Abstract
Spirulina platensis is having high nutritive value due to pigments such as chlorophyll-a, phycobiliproteins (especially phycocyanins) and carotenoids. In our present work, C-phycocyanin (C-PC) was extracted from dry biomass of Spirulina platensis. C-PC being heat sensitive, reiterates the need for additional protection during drying (micro encapsulation). Accordingly, a novel method employing aqueous two phase systems (ATPSs) as carrier materials to achieve double encapsulation was studied for the first time. PEG 4000/Potassium phosphate and PEG 6000/Dextran were used at already standardized tie line length, at different volume ratios (by varying the total phase composition). ATPS at each volume ratio acted as different carrier materials offering varied degree of heat protection during double encapsulation while maltodextrin, being the conventional carrier material, was used for comparison. The best results of spray dried powders using PEG (4% w/w)/Potassium phosphate salt (18%, w/w) and PEG (6%)/Dextran (10%, w/w) phase systems as carrier materials were compared with conventional encapsulation (MDX as a carrier material) and freeze dying as control. PEG/Dextran as a carrier material with volume ratio of 0.25 resulted in the highest retention of blue colour (b*value), purity (0.43) as well as yield (YEP) of 94.99% w/w of C-PC, which could be stored for 6 months without much reduction from initial powder characteristics. From the overall results, it can be concluded that ATPS can be used as an effective carrier material for double encapsulation of biomolecules such as C-PC with additional benefit of enhancing the purity.
Keywords: Aqueous biphasic system, Phycobiliproteins, Freeze drying, Spray drying, Microencapsulation
Introduction
The study of spirulina has been gaining importance as it is used as nutritional supplement in food, feed, pharmaceutical and cosmetic industry. It contains many Phytonutrients such as Phycocyanins, Beta-carotene, Chlorophylls, Xanthophyl etc. Among them, phycocyanins (phycocyanin, C-PC and Allo-phycocyanin, A-PC) are high value low volume compounds, as they are reported to have a variety of pharmacological properties such as anti-aging, antioxidant, anti-inflammatory, neuroprotective and hepatoprotective properties (Sekar and Chandramohan 2008; Ge et al. 2006; Romay et al. 2003; Reddy et al. 2003). The phycocyanins (C-PC and A-PC) find applications in diagnostics (as florescent probes), cosmetics and also in food processing as a natural food colourant, a substitute for synthetic dyes (Minkova et al. 2003). The applications of C-phycocyanin (C-PC) are dependent on its purity value, C-PC with purity (ratio of the absorbance at 620 nm to that at 280 nm, A620/A280) 0.7 is considered as food grade, purity of 3.9 reactive grade and greater than 4.0 is considered analytical grade (Rito-Palomares et al. 2001).
In the chain of unit operations of downstream processing (DSP), drying of final product comes towards the end. Drying is an important step as it extends the shelf life and promotes ease of transportation, storage and end use of the product. The challenge lies in the retention of bioactive properties as drying is a thermal process and C-phycocyanin (C-PC) is heat labile biomolecules. It was reported that phycocyanin (C-PC) is highly unstable at 45 °C and above (Sarada et al. 1999).
Spray drying by employing different carrier materials is one of the most popular microencapsulation method for extension of shelf life of biomolecules. Other methods for encapsulation are freeze drying and cocrystallization (Chandralekha et al. 2016; Dewi et al. 2016; López-Córdoba et al. 2016). For drying of heat-sensitive materials (like phycocyanins), freeze drying employing carrier materials is the most suitable method (Dewi et al. 2017a, b, Dewi et al. 2019). However, it is a cost-intensive and time consuming unit operation. It may be noted that DSP contributes about 50–80% of the total cost of the final product, and often many processes do not see the light of the day due to such high costs. Spray drying is one economically attractive alternative for the encapsulation of bioactive molecules (Desobry et al. 1997).
There are a few literature reports on spray drying of C-phycocyanin (C-PC). It was reported that carrier material plays an important role in encapsulation by spray drying in case of yeast (Chandralekha et al. 2016) and on phycocyanins (Dewi et al. 2016; Dewi et al. 2017a, b; Kurniasih et al. 2018). However, spray drying has its own disadvantages. For instance, though spray drying takes place at wet bulb temperature, heat damage occurs when the powder remains in contact with hot air until it is separated in the cyclone. Such damage is relatively more for thermolabile biomolecule like C-PC. A possible solution for reducing thermal damage to C-PC is by providing extra protection by double encapsulation during drying.
Accordingly, a novel concept of employing aqueous two phase systems as a carrier material for achieving double microencapsulation of C-phycocyanin (C-PC) during spray drying was explored for the first time. The aim of using the two phase system is to get the double layer protection (additional protection) for C-phycocyanin (C-PC). Polymer/polymer and polymer/salt type phase systems were employed as carrier materials in spray and freeze drying. Spray drying with MDX alone (single layer) as a carrier material was used for comparison between the concepts of single and double encapsulation. Freeze drying served as a control for spray drying method. The dried powder was analyzed for yield, purity, fluorescence, moisture content, colour, flow properties and shelf life of C-phycocyanin (C-PC).
Materials and method
Materials
The dry biomass of Spirulina platensis was purchased from the Parry Nutraceuticals, Chennai, PEG 4000 and PEG 6000 from Himedia Laboratories, Mumbai, Potassium phosphate salts (KH2PO4 and K2HPO4) from Merck, Mumbai, Maltodextrin (MDX) from Loba chemicals, Mumbai and Dextran T70 from Pharmacosmos, Holbæk.
All other chemicals used also were of analytical grade.
Methods
Extraction
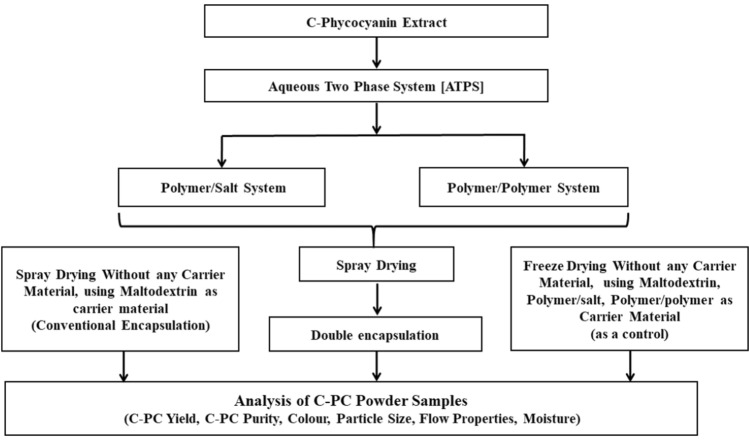
Extraction was carried out at standardized conditions of homogenization method from our earlier study (Tavanandi et al. 2018). 1 gm of dry biomass was soaked in potassium phosphate buffer (at 1:8 S/L ratio, w/v) for 120 min. The suspension was subjected to homogenization (MICRA D-9, Germany) at 15,000 RPM for 6 min and it was centrifuged at 11,200 × g for 30 min at 4 °C. Supernatant was taken for spectrophotometric analyses. The supernatant thus obtained was used for encapsulation experiments and the overall work plan is presented in Fig. 1.
Fig. 1.
Overall work plan
Aqueous two phase system as a carrier material
Aqueous two phase system (ATPS) was employed as a carrier material for double encapsulation of C-phycocyanin (C-PC). PEG 4000/Potassium phosphate, PEG 6000/Dextran and PEG 6000/MDX phase systems were used, which offers additional benefit of purification (due to partitioning between phases). Considering one tie line length, different volume ratios (VR by varying the total phase composition along that tie line length) of each ATPS were studied. The ATPSs were subjected to microencapsulation by different drying methods.
Maltodextrin as a carrier material
Maltodextrin, which is the most used carrier material in micro encapsulation, was used as a carrier material in order to compare with ATPS as a carrier material. Feed was prepared by adding 15% w/w MDX with crude extract and it was subjected for spray drying.
Double encapsulation
Freeze drying
Freeze drying was used as control for spray drying method. The feed was prepared by adding predetermined weighed quantities of polymer/salt and polymer/polymer from previous section. The phase system was equilibrated for 2 h using magnetic stirrer and kept overnight for phase formation.
After phase formation, the extracting phase containing C-phycocyanin (C-PC) was subjected to freeze drying (Martin Christ, Osterode am Harz, capacity of condenser 16 kg, temperature −20 ºC to + 5 ºC, maximum timing of 20 h). The samples were frozen at −20 ºC for 24 h followed by drying at −51 ºC for 14 h under pressure less than 0.12 mbar. The freeze dried powder was collected and stored in polyethylene-terephthalate laminate pouches at room temperature (27 ± 2 ºC) in desiccator.
Spray drying
The feed preparation of spray drying is same as freeze drying. The spray dryer (LSR-48 Mini spray drier, JISL, Mumbai) was used for spray drying experiments at the following conditions, inlet air temperature 120 ± 2 ºC, outlet air temperature 60 ± 2 ºC and feed flow rate of 2 mL/min while stirring continuously using magnetic stirrer. The powder samples were collected and stored at room temperature (27 ± 2 ºC) in desiccators.
Spectroscopy
The absorbance of C-phycocyanin (C-PC) was determined using UV/Vis microplate spectrophotometer (Thermoscientific, multiskan sky, Waltham) by measuring the optical density at 620 nm (λmax for C-PC), 650 nm (λmax for A-PC) and 280 nm (λmax for total protein). The concentration and purity of C-PC were calculated by the following equations (Marsac and Houmard, 1988; Bennett and Bogorad 1973).
| 1 |
| 2 |
The yield in a given phase (top or bottom) of ATPS was calculated by the following formula
| 3 |
| 4 |
where, ‘Y’ is the yield in the extracting phase before drying (C-PC rich top or bottom phases).
‘YEP’ is the yield in the extracting phase of the resuspended powder.
Colour measurement
In order to measure the colour intensity, the samples (feed, dried powder as well as resuspended powder) were analyzed for color using a colorimeter (Konica Minolta CM-5, Japan). The L*, a* and b* values were measured by using illuminant D65 lamp.
The three values L*, a* and b* represents lightness, redness or greenness and blue colour respectively. The L* value which ranges from 0 to100 (black to white), between red and green (a*, negative values indicate green while positive values indicate red) and its position between yellow and blue (b*, negative values indicate blue and positive values indicate yellow).
Moisture content
The moisture content of dried samples was measured using IR moisture analyzer (MA37, Sartorius, Gottingen). 2 g of sample taken in an aluminum pan and subjected to temperature of 80 ± 2 °C until constant weight was recorded. The results were expressed in terms of moisture content (%) on dry basis (d.b) of the sample.
Particle size analysis
Particle size of dried powder was analyzed by using Microtrac Turbo trac dry powder dispersion system (Bluewave, USA) equipped with laser dispersion particle size analyzer (S3500 series, USA). The particles over a range of 0.25–3000 µm can be measured using the instrument. For the analysis, 1 g of sample was loaded for each measurement. All the measurements were carried out in triplicates.
Flow properties
In order to measure the flowability of the dried (spray and freeze dried) powder, tap density tester (Elector lab make, ETD-1020) was used. Volume occupied by powder before (bulk volume) and after tapping (tapped volume) was recorded to calculate the Carr’s index (CI) and Hausner ratio (HR) using the following equations (Carr 1965; Hausner 1975)
| 5 |
where ‘VB’ is the bulk volume and ‘VT’ is the tapped volume of the dried powder
| 6 |
Fluorimetry analysis
The fluorescence spectra of C-phycocyanin (C-PC) dried powder samples were measured by using spectrofluorometer (Shimadzu, RF-5301Pc). The samples were subjected to the excitation wavelength of 620 nm and emission wavelength range of 600–700 nm.
Storage studies
The dried samples were stored in Aluminium polyethylene-terephthalate laminate pouches at room temperature (27 ± 2 °C) in desiccators under sterile conditions. C-phycocyanin (C-PC) purity, yield and powder characteristics were checked in regular interval for 6 months. The results of the different analyses (Purity, Yield, Color, Fluorescence etc.) performed vary significantly with the change of state of the sample. That is, the results of analyses observed for phase system samples will not be same as that of dried powder samples, making it not suitable for comparison. As a result the dried powders were resuspended in buffer and allowed for phase separation so as to compare with the initial readings before drying.
Results and discussion
Double encapsulation phenomenon
Primary extraction of C-PC was performed by homogenization of dry biomass (pre-soaked) and the extract with C-PC purity of 0.41 thus obtained was used for ATPS based double encapsulation experiments. In order to find out the effect of carrier material, polymer/salt (P/S) and polymer/polymer (P/P) aqueous two phase systems were examined for their suitability for double encapsulation.
In aqueous two phase system, the compound/biomolecule more preferentially partitions to one of the phases. On stirring, the preferred phase containing biomolecule gets dispersed into the other phase (polymer or salt). Depending on the volume ratio, one of the phases forms dispersed phase and the other forms continuous phase. This dispersion was subjected to spray drying while continuously stirring (to prevent the phase demixing) results in double encapsulation. This phenomenon is equally applicable for both polymer/polymer and polymer/salt type aqueous two phase systems.
For achieving effective double encapsulation, one of the most important process parameters, that is, the phase volume ratio needs to be standardized by varying total phase composition of ATPS on a selected tie line. This not only determines the efficacy of double encapsulation but also results in increased purity with good yield of C-PC during the extraction using ATPSs. From earlier studies, the tie line length of 33.53, 21.40 and 71% in case of PEG/Potassium phosphate salt (Patil and Raghavarao 2007), PEG/Dextran (Zaslavsky 1995) and PEG/Maltodextran (Srinivas et al. 2000) systems, respectively were selected for studying the effect of volume ratio. The details of different phase systems and volume ratios (VR) are described below in the following sub sections.
Effect of phase volume ratio
Polymer/salt system
In our earlier study (Patil and Raghavarao 2007), 33.53% TLL was arrived as the most suitable tie line (TL) for differential partitioning of C-PC in PEG/Potassium phosphate salt phase system and hence the same TL was considered for the present work. The effect of volume ratio, by selecting different total phase compositions on this %TLL (there by varying the volume ratio) was studied and the results are presented in Table 1.
Table 1.
Effect of phase volume ratio on double encapsulation
| Sl no | Phase composition (w/w %)/ carrier materials | Volume ratio | Before drying | After drying | ||||
|---|---|---|---|---|---|---|---|---|
| C-PC Purity (Top phase) | C-PC Purity (Bottom phase) | C-PC yield in top phase (%w/w) | C-PC yield in bottom phase (%w/w) | C-PC Purity (extracting phase) | C-PC yield in extracting phase (%w/w) | |||
| PEG 4000/potassium phosphate system | ||||||||
| 1 | PEG/Salt (PEG 4% and salt 18%) | 0.29 | 0.88 ± 0.04 | 0.18 ± 0.002 | 81.44 ± 0.65 | 14 ± 0.35 | 0.5 ± 0.02 | 78.93 ± 1.5 |
| 2 | PEG/Salt (PEG 10% and Salt 16%) | 0.09 | 0.76 ± 0.02 | 0.17 ± 0.01 | 25.05 ± 0.43 | 22.55 ± 0.9 | 0.47 ± 0.04 | 43.53 ± 0.75 |
| 3 | PEG/Salt (PEG 13% and Salt 14%) | 0.8 | 0.54 ± 0.17 | 0.08 ± 0.01 | 93.11 ± 0.51 | 5.41 ± 0.15 | 0.41 ± 0.01 | 60.36 ± 1.7 |
| PEG 4000/Dextran system | ||||||||
| 4 | PEG/Dextran (PEG 8% and dextran 5%) | 1.33 | 0.65 ± 0.03 | 0.44 ± 0.002 | 42.51 ± 0.41 | 37.07 ± 1.56 | 0.4 ± 0.02 | 100 ± 1.62 |
| 5 | PEG/Dextran (PEG 6% and dextran 10%) | 0.25 | 0.41 ± 0.001 | 0.55 ± 0.05 | 9.74 ± 0.08 | 81.83 ± 2.48 | 0.43 ± 0.01 | 94.98 ± 1.4 |
| 6 | PEG/Dextran (PEG 4% and dextran 13%) | 2.33 | 0.43 ± 0.001 | 0.43 ± 0.02 | 58.24 ± 0.2 | 40.91 ± 0.65 | 0.4 ± 0.01 | ~ 100 ± 1.7 |
| PEG 4000/Maltodextrin system | ||||||||
| 7 | PEG/MDX (PEG 9.3% and MDX 36%) | No phase formation | – | – | – | – | – | – |
| 8 | PEG/MDX (PEG 12.3% and MDX 30%) | – | – | – | – | – | – | – |
| Maltodextrin Alone (conventional microencapsulation) | ||||||||
| 9 | MDX alone | – | 0.33 ± 0.02 | 22.45 ± 0.02 mg/g.db | 0.32 ± 0.03 | 72.67 ± 0.34 | ||
Values are averages±standard deviation from three replicate (n=3) analyses
*Crude extract has C-PC purity of 0.38
In the polymer/salt two phase system, C-PC partitioned to the top phase (polymer rich) while the contaminant proteins to the bottom phase (salt phase). Total phase compositions on a given tie line affects significantly on volume ratio and in turn the volume of the extracting phase. This decides the differential partitioning of the C-PC and contaminant proteins between the phases affecting the yield and purity of C-PC in the extracting phase. The major reason for the differential partitioning is determined by the solubility of the desired biomolecule in one of the phases and the volume exclusion effect of the phases towards either desired biomolecule or contaminant proteins (Patil and Raghavarao 2007).
It can be seen from the table that the yield increased (25.05–93.11% w/w) with an increase in volume ratio (0.09–0.8). On the other hand, purity followed the opposite trend. Purity was found to be the lowest at the highest volume ratio (0.8). The increase in yield can be attributed to increase in volume of extracting phase. The low C-PC purity at higher volume ratio could be due to partitioning of contaminant proteins to the top phase with increasing volume of extracting phase. Similar results were obtained by Narayan and Raghavarao (2007) in extraction and purification of C-phycocyanin employing ATPS (Narayan and Raghavarao 2007).
In order to check the effect of different phase volume ratio of PEG/Potassium phosphate salt system as a carrier material for double encapsulation of C-PC, the phase system with different volume ratios were subjected for spray drying.
Polymer/polymer system
For PEG/Dextran system, a tie line (TLL 21.4%) was selected from the literature (Zaslavsky 1995). The results at 3 volume ratios (with corresponding total phase compositions) are presented in Table 1. It can be seen from the table, that C-PC partitioned relatively more selectively to the bottom phase. Further, the purity and yield in the bottom phase decreased with an increase in volume ratio, resulting in the highest purity of 0.55 and yield of 81.83% w/w and at the lowest volume ratio (0.25). This can be attributed to high volume of extracting phase (bottom phase).
In case of PEG/Maltodextin system, 71% TLL was selected from the literature (Srinivas et al. 2000). The 2 volume ratios (with corresponding total phase compositions given in Table 1) studied did not form phase.
Maltodextrin as a carrier material
In order to compare, MDX alone was used as a carrier material for encapsulation (conventional encapsulation) of C-PC and the results are presented in Table 1. It can be seen from the table that the purity and yield of C-PC were 0.33 and 22.45 mg/g d.b, respectively, which are much lower than purity and yield obtained in C-PC rich phase in ATPS. The increased purity of C-PC in ATPS is a result of purification achieved because of differential partitioning of C-PC and contaminant proteins between the phases.
Freeze drying
Freeze drying of C-PC without carrier material
Freeze drying of C-PC without any carrier material was carried out for the purpose of comparison with freeze dried sample with carrier materials in order to understand the effect of carrier material. Freeze drying of C-PC without any carrier material resulted in C-PC purity of 0.41 and yield of 96.49% w/w. Low drying temperatures involved in this type of drying resulted in higher retention of C-PC.
Freeze drying of C-PC with different carrier materials
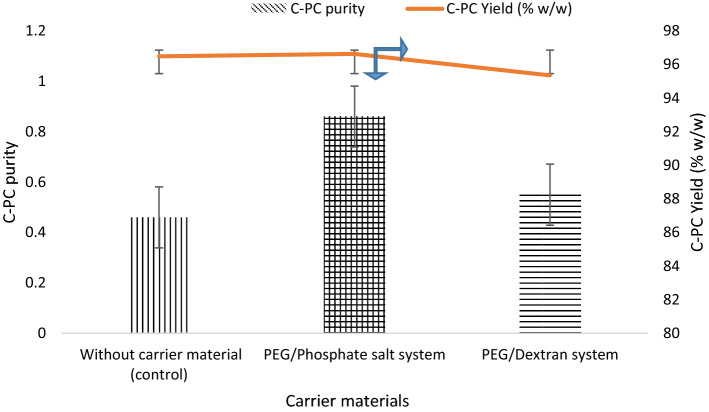
Freeze drying was carried out as a control for spray drying method. The phase system which resulted in the highest C-PC purity and yield in both P/S and P/P phase systems were subjected to freeze drying. The results are consolidated in Fig. 2. It can be seen from the figure that the increase in purity (0.86 and 0.54) as well as yield (96.63 and 95.35% w/w) could be observed in freeze dried C-PC with both PEG/Potassium phosphate salt and PEG/Dextran phase systems as a carrier material, respectively. This can be attributed to milder processing conditions associated with freeze drying when compared to spray drying. Increased purity in case of samples with ATPSs as carrier materials is due the purification process taking place before freeze drying which otherwise is not the case with the samples without ATPSs.
Fig. 2.
C-PC Yield and purity in freeze dried encapsulated powder samples
Freeze drying is reported to be the best drying method for heat sensitive biomolecules. Freeze drying of C-PC with different carrier materials such as κ-Carrageenan, Na-alginate, maltodextrin and their combinations were reported where combination of carrier materials resulted in better retention of blue colour in dried sample (Dewi et al. 2017). High cell survival rate in microorganisms (Wang et al. 2004; Chandralekha et al. 2017) and retention of enzyme activity (Jesus et al. 2014) were observed in freeze dried powders however, it becomes economically unviable for large-scale production.
Spray drying
Spray drying of C-PC without carrier materials
Spray drying was carried out without any carrier material in order to find out the effect of carrier material. C-PC spray dried without any carrier material resulted in a purity of 0.4 and yield of 51.2% w/w. The lower yield observed is a result of hygroscopic nature of powder sticking to the walls of spray drier and cyclone. Hence, further analysis of this sample could not be carried out.
Spray drying of C-PC using different carrier materials
Spray drying of C-PC using ATPS as a carrier material was carried out and the results are presented in Table 1. It can be seen from the table that among the three volume ratios (VR) in case of PEG/Potassium phosphate salt phase system, the highest purity (0.5) and yield (YEP of 78.93%, w/w) were obtained in the top phase of volume ratio 0.3 (4% PEG and 18% Salt).
The high purity and yield could be due the low volume ratio, where salt rich phase of higher volume formed the continuous phase. Higher volume of salt phase gives more protection to C-PC that is partitioned in the PEG rich dispersed phase. The higher retention of C-PC is also reflected in the colour value of the powder. The highest –b* value (-17.89), indicating blueness of powder is a confirmation for the claim. It can be seen from Table 1 that C-PC yield in the top phase was the highest (93.11%, w/w) at the highest VR before drying, however after drying the C-PC yield (YEP) in the extracting phase reduced to 60.36% w/w. The reduction in yield could be due to relatively high volume of C-PC rich phase, resulting in degradation of C-PC. It was reported that, in case of PEG/salt system, at higher volume ratio, the PEG rich top phase forms the continuous phase and the salt phase as dispersed phase (Narayan et al. 2011).
In case of PEG/Dextran phase system, even though the purity and yield were lower compared to PEG/Potassium phosphate salt system before drying, the retention of yield after drying (YEP) is high compared to PEG/Potassium phosphate salt system. This can be attributed to the high degree of double encapsulation or double layer protection in case of PEG/Dextran system compared to PEG/salt system. Among different volume ratios (total phase compositions) of PEG/Dextran system, the highest C-PC purity (0.43) and yield (YEP of 94.99%, w/w) was observed in the lowest volume ratio (0.25). An image of the double encapsulated powder obtained after spray drying (with PEG/Dextran system) is presented as Fig. 3. Mastiani et al. (2019) reported the use of P/S and P/P phase system for encapsulation by forming micro droplets. Effect of spray drying temperatures of phycocyanins using MDX and carrageenan as a carrier materials was studied where drying temperature of 90 ºC resulted in higher concentration of phycocyanin (Purnamayati et al. 2017). Microencapsulation of C-phycocyanin was carried out by extrusion technology using sodium alginate as a carrier material in order to improve stability of C-phycocyanin (Pradeep et al. 2019).
Fig. 3.

Double encapsulated C-PC powder (with PEG/Dextran system)
When maltodextrin alone was used as a carrier material, C-PC purity and yield of 0.32 and 72.67% w/w, respectively, were observed which are much lower than those obtained from double encapsulated powder samples with P/S and P/P phase systems as a carrier material. Thus, it can be inferred that ATPS system as a carrier material is more effective for double encapsulation of biomolecules compared to hitherto reported conventional carrier materials employed for microencapsulation.
Colour analysis
As phycocyanin is a protein pigment complex, the colour analysis values plays an important role in inferring retention/degradation of C-PC. The high and low value of b* signifies the degradation and retention of C-PC respectively. The powder in which the C-PC retained is relatively higher will exhibit higher blue shade. Color analysis studies indicates a clear relation between the C-PC content in the powder and degree of blueness of powder obtained for a given drying method. The objective is to achieve a powder with higher blue shade, that means, –ve values of b* is preferred. The colour analysis of C-PC double encapsulated with ATPS systems (P/P and P/S) before drying, dried powder and after resuspending the dried powder (freeze and spray dried) was carried out and the results are presented in the Table 2.
Table 2.
Colour analysis of double encapsulated C-PC powder samples
| Samples | Before drying | Dried powder | Resuspension | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phase composition/carrier materials | L*(D65) | a*(D65) | b*(D65) | L*(D65) | a*(D65) | b*(D65) | L*(D65) | a*(D65) | b*(D65) | |
| Freeze drying | ||||||||||
| P/S phase system | PEG and salt (PEG 4% and salt 18%) | 76.1 ± 0.4 | −30.35 ± 2.0 | −23.67 ± 1.0 | 41.6 ± 2.1 | −13.32 ± 0.90 | −10.28 ± 1.0 | 78.2 ± 1.12 | −27.5 ± 1.20 | −21.7 ± 2.1 |
| P/P phase system | PEG Dextran (PEG 6% and dextran10%) | 94.49 ± 1.01 | −7.82 ± 1.5 | −6.12 ± 1.1 | 35.41 ± 2.06 | −10 ± 0.98 | −21.29 ± 1.02 | 95.00 ± 2.0 | −7.15 ± 0.89 | −6.03 ± 1.02 |
| P/S | Spray drying | |||||||||
| TP | PEG/Salt (PEG 4% and salt 18%) | 76.1 ± 0.4 | −30.35 ± 0.84 | −23.67 ± 1.4 | 54.89 ± 2.65 | −17.23 ± 1.4 | −5.77 ± 0.48 | 80.24 ± 3.5 | −25.14 ± 2.0 | −17.89 ± 1.2 |
| TP | PEG/Salt (PEG 10% and Salt 16%) | 88.03 ± 0.46 | −15.9 ± 0.44 | −16.82 ± 0.94 | 48.16 ± 2.0 | −16.24 ± 0.94 | −8.85 ± 0.54 | 94.32 ± 3.5 | −7.75 ± 0.14 | −6.46 ± 0.9 |
| TP | PEG/Salt (PEG 13% and Salt 14%) | 85.81 ± 0.51 | −19.76 ± 0.68 | −15.24 ± 0.87 | 38.55 ± 2.15 | −15.55 ± 0.89 | −8.83 ± 0.62 | 93.05 ± 3.5 | −9.13 ± 0.73 | −6.92 ± 0.87 |
| P/P | ||||||||||
| TP | PEG/Dextran (PEG 8% and dextran 5%) | 96.68 ± 0.41 | −4.86 ± 1.51 | −4.59 ± 0.84 | 43.55 ± 3.5 | −16.28 ± 1.1 | −15.31 ± 1.4 | 97.38 ± 3.5 | −4.15 ± 0.84 | −3.61 ± 0.78 |
| BP | 93.9 ± 0.21 | −8.53 ± 0.9 | −5.58 ± 0.45 | 94.91 ± 4.15 | −7.74 ± 0.73 | −5.68 ± 0.94 | ||||
| TP | PEG/Dextran (PEG 6% and dextran 10%) | 97.71 ± 0.39 | −3.28 ± 0.54 | −3.02 ± 0.48 | 57.98 ± 3.78 | −20.68 ± 1.11 | −16.73 ± 1.23 | 98.31 ± 4.1 | −2.57 ± 0.54 | −1.9 ± 0.54 |
| BP | 94.49 ± 0.41 | −7.82 ± 0.51 | −6.12 ± 0.64 | 94.11 ± 3.62 | −8.64 ± 0.47 | −6.02 ± 0.98 | ||||
| TP | PEG/Dextran (PEG 4% and dextran 13%) | 95.41 ± 0.38 | −6.53 ± 0.48 | −4.61 ± 0.41 | 54.33 ± 3.57 | −19.39 ± 0.99 | −17.95 ± 0.97 | 95.88 ± 2.95 | −6.32 ± 0.34 | −4.67 ± 0.77 |
| BP | 97.46 ± 0.47 | −3.9 ± 0.5 | −3.75 ± 0.33 | 97.17 ± 3.15 | −4.54 ± 0.23 | −4.32 ± 0.84 | ||||
| Maltodextrin (15%) | 95.78 ± 0.64 | −6.19 ± 0.6 | −4.42 ± 0.54 | 66.49 ± 3.25 | −19.29 ± 0.89 | −14.66 ± 0.94 | 95.76 ± 3.0 | −5.85 ± 0.41 | −4.14 ± 0.96 | |
| Crude extract | 91.1 ± 0.22 | −13.03 ± 0.44 | −9.64 ± 0.39 | |||||||
Values are averages±standard deviation from three replicate (n = 3) analyses
TP Top phase, BP bottom phase
It was observed that, in case of P/S system the majority of the desired protein (C-PC) partitioned to top phase, which implies selective partitioning of the C-PC to the top phase. However, in case of P/P phase system the C-PC partition in both the phases (selective partitioning does not take place properly). It can be seen from the table that in case of P/S system the intensity of blue colour (b* value) are higher in the top phase compared to P/P system. The highest b* value (blue colour) was observed in case of P/S system (PEG 4% and salt 14%). The b* value was found to be -23.67 before subjecting to spray and freeze drying. However, after resuspending the dried powder in buffer, the b* value in the top phase was observed to be -21.7 and -17.89 in case of freeze and spray dried samples, respectively. The reduction of b* value in freeze drying (8%) compared to spray drying (32.3%) is due to low/mild temperature involved during freeze drying, resulting in the minimal degradation of C-PC.
In case of P/P phase system as a carrier material, the b* value of the encapsulated C-PC powder sample are higher compared to before drying as well as after resuspending the powder, irrespective of the phase compositions used. This could be due to low selective partitioning, where C-PC partition in both the phases and when it is dried, the intensity of blue colour (b*) in both phases reflects in the dried powder. The lowest b* value (blue colour) was observed to be -6.12 at the bottom phase in case of PEG 6% and dextran 10% before subjecting it to drying. The percentage reduction in b* values was found to be 1.47 and 1.63% in case of freeze and spray dried sample respectively, which can be inferred as an effective encapsulating agent of PEG/Dextran phase system as a carrier material for double encapsulation. When maltodextrin (MDX) alone was used as a carrier material, the b* value before drying was found to be -4.42 and it reduced to −4.14 after drying and resuspension (6.33% reduction). Thus, from the results it can be inferred that b* value of C-PC with MDX alone as a carrier material is relatively higher than the C-PC with both P/P and P/S as a carrier material indicating effectiveness of double encapsulation in retaining of biomolecule compared to conventional encapsulation.
Moisture content
Moisture content of the powder plays an important role when it comes to shelf life of dried powder. The moisture content analysis of both spray and freeze dried powder (with the best carrier material) were carried out and the results are presented in Table 3. It can be seen from the table that moisture content of spray dried samples with different carrier materials (PEG/Potassium phosphate salt and PEG/Dextran phase system and MDX alone) ranged between 3.2 and 5.78%. The moisture content of freeze dried samples with different carrier materials ranged between 3.98% and 5.2%. Accordingly, all the powders obtained from freeze and spray drying can be considered as stable as the moisture content less than 10% was categorized as microbiologically stable (Hari et al., 2013).
Table 3.
Moisture content and flow properties of double encapsulated C-PC powder samples
| Ser. No | Samples | Moisture (%,w/w) |
Flow properties | |
|---|---|---|---|---|
| Carr’s index* | Hausner’s ratio | |||
| Freeze drying | ||||
| 1 | PEG and salt (PEG 4% and salt 18%) | 5.2 ± 0.26 | 22 ± 1.1 | 1.13 ± 0.06 |
| 2 | PEG Dextran (PEG 6% and dextran10%) | 3.98 ± 0.2 | 30 ± 1.5 | 1.42 ± 0.07 |
| Spray drying | ||||
| 3 | PEG/Salt (PEG 4% and salt 18%) | 3.95 ± 0.19 | 35 ± 1.8 | 1.5 ± 0.075 |
| 4 | PEG/Salt (PEG 10% and Salt 16%) | 3.2 ± 0.16 | 33 ± 1.7 | 1.49 ± 0.75 |
| 5 | PEG/Salt (PEG 13% and Salt 14%) | 4.09 ± 0.20 | 38 ± 1.9 | 1.61 ± 0.08 |
| 6 | PEG/Dextran (PEG 8% and dextran 5%) | 5.12 ± 0.26 | 40 ± 2 | 1.6 ± 0.08 |
| 7 | PEG/Dextran (PEG 6% and dextran 10%) | 5.38 ± 0.27 | 39 ± 1.95 | 1.64 ± 0.07 |
| 8 | PEG/Dextran (PEG 4% and dextran 13%) | 4.98 ± 0.25 | 41 ± 2.05 | 1.69 ± 0.08 |
| 9 | Maltodextrin | 5.78 ± 0.29 | 40 ± 2 | 1.6 ± 0.08 |
Values are averages±standard deviation from three replicate (n = 3) analyses
* Range of Carr’s index value with its respective flow characteristics, 5–15: Excellent flow, 16–18: Good flow, 19–21: Moderately good flow, 22–35: Poor flow, 36–40: Very poor flow
Flow properties
The flow properties of spray and freeze dried powders were evaluated by measuring Carr’s index and Hausner ratio and the results are presented in Table 3. It can be seen from the table that the flow characteristics of dried C-PC were different with different carrier materials. The highest CI value (40) was observed when MDX alone was used as a carrier material. This could be due to the hygroscopic nature of MDX. In order to increase the flow properties of the powder, addition of anti-caking agent’s like silicon dioxide was reported (Amrutha et al. 2014).
Particle size analysis
The particle size analysis was carried out for the dried samples (freeze and spray dried) which resulted in the highest yield and purity in both P/S and P/P phase system. The particle size of spray and freeze dried samples were analysed and the values are presented in Table 4. It can be seen from the table that the particle size ranged between 8.77 and 380.5 µm. All the dried powders (both freeze and spray dried) obtained were not uniform in size.
Table 4.
Particle size of double encapsulated CPC powder samples
| Ser. no | Sample | Particle size (µm) |
|---|---|---|
| 1 | Freeze-dried without carrier materials |
78.3%−43 21.7%–245.1 |
| 2 | Freeze-dried (4% PEG & 18% Salt) |
79.5%–366.1 20.5%–111 |
| 3 | Freeze-dried (10% PEG & 16% Dextran) |
78.3%–316 21.7%–110.9 |
| 4 | Spray-dried (4% PEG & 18% Salt) |
56.3%–342.4 43.7%–74.3 |
| 5 | PEG/Salt (PEG 10% and Salt 16%) |
61.2%–349.6 48.8%–75.4 |
| 6 | PEG/Salt (PEG 13% and Salt 14%) |
76.3%–380.5 23.7%–79.3 |
| 7 | PEG/Dextran (PEG 8% and dextran 5%) |
58.2%–8.77 41.8%–182.6 |
| 8 | Spray-dried (6% PEG & 10% dextran) |
71.1%–9.37 28.9%–141.8 |
| 9 | PEG/Dextran (PEG 4% and dextran 13%) |
75.2%–11.2 24.8%–153.5 |
| 10 | Maltodextrin |
81.1%–18.7 18.9%–112.3 |
All the feed solutions were spray dried under the same conditions, and the variations in particle size among the C-PC encapsulated powders samples with different carrier materials may be attributed to the different film-forming and gelling properties of the wall materials used for spray drying. Thus, the carrier material used had significant effect on the particles size of the dried powders.
Storage studies
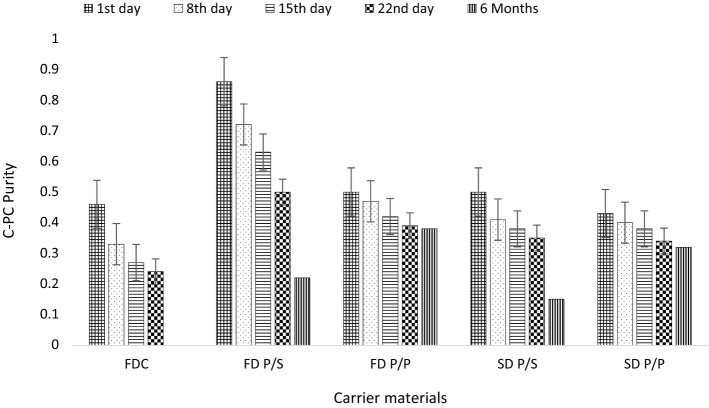
Storage studies were carried out for spray and freeze dried samples which resulted in the highest yield and purity in both P/S and P/P phase system for the period of 6 months at room temperature (27 ± 2 °C). C-PC purity and powder characteristics (moisture content, colour analysis, flow properties) were analysed regularly at each interval (weekly for 3 weeks and after 6 months) and fluorescence spectra was checked after 6 months of storage. The results of the storage studies are presented in (Figs. 4, 5, 6 and Tables 5, 6). It can be seen from the Fig. 4 that C-PC purity decreases slightly, where the decrease percentage range from 20.93 to 47.83%, with storage time till 3 weeks and 24–74.4% till 6 months of storage. Freeze and spray dried samples with PEG/Dextran as a carrier material resulted in lower reduction of purity i.e. 24 and 25.58%, respectively till 6 months of storage. The % reduction of C-PC purity in P/S is more than P/P phase system as a carrier material, indicating P/P phase system as a better carrier material compared to P/S phase system. The reduction of purity with storage time could be due to degradation of C-PC with time (reduction in A620).
Fig. 4.
Purity of encapsulated C-PC powder samples during storage. FDC freeze dried w/o carrier material; FD P/S freeze dried PEG/salt aqueous two phase system; FD P/P freeze dried PEG/dextran; SD P/S spray dried PEG/salt; SD P/P spray dried PEG/dextran
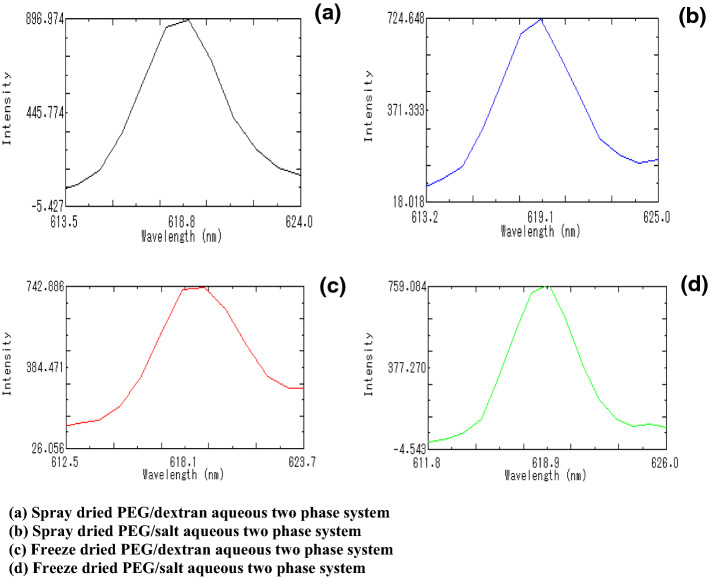
Fig. 5.
Fluorescence spectra of C-PC encapsulated powder samples after 6 months storage (protein concentration used was 0.8 mg/ml for all the resuspended dried powder samples). a Spray dried PEG/dextran aqueous two phase system. b Spray dried PEG/salt aqueous two phase system. c Freeze dried PEG/dextran aqueous two phase system.d Freeze dried PEG/salt aqueous two phase system
Fig. 6.
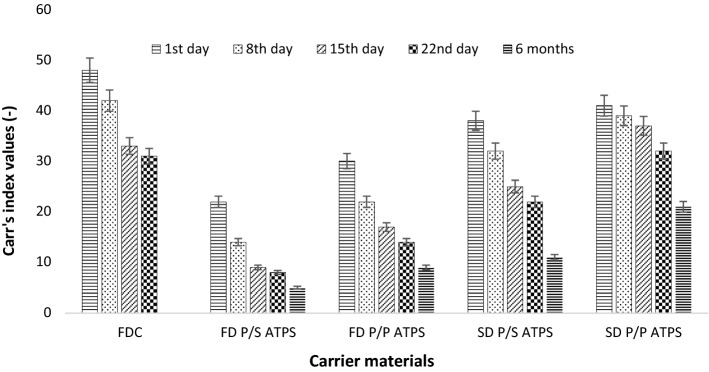
Flow properties of encapsulated C-PC powder samples during storage. FDC freeze dried w/o carrier material; FD P/S ATPS freeze dried PEG/salt aqueous two phase system; FD P/P ATPS freeze dried PEG/dextran aqueous two phase system; SD P/S ATPS spray dried PEG/salt aqueous two phase system; SD P/P ATPS spray dried PEG/dextran aqueous two phase system
Table 5.
Moisture Content of double encapsulated C-PC powder samples during storage
| Sample | % Moisture (1st day) | % Moisture (8th day) | % Moisture (2 weeks) | % Moisture (3 weeks) | % Moisture (6 months) |
|---|---|---|---|---|---|
| FDC | 11.60 ± 0.57 | 12.30 ± 0.9 | 14.02 ± 0.75 | 15.68 ± 0.45 | – |
| FD P/S ATPS | 5.2 ± 0.26 | 7.97 ± 0.7 | 8.67 ± 0.62 | 9.27 ± 0.23 | 10.2 ± 0.52 |
| FD P/P ATPS | 3.98 ± 0.2 | 4.97 ± 0.51 | 7.09 ± 0.84 | 8.97 ± 0.8 | 9.45 ± 0.41 |
| SD P/S ATPS | 4.09 ± 0.20 | 5.88 ± 0.57 | 6.46 ± 0.32 | 8.92 ± 0.87 | 9.25 ± 0.27 |
| SD P/P ATPS | 5.38 ± 0.27 | 6.18 ± 0.62 | 6.98 ± 0.25 | 7.24 ± 0.72 | 7.9 ± 0.31 |
Values are averages±standard deviation from three replicate (n = 3) analyses
FDC freeze dried w/o carrier material, FD P/S freeze dried polymer/salt aqueous two phase system, FD P/P freeze dried polymer/polymer, SD P/S spray dried polymer/salt, SD P/P spray dried polymer/polymer
Table 6.
Colour analysis of double encapsulated C-PC powder samples (resuspended) during storage
| 1st day | 8th day | 15th day | 22nd day | 6 months | 1st day | 8 th day | 15th day | 22nd day | 6 months | 1st day | 8th day | 15th day | 22nd day | 6 months | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | L* | L* | L* | L* | L* | a* | a* | a* | a* | a* | b* | b* | b* | b* | b* |
| FDC | 39.39 | 37.73 ± 0.71 | 31.95 ± 0.91 | 30.02 ± 0.67 | − | −16.3 | −15.79 ± 0.41 | −14.18 ± 0.81 | −12.57 ± 0.73 | – | −6.99 ± 0.71 | −6.76 ± 0.56 | −6.19 ± 0.58 | −5.67 ± 0.62 | – |
| FD P/S ATPS | 78.2 ± 1.12 | 70.21 ± 0.75 | 68.34 ± 1.1 | 66.47 ± 0.59 | 64 ± 1.71 | −27.5 ± 1.20 | −27.11 ± 0.63 | −27.05 ± 1.7 | −26.21 ± 1.1 | −4.55 ± 0.75 | −21.7 ± 2.1 | −17.55 ± 0.98 | −15.47 ± 0.61 | −13.28 ± 0.98 | 0.85 ± 0.21 |
| FD P/P ATPS | 95.00 ± 2.0 | 90.7 ± 0.33 | 88.59 ± 0.97 | 85.07 ± 0.95 | 83 ± 1.02 | −7.15 ± 0.89 | −7.11 ± 0.74 | −6.99 ± 0.62 | −6.42 ± 0.47 | −5.76 ± 0.85 | −6.03 ± 1.02 | −5.98 ± 0.47 | −4.03 ± 0.45 | −3.84 ± 0.79 | −5.06 ± 0.3 |
| SD P/S ATPS | 80.24 ± 3.5 | 78.5 ± 0.62 | 76.47 ± 0.88 | 74.03 ± 0.87 | 62.07 ± 1.21 | −25.14 ± 2.0 | −24.08 ± 0.8 | −24.02 ± 0.91 | −23.23 ± 0.71 | −2.06 ± 0.42 | −17.89 ± 1.2 | −10.9 ± 0.54 | −5.16 ± 0.28 | −2.91 ± 0.35 | −0.98 ± 0.41 |
| SD P/P ATPS | 94.11 ± 3.62 | 88.45 ± 0.84 | 85.52 ± 0.64 | 82.13 ± 0.79 | 75.08 ± 0.98 | −8.64 ± 0.47 | −8.5 ± 0.52 | −8.35 ± 0.54 | −8.06 ± 0.53 | −2.2 ± 0.29 | −6.02 ± 0.98 | −6.00 ± 0.81 | −5.96 ± 0.43 | −5.88 ± 0.86 | −1.56 ± 0.12 |
Values are averages±standard deviation from three replicate (n=3) analyses
FDC freeze dried w/o carrier material, FD P/S freeze dried polymer/salt aqueous two phase system, FD P/P freeze dried polymer/polymer, SD P/S spray dried polymer/salt; SD P/P spray dried polymer/polymer
The moisture content of freeze and spray dried samples increases with storage period (Table 5). The degree of moisture absorption with time is higher in case of freeze dried sample compared to spray dried sample even though initially showed the similar moisture contents. The increase in moisture content (% d.b) in case of spray dried P/S and P/P as a carrier material after 6 months was found to be 55 and 31.9% d.b, respectively, indicating the hygroscopic nature of the powder obtained by using P/S phase system as a carrier material.
The colour analysis was carried out at regular interval during the storage and the results are presented in Table 6. It can be seen from table that, the decrease in the lightness (L* value) of C-PC powder with PEG/Dextran as a carrier material were 12.63 and 20.22% for freeze and spray dried sample respectively with storage time (up to 6 months). The greenness value (-a* value) also reduced during storage time irrespective of the carrier material and drying method used. There is a significant reduction in blue colour (-b* value) in freeze and spray dried C-PC with PEG/Potassium phosphate salt as a carrier material (96.08 and 94.52%, respectively) even though it resulted in the much higher -b* value initially after drying. However, freeze and spray dried C-PC with PEG/Dextran as a carrier material resulted in minimum reduction of -b* value (16.08 and 74.08% respectively) during storage time (up to 6 months) compared to PEG/Potassium phosphate salt phase system. The C-PC powder samples after 6 months of storage were checked for its fluorescence and the results are presented in Fig. 5. It can be seen from Fig. 5 that the fluorescent intensity of C-PC powder samples was observed to be retained and the emission wavelength are relatively similar in all the C-PC powder samples after the storage of 6 months.
It can be seen from Fig. 6 that the % reduction in flow properties during storage of the freeze dried sample was observed to be higher (70%) than spray dried sample (48.78%) with PEG/Dextran as a carrier material. This could be due to more porous surface properties of freeze dried sample compared to that of spray dried sample.
Considering all the results (colour, purity and fluorescence intensity spectrum) with respect to retention of C-PC, double encapsulation by spray drying of C-PC with PEG/Dextran as a carrier material resulted in the highest result in terms of protection for biomolecules even after storage of 6 months.
Conclusions
Double encapsulation of C-phycocyanin (C-PC) was carried out successfully by spray drying using Aqueous two phase systems as a carrier material for the first time. The results of double encapsulation obtained using polymer/salt (4% PEG and 18% salt, w/w) and polymer/polymer (6% PEG and 10% dextran, w/w) phase systems as a carrier material were compared with conventional microencapsulation (MDX alone as a carrier material) and Freeze dying (control). The C-PC purity of 0.5 and 0.43 and yield of 78.93 and 94.99% w/w were observed after drying in case of PEG/Potassium phosphate salt and PEG/Dextran phase system, respectively which are much higher than the purity and yield (0.32 and 72.69% w/w) obtained from the sample with MDX as a carrier material. The results were better in case of PEG/Dextran system as a carrier material when compared to PEG/Potassium phosphate salt systems. PEG/Dextran as a carrier material with volume ratio of 0.25 resulted in the highest retention of blue colour (b*value), purity (0.43) as well as yield (YEP) of 94.99% w/w of C-PC. From the overall results, it can be concluded that ATPSs can be used as an effective carrier material for double encapsulation with additional benefit of enhancing the purity of biomolecules. The dry C-PC powder obtained could be stored for 6 months without much changes in the powder characteristics.
Acknowledgments
The authors Miss. A Chandralekha, Mr. Prashanth HS and Mr. Hrishikesh A Tavanandi gratefully acknowledge the Director, CSIR-CFTRI, for providing the infrastructural facilities at the institute. Miss. A Chandralekha and Mr. Hrishikesh Tavanandi acknowledges Council of Scientific and Industrial Research, Government of India for providing the fellowship.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Amrutha N, Hebbar HU, Prapulla SG, Raghavarao KSMS. Effect of additives on quality of spray-dried fructooligosaccharide powder. Dry Technol. 2014;32:1112–1118. doi: 10.1080/07373937.2014.886257. [DOI] [Google Scholar]
- Antelo FS, Costa JA, Kalil SJ. Thermal degradation kinetics of the phycocyanin from Spirulina platensis. Biochem Eng J. 2008;41:43–47. doi: 10.1016/j.bej.2008.03.012. [DOI] [Google Scholar]
- Bennett B. Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol. 1973;58(2):419–435. doi: 10.1083/jcb.58.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr RL. Evaluating flow properties of solids. Chem Eng. 1965;72:163–168. [Google Scholar]
- Chandralekha A, Tavanandi AH, Amrutha N, Hebbar HU, Raghavarao KSMS, Gadre R. Encapsulation of yeast (Saccharomyces cereviciae) by spray drying for extension of shelf life. Dry Technol. 2016;34:1307–1318. doi: 10.1080/07373937.2015.1112808. [DOI] [Google Scholar]
- Chandralekha A, Rani A, Tavanandi HA, Amrutha N, Hebbar U, Raghavarao KSMS. Role of carrier material in encapsulation of yeast (Saccharomyces cerevisiae) by spray drying. Dry Technol. 2017;35:1029–1042. doi: 10.1080/07373937.2016.1230626. [DOI] [Google Scholar]
- Desobry SA, Netto FM, Labuza TP. Comparison of spray-drying, drum-drying and freeze-drying for β-carotene encapsulation and preservation. J Food Sci. 1997;62:1158–1162. doi: 10.1111/j.1365-2621.1997.tb12235.x. [DOI] [Google Scholar]
- De Jesus SS, Maciel Filho R. Drying of α-amylase by spray drying and freeze-drying-a comparative study. Braz J Chem Eng. 2014;31:625–631. doi: 10.1590/0104-6632.20140313s00002642. [DOI] [Google Scholar]
- Dewi EN, Purnamayati L, Kurniasih RA. Antioxidant activities of phycocyanin microcapsules using maltodextrin and carrageenan as coating materials. J Teknol. 2016;78:4–2. [Google Scholar]
- Dewi EN, Kurniasih RA, Purnamayanti L. Physical Properties of Spirulina Phycocyanin Microencapsulated with Maltodextrin and Carrageenan. Philipp J Sci. 2017;147:201–207. [Google Scholar]
- Dewi EN, Purnamayati L, Kurniasih RA (eds) (2017b) Physical characteristics of phycocyanin from spirulina microcapsules using different coating materials with freeze drying method. IOP Conference Series: Earth and Environmental Science. IOP Publishing.
- Dewi EN, Purnamayati L, Kurniasih RA, Sabdono A. (2019) Microencapsulation of phycocyanin as influenced by different wall materials and drying methods. CJFS.
- Ge B, Qin S, Han L, Lin F, Ren Y. Antioxidant properties of recombinant allophycocyanin expressed in Escherichia coli. J Photochem Photobiol B. 2006;84:175–180. doi: 10.1016/j.jphotobiol.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Hausner HH. Friction conditions in a mass of metal powder. Int J Powder Metall. 1967;3:7–13. [Google Scholar]
- Hari S, Reginold J, Sivaraman K. Production and characterization of sugarcane juice powder. J Sugarcane Res. 2013;3:20–34. [Google Scholar]
- Kurniasih RA, Purnamayati L, Amalia U, Dewi EN. Formulation and characterization of phycocyanin microcapsules within maltodextrin-alginat. agriTECH. 2019;38:23–29. doi: 10.22146/agritech.16752. [DOI] [Google Scholar]
- Pradeep HN, Nayak CA. Enhanced stability of C-phycocyanin colorant by extrusion encapsulation. J Food Sci Technol. 2019;56:4526–4534. doi: 10.1007/s13197-019-03955-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Córdoba A, Gallo L, Bucalá V, Martino M, Navarro A. Co-crystallization of zinc sulfate with sucrose: a promissory strategy to render zinc solid dosage forms more palatable. J Food Eng. 2016;170:100–107. doi: 10.1016/j.jfoodeng.2015.09.024. [DOI] [Google Scholar]
- Mastiani M, Firoozi N, Petrozzi N, Seo S, Kim M. Polymer-salt aqueous two-phase system (AtpS) micro-droplets for cell encapsulation. Sci Rep. 2019;9:1–9. doi: 10.1038/s41598-019-51958-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkova KM, Tchernov AA, Tchorbadjieva MI, Fournadjieva ST, Antova RE, Busheva MC. Purification of C-phycocyanin from Spirulina (Arthrospira) fusiformis. J Biotechnol. 2003;102:55–59. doi: 10.1016/S0168-1656(03)00004-X. [DOI] [PubMed] [Google Scholar]
- Narayan AV, Raghavarao KSMS (2007) Extraction and purification of Cphycocyanin from Spirulina platensis employing aqueous two phase systems. Int J Food Eng, pp 1556–3758
- Narayan AV, Madhusudhan MC, Raghavarao KSMS. Demixing kinetics of phase systems employed for liquid–liquid extraction and correlation with system properties. Food Bioprod Process. 2011;89:251–256. doi: 10.1016/j.fbp.2010.11.014. [DOI] [Google Scholar]
- Patil G, Raghavarao KSMS. Aqueous two phase extraction for purification of C-phycocyanin. Biochem Eng J. 2007;34:156–164. doi: 10.1016/j.bej.2006.11.026. [DOI] [Google Scholar]
- Purnamayati L, Dewi E, Kurniasih R (2018) Phycocyanin stability in microcapsules processed by spray drying method using different inlet temperature. IOP Conference Series: Earth and Environmental Science, IOP Publishing.
- Reddy MC, Subhashini J, Mahipal SVK, Bhat VB, Reddy PS, Kiranmai G, Madyastha KM, Reddanna P. C-Phycocyanin, a selective cyclooxygenase-2 inhibitor, induces apoptosis in lipopolysaccharide-stimulated RAW 264.7 macrophages. Biochem Biophys Res Commun. 2003;304:385–392. doi: 10.1016/S0006-291X(03)00586-2. [DOI] [PubMed] [Google Scholar]
- Romay CH, Gonzalez R, Ledon N, Remirez D, Rimbau V. C-phycocyanin: a biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr Protein Pept Sci. 2003;4:207–216. doi: 10.2174/1389203033487216. [DOI] [PubMed] [Google Scholar]
- Rito-Palomares M, Nunez L, Amador DJ. Practical application of aqueous two-phase systems for the development of a prototype process for C-phycocyanin recovery from Spirulina maxima. J Chem Technol Biotechnol. 2001;76:1273–1280. doi: 10.1002/jctb.507. [DOI] [Google Scholar]
- Sarada R, Pillai MG, Ravishankar G. Phycocyanin from Spirulina sp: influence of processing of biomass on phycocyanin yield, analysis of efficacy of extraction methods and stability studies on phycocyanin. Process Biochem. 1999;34:795–801. doi: 10.1016/S0032-9592(98)00153-8. [DOI] [Google Scholar]
- Sekar S, Chandramohan M. Phycobiliproteins as a commodity: trends in applied research, patents and commercialization. J Appl Phycol. 2008;20:113–136. doi: 10.1007/s10811-007-9188-1. [DOI] [Google Scholar]
- Srinivas N. (2000) Aqueous Two-phase extraction for the downstream processing of enzymes. University of Mysore
- Tandeaude Marsac N, Houmard J. Complementary chromatic adaptation: physiological conditions and action spectra. Methods Enzymol. 1988;167:318–328. doi: 10.1016/0076-6879(88)67037-6. [DOI] [Google Scholar]
- Tavanandi HA, Mittal R, Chandrasekhar J, Raghavarao KSMS. Simple and efficient method for extraction of C-Phycocyanin from dry biomass of Arthrospira platensis. Algal Res. 2018;31:239–251. doi: 10.1016/j.algal.2018.02.008. [DOI] [Google Scholar]
- Wang YC, Yu RC, Chou CC. Viability of bifidobacterial isolates from infant stool active lactic acid bacteria and bifidobacteria in fermented soymilk after drying, subsequent rehydration and storage. Int J Food Microbiol. 2004;93:209–217. doi: 10.1016/j.ijfoodmicro.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Zaslavsky BY. Aqueous two-phase partitioning: physical chemistry and bioanalytical applications. New York: Marcel Dekkar Inc; 1995. [Google Scholar]