580, 642 nm in aqueous solution. Disappearance of peak at 467 nm, gradual reduction of peak at 596 nm and increase in the intensity of the absorption and red-shifted by 7 nm. The fluorescence emission intensity (at 665 nm) of SQ increased and emission (at 440 nm) of pyrene gradually reduced upon addition of spermine. The fluorescence intensity increased linearly with the concentration (20-100 mM) of spermine with a detection limit of 4.73 mM. This approach was applied for the analysis of spermine in buffer solution and urine (Tu et al. 2016).
Malik et al. (2016) demonstrated the detection of spermine using aggregation-induced FRET via polymer-surfactant complexation. Water-soluble cationic conjugated polymer [9,9-bis(6′-methyl imidazolium bromide)hexyl)-fluorene-co-4,7-(2,1,3-benzothiadiazole)] (PFBT-MI) was designed and synthesized. PFBT-MI displayed a new peak (at 545 nm) upon addition of SDS and SDBS in the aqueous solution with the detection limit of 0.12 µM and 0.13 µM, respectively. Naked eye detection (blue color changes to yellowish green) of common anionic surfactants (SDS/SDBS) as a result of FRET from the donor (fluorene) to the acceptor (benzothiadiazole units) in the aqueous solution. The polymer–surfactant nanoaggregates (PFBT–MI–SDS) thus formed via electrostatic as well as hydrophobic interactions is used for the sensitive detection of spermine. Addition of spermine results in formation of PFBT-MI-SDS-spermine complex leads to quenching of emission at 545 nm and detection limit of spermine was 66 ppb (0.33 μM). This approach would enable to detect the spermine in urine for the early diagnosis of cancer (Malik et al. 2016).
Wang et al. (2017) reported the sensitive detection of histamine by using the fluorescent sensing probe based on the molecular imprinting ionic liquid (IL)-modified quantum dots (QD). The sensing probe was synthesized via a facile one pot route by modifying the surface of CdSe/ZnS quantum dots with IL (1-vinyl-3-butyl-imidazolium hexafluorophosphate) through electrostatic interactions. The fluorescence probe displayed enhancement in the fluorescence intensity upon addition of histamine. The fluorescence intensity of the probe increased linearly with the concentration (0.449–2.249 mM) of histamine with a detection limit of 0.11 mM. The reproducibility of the probe was reported with acceptable relative standard deviation (2.8%). This approach was applied for the real sample analysis of histamine in canned fish (Wang et al. 2017).
The reported methods to detect the presence of BAs in food products have been highlighted in this section. Bueno-Solano et al. (2012) determined the level of representative BAs (histamine, and tyramine) in shrimp based products (dry protein powder, liquid protein hydrolysate and shrimp head flour) using HPLC techniques. BAs were extracted and derivatized using trichloroacetic acid and o-phthaldialdehyde, respectively, and separated using an analytical column. The results showed that about 84.29 and 98.68% of histamine and tyramine could be recovered from the shrimp samples, respectively. The study confirmed that the described HPLC method was sensible, reliable with reproducible results, which could be used to detect the BAs in shrimp and fish products (Bueno-Solano et al. 2012). Likely, the pre-column (core–shell particle column) derivatization with dansyl-chloride and HPLC separation method has been demonstrated to detect the 11 BAs in beverages by Preti group. This improved method was less time (the run time was 13 min) and eluent consuming process with an acceptable result. The quick HPLC based detection method could be used for the systematic analysis of food samples for BAs contamination (Preti et al. 2015).
Detection of preservatives, BAs in fish and meat product has been explained by De Dea Lindner group using liquid chromatography-tandem mass spectrometry-based method, and the method feasibility has been validated (Molognoni et al. 2018). The various food samples (fermented and cooked meat and fish products) were extracted using extraction solution containing methanol: acetonitrile: water at a ratio of 45:45:10 and 0.1% acetic acid, and separated using diisopropyl-3-aminopropyl silane bound to a hydroxylated silica column. The results showed that the developed method detected the BAs and preservatives in fish and meat product, concurrently (Molognoni et al. 2018). Recently, Ekici and Omer (2018) also reported that the HPLC method is effective in detecting and quantifying the BAs in fermented sausages.
Ishimaru et al. (2019) developed a column switching (changing the flow direction)-HPLC method to detect and measure the BAs in fish and fermented foods. The samples were extracted using 0.4 M perchloric acid. The extracts were separated using a Develosil XG-C30M-5 column with mobile phase consist of acetonitrile and water. After 20 min of initial separation, the flow direction was changed to remove the impurities in the samples. The method was validated to detect seven BAs in the food samples namely tyramine, tryptamine, histamine, 2-phenylethylamine, cadaverine, putrescine, and spermidine. Since the study results are highly reproducible, column switching-HPLC method could be used to detect the BAs in fish, and fermented food products.
The detection of many BAs in food materials by a single technique aid the manufacturers to ensure the quality of the foods rapidly. Mayr and Schieberle (2012) demonstrated the stable-isotope dilution assay to determine the several BAs and polyamine in food samples by LC–MS/MS techniques, simultaneously. The results showed that about 14 BAs could be quantified simultaneously. Especially, tyramine and putrescine content in salami has been detected, and spermine was detected in tuna and salami samples (Mayr and Schieberle 2012). Similarly, a colorimetric method has been developed by Lee et al. (2013) using Cu2+-containing monoamine oxidase and flavin adenine dinucleotide-containing putrescine oxidase (a bifunctional enzyme fusion) to detect the BAs in food samples. The method can detect monoamines and putrescine simultaneously, which could be used for the early screening of the BAs contamination in the food at production, processing, and transportation of the foods (Lee et al. 2013).
Even though several chemosensors are available to detect the BAs, HPLC, LC–MS, and colorimetric methods are the commonly used methods in several studies and further improvements are happening in those techniques to reduce the analyzing time and to improve the accuracy.
Prevention of BAs formation
Impact of starter culture on BAs content
The use of characterized, decarboxylase-negative, amine oxidase-positive starter culture (single or mixed strains), and microbes capable of degrading BAs could effectively control undesirable chemical reactions and improve the quality of fermented meat and fish products regarding BAs load (Alvarez and Moreno-Arribas 2014; Lorenzo et al. 2017). The role of starter culture in the management of BAs content of fermented foods have been detailed as follows.
The use of mixed starter cultures (L. casei, Streptococcus lactis, Saccharomyces cerevisiae Hansen, and Monascus anka) for the production of fermented bighead carp surimi and fermented grass carp muscles significantly reduced the formation of BAs, especially histamine, tyramines, spermine and spermidine, when compared to the control (Zhong-Yi et al. 2010). The pre-inoculation of mixed starters with grass carp muscles for 12 h effectively reduced the total volatile basic nitrogen and BAs (Liu et al. 2010). The starter culture reduced the pH of the sample, which further prevented the growth of contaminating microorganisms residing in the raw materials (Zhong-Yi et al. 2010; Liu et al. 2010). Suan yu (Chinese fermented fish product) prepared with LAB starter cultures (L. plantarum 120, S. cerevisiae 31, and S. xylosus 135 or S. cerevisiae 22, S. xylosus 135, and Pediococcus pentosaceus 220) exhibited low BAs content than that of the control. The pH value, total volatile base nitrogen, and microbial contamination were found to be reduced in Suan yu samples prepared with a starter culture. The results suggested that the decarboxylase-negative starter culture suppress the formation of BAs during fermentation and storage of food products (Xia et al. 2013).
Bover-Cid et al. (2001) investigated the effect of L. sakei CTC494 (amine negative starter culture) on the BAs formation in the fermented pork sausages. Fermented pork sausages were prepared with and without starter L. sakei CTC494 and matured for 21 days. The sausages made from good quality raw materials (frozen samples) with CTC494 showed a minimal amount of BAs (5–15 mg/kg of the sample) compared to the respective control. Whereas, sausages made with raw materials stored in the refrigerator and starter culture showed relatively high content of putrescine, cadaverine, and tyramine, comparable to uncontrolled fermented samples. The study suggested that the quality of raw materials in terms of microbial load play a critical role in preventing or reducing the BAs formation in sausages along with the potential starter culture (Bover-Cid et al. 2001).
Zaman et al. (2011) studied the impact of the use of amino oxidase-positive strains Bacillus amyloliquefaciens FS05 and Staphylococcus carnosus FS19 for the production of fermented fish sauce. The changes in pH and salt concentration during the fermentation process was not significantly different among the samples. The level of BAs was found to be increased gradually during fermentation, but the samples with either FS05 or FS19 showed reduced BAs (15.9, and 12.5%, respectively) when compared to the control samples. The study suggested that the use of amino oxidase-positive starter might suppress the accumulation of BAs in fermented fish sauces (Zaman et al. 2011).
The fermented Turkish sausages made with L. casei, or L. acidophilus or both as starter culture showed low BAs content than that of the control samples. The concentration of BAs was found to be changed during manufacturing and storage for 8 months; particularly histamine, cadaverine, and putrescine levels were varied in the fermented Turkish sausages. The microbiological examination revealed that the fermented Turkish sausages were not contaminated with representative pathogens and coliforms. Overall, the results suggested that the use of starter culture effectively controls the BAs formation during production and storage of sausages (Ergönül and Kundakçi 2011).
The use of decarboxylase negative (L. sakei BCC 102, Debaryomyces hansenii BCC 106), and γ-aminobutyric acid-producing (P. pentosaceus HN8 and L. namurensis NH2) starter strains have successfully reduced the BAs development in nham production (Limsuwan et al. 2007; Kantachote et al. 2016). Starter culture effectively dropped the pH of the meat batter that prevents the microbial contamination and suppressed the pathogenic growth, thereby, reduced the formation BAs in sausages (Limsuwan et al. 2007). The addition of HN8 and NH2 starters improved the quality of nham in terms of GABA content and reduced level of BAs, and cholesterol (Kantachote et al. 2016).
Seong group investigated the effect of five commercial starter culture mixtures (S1: Staphylococcus carnosus, S. xylosus, Debaryomyces hansenii, and L. curvatus; S2: S. carnosus, and L. sakei; S3: P. pentosaceus, and S. carnosus; S4: P. acidilactici; S5: S. xylosus, and L. plantarum) on quality, BAs content and lipid oxidation during the production of fermented sausage. Tyramine and putrescine were detected in all the samples, and the pH of the sample was correlated with putrescine content. Except S1 or S2 inoculated sausage samples, all other samples exhibited high tyramine content. The sausages prepared with starter culture showed high lipid oxidation and better sensory acceptability. The results suggested that the use of S. carnosus, and L. sakei as a starter for the production of sausages significantly improved the quality of sausage (Ba et al. 2016). Domínguez et al. (2016) studied the impact of commercial starter culture on the quality of dry-cured foal sausage. The commercial starter mixtures such as CX (S. carnosus, S. xylosus, and P. pentosaceus), FL (D. hansenii, and S. xylosus), and TH (P. pentosaceus, and S. xylosus) were used in the study. The increased viable LAB load and decreased Enterobacteriaceae count were observed in starter-fermented sausages compared to spontaneously fermented samples. The starter fermented batch showed a relatively high amount of BAs compared to control, but the level of concentration doesn’t reach the lethal level. The results suggest that selection of starter culture is critical to control the BAs accumulation and quality improvement of foal sausages.
Likely, Xie et al. (2015) studied the effect of the use of L. plantarum, or S. xylosus or both as a starter for the production of fermented sausages on BAs content. About 25, 22, and 21% of reduction of histamine, cadaverine, and tyramine, were observed in S. xylosus fermented sausage samples, respectively. The mixed starter sample showed a significant reduction in tryptamine, phenylethylamine, putrescine, cadaverine, histamine, and tyramine when compared to other samples. The results suggested that the use of multiple potent starter culture considerably improved the quality of the sausages by reducing the BAs accumulation.
Generally, Chinese traditional fermented freshwater fish products (CTFP) was prepared by spontaneous fermentation, and tyramine and putrescine are the common BAs found in it. The impact of the use of starter cultures (L. plantarum 120, S. cerevisiae 2018, S. xylosus 135, or mixed culture) on quality of the fish product has been studied. S. xylosus 135, and L. plantarum 120 effectively suppressed the accumulation of putrescine while tyramine was controlled by the mixed starter culture. Additionally, the use of starter culture reduced the cadaverine, spermidine and spermine load in CTFP. The BAs content was correlated with α‐amino nitrogen contents and protease activities. The results suggested that the use of specific starters could improve the quality of CTFP, especially in terms of BAs load (Liao et al. 2018).
Recently Kim et al. (2019) reported that use of Tetragenococcus halophilus MJ4 effectively suppressed the BAs accumulation, especially cadaverine, in saeu-jeot (fermented shrimp product) during fermentation. The study also reported that MJ4 strain reduced the load of Tetragenococcus muriaticus, one of the predominant contaminant of saeu-jeot with BA producing capability. The results recommended that T. halophilus MJ4 could be used as a potent starter for the production of saeu-jeot.
The addition of bacteriocin producing bacterial strains could inhibit the growth of amino biogenic bacteria, thereby reducing the accumulation of BAs in fermented foods (Tabanelli et al. 2014). The pre-treatment of smoked pork sausages with LAB strains that are capable of producing inhibitor substance against pathogenic microbes has reduced the formation of BAs. The treatment stage (i.e., before and after the smoking process) influenced the level of BAs, and type of BAs in sausages. The study confirmed that use of LAB strains (Pediococcus acidilactici KTU05-7, P. pentosaceus KTU05-9 and L. sakei KTU05-6) effectively reduced the microbial contamination, BAs and polycyclic aromatic hydrocarbons content in cold fermented pork sausages, and also improved the sensory quality (Mozuriene et al. 2016; Bartkiene et al. 2017). Similarly, use of P. pentosaceus or L. sakei as a starter with S. xylosus successfully reduced the BAs load in dry-fermented sausages. The study argued that the selection of appropriate starter and optimal processing conditions are the critical production steps, which could improve the quality of the products in terms of BAs (Pasini et al. 2018).
Effect of physical, chemical treatment and processing conditions on BAs content
Rabie et al. (2010) studied the effect of γ-irradiation on BAs content in Egyptian fermented sausages during storage. Cadaverine and tyramine are the major BAs found in control and irradiated samples, respectively, and histamine was found at a lethal level in control samples. The results clearly showed that the radiation process significantly reduced the total BAs and histamine levels in sausages. The data suggest that γ-irradiation could be a physical treatment, which could suppress the BAs formation during sausage storage (Rabie et al. 2010).
The high hydrostatic pressure treatment (500 MPa/10 min) reduced the BAs content, especially putrescine and cadaverine, and improved the microbial quality of Hungarian dry fermented sausages during one-month storage at 8 °C. The organoleptic quality of the sausage was not significantly affected by the pressure treatment. Thus, the results appealed that hydrostatic pressure treatment could improve the quality of sausages and reduce the BAs load during storage (Simon-Sarkadi et al. 2012). Likely, use of specific starter cultures (L. sakei and S. xylosus strains) and high hydrostatic pressure treatment considerably reduced the formation of BAs and improved the microbiological value of the fermented sausages, especially to eliminate the Salmonella spp. contamination (Garriga et al. 2005).
Recently, Wójciak et al. (2019) reported that the ultra-sonication process significantly reduced the formation of BAs (cadaverine, histamine, tyramine, and putrescine) in the dry-fermented beef product, and there was no impact on the growth of LAB during the ripening stage.
The storage temperature greatly influences the formation of BAs by promoting microbial growth. The storage of salamis sausages at 15 °C exhibited high BAs content compared to the samples stored at 5 °C (Kameník et al. 2012b). Whereas, the fermentation process at elevated temperature reduced the BAs formation in the fish product. The fermentation of fish at 25 °C for the first 10 days, followed by at 30 °C for 18 days significantly endorsed the LAB growth, suppressed the pathogenic growth, and BAs formation. In addition, the elevated temperature aided the development of volatile flavor compounds in the fermented fish product. The study results concluded that the fermentation of low-salted fish at elevated temperature reduced the BAs content and enhanced the overall quality of the product (Xu et al. 2019).
Dry fermented sausages stored under modified atmospheric condition (100% N2 or 70% N2/30% CO2) with different aw content affected the aroma profile and BAs content. Especially, high aw value directly associated with high accumulation of BAs in sausages during storage. The study results recommend that care should to taken while processing and packing of sausages in terms of aw value, which significantly affects the quality of the products, regarding BAs content, irrespective of storage atmospheric condition (Tabanelli et al. 2013).
The salt (NaCl) concentration, temperature, and glucose content in the sausage significantly influenced the growth of Enterobacteriaceae species, which was associated with putrescine and cadaverine accumulation during storage. The condition facilitates the growth of LAB. Thus, the use of optimal LAB starter might prevent BAs formation and improve the quality of the sausages (Bover-Cid et al. 2009).
The preparation of sucuk (fermented sausage) prepared with a mixed starter culture (L. sake, P. pentosaceus, S. carnosus, and S. xylosus), nitrite, and nisin reduced the BAs load. Tyramine, tryptamine, histamine, cadaverine, putrescine, and 2-phenylethylamine content was reduced by the addition of nitrite. The tryptamine level was reduced in the presence of nisin while cadaverine, putrescine and spermidine levels were increased. The results further showed that the addition of nisin reduced the availability of nitrite. The study suggested that the use of a combination of compactable chemicals might improve the quality of sucuk (Kurt and Zorba 2010).
The addition of tea polyphenols and 6-gingerol (0.15% each) significantly prevented the formation of BAs in shrimp paste during storage when compared to control. Moreover, significant inhibition of lipid and protein oxidation, and microbial growth was observed in tea polyphenols and 6-gingerol added samples. The study claimed that the mixture of tea polyphenols and 6-gingerol could be used as a potent protective agent to preserve the fermented foods during storage at ambient temperature (Cai et al. 2015) (Fig. 4).
Fig. 4.
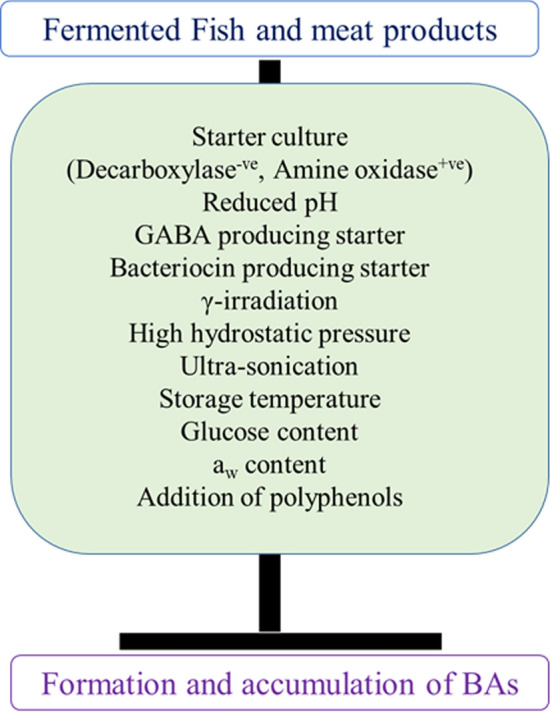
Factors aids to prevent or reduce the formation and accumulation of biogenic amines in fermented fish and meat products
Safety regulations
Nout (1994) reported that approximately 1000 ppm is considered as the hazardous level of total biogenic amines. Histamine is the only BA that is regulated by law. The joint FDA/WHO meeting reported that 50 mg of histamine is considered as the no-observed-adverse-effect level (NOAEL), which is the appropriate hazard level. Healthy individuals are expected to have no symptoms associated with histamine poisoning, upon consumption of fish products containing 50 mg of histamine (FAO/WHO 2013). The joint FDA/WHO meeting reported that food business operators who apply good hygienic practices and hazard analysis and critical control point (HACCP) can achieve a histamine level < 15 mg/kg in fish products, based on data obtained from industry (using a test method with a lower detection limit of 15 mg/kg) (FAO/WHO 2013). Fermented fish sauces are consumed in small quantities as few grams of fish sauce per servings, and people in countries such as Thailand and Vietnam are known for the daily consumption of fish sauces at highest rate. Therefore, the Standard for Fish Sauce were revised by Codex (31st Session of the Codex Committee on Fish and Fishery Products) up to 20 mg of histamine per 100 g of fermented fish sauces is considered as acceptable level for consumer protection.
EFSA (European Food Safety Authority) Panel on Biological Hazards (BIOHAZ) published a Scientific opinion (Scientific opinion on risk based control of biogenic amine formation in fermented foods) by qualitatively evaluating the risk levels of biogenic amines that are present in the fermented foods using the data obtained from reported toxicological studies on dietary amines, clinical studies, and from European Union-related consumption data, reports and surveys (EFSA Panel on BIOHAZ 2011). Based on the studies, level of biogenic amines exposure was considered as safe when an individual has no adverse effects upon consumption of fermented foods. About 50 mg of histamine per meal (meal containing fermented foods) was interpreted as safe level for a healthy individual. Since low detectable levels of histamine might cause adverse effects in individuals with histamine tolerance, histamine levels below detectable limits are interpreted as safe for individuals with histamine tolerance. About 6 mg of tyramine per meal was interpreted as safe level for an individual treated with classical monoamino oxidase inhibitor (MAOI) drugs, and 50 mg of tyramine per meal for an individual under treatment with third generation MAOI drugs (RIMA, reversible inhibitors of MAO-A). About 600 mg of tyramine per meal (meal containing fermented foods) was interpreted as safe level for a healthy individual (who doesn’t use MAOI drugs).
Latorre-Moratalla et al. (2017) assessed the risk of biogenic amines (histamine and tyramine) exposure in Spanish population upon consuming dry fermented sausages (chorizo, salami, salchichόn, and sobrasada). Based on the assessment of histamine and tyramine levels in 87 samples of chorizo, 18 samples of salami, 357 samples of salchichόn, and 12 samples of sobrasada, and based on the data of consumption of dry fermented sausages (chorizo, salami, salchichόn, and sobrasada) obtained from Spanish national dietary survey that was conducted with 3000 individuals (1500 females, males; Age = 18-64 years) by Spanish Agency for Consumer Affairs, Food Safety and Nutrition (AECOSAN 2011), about 1.39 mg of histamine per meal and about 6.2 mg of tyramine per meal were estimated as the average exposure levels of biogenic amines per individual upon intake of fermented sausages. The probability of exposure risk to exceed the threshold limit of histamine and tyramine in a meal (containing dry fermented sausages) was estimated to be negligible in healthy individuals. Approximately, 7000 individuals (with histamine tolerance) per million were estimated that they could be at risk of suffering adverse effects upon consumption of dry fermented sausages. The probability of exposure risk to exceed the threshold limit of tyramine (6 mg per meal per person) was estimated to be 34% in individuals (Latorre-Moratalla et al. 2017).
Cadayong and Barraquio (2014) investigated the risk for human exposure to biogenic amines (histamine and tyramine) upon consumption of fermented sausages (chorizo, salami, and pepperoni). Based on the survey conducted in Southern Luzon, about 2.62 g of chorizo that contains 0.78 ppm of histamine, about 0.91 g of salami that contains 0.79 ppm of histamine, 0.45 ppm of tyramine, and about 2.71 g of pepperoni that contains 0.26 ppm of histamines were determined as the average daily intake amount by an individual. About 5.09 g of fermented sausage was interpreted to cause low risk upon consumption (Cadayong and Barraquio 2014).
Concluding remarks
BAs accumulation occurs in high concentrations in meat sausages, when compared to other meat and fish products, due to the availability of free amino acids and microbial activity. The contaminating microbes, especially decarboxylase-positive microorganisms, are the major reason for BAs formation in fermented meat product. The quality of raw materials, availability of free amino acids, high temperature, high moisture content, salt concentration, food additives, long fermentation duration, storage conditions, the diameter of the sausage casing, and cooking methods are the influencing factors of BAs formation in fermented fish and meat products.
However, the factors influencing the BAs formation in fermented fish and meat products are well-identified; the complete prevention of occurrence of BAs is difficult. The literature suggested that the use of potent starter cultures (decarboxylase-negative, amino oxidase-positive strains; bacteriocin and GABA producing strains), use of hygienic raw materials, improved processing (γ-irradiation, high hydrostatic pressure, and ultra-sonication), fermentation (glucose content, pH, and aw content) and storage conditions (temperature, and humidity) could successfully prevent or reduce the BAs formation in fermented fish and meat products. Based on the joint FDA/WHO meeting report, food business operators who apply good hygienic practices and hazard analysis and critical control point (HACCP) can achieve a histamine level < 15 mg/kg in fish products.
Collectively, the studies suggested that regular monitoring of BAs content during the production and storage period is advisable to ensure the quality of the foods. Further, studies are required on automation in food production and processing, and the rapid screening methods to detect the BAs load in food material at any stage of the production process.
Acknowledgements
The authors gratefully acknowledge the Chiang Mai University, Chiang Mai, Thailand. The current research was partially supported by Chiang Mai University.
Author contributions
B.S.S. contributed to the conception and design, acquisition, manuscript preparation, and critical revision of the manuscript. P.K. and C.C. were involved in the review and finalization of the manuscript. All the authors agree with the content of the manuscript.
Funding
This research received no external funding.
Compliance with ethical standards
Conflict of interest
The authors declare that there are no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 31st Session of the Codex Committee on Fish and Fishery Products. https://ec.europa.eu/food/sites/food/files/safety/docs/codex_ccffp_31_eu_positions_complil_en.pdf. Cited 19 Feb 2020
- Alvarez MA, Moreno-Arribas MV. The problem of biogenic amines in fermented foods and the use of potential biogenic amine-degrading microorganisms as a solution. Trends Food Sci Tech. 2014;39(2):146–155. [Google Scholar]
- Alves SP, Alfaia CM, Škrbić BD, Živančev JR, Fernandes MJ, Bessa RJB, Fraqueza MJ. Screening chemical hazards of dry fermented sausages from distinct origins: biogenic amines, polycyclic aromatic hydrocarbons and heavy elements. J Food Compos Anal. 2017;59:124–131. [Google Scholar]
- Ba HV, Seo HW, Kim JH, Cho SH, Kim YS, Ham JS, et al. The effects of starter culture types on the technological quality, lipid oxidation and biogenic amines in fermented sausages. LWT Food Sci Technol. 2016;74:191–198. [Google Scholar]
- Bargossi E, Gardini F, Gatto V, Montanari C, Torriani S, Tabanelli G. The capability of tyramine production and correlation between phenotypic and genetic characteristics of Enterococcus faecium and Enterococcus faecalis strains. Front Microbiol. 2015;6:1371. doi: 10.3389/fmicb.2015.01371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkiene E, Bartkevics V, Mozuriene E, Krungleviciute V, Novoslavskij A, Santini A, Rozentale I, Juodeikiene G, Cizeikiene D. The impact of lactic acid bacteria with antimicrobial properties on biodegradation of polycyclic aromatic hydrocarbons and biogenic amines in cold smoked pork sausages. Food Control. 2017;71:285–292. [Google Scholar]
- Bover-Cid S, Holzapfel WH. Improved screening procedure for biogenic amine production by lactic acid bacteria. Int J Food Microbiol. 1999;53(1):33–41. doi: 10.1016/s0168-1605(99)00152-x. [DOI] [PubMed] [Google Scholar]
- Bover-Cid S, Hugas M, Izquierdo-Pulido M, Vidal-Carou MC. Reduction of biogenic amine formation using a negative amino acid-decarboxylase starter culture for fermentation of fuet sausages. J Food Protect. 2000;63(2):237–243. doi: 10.4315/0362-028x-63.2.237. [DOI] [PubMed] [Google Scholar]
- Bover-Cid S, Izquierdo-Pulido M, Vidal-Carou MC. Effectiveness of a Lactobacillus sakei starter culture in the reduction of biogenic amine accumulation as a function of the raw material quality. J Food Protect. 2001;64(3):367–373. doi: 10.4315/0362-028x-64.3.367. [DOI] [PubMed] [Google Scholar]
- Bover-Cid S, Torriani S, Gatto V, Tofalo R, Suzzi G, Belletti N, Gardini F. Relationships between microbial population dynamics and putrescine and cadaverine accumulation during dry fermented sausage ripening. J Appl Microbiol. 2009;106(4):1397–1407. doi: 10.1111/j.1365-2672.2008.04108.x. [DOI] [PubMed] [Google Scholar]
- Bueno-Solano C, López-Cervantes J, Sánchez-Machado DI, Campas-Baypoli ON. HPLC determination of histamine, tyramine and Amino acids in shrimp by-products. J Braz Chem Soc. 2012;23(1):96–102. [Google Scholar]
- Cadayong BP, Barraquio VL. Human exposure risk assessment to biogenic amines in cheeses and fermented sausages in Southern Luzon. Philipp J Sci. 2014;143(2):107–112. [Google Scholar]
- Cai L, Liu S, Sun L, Wang Y, Ji H, Li J. Application of tea polyphenols in combination with 6-gingerol on shrimp paste of during storage: biogenic amines formation and quality determination. Front Microbiol. 2015;6:981. doi: 10.3389/fmicb.2015.00981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra S, Singh J, Kaur H, Singh N, Kaur N. Estimation of biogenic amines and biothiols by metal complex of fluorescent organic nanoparticles acting as single receptor multi-analyte sensor in aqueous medium. Sens Actuat B. 2015;220:295–301. [Google Scholar]
- Chopra S, Singh J, Kaur H, Singh H, Singh N, Kaur N. Selective chemosensing of spermidine based on fluorescent organic nanoparticles in aqueous media via a Fe3+ displacement assay. New J Chem. 2015;39(5):3507–3512. [Google Scholar]
- Chopra S, Singh A, Venugopalan P, Singh N, Kaur N. Organic nanoparticles for visual detection of spermidine and spermine in vapors and aqueous phase. ACS Sustain Chem Eng. 2017;5(2):1287–1296. [Google Scholar]
- Chung BY, Park SY, Byun YS, Son JH, Choi YW, Cho YS, Kim HO, Park CW. Effect of different cooking methods on histamine levels in selected foods. Ann Dermatol. 2017;29(6):706–714. doi: 10.5021/ad.2017.29.6.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciçek Ü, Tokatli K. Biogenic amine formation in “Bez Sucuk”, a type of Turkish traditional fermented sausage produced with different meat: fat ratios. Korean J Food Sci An. 2018;38(1):152–161. doi: 10.5851/kosfa.2018.38.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez R, Munekata PE, Agregán R, Lorenzo JM. Effect of commercial starter cultures on free amino acid, biogenic amine and free fatty acid contents in dry-cured foal sausage. LWT Food Sci Technol. 2016;71:47–53. [Google Scholar]
- Eerola HS, Roig Sagués AX, Hirvi T. Biogenic amines in Finnish dry sausages. J Food Saf. 1998;18(2):127–138. [Google Scholar]
- EFSA Panel on Biological Hazards (BIOHAZ) Scientific Opinion on Scientific Opinion on risk based control of biogenic amine formation in fermented foods. EFSA J. 2011;9(10):2393. doi: 10.2903/j.efsa.2011.2393. [DOI] [Google Scholar]
- Ekici K, Omer AK. Detection of common biogenic amines in fermented sausage produced in Turkey. Data in Brief. 2018;20:1360–1362. doi: 10.1016/j.dib.2018.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdag D, Merhan O, Yildiz B. Biochemical and pharmacological properties of biogenic amines. In: Proestos C, editor. Biogenic amines. 1. London: IntechOpen; 2018. pp. 1–14. [Google Scholar]
- Ergönül B, Kundakçi A. Microbiological attributes and biogenic amine content of probiotic Turkish fermented sausage. J Verbrauch Lebensm. 2011;6(1):49–56. [Google Scholar]
- Erkmen O, Bozkurt H. Quality characteristics of retailed sucuk (Turkish Dry-Fermented Sausage) Food Technol Biotechnol. 2004;42(1):63–69. [Google Scholar]
- Ezzat MA, Zare D, Karim R, Ghazali HM. Trans- and cis-urocanic acid, biogenic amine and amino acid contents in Ikan pekasam (fermented fish) produced from Javanese carp (Puntius gonionotus) and black tilapia (Oreochromis mossambicus) Food Chem. 2015;172:893–899. doi: 10.1016/j.foodchem.2014.09.158. [DOI] [PubMed] [Google Scholar]
- FAO/WHO [Food and Agriculture Organization of the United Nations/World Health Organization] (2013). Public health risks of histamine and other biogenic amines from fish and fishery products. Meeting report
- Ferreira IM, Pinho O. Biogenic amines in Portuguese traditional foods and wines. J Food Prot. 2006;69(9):2293–2303. doi: 10.4315/0362-028x-69.9.2293. [DOI] [PubMed] [Google Scholar]
- Gardini F, Özogul Y, Suzzi G, Tabanelli G, Özogul F. Technological factors affecting biogenic amine content in foods: a review. Front Microbiol. 2016;7:1218. doi: 10.3389/fmicb.2016.01218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garriga M, Marcos B, Martín B, Veciana-Nogués MT, Bover-Cid S, Hugas M, Aymerich T. Starter cultures and high-pressure processing to improve the hygiene and safety of slightly fermented sausages. J Food Protect. 2005;68(11):2341–2348. doi: 10.4315/0362-028x-68.11.2341. [DOI] [PubMed] [Google Scholar]
- González-Tenorio R, Fonseca B, Caro I, Fernández-Diez A, Kuri V, Soto S, Mateo J. Changes in biogenic amine levels during storage of Mexican-style soft and Spanish-style dry-ripened sausages with different aw values under modified atmosphere. Meat Sci. 2013;94(3):369–375. doi: 10.1016/j.meatsci.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Hussain MA, Dawson CO. Economic impact of food safety outbreaks on food businesses. Foods. 2013;2(4):585–589. doi: 10.3390/foods2040585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonić P, Tasić T, Petrović L, Škaljac S, Jokanović M, Mandić A, Ikonić B. Proteolysis and biogenic amines formation during the ripening of Petrovská klobása, traditional dry-fermented sausage from Northern Serbia. Food Control. 2013;30(1):69–75. [Google Scholar]
- Ishimaru M, Muto Y, Nakayama A, Hatate H, Tanaka R. Determination of biogenic amines in fish meat and fermented foods using column-switching high-performance liquid chromatography with fluorescence detection. Food Anal Methods. 2019;12(1):166–175. [Google Scholar]
- Jiang W, Xu Y, Li C, Dong X, Wang D. Biogenic amines in commercially produced Yulu, a Chinese fermented fish sauce. Food Addit Contam Part B Surveill. 2014;7(1):25–29. doi: 10.1080/19393210.2013.831488. [DOI] [PubMed] [Google Scholar]
- Kameník J, Standarová E, Saláková A, Vorlová L. Contents of biogenic amines and polyamines in mould-fermented sausages. J Food Nutr Res. 2012;51(3):164–172. [Google Scholar]
- Kameník J, Saláková A, Bořilová G, Pavlík Z, Standarová E, Steinhauser L. Effect of storage temperature on the quality of dry fermented sausage poličan. Czech J Food Sci. 2012;30:293–301. [Google Scholar]
- Kanki M, Yoda T, Tsukamoto T, Baba E. Histidine decarboxylases and their role in accumulation of histamine in tuna and dried saury. Appl Environ Microbiol. 2007;73(5):1467–1473. doi: 10.1128/AEM.01907-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantachote D, Ratanaburee A, Sukhoom A, Sumpradit T, Asavaroungpipop N. Use of γ-aminobutyric acid producing lactic acid bacteria as starters to reduce biogenic amines and cholesterol in Thai fermented pork sausage (Nham) and their distribution during fermentation. LWT Food Sci Technol. 2016;70:171–177. [Google Scholar]
- Kaur G, Raj T, Kaur N, Singh N. A Biginelli-based organic nanoprobe for simultaneous estimation of tyramine and 1,2-diaminopropane: application in real samples. New J Chem. 2016;40(12):10536–10544. [Google Scholar]
- Kaur N, Chopra S, Singh G, Raj P, Bhasin A, Sahoo SK, Kuwar A, Singh N. Chemosensors for biogenic amines and biothiols. J Mater Chem B. 2018;6:4872–4902. doi: 10.1039/c8tb00732b. [DOI] [PubMed] [Google Scholar]
- Kim T, Kim Y. Analyte-directed formation of emissive excimers for the selective detection of polyamines. Chem Commun. 2016;52(70):10648–10651. doi: 10.1039/c6cc05761f. [DOI] [PubMed] [Google Scholar]
- Kim KH, Lee SH, Chun BH, Jeong SE, Jeon CO. Tetragenococcus halophilus MJ4 as a starter culture for repressing biogenic amine (cadaverine) formation during saeu-jeot (salted shrimp) fermentation. Food Microbiol. 2019;82:465–473. doi: 10.1016/j.fm.2019.02.017. [DOI] [PubMed] [Google Scholar]
- Köse S, Koral S, Tufan B, Pompe M, Scavniçar A, Koçar D. Biogenic amine contents of commercially processed traditional fish products originating from European countries and Turkey. Eur Food Res Technol. 2012;235(4):669–683. [Google Scholar]
- Kuley E, Özogul F, Özogul Y, Akyol I. The function of lactic acid bacteria and brine solutions on biogenic amine formation by foodborne pathogens in trout fillets. Food Chem. 2011;129(3):1211–1216. doi: 10.1016/j.foodchem.2011.05.113. [DOI] [PubMed] [Google Scholar]
- Kurt S, Zorba O. Biogenic amine formation in Turkish dry fermented sausage (sucuk) as affected by nisin and nitrite. J Sci Food Agric. 2010;90(15):2669–2674. doi: 10.1002/jsfa.4138. [DOI] [PubMed] [Google Scholar]
- Latorre-Moratalla ML, Bover-Cid S, Vidal-Carou MC. Technological conditions influence aminogenesis during spontaneous sausage fermentation. Meat Sci. 2010;85(3):537–541. doi: 10.1016/j.meatsci.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Latorre-Moratalla ML, Bover-Cid S, Bosch-Fusté J, Vidal-Carou MC. Influence of technological conditions of sausage fermentation on the aminogenic activity of L. curvatus CTC273. Food Microbiol. 2012;29(1):43–48. doi: 10.1016/j.fm.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Latorre-Moratalla ML, Comas-Basté O, Bover-Cid S, Vidal-Carou MC. Tyramine and histamine risk assessment related to consumption of dry fermented sausages by the Spanish population. Food Chem Toxicol. 2017;99:78–85. doi: 10.1016/j.fct.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Lee JI, Jang JH, Yu MJ, Kim YW. Construction of a bifunctional enzyme fusion for the combined determination of biogenic amines in foods. J Agric Food Chem. 2013;61(38):9118–9124. doi: 10.1021/jf403044m. [DOI] [PubMed] [Google Scholar]
- Leng P-Q, Zhao F-L, Yin B-C, Ye B-C. A novel, colorimetric method for biogenic amine detection based on arylalkylamine N-acetyltransferase. Chem Commun. 2015;51(41):8712–8714. doi: 10.1039/c5cc02370j. [DOI] [PubMed] [Google Scholar]
- Liao E, Xu Y, Jiang Q, Xia W. Effects of inoculating autochthonous starter cultures on biogenic amines accumulation of Chinese traditional fermented fish. J Food Process Pres. 2018;42(8):e13694. [Google Scholar]
- Limsuwan S, Visessanguan W, Kongkiattikajorn J. The effects of starter cultures on biogenic amine and free amino acid contents in Nham during fermentation. Kaset J Nat Sci. 2007;41:363–372. [Google Scholar]
- Liu ZY, Li ZH, Zhong PP, Zhang P, Zeng MQ, Zhu CF. Improvement of the quality and abatement of the biogenic amines of grass carp muscles by fermentation using mixed cultures. J Sci Food Agri. 2010;90(4):586–592. doi: 10.1002/jsfa.3852. [DOI] [PubMed] [Google Scholar]
- Lorenzo JM, Munekata PES, Domínguez R. Role of autochthonous starter cultures in the reduction of biogenic amines in traditional meat products. Curr Opin Food Sci. 2017;14:61–65. [Google Scholar]
- Maksimovic AZ, Zunabovic-Pichler M, Kos I, Mayrhofer S, Hulak N, Domig KJ, Mrkonjic Fuka M. Microbiological hazards and potential of spontaneously fermented game meat sausages: a focus on lactic acid bacteria diversity. LWT Food Sci Technol. 2018;89:418–426. [Google Scholar]
- Malik AH, Hussain S, Iyer PK. Aggregation-induced FRET via polymer–surfactant complexation: a new strategy for the detection of spermine. Anal Chem. 2016;88(14):7358–7364. doi: 10.1021/acs.analchem.6b01788. [DOI] [PubMed] [Google Scholar]
- Marcobal A, Martín-Álvarez PJ, Moreno-Arribas MV, Muñoz R. A multifactorial design for studying factors influencing growth and tyramine production of the lacticacid bacteria Lactobacillus brevis CECT 4669 and Enterococcus faecium BIFI-58. Res Microbiol. 2006;157(5):417–424. doi: 10.1016/j.resmic.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Mayr CM, Schieberle P. Development of stable isotope dilution assays for the simultaneous quantitation of biogenic amines and polyamines in foods by LC–MS/MS. J Agric Food Chem. 2012;60(12):3026–3032. doi: 10.1021/jf204900v. [DOI] [PubMed] [Google Scholar]
- Molognoni L, Daguer H, de Sá Ploêncio LA, De Dea Lindner J. A multi-purpose tool for food inspection: simultaneous determination of various classes of preservatives and biogenic amines in meat and fish products by LC–MS. Talanta. 2018;178:1053–1066. doi: 10.1016/j.talanta.2017.08.081. [DOI] [PubMed] [Google Scholar]
- Moon JS, Kim Y, Jang KI, Han NS, Cho KJ, Yang SJ, Yoon GM, Kim SY. Analysis of biogenic amines in fermented fish products consumed in Korea. Food Sci Biotechnol. 2010;19(6):1689–1692. [Google Scholar]
- Moreno-Arribas V, Lonvaud-Funel A. Purification and characterization of tyrosine decarboxylase of Lactobacillus brevis IOEB 9809 isolated from wine. FEMS Microbiol Lett. 2001;195(1):103–107. doi: 10.1111/j.1574-6968.2001.tb10505.x. [DOI] [PubMed] [Google Scholar]
- Mozuriene E, Bartkiene E, Krungleviciute V, Zadeike D, Juodeikiene G, Damasius J, Baltusnikiene A. Effect of natural marinade based on lactic acid bacteria on pork meat quality parameters and biogenic amine contents. LWT Food Sci Technol. 2016;69:319–326. [Google Scholar]
- Nakamura M, Sanji T, Tanaka M. Fluorometric sensing of biogenic amines with aggregation-induced emission-active tetraphenylethenes. Chem Eur J. 2011;17:5344–5349. doi: 10.1002/chem.201003285. [DOI] [PubMed] [Google Scholar]
- Nout MJR. Fermented foods and food safety. Food Res Int. 1994;27(3):291–298. [Google Scholar]
- Oshikawa Y, Furuta K, Tanaka S, Ojida A. Cell surface-anchored fluorescent probe capable of real-time imaging of single mast cell degranulation based on histamine-induced coordination displacement. Anal Chem. 2016;88:1526–1529. doi: 10.1021/acs.analchem.5b04758. [DOI] [PubMed] [Google Scholar]
- Özogul F, Hamed I. The importance of lactic acid bacteria for the prevention of bacterial growth and their biogenic amines formation: a review. Crit Rev Food Sci Nutr. 2018;58(10):1660–1670. doi: 10.1080/10408398.2016.1277972. [DOI] [PubMed] [Google Scholar]
- Parente E, Martuscelli M, Gardini F. Evolution of microbial populations and biogenic amine production in dry sausages produced in Southern Italy. J Appl Microbiol. 2001;90(6):882–891. doi: 10.1046/j.1365-2672.2001.01322.x. [DOI] [PubMed] [Google Scholar]
- Pasini F, Soglia F, Petracci M, Caboni MF, Marziali S, Montanari C, Gardini F, Grazia L, Tabanelli G. Effect of fermentation with different lactic acid bacteria starter cultures on biogenic amine content and ripening patterns in dry fermented sausages. Nutrients. 2018;10(10):1497. doi: 10.3390/nu10101497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho O, Ferreira OIMPLV, Mendes E, Oliveira BM, Ferreira M. Effect of temperature on evolution of free amino acid and biogenic amine contents during storage of Azeitao cheese. Food Chem. 2001;75(3):287–291. [Google Scholar]
- Preti R, Antonelli ML, Bernacchia R, Vinci G. Fast determination of biogenic amines in beverages by a core-shell particle column. Food Chem. 2015;187:555–562. doi: 10.1016/j.foodchem.2015.04.075. [DOI] [PubMed] [Google Scholar]
- Rabie MA, Siliha H, El-Saidy S, El-Badawy AA, Malcata FX. Effects of γ-irradiation upon biogenic amine formation in Egyptian ripened sausages during storage. Innov Food Sci Emerg Technol. 2010;11(4):661–665. [Google Scholar]
- Rabie MA, Elsaidy S, El-Badawy AA, Siliha H, Malcata FX. Biogenic amine contents in selected Egyptian fermented foods as determined by ion-exchange chromatography. J Food Protect. 2011;74(4):681–685. doi: 10.4315/0362-028X.JFP-10-257. [DOI] [PubMed] [Google Scholar]
- Rabie MA, Peres C, Malcata FX. Evolution of amino acids and biogenic amines throughout storage in sausages made of horse, beef and turkey meats. Meat Sci. 2014;96(1):82–87. doi: 10.1016/j.meatsci.2013.05.042. [DOI] [PubMed] [Google Scholar]
- Santiyanont P, Chantarasakha K, Tepkasikul P, Srimarut Y, Mhuantong W, Tangphatsornruang S, Zo YG, Chokesajjawatee N. Dynamics of biogenic amines and bacterial communities in a Thai fermented pork product Nham. Food Res Int. 2019;119:110–118. doi: 10.1016/j.foodres.2019.01.060. [DOI] [PubMed] [Google Scholar]
- Seto D, Soh N, Nakano K, Imato T. An amphiphilic fluorescent probe for the visualization of histamine in living cells. Bioorg Med Chem Lett. 2010;20(22):6708–6711. doi: 10.1016/j.bmcl.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Seto D, Soh N, Nakano K, Imato T. Selective fluorescence detection of histamine based on ligand exchange mechanism and its application to biomonitoring. Anal Biochem. 2010;404(2):135–139. doi: 10.1016/j.ab.2010.04.038. [DOI] [PubMed] [Google Scholar]
- Simon-Sarkadi L, Pásztor-Huszár K, Dalmadi I, Kiskó G. Effect of high hydrostatic pressure processing on biogenic amine content of sausage during storage. Food Res Int. 2012;47(2):380–384. [Google Scholar]
- Singh G, Mangat SS, Sharma H, Singh J, Arora A, Pannu APS, Singh N. Design and syntheses of novel fluorescent organosilicon-based chemosensors through click silylation: detection of biogenic amines. RSC Adv. 2014;4:36834–36844. [Google Scholar]
- Suzzi G, Gardini F. Biogenic amines in dry fermented sausages: a review. Int J Food Microbiol. 2003;88(1):41–54. doi: 10.1016/s0168-1605(03)00080-1. [DOI] [PubMed] [Google Scholar]
- Tabanelli G, Torriani S, Rossi F, Rizzotti L, Gardini F. Effect of chemico-physical parameters on the histidine decarboxylase (HdcA) enzymatic activity in Streptococcus thermophilus PRI60. J Food Sci. 2012;77(4):M231–M237. doi: 10.1111/j.1750-3841.2012.02628.x. [DOI] [PubMed] [Google Scholar]
- Tabanelli G, Montanari C, Grazia L, Lanciotti R, Gardini F. Effects of aw at packaging time and atmosphere composition on aroma profile, biogenic amine content and microbiological features of dry fermented sausages. Meat Sci. 2013;94(2):177–186. doi: 10.1016/j.meatsci.2013.01.018. [DOI] [PubMed] [Google Scholar]
- Tabanelli G, Montanari C, Bargossi E, Lanciotti R, Gatto V, Felis G, Torriani S, Gardini F. Control of tyramine and histamine accumulation by lactic acid bacteria using bacteriocin forming lactococci. Int J Food Microbiol. 2014;190:14–23. doi: 10.1016/j.ijfoodmicro.2014.08.023. [DOI] [PubMed] [Google Scholar]
- Tu J, Sun S, Xu Y. A novel self-assembled platform for the ratiometric fluorescence detection of spermine. Chem Commun. 2016;52(5):1040–1043. doi: 10.1039/c5cc07861j. [DOI] [PubMed] [Google Scholar]
- Wang Q-H, Fang G-Z, Liu Y-Y, Zhang D-D, Liu J-M, Wang S. Fluorescent sensing probe for the sensitive detection of histamine based on molecular imprinting ionic liquid-modified quantum dots. Food Anal Methods. 2017;10:2585–2592. [Google Scholar]
- Wójciak KM, Solska E. Evolution of free amino acids, biogenic amines and N-nitrosoamines throughout ageing in organic fermented beef. Acta Sci Pol Technol Aliment. 2016;15(2):191–200. doi: 10.17306/J.AFS.2016.2.19. [DOI] [PubMed] [Google Scholar]
- Wójciak KM, Stasiak DM, Stadnik J, Ferysiuk K, Kononiuk A. The influence of sonication time on the biogenic amines formation as a critical point in uncured dry-fermented beef manufacturing. Int J Food Sci Technol. 2019;54(1):75–83. [Google Scholar]
- Xia XZW, Yang F, Jiang Q. Changes of biogenic amines in Chinese low-salt fermented fish pieces (Suan yu) inoculated with mixed starter cultures. Int J Food Sci Technol. 2013;48(4):685–692. [Google Scholar]
- Xie C, Wang HH, Nie XK, Chen L, Deng SL, Xu XL. Reduction of biogenic amine concentration in fermented sausage by selected starter cultures. CYTA J Food. 2015;13(4):491–497. [Google Scholar]
- Xu Y, He L, Xia W, Jiang Q, Yang F, Gao P, Wang B. The impact of fermentation at elevated temperature on quality attributes and biogenic amines formation of low-salt fermented fish. Int J Food Sci Technol. 2019;54(3):723–733. [Google Scholar]
- Yongsawatdigul J, Rodtong S, Raksakulthai N. Acceleration of Thai fish sauce fermentation using proteinases and bacterial starter cultures. J Food Sci. 2007;72(9):M382–M390. doi: 10.1111/j.1750-3841.2007.00532.x. [DOI] [PubMed] [Google Scholar]
- Zaman MZ, Abu Bakar F, Jinap S, Bakar J. Novel starter cultures to inhibit biogenic amines accumulation during fish sauce fermentation. Inter J Food Microbiol. 2011;145(1):84–91. doi: 10.1016/j.ijfoodmicro.2010.11.031. [DOI] [PubMed] [Google Scholar]
- Zhong-Yi L, Zhong-Hai L, Miao-Ling Z, Xiao-Ping D. Effect of fermentation with mixed starter cultures on biogenic amines in bighead carp surimi. Inter J Food Sci Technol. 2010;45(5):930–936. [Google Scholar]