Abstract
The encapsulation of ascorbic acid within chitosan nanoparticles (CHNs), embedded in a fibrous structure of a dexamethasone (Dex)-loaded PCL scaffold, provides a new plan for osteogenic differentiation of mesenchymal stem cells. This electrospun PCL fibrous scaffold can release Dex, as bone differentiation initiator, and ascorbic acid, as bone differentiation enhancer, in an approximately sustained release pattern for about 2 weeks. Ascorbic acid-loaded CHNs were prepared by electrospraying a mixture of chitosan and ascorbic acid, and Dex-containing PCL fibers were prepared by electrospinning a mixture of PCL and Dex. The final PCL/chitosan bilayer scaffolds were obtained by the sequential employment of electrospinning and electrospraying methods. Scanning electron microscopy (SEM) and Fourier transform infrared spectroscopy (FTIR) confirmed that the CHNs were successfully incorporated into the fibrous PCL matrix. The improved proliferation of hMSCs cultured on the PCL/chitosan scaffolds was also verified. Osteogenic assays showed an increase in alkaline phosphatase activity and mineral deposits. The expression of bone-specific genes also confirmed the osteogenic differentiation of cells cultured on these PCL/chitosan bilayer scaffolds. Dual-drug-loaded PCL/chitosan scaffold enhanced the osteoblast differentiation of hMSC cells and can be served as a potential scaffold for bone tissue engineering.
Keyword: PCL scaffolds, Chitosan nanoparticles, Electrospray, Electrospinning
Introduction
Tiny bone defects are usually self-healed; whereas some serious bone defects resulting from tumor removal, skeletal injuries or complete hinge substitution need further therapeutic techniques. Bone tissue engineering, as a novel therapeutic method, attempts to eliminate the limitations of traditional techniques such as autograft and allograft in the treatment of serious bone defects. A common approach in bone tissue engineering is in vitro differentiation of stem cells in the presence of some differentiation factors such as ascorbic acid (Cuaranta-Monroy et al. 2014) and dexamethasone (Dex) (Li et al. 2014) for several weeks.
Ascorbic acid plays an important role as an auxiliary factor in the hydroxylation of proline and lysine residues in collagen (Langenbach and Handschel et al. 2013; Li and Wu 2018). Investigations show that the presence of ascorbic acid increases the expression of genes associated with the mitosis while the absence of ascorbic acid decreases alkaline phosphatase expression and inhibits calcium accumulation (Vater et al. 2011). Usually, 50–500 micro-molar ascorbic acid solutions are used in bone phenotype induction for mesenchymal stem cells (MSCs) (Langenbach and Handschel et al. 2013). Dex, as a synthetic glucocorticoid, plays a principal role in bone differentiation. If Dex is completely eliminated from the human MSCs culture, differentiation does not occur. Hamidouche et al. stated that Dex induces bone differentiation of MSCs by increasing the transcription of FHL2, a member of the LIM protein superfamily, the expression of which induces bone phenotype acquisition (Hamidouche et al. 2008).
One of the principal parts of bone tissue engineering is biodegradable scaffolds. These scaffolds are porous beds with a structure like that of an extracellular matrix (ECM), directing the cell growth towards the formation of the desired tissue (Wismer et al. 2014; Hoshiba and Gong et al. 2018). In the conventional approaches of tissue engineering, separated and proliferated cells are seeded on scaffolds, and prior to implantation in the patient body, they undergo their differentiation period in the cell culture medium containing stimulant molecules. This differentiation period is about 21 days in the case of bones. Employing such conventional approaches is not appropriate, especially when the importance of therapeutic duration becomes more pronounced. If it is possible to eliminate the in vitro cell culture stage from the development steps of an engineered tissue, the therapeutic duration is minimized. However, what makes the removal of such a stage difficult is the lack of a sufficient amount of differentiation factors in the human body. One of the proposed solutions to overcome these problems is the direct injection of these molecules. Although the direct injection can provide the required molecules for the differentiation step, fast diffusion and subsequent migration from the damage location require a continuous injection of an enormous amount of these factors. To overcome this issue, a novel approach entitled “releasing scaffolds” was introduced in tissue engineering (Madry et al. 2013). These scaffolds accelerate the in vivo tissue formation process and decrease the amount of drugs and/or stimulant differentiation factors by their local release in comparison with direct injection. Drugs and biomolecules could be directly released from the scaffolds and/or micro/nanoparticles incorporated into the scaffolds. Micro/nanoparticles are used to transfer drugs and proteins for a long time. Scaffolds containing stabilized micro/nanoparticles inhibit/decrease the drug burst release. Moreover, different biological factors could be released in a controlled manner while the release kinetics of each factor can be controlled separately via the individual formulation of the micro/nanoparticle. Zahiri et al. (2020) fabricated curcumin (Cur) loaded chitosan nanoparticle (CNs) in hybrid PCL/gelatin fibrous mesh with endometrium stem cells (EnSCs) to evaluate the in vivo wound healing ability of the fabricated scaffolds. The electrospun hybrid scaffold seeded with EnSCs showed desirable biocompatibility with the host immune system and wound healing ability in a full-thickness excisional animal model. Compared with the CNs/Cur carrier, the release rate of pure Cur from the fiber mat was very fast, confirming the ability of controlled drug release by the CNs carrier Zahiri et al. (2020).
Omidvar et al. (2016) studied the sustained release of Dex from the PCL nanofiber scaffold for bone differentiation of MSCs. The entrapment of Dex in chitosan microparticles and its incorporation in PCL fibers lead to the sustained release of Dex over a period of 14 days. The presence of this drug in cell culture results in bone differentiation induction and cell proliferation enhancement (Costa et al. 2015). Rasti Boroojeni et al. (2019) studied dexamethasone sodium phosphate (DEXP)-loaded chitosan nanoparticles embedded in poly-ε-caprolacton (PCL) and gelatin electrospun nanofiber scaffold for the treatment of the nervous system. The results showed that nanoparticles embedded in the nanofiber scaffold provided a more controlled release pattern of the loaded drug. Such scaffolds with the capability of sustained release of growth and differentiation factors can be the right candidates for therapeutic purposes through tissue engineering. More control on the growth, proliferation, and differentiation of cells could be achieved by a scaffold with the possibility of releasing two different drugs with two distinct release profiles. Li et al. (2015) utilized both Dex and bone morphogenetic protein-2 (BMP-2) in an electrospun nanofiber scaffold simultaneously for the bone differentiation of MSCs and the resolution of mouse skull major bone defects. They first loaded BMP-2 into bovine serum albumin (BSA) nanoparticles and then being loaded into the electrospun nanofibers to both maintaining the bioactivity of BMP-2 and having a more sustained drug release. Dex was directly loaded into the nanofibers to have a faster release profile associated with burst release.
Bilayer electrospinning is a novel approach that has been recently employed in the fabrication of tissue engineering scaffolds (Rajzer et al. 2014). Utilization of the preferred characteristics of each layer and the possibility of separate loading of drugs and biomolecules in every layer are the main advantages of these scaffolds. Vakilian et al. (2015) prepared three-layered PCL containing BSA-loaded chitosan nanoparticles and poly-L lactic acid (PLLA) hybrid nanofiber (PCL/NP-PLLA) mesh as the scaffold. According to the BSA release profile, the multi-layered structure of nanofibers with two barrier layers provided a programmable release pattern of the loaded protein.
In this study, PCL electrospun bilayer scaffolds containing chitosan nanoparticles (CHNs) were fabricated for the first time for the controlled delivery of Dex and ascorbic acid. Dex initiated bone differentiation of bone marrow MSCs and ascorbic acid contributed to the completion of this process. Dex was directly loaded into the PCL fibers through electrospinning, while ascorbic acid was loaded into CHNs through electrospraying and then dispersed within the PCL nanofibers. Sequential employment of electrospinning and electrospraying methods to formulate the CHN-embedded PCL bilayer scaffolds can be nominated as another unique feature of this study. The properties of the PCL/chitosan bilayer scaffolds were characterized by SEM and tensile strength analysis. The ability of these new scaffolds to control human-MSC (hMSCs) adhesion, proliferation, and bone differentiation was also investigated.
Materials and methods
Materials
Chitosan with average molecular weight and PCL (70–90 kDa) were purchased from Sigma Aldrich Company (USA). Chloroform, dimethylformamide (DMF), and acetic acid were purchased from Merck Company (Germany). Dexamethasone (Dex) and ascorbic acid (AA) were prepared by Temad Company (Iran).
Preparation of CHNs
The electrospraying method was used for the preparation of CHNs due to its good characteristics like being single-stage, being economic, and having high efficiency of drug loading. Similar to electrospinning, in the electrospraying technique, electrostatic forces were employed for the fabrication of particles; however, the difference is that the lower concentration of polymeric solution led to rupturing of the charged jet into the charged particles. Therefore, the nano/microparticles were collected at the collector surface. To prepare CHNs, 1% (w/v) chitosan solution was prepared by addition of 0.1 g chitosan to the mixture of 9-mL distilled water and 1-mL pure acetic acid. The obtained solution was stirred on a magnetic stirrer at the rate of 500 rpm for 24 h. To prepare ascorbic acid-loaded CHNs (AA-CHNs), 0.05 g ascorbic acid was added to the above-mentioned solution and stirring process was continued for a further 30 min. The injection into the electrospinning device (Fanavarn Nanomeghyas ES1000-Iran) was carried out with the feeding rate of 1.2 mL/h towards the rotating drum (200 rpm), which was placed in a constant distance of 6 cm from the needle tip (the internal diameter of the utilized needle was 0.8 mm). The voltage difference between the needle tip and drum was held at 13 kV.
To investigate the morphology of the collected particles on the aluminum foil, circles with 1.5 cm diameter of aluminum foil were separated and after gold coating, were evaluated using scanning electron microscopy (SEM, Phillips XL30—USA) under 30 kV voltage. The SEM micrographs were analyzed to measure the size of the particles using the Image J-software (National Institute of Health).
Preparation of PCL nanofibers
To prepare the electrospinning solution, 0.96 g PCL was completely dissolved in an 8-mL solvent (a mixture of chloroform and DMF, with the ratio of 3–1) by 2-h stirring with 300 rpm on a magnetic stirrer. For preparing Dex-containing PCL (PCL-Dex) fibers, 0.04 g Dex was added to the PCL solution and the stirring process was continued for 30 min. The polymeric solutions were poured in 12-mL SUPA syringe (with 0.8-mm needle). The electrospinning process, based on our pre-experiments (Omidvar et al. 2016), was performed at a distance of 17 cm from the tip to collector and a feeding rate of 1 mL/h through a 21G stainless steel needle at 350 rpm drum rotating speed. To determine the optimum voltage for conducting uniform and rupture-free fibers, voltages of 15, 17, 19, and 21 kV were selected for optimization of the Dex-free fibers while voltages of 15, 16, and 17 kV were selected for optimization of the PCL-Dex fibers. Lower voltages were employed for the PCL-Dex fibers considering the higher electrical conductivity of the PCL solution in the presence of Dex. The morphology of the electrospun nanofibers was investigated using a scanning electron microscope (SEM, Phillips XL30—USA). The average diameter in each case was calculated and reported by Image-J software from at least 50 randomly selected fibers. The optimum voltage values were identified through qualitative investigation of SEM micrographs.
Preparation and characterization of PCL/chitosan scaffolds
Firstly, 2.4 mL of the Dex-containing PCL solution was electrospun to form the first PCL-Dex layer on the rotating drum at the optimum condition, i.e., 16 kV voltage. Then, 6 mL of the ascorbic acid-containing chitosan solution was electrosprayed at the optimum condition to form and distribute AA-CHNs on the first PCL-Dex layer. Finally, the second PCL-Dex layer was electrospun from 2.4 mL of the Dex-containing PCL solution, exactly the same as the first layer. To investigate the effect of the presence of CHNs between the PCL fibers four scaffolds with different structure were prepared as described in Table 1.
Table 1.
Composition of the scaffolds
| Scaffold no | Scaffold’s layer arrangement | No. of scaffold layers | Scaffold description |
|---|---|---|---|
| 1 | PCL | 1 | One PCL layer |
| 2 | PCL-Dex/CHNs/PCL-Dex | 2 | CHNs embedded between tow Dex-loaded PCL layers |
| 3 | PCL/AA-CHNs/PCL | 2 | Ascorbic-loaded CHNs embedded between two PCL layers |
| 4 | PCL-Dex/AA-CHNs/PCL-Dex | 2 | Ascorbic-loaded CHNs embedded between two Dex-loaded PCL layers |
To illustrate the presence and distribution of CHNs on and among the PCL fibers, SEM analysis (SEM, Phillips XL30—USA, at a working distance of 8.5–10.5 mm and voltage of 25.0 kV) and FTIR spectra (Perkin Elmer Spectrometer Frontier-America, with KBr pellets in the 400–4000 cm−1 range, with a resolution of 1 cm−1) were recorded.
In vitro release studies
Prepared scaffolds were weighed and soaked in 5 mL of phosphate buffer saline (PBS) in triplicate. The samples were incubated at 37 °C shaking at 80 rpm for 2 weeks. At the predetermined times, 1 mL of the released solution was collected and replaced with fresh PBS. The cumulative amounts of Dex and AA released from fibers were determined by UV analysis at 235 and 274 nm, respectively (Spectrophotometer UV-160A, Shimadzu, Japan). All measurements were performed triplicate; data are reported as means ± SD.
Cell studies
Cell proliferation and culture on scaffolds
Human bone marrow stem cells were separated from the patients’ bone marrows, who volunteered for stem cell transplantation according to the Ethics Committee of the Royan Institute (Tehran, Iran). Cells from the third to fourth passage were used for further examination. The hMSCs were seeded on the prepared scaffold and punched as discs of 1.5 cm diameter at a number of 2 × 105 cell/scaffold.
Proliferation and viability assessment of hMSCs
The viability of cells cultured on the scaffolds and their proliferation was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay after 1, 7, and 14 days. The MTT method is principally based on the ability of living cells to the reduction of tetrazolium compound into a purple formazan solution. For this purpose, the culture medium on the fibrous scaffolds was first removed and then replaced by a 500-µl fresh culture medium. Thereafter, a 100-µl MTT solution was added to every well. The samples were held in an incubator for 2 h (at the temperature of 37 °C and air atmosphere with 5% CO2). Subsequently, the upper medium was discharged and the produced formazan crystals were dissolved by dimethyl sulfoxide (DMSO). Then, the optical absorption of the obtained purple solution was measured by spectrophotometry (λ = 570 nm).
Morphology and distribution of cells on scaffolds
SEM analysis was carried out to investigate the morphology and distribution of the cultured hMSCs on the scaffolds. At the 7th day of cell culture, the samples were fixed in 2.5% glutaraldehyde solution in refrigerator (4 °C). Then, the samples were dehydrated with increasing the ethanol concentration (30, 50, 70, 80, 90, and 100%). Thereafter, the samples were coated with nanometric layer of gold and then evaluated by SEM.
Investigation of alkaline phosphates activity of cells
For investigation of alkaline phosphatase activity of the cultured hMSCs in the days 7 and 14, the cells on the constructs were rinsed with PBS solution and sonicated within 250-µl assay buffer. The substrate solution (0.2 mL of p-nitrophenyl phosphate (pNPP), Biovision) was mixed with 0.2 mL of the cell lysate. After preserving for 1 h at room temperature, the spectrophotometry analysis was carried out at 405-nm wavelength to measure the absorption amount of the samples. In this method, alkaline phosphatase enzyme catalyzes the transformation of color-less para-nitrophenyl phosphate into yellowish para nitrophenyl; hence, by measuring the adsorption of the formed nitrophenyl, the alkaline phosphatase activity value can be identified. Alkaline phosphatase activity was normalized based on the total proteins existing in the samples. The total proteins in the samples were also identified by the BCA method.
Real-time (RT-PCR) for gene expression analysis
Osteogenic differentiation was measured by the expression of bone-related genes such as osteopontin (OPN), collagen type I (COL I) and RUNX2 in the day 14. In brief, total RNA was isolated with the RNeasy MicroKit (Qiagen). The reverse-transcription reaction was performed with 2 mg total RNA using random hexamer as a primer and RevertAid M-MuLV Reverse Transcriptase kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. Moreover, cDNA was amplified by specific COLI (forward: 5′ ATGCCTGGTGAACGTGGT3′, reverse: 5′ AGGAGAGCCATCAGCACCT3′), OPN (forward:5′ GCCGAGGTGATAGTGTGGTT3′, reverse: 5′ TGAGGTGATGTCCTCGTCTG3′), RUNX2 (forward: 5′ ATGACACTGCCACCTCTGA3′, reverse: 5′ ATGAAATGCTTGGGAACTGC 3′) and GAPDH (forward: 5′CTCATTTCCTGGTATGACAACGA3′, reverse: 5′ CTTCCTCTTGTGCTCTTGCT3′) primers and Power SYBRVR Green PCR Master Mix in ABI (Applied Biosystems). Gene expression levels were determined by the 2−(ΔΔCT) method with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as reference.
Statistical analysis
Experimental data are represented as mean standard deviation) mean ± SD). The statistical differences among each set were investigated by t test analysis and statistical significance was presented as P < 0.05. Each experiment was repeated three times.
Results and discussion
Characterization of CHNs
CHNs with and without ascorbic acid were prepared using the electrospray method. Based on Fig. 1, both CHNs and AA-CHNs were obtained successfully with an almost spherical shape. Interestingly, they were obtained in two different sizes. The greater particles that were less in number are called “primary droplets” and the finer particles that were numerous in number are known as “satellite droplets” (Hartman et al. 2000). Both the primary and satellite particles represented a uniform distribution. The reason for the formation of two types of particles with two distinct sizes in the electrospraying method was well described by Hong et al. (2008) and Hartman et al. (1997). They showed that after rupturing of the stable cone jet, due to reciprocal electrical interactions between charged particles and electrostatic inertia effects, the fast separation of primary and satellite droplets will occur. Hence, after the formation of the particles, two distinguished spraying regions were observed: the internal core of the cone jet that was the precursor of the primary microparticles and the external parts of the cone that was the precursor of the satellite and finer microparticles. For CHNs, the primary microparticles, which formed about 15% of all the microparticles, had an average diameter of 1.12 ± 0.09 µm, whereas the satellite microparticles, which formed about 85% of all the microparticles, had an average diameter of 285 ± 7 nm (Table 2). For AA-CHNs, the primary microparticles, which constituted about 20% of all the droplets, had an average diameter of 1.20 ± 0.1 µm, while the satellite microparticles, which constituted about 80% of all the microparticles, had an average diameter of 273 ± 5 nm (Table 2). All the subset groups had distorted, rough, and nearly spherical surfaces.
Fig. 1.
SEM micrographs of a CHNs and b AA-CHNs, (magnification × 1000, scale bar 20 μm)
Table 2.
Characteristics of chitosan nanoparticles
| Sample | Starting Solution | Obtained nanoparticles | |||||
|---|---|---|---|---|---|---|---|
| Chitosan solution concentration (mg/ml) | Ascorbic acid solution Concentration (mg/ml) | Satellite droplets | Primary droplets | Geometry | |||
| Number of particles (%) | Mean particles diameter (nm) | Number of particles (%) | Mean particles diameter (μm) | ||||
| CHNs | 100 | – | 85 | 285 ± 7 | 15 | 1.12 ± 0.09 | Nearly spherical |
| AA-CHNs | 100 | 50 | 80 | 273 ± 5 | 20 | 1.20 ± 0.1 | Nearly spherical |
Characterization of PCL fibers
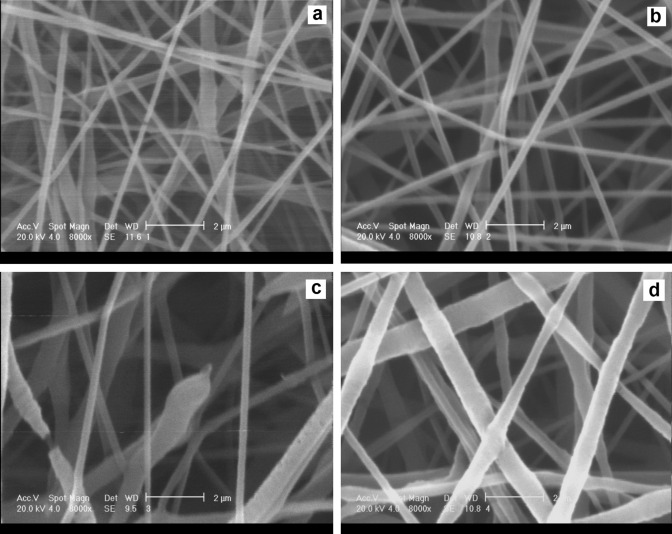
The effect of the applied voltages on the diameter of the resulting PCL fibers is presented in Fig. 2. The process was optimized towards smaller and more homogeneous fiber diameters. As the voltage increased from 15 to 21 kV, the diameter of the electrospun PCL fibers increased (Fig. 2). This is due to the increased electrostatic force, driving the solution from the needle tip towards the rotating drum; increasing this force tended to increase the mass of the solution discharged from the needle tip (Wutticharoenmongkol et al. 2006). Figure 2a shows that the PCL fibers obtained at conditions of 15 kV were not sufficiently uniform and had some ruptures and fractures. The PCL fibers obtained at 17 kV had a uniform distribution of diameter, and no fracturing or rupturing was observed in their structure (Fig. 2b). When the voltage increased to 19 kV, it was found that the PCL fibers were strongly non-uniform and some of them were ruptured (Fig. 2c). This discontinuity may be due to the instability of the established polymeric cone at the needle tip at the voltages higher than the optimum value (Sill and Von Recum et al. 2008). At the voltage of 21 kV, the non-uniform distribution of large fibers was clearly observed (Fig. 2d).
Fig. 2.
SEM micrographs for PCL nanofibers electrospun at the voltage of a 15 kV, b 17 kV, c 19 kV, and d 21 kV, (magnification × 8000, scale bar 2 μm)
Therefore, the voltage of 17 kV was selected as the optimum voltage for electrospinning of the drug-free PCL scaffolds. The average diameter of the PCL fibers in Fig. 2b was measured as about 320 ± 4 nm.
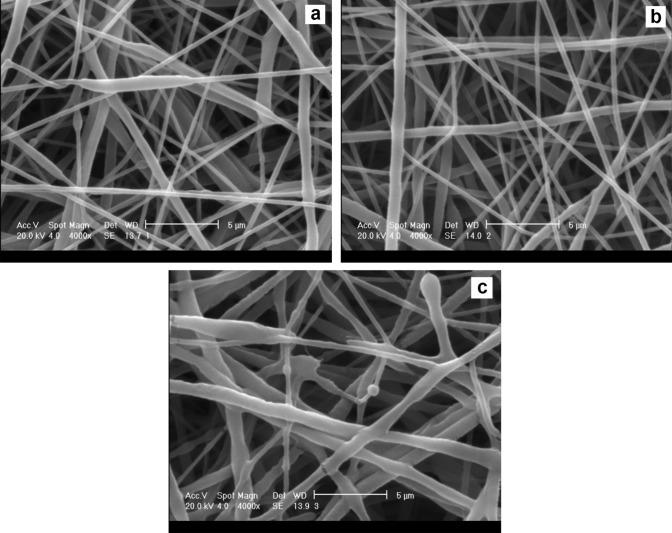
The effect of the voltages applied to the diameter of the resulting PCL-Dex fibers is represented in Fig. 3. The process was again optimized towards smaller and more homogeneous fiber diameters. Figure 3 shows that the diameter of the electrospun PCL-Dex fibers increased as the voltage increased from 15 to 17 kV. At the voltage of 15 kV, a lack of sufficient electrical energy for the synthesis of a stable fiber led to developing a non-uniform and shapeless PCL-Dex fiber (Fig. 3a). The PCL-Dex fibers obtained at 16 kV had harmony in their shape with a uniform distribution of diameter, and no fracturing or rupturing was observed in their structure (Fig. 3b). At the voltage of 17 kV, in addition to the instability of the polymeric cone and rupturing of the fibers, the non-uniform distribution of the PCL-Dex fibers was also observed (Fig. 3c). Therefore, the voltage of 16 kV was presented as the optimum voltage to electrospin the PCL-Dex fibers. In this voltage, the average diameter of the fibers was measured as 370 ± 6 nm (Fig. 3b).
Fig. 3.
SEM micrographs for PCL-Dex nanofibers electrospun at the voltage of a 15 kV, b 16 kV, and c 17 kV, (magnification × 4000, scale bar 5 μm)
Characterization of PCL/chitosan scaffolds
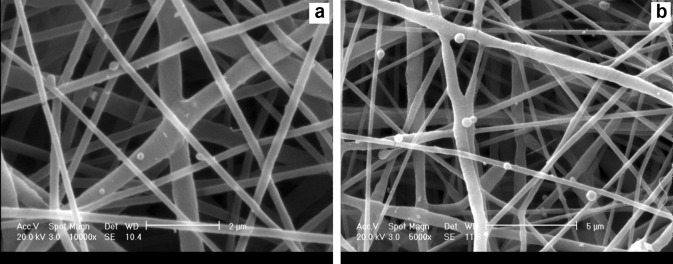
The microstructure of the PCL/chitosan bilayer scaffolds was observed by SEM, illustrating the random distribution of AA-CHNs among the two PCL-Dex electrospun fibrous layers (Fig. 4). Evidently, the CHNs were well distributed and adhered to the PCL-Dex nanofibers, without any change in their spherical structure or any increase in the PCL nanofiber diameters. The electrospinning of the second layer of the PCL-Dex nanofibers enhanced the adhesion and entrapment of these nanoparticles. Employing this approach may also reduce the probability of separation, segregation, and departure of drug-containing CHNs from these constructs during cell culture and release periods.
Fig. 4.
SEM micrographs showing the distribution of AA-CHNs on electrospun PCL-Dex nanofibers, (magnification × 5000, scale bar 5 μm)
FTIR spectra recorded for CHNs, AA-CHNs, PCL, PCL-Dex and a bilayer PCL/chitosan scaffold are presented in Fig. 5. FTIR spectra of the PCL/chitosan scaffold showed the presence of individual polymers in the bilayer scaffold after electrospinning. The bands appearing at 1660 cm−1 and 1190 cm−1 of the PCL/chitosan scaffold spectra could be assigned to the C = O stretching of amide I and asymmetric C–O–C stretching of chitosan, respectively. In addition, the 2915 and 2967 cm−1 adsorption bands in the PCL/chitosan spectra were intensified in comparison with the PCL spectra. This intensification may be due to the addition of 2924 and 2947 cm−1 adsorption bands of chitosan. The results of FTIR studies confirmed that CHNs were successfully incorporated into the fibrous PCL matrix.
Fig. 5.
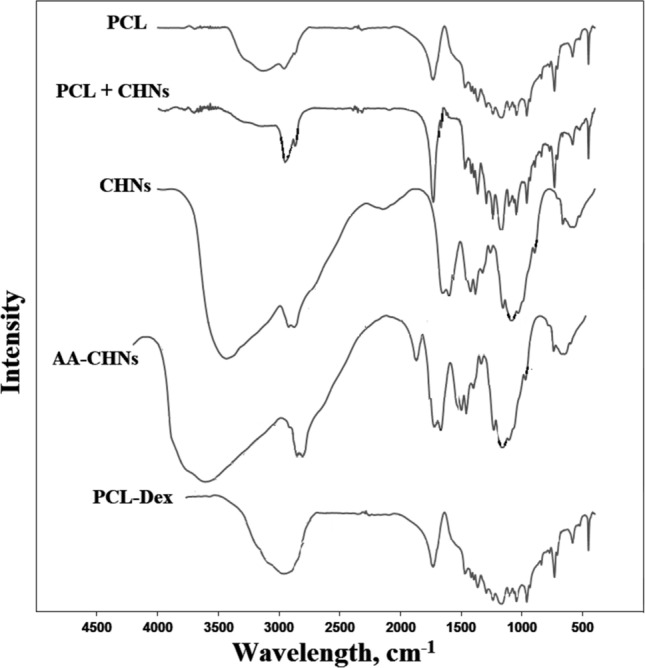
FTIR spectra for PCL, CHNs, PCL + CHNs, AA/CHNs and PCL-Dex scaffolds
Upon comparing the CHNs and AA-CHNs FTIR, the formation of AA-CHNs can be proven by the appearance of absorption bands of C = O of AA at 1761 cm−1. In addition, a higher reading of OH in 3520 cm−1 in AA-CHNs FTIR can be attributed to extra OH groups of AA.
The PCL-DEX FTIR spectrum had no important fluctuations compared to the PCL FTIR due to low dexamethasone content (4.1% by wt). Higher reading of OH in 3225 cm−1 in PCL-Dex FTIR can be attributed to extra OH groups of Dex.
In vitro release study
The release profile of Dex from PCL scaffolds was followed for 20 days and the results are illustrated in Fig. 6. The release profile of Dex exhibited a burst of about 30% in 12 h, then maintained a slow-release rate (57% up to day 13), and finally reached a saturated point from day 13. The burst release of Dex from PCL-Dex fibers can be attributed to the distribution pattern of Dex within the fibers. There is a short diffusional path for those amounts of loaded Dex located on the surface, causing a burst release of the drug within a short period. On the other hand, the high solubility of the drug in the release media (about 25 mg/mL) could be the logical stimulator for this fast release.
Fig. 6.
In vitro cumulative release pattern of Dex and AA (n = 3)
As it can be seen, Dex release was slowly continued for 13 days and finally stopped after day 13. Since PCL is a semi-crystalline polymer, this phenomenon can be attributed to the entrapment of Dex within crystalline regions. Part of the loaded Dex located on the surface or in the amorphous regions of the polymeric fibers can easily penetrate the medium within the pores. However, the crystalline regions prevent the absorption of water into the fibers, and the remaining drug becomes entrapped within the fibers. Thus, the residual drugs are trapped in the polymer and can only be released after the degradation of the entire structure.
The release profile of AA from CHN nanoparticles was also followed for 20 days and the results are displayed in Fig. 6. The release profile of AA exhibited a small burst of about 25% on the first day and then maintained a slow-release rate (from day 1 to day 13) and reached a saturated point on day 13. In the case of release from the surface of nanoparticles, surface-absorbed AA immediately dissolves when it comes into contact with the release medium, which leads to a burst of AA.
Cell studies of PCL/chitosan scaffolds
To investigate the interaction between hMSCs and the prepared scaffolds, MTT analysis was performed for the cultured cell on all nanofibrous scaffolds in 7 and 14 days. The results of their proliferative activity are presented in Fig. 7. Cell activities and the number of living cells in all the four groups of scaffolds increased 7 and 14 days after culturing. This means that none of the scaffolds had toxic effects, and they even facilitated the growth and activity of the cells.
Fig. 7.
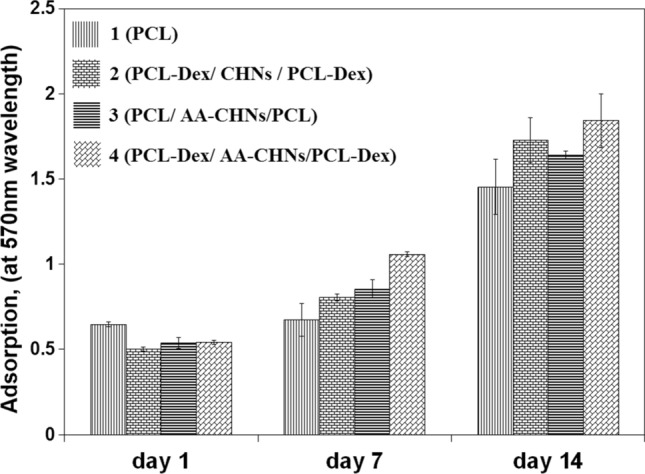
MTT analysis results of nanofibrous scaffolds at 1st, 7th, and 14th days after cell culture
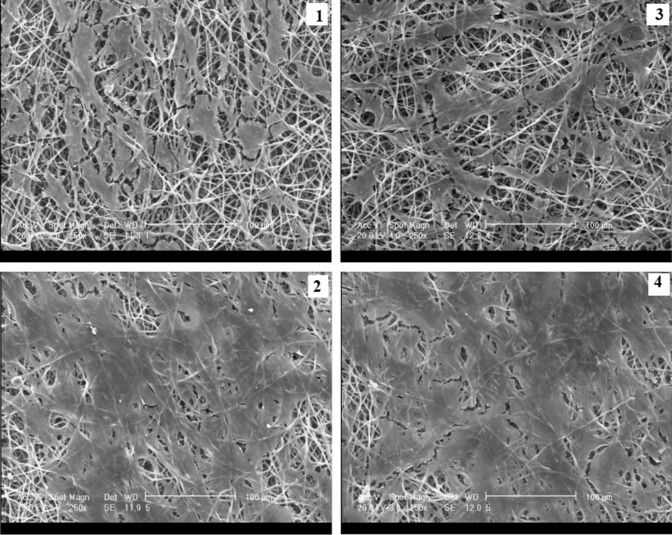
The morphology and distribution of the cultured hMSCs on all the nanofibrous scaffolds were studied on day 7 by the use of SEM micrographs (Fig. 8). Based on the micrographs, the adhesion and distribution of the cells were greater on the scaffolds containing CHNs (i.e., scaffolds No. 2, 3 and 4) than on the scaffolds without CHNs (i.e., scaffold No. 1). This may be attributed to the increase of hydrophilicity of the scaffolds, and therefore, to the easier penetration of water into the PCL/chitosan scaffolds and more adhesion tendency of the cells to the PCL/chitosan scaffolds, as compared to the PCL scaffolds. The presence of hydrophilic groups in the chitosan chain resulted in hydrogen bond formation with water and improved the hydrophilicity of the PCL/chitosan scaffolds. Meanwhile, the PCL scaffolds without CHNs showed a higher hydrophobicity because of the presence of –CH2 groups in the PCL fibers.
Fig. 8.
SEM micrographs of cell distribution at seventh day on the scaffolds: (1) PCL, (2) PCL-Dex/CHNs/PCL-Dex, (3) PCL/AA-CHNs/PCL, and (4) PCL-Dex/AA-CHNs/PCL-Dex, (magnification × 250, Scale bar 100 μm)
The differentiation of hMSCs seeded on all four scaffolds was also investigated by monitoring the ALP activity. The secretion of ALP (Fig. 9) was investigated by the pNPP assay on days 7 and 14. The ALP activity was directly related to the formation of mineralized substances on the scaffold substrate. The highest level of this activity occurred in the bone regeneration process. This object was considered as a suitable criterion for identifying the bone differentiation of MSCs. A comparison of the ALP activity of all the four experimental groups on days 7 and 14 revealed that scaffold No. 1, which did not contain any ascorbic acid and Dex, had the lowest ALP activity. Moreover, no ALP activity increase was observed on day 14 for this scaffold.
Fig. 9.
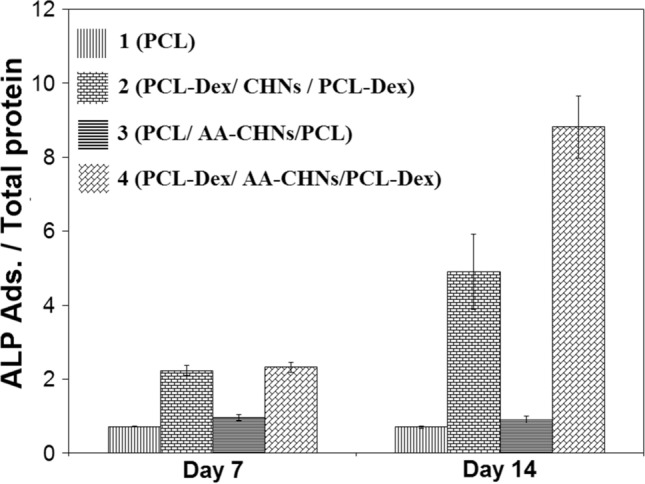
Alkaline phosphatase activity of cells on nanofibrous scaffolds at 7th and 14th days of culturing
It was also observed that scaffold No. 3, which was free of Dex and only contained ascorbic acid, showed no clear and distinguished ALP activity. Ascorbic acid was associated with two other important molecules, i.e., Dex and b-glycerol phosphate, which were added to the bone differentiation culture medium. Regarding the ALP activity of scaffold No. 3, the role of ascorbic acid in the inception and initiation of the bone differentiation process was very weak. Ascorbic acid’s presence could not cause the differentiation of hMSCs into the bone cells, and it seems that the presence of Dex as the initiator of differentiation was vital. Dex, as a synthesized glucocorticoid, plays a principal role in the differentiation of hMSCs into the bone (Coelho et al. 2000; Ghali et al. 2015); if Dex is completely eliminated from the cell culture medium, no differentiation occurs (Herbertson and Aubin et al. 1995; Porter et al. 2003).
It was also observed that the ALP activity of the cultured cells on scaffolds No. 2 and 4 on day 14 had consumable increment in comparison with the day 7. This ALP activity increase, caused by the continuous release and presence of Dex from these scaffolds, can be regarded as the main role of this drug in the differentiation of hMCSCs into bone cells. Considering the higher ALP activity of the cells on scaffold No. 4 containing both Dex and ascorbic acid, it can be deduced that ascorbic acid, in the presence of Dex and after the initiation of the differentiation process by Dex, can also proceed and enhance bone differentiation in such a way that the ALP activity in this group is 80% higher than that in the scaffold No. 2.
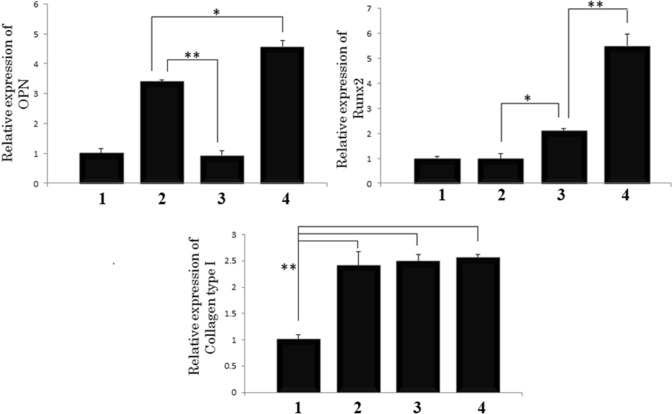
To determine the osteogenic differentiation of MSCs on the scaffolds, the gene expression of RunX2, OPN and COLI was examined on day 14. The relative gene expression was normalized in contrast to the GAPDH as the house-keeping gene and compared to the cells cultured on scaffold No. 1 (the PCL scaffold as the negative control). Figure 10 shows that the expression of OPN and RunX2 in scaffold No. 4 was significantly higher than that in the other groups. In this study, the up-regulation in OPN and RunX2 expression for MSCs cultured on the scaffolds releasing Dex and ascorbic acid demonstrated that the sustained release of Dex and ascorbic acid can facilitate the differentiation of hMSCs into osteoblasts. The results indicated that the expression of COLI was higher in all the groups than in the control group. Ascorbic acid acted as a co-factor for hydroxylation proline and lysine residue in the collagens.
Fig. 10.
Expression of OPN, Runx2 and Collagen type I for scaffolds: (1) PCL, (2) PCL-Dex/CHNs/PCL-Dex, (3) PCL/AA-CHNs/PCL, and (4) PCL-Dex/AA-CHNs/PCL-Dex. The data represent mean standard deviation (n = 3)
Conclusion
CHN-embedded PCL bilayer scaffolds were prepared with the capability of controlled release of Dex and ascorbic acid as two bone induction factors. For this purpose, ascorbic acid-containing CHNs prepared by the electrospraying method were distributed among two Dex-containing PCL electrospun fibrous layers. The introduction of CHNs improved the mechanical properties and bioavailability of the PCL scaffolds. The cell viability experiment confirmed the nontoxicity of the PCL/chitosan scaffolds. The in vitro biological evaluation showed that our PCL/chitosan scaffold enhanced the osteoblast differentiation of hMSC cells, making it a potential scaffold for bone tissue engineering.
Acknowledgements
The authors declare that no funding has been received for the conduct of this study. The authors would like to thank Tarbiat Modares University and Royan Institute for Stem Cell Biology and Technology for their technical maintenance.
Funding
This study was funded by Tarbiat Modares University and Royan Institute for Stem Cell Biology and Technology.
Declarations
Conflict of interest
Ameneh Seddighian declares that she has no conflict of interest. Fariba Ganji declares that she has no conflict of interest. Mohamadreza Baghaban-Eslaminejad declares that she has no conflict of interest. Fatemeh Bagheri declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fariba Ganji, Email: fganji@modares.ac.ir.
Mohamadreza Baghaban-Eslaminejad, Email: eslami@royaninstitute.org.
References
- Allahyarzadeh MH, Aliofkhazraei M, Rezvanian AR, Torabinejad V, Sabour Rouhaghdam AR. Ni-W electrodeposited coatings: characterization, properties and applications. Sur Coat Tech. 2016;307:978–1010. doi: 10.1016/j.surfcoat.2016.09.052. [DOI] [Google Scholar]
- Boroojeni FR, Mashayekhan S, Abbaszadeh HA. The controlled release of dexamethasone sodium phosphate from bioactive electrospun PCL/gelatin nanofiber scaffold. Iran J Pharma Res. 2019;18:111–124. [PMC free article] [PubMed] [Google Scholar]
- Coelho M, Fernandes M. Human bone cell cultures in biocompatibility testing. Part II: effect of ascorbic acid, β-glycerophosphate and dexamethasone on osteoblastic differentiation. Biomaterials. 2000;21:1095–1102. doi: 10.1016/S0142-9612(99)00192-1. [DOI] [PubMed] [Google Scholar]
- Costa PF, Puga AM, Díaz-Gomez L, Concheiro A, Busch DH, Alvarez-Lorenzo C. Additive manufacturing of scaffolds with dexamethasone controlled release for enhanced bone regeneration. Int J Pharm. 2015;496:541–550. doi: 10.1016/j.ijpharm.2015.10.055. [DOI] [PubMed] [Google Scholar]
- Cuaranta-Monroy I, Simandi Z, Kolostyak Z, Doan-Xuan QM, Poliska S, Horvath A, Nagy G, Bacso Z, Nagy L. Highly efficient differentiation of embryonic stem cells into adipocytes by ascorbic acid. Stem Cell Res. 2014;13:88–97. doi: 10.1016/j.scr.2014.04.015. [DOI] [PubMed] [Google Scholar]
- Ghali O, Broux O, Falgayrac G, Haren N, van Leeuwen JP, Penel G, Hardouin P, Chauveau C. Dexamethasone in osteogenic medium strongly induces adipocyte differentiation of mouse bone marrow stromal cells and increases osteoblast differentiation. BMC Cell Biol. 2015;16:9. doi: 10.1186/s12860-015-0056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidouche Z, Haÿ E, Vaudin P, Charbord P, Schüle R, Marie PJ, Fromigué O. FHL2 mediates dexamethasone-induced mesenchymal cell differentiation into osteoblasts by activating Wnt/β-catenin signaling-dependent Runx2 expression. FASEB J. 2008;22:3813–3822. doi: 10.1096/fj.08-106302. [DOI] [PubMed] [Google Scholar]
- Hartman RPA, Borra JP, Brunner DJ, Marijnissen JC, Scarlett B. The evolution of electrohydrodynamic sprays produced in the cone-jet mode, a physical model. J Electrostat. 1997;47:143–170. doi: 10.1016/S0304-3886(99)00034-0. [DOI] [Google Scholar]
- Hartman RPA, Brunner DJ, Camelot DMA, Marijnissen JCM, Scarlett B. Jet break-up in electrohydrodynamic atomization in the cone-jet mode. J Aerosol Sci. 2000;31:65–95. doi: 10.1016/S0021-8502(99)00034-8. [DOI] [Google Scholar]
- Herbertson A, Aubin JE. Dexamethasone alters the subpopulation make-up of rat bone marrow stromal cell cultures. J Bone Miner Res. 1995;10:285–294. doi: 10.1002/jbmr.5650100216. [DOI] [PubMed] [Google Scholar]
- Hong Y, Li Y, Yin Y, Li D, Zou G. Electrohydrodynamic atomization of quasi-monodisperse drug-loaded spherical/wrinkled microparticles. J Aerosol Sci. 2008;39:525–536. doi: 10.1016/j.jaerosci.2008.02.004. [DOI] [Google Scholar]
- Hoshiba T, Gong J. Fabrication of cell-derived decellularized matrices on three-dimensional (3D)-printed biodegradable polymer scaffolds. Microsyst Technol. 2018;24:613–617. doi: 10.1007/s00542-017-3470-1. [DOI] [Google Scholar]
- Langenbach F, Handschel J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res Ther. 2013;4:117. doi: 10.1186/scrt328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Wu G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids. 2018;50:29–38. doi: 10.1007/s00726-017-2490-6. [DOI] [PubMed] [Google Scholar]
- Li T, Li H, Li T, Fan J, Zhao RC, Weng X. MicroRNA expression profile of dexamethasone-induced human bone marrow-derived mesenchymal stem cells during osteogenic differentiation. J Cell Biochem. 2014;115:1683–1691. doi: 10.1002/jcb.24831. [DOI] [PubMed] [Google Scholar]
- Li L, Zhou G, Wang Y, Yang G, Ding S, Zhou S. Controlled dual delivery of BMP-2 and dexamethasone by nanoparticle-embedded electrospun nanofibers for the efficient repair of critical-sized rat calvarial defect. Biomaterials. 2015;37:218–229. doi: 10.1016/j.biomaterials.2014.10.015. [DOI] [PubMed] [Google Scholar]
- Madry H, Rey-Rico A, Venkatesan JK, Johnstone B, Cucchiarini M. Transforming growth factor beta-releasing scaffolds for cartilage tissue engineering. Tissue Eng Part B Rev. 2013;20:106–125. doi: 10.1089/ten.teb.2013.0271. [DOI] [PubMed] [Google Scholar]
- Omidvar N, Ganji F, Eslaminejad MB. In vitro osteogenic induction of human marrow-derived mesenchymal stem cells by PCL fibrous scaffolds containing dexamethazone-loaded chitosan microspheres. J Biomed Mater Res Part A. 2016;104:1657–1667. doi: 10.1002/jbm.a.35695. [DOI] [PubMed] [Google Scholar]
- Porter RM, Huckle WR, Goldstein AS. Effect of dexamethasone withdrawal on osteoblastic differentiation of bone marrow stromal cells. J Cell Biochem. 2003;90:13–22. doi: 10.1002/jcb.10592. [DOI] [PubMed] [Google Scholar]
- Rajzer I, Menaszek E, Kwiatkowski R, Planell JA, Castano O. Electrospun gelatin/poly (ε-caprolactone) fibrous scaffold modified with calcium phosphate for bone tissue engineering. Mater Sci Eng, C. 2014;44:183–190. doi: 10.1016/j.msec.2014.08.017. [DOI] [PubMed] [Google Scholar]
- Sill TJ, Von Recum HA. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials. 2008;29:1989–2006. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Torabinejad V, Aliofkhazraei M, Sabour-Rouhaghdam A, Allahyarzadeh MH, Kasama T, Alimadadi H. Mechanical properties of multilayer Ni-Fe and Ni-Fe-Al2O3 nanocomposite coating. Mat Sci Eng: A. 2017;700:448–456. doi: 10.1016/j.msea.2017.06.009. [DOI] [Google Scholar]
- Vakilian S, Mashayekhan S, Shabani I, Khorashadizadeh M, Fallah A, Soleimani M. Structural stability and sustained release of protein from a multilayer nanofiber/nanoparticle composite. Int J Bio Macro. 2015;75:248–257. doi: 10.1016/j.ijbiomac.2015.01.051. [DOI] [PubMed] [Google Scholar]
- Vater C, Kasten P, Stiehler M. Culture media for the differentiation of mesenchymal stromal cells. Acta Biomater. 2011;7:463–477. doi: 10.1016/j.actbio.2010.07.037. [DOI] [PubMed] [Google Scholar]
- Wismer N, Grad S, Fortunato G, Ferguson SJ, Alini M, Eglin D. Biodegradable electrospun scaffolds for annulus fibrosus tissue engineering: effect of scaffold structure and composition on annulus fibrosus cells in vitro. Tis Eng Part A. 2014;20:672–682. doi: 10.1089/ten.TEA.2012.0679. [DOI] [PubMed] [Google Scholar]
- Wutticharoenmongkol P, Sanchavanakit N, Pavasant P, Supaphol P. Preparation and characterization of novel bone scaffolds based on electrospun polycaprolactone fibers filled with nanoparticles. Macromol Biosci. 2006;6:70–77. doi: 10.1002/mabi.200500150. [DOI] [PubMed] [Google Scholar]
- Zahiri M, Khanmohammadi M, Goodarzi A, Ababzadeh S, Sagharjoghi Farahani M, Mohandesnejad S, Bahrami N, Nabipour I, Ai J. Encapsulation of curcumin loaded chitosan nanoparticle within poly (ε-caprolactone) and gelatin fiber mat for wound healing and layered dermal reconstitution. Int J Bio Macro. 2020;153:1241–1250. doi: 10.1016/j.ijbiomac.2019.10.255. [DOI] [PubMed] [Google Scholar]