Abstract
High level carbapenem and extensively drug resistant (XDR) Escherichia coli strain N7, which produces a variant of New Delhi metallo-β-lactamase (NDM-5), was isolated from the influent of the Jungnang wastewater treatment plant located on Han River, Seoul, South Korea. Phenotypic and genotypic resistances to carbapenem were tested using agar and broth dilution methods, and polymerase chain reaction. Whole-genome sequencing was performed to characterize the genetic structure of strain N7. E. coli strain N7, which harbors the blaNDM–5 gene, showed high level of carbapenem resistance at concentrations of doripenem (512 mg/L) and meropenem (256 mg/L), and XDR to 15 antibiotics. Based on the genomic sequence analysis, two plasmids, a hybrid IncHI2/N-type and an IncX3 type, were present. The former contains a cluster (blaNDM–5-bleMBL-trpF-dsbD) bracketed by multi-insertional sequences, IS3000, ISAba125, IS5, and IS26. The latter carries the following resistance genes: blaCTX–14, aac(3)-IV, aadA1, aadA2, aph(3′)-Ia, aph(4)-Ia, sul1, sul2, sul3, dfrA12, fosA3, oqxA, oqxB, mph(A), and floR, and cmlA1. The chromosome, contig3, and contig5 also carry blaCTX–64 and mdf(A), tet(A), and erm(B), tet(M) and aadA22, respectively. Strain N7 also harbors virulence factors such as fimH, flu, ecpABCDE, sfmA, hlyE, and gadA. This study demonstrates the emergence of high level carbapenem resistant XDR E. coli strain N7 containing blaNDM–5 in aquatic environment, Seoul, South Korea. Due to the presence of mobile genetic elements, this strain could horizontally transfer resistance genes, including blaNDM–5 to environmental bacteria. Thus, it is necessary to conduct continuous surveillance for carbapenem resistance in various aquatic environments.
Keywords: carbapenem resistance, extensively drug resistance, blaNDM gene, wastewater treatment plants, Escherichia coli, horizontal gene transfer, aquatic environment
Introduction
Carbapenem-resistant Enterobacteriaceae (CRE) is one of the most critical pathogens, together with carbapenem-resistant Acinetobacter baumannii and Pseudomonas aeruginosa, and has been clinically issued with growing concerns in need of new antibiotics (Tacconelli et al., 2017). CRE can produce several enzymes belonging to the class of New Delhi metallo-β-lactamase (NDM) to hydrolyze carbapenems (Doi and Paterson, 2015). Since the first report of NDM (Yong et al., 2009), a series of NDM variants, which possess distinct hydrolytic activity against β-lactams (blaNDM) from NDM-1 to NDM-29, have been identified with the clinical evolution of NDM (Cheng et al., 2018). In particular, NDM-5 producing Escherichia coli shows higher level of resistance to carbapenems compared to previously reported NDM-1 producing bacteria (Hornsey et al., 2011).
The first occurrence of NDM-5 producing E. coli EC405 was reported in a patient in the United Kingdom in 2011, and it showed a high level of resistance to cephalosporins, carbapenems, aminoglycosides, and quinolones, while being susceptible to colistin and tigecycline (Hornsey et al., 2011). Following this discovery, two carbapenemase-producing Enterobacteriaceae (NDM-5 producing E. coli and NDM-1 producing Klebsiella pneumoniae) showing distinct hydrolytic activity against imipenem were isolated from a traveler from Bangladesh in 2013 and Indonesia in 2014, respectively (Nakano et al., 2014). Subsequently, in South Korea, NDM-9 and NDM-5 producing Klebsiella variicola and E. coli strains were recovered from a river in 2017 (Di et al., 2017) and patients in 2018 (Jhang et al., 2018), respectively, suggesting that environmental and clinical NDM-producing bacteria are in circulation.
The blaNDM genes have been predominantly found in opportunistic pathogenic bacteria displaying resistance to multiple antimicrobials, particularly, Enterobacteriaceae, such as E. coli, Klebsiella sp., and Enterobacter sp. (Bush, 2010). Since the isolation of clinical NDM-1 producing Acinetobacter spp. and Pseudomonas spp. in 2012 (Bharadwaj, 2012), the occurrence of NDM-producing bacteria has been on the rise in various aquatic environments including river stream, wastewater treatment plants (WWTPs), and tap water (Walsh et al., 2011; Luo et al., 2013; Di et al., 2017). WWTPs have been suggested as potential hot spots for antibiotic resistance (Karkman et al., 2018). Contamination determinants from households, hospitals, farms, and other non-point source pollutions may play a role in selective pressure for the increase in antibiotic resistance, escalating antibiotic resistance that enables the development of multi-drug resistant (MDR), extensively drug resistant (XDR), and/or pan-drug resistant (PDR) bacteria, which make it increasingly difficult to treat infections.
In this study, we report the emergence of pathogenic, and highly carbapenem-resistant and XDR E. coli strain N7, isolated from the urban influent of Jungnang WWTP on the Han River located in Seoul, the capital city of South Korea. Whole-genome sequencing analyses of E. coli strain N7 indicated that 23 antibiotic resistance genes (ARGs) including blaNDM–5, a variant of NDM, were present in chromosome, plasmids, and contigs. Among them, seventeen were carried on two plasmids, which were formulated structurally in a manner of well-known conserved clusters with either class 1 integron and/or insertional sequences (ISs), suggesting that E. coli strain N7 can act as a carrier of ARGs in the aquatic environment.
Materials and Methods
Isolation and Identification of Carbapenem-Resistant Bacteria From a WWTP
The influent sample was collected from the Jungnang (JN) WWTP on the Han River, Seoul, South Korea in May of 2018 by using sterile bottles. After collection, the samples were immediately shipped to the laboratory under cool conditions (4°C) and filtered through a 0.22 μm pore size membrane filter (Advantec, Tokyo, Japan). The membranes were suspended in 10 mL of Mueller-Hinton (MH) broth (MBCell, Seoul, South Korea), thoroughly vortexed, and then processed with a serial dilution up to 10–3 times (100, 10–1, 10–2, and 10–3). A 100 μL of sample of the MH broth was spread on mSuperCARBA (CHROMagar, France) agar plates and the plates were incubated at 37°C for 48 h. After incubation, the colonies on the plates were streaked on new MH agar plates containing 8 mg/L of meropenem to obtain a single colony of presumptive carbapenemase-producing bacteria. The isolate grown on the plates were taxonomically identified using 16S rDNA gene sequencing (Macrogen, Seoul, South Korea).
Phenotypic and Genotypic Resistance Test
Eleven carbapenem resistance genes (blaIMP, blaVIM, blaNDM, blaSPM, blaAIM, blaDIM, blaGIM, blaSIM, blaKPC, blaBIC, and blaOXA–48) (Poirel et al., 2011) were screened using PCR detection from the presumptive carbapenemase-producing bacteria. The amplicons were sequenced (Macrogen) and identified using NCBI BLAST1. For the screened carbapenemase-producing bacteria, MDR to 16 antibiotics was determined using Kirby-Bauer disk diffusion, and resistance to colistin was determined using broth dilution methods. For MDR, the following antibiotic disks were used: ampicillin-sulbactam (10/10 μg), cefotaxime (30 μg), ceftazidime (30 μg), chloramphenicol (30 μg) ciprofloxacin (5 μg), colistin (2 mg/L), doripenem (10 μg), fosfomycin (200 μg), gentamicin (10 μg), levofloxacin (5 μg), meropenem (10 μg), netilmicin (10 μg), piperacillin (100 μg), tetracycline (30 μg), tobramycin (10 μg), and trimethoprim-sulfamethoxazole (1.25/23.75 μg) (Liofilchem, Roseto degli Abruzzi, Italy). Resistance to the antibiotics was determined according to the Clinical and Laboratory Standards Institute (CLSI) guideline (Clinical Laboratory Standars and Institue, 2016). Subsequently, MICs of 16 antibiotics for E. coli strain N7 were evaluated using the broth dilution method (Hasselmann, 2003).
Whole Genome Sequencing
The genome was constructed de novo using PacBio sequencing data (Pacific Biosciences, Menlo Park, CA, United States). Sequencing analysis was performed at Chunlab Inc. (Seoul, South Korea). PacBio sequencing data were assembled with PacBio SMRT Analysis 2.3.0 using the HGAP2 protocol (Pacific Biosciences). The resulting contigs from PacBio sequencing data were circularized using Circulator 1.4.0 (Sanger Institute, Hinxton, Cambridgeshire, United Kingdom) (Yoon et al., 2017). Circular maps for plasmid structures and linear maps generated by Circulator 1.4.0 and geneCo (Jung et al., 2019), respectively, were manually modified. The chromosomal and plasmid origins of replication were identified using DoriC 5.0 and the plasmid types were determined by PlasmidFinder 1.3, using FASTA file (Carattoli et al., 2014). ARGs were identified using ResFinder (Zankari et al., 2012). Multi-locus sequence type (MLST) was determined by sequences of seven housekeeping genes (adk, fumC, gyrB, icd, mdh, purA, and recA) according to a previous description (Clermont et al., 2000). The WGS data were deposited in GenBank under the accession JABWPS000000000.
Serotyping and Virulence Determinants
Carbapenemase-producing E. coli strain was serotyped with four O-antisera (O26, O111, O146, and O157) (SSI Diagnostica, Hillerød, Denmark) by incubation in MH broth for 16 h, boiling at 95°C for 15 min. Equal volume of the lysate and antisera were mixed in a 96-well culture plate, and then incubated at 52°C overnight. The agglutination of O-antigen and O-antisera was visually checked according to a previously described protocol (SSI Diagnostica). Virulence genes and serotype were determined from WGS data using VirulenceFinder and SerotypeFinder 2.0 (Carattoli et al., 2014; Joensen et al., 2015).
Results
Isolation and Identification of Carbapenem-Resistant Bacteria
Among the 50 isolates from the influent of JN WWTP, 24 isolates were presumptive carbapenem-resistant bacteria. The PCR detection of 11 carbapenemase genes (blaIMP, blaVIM, blaNDM, blaSPM, blaAIM, blaDIM, blaGIM, blaSIM, blaKPC, blaBIC, and blaOXA–48) revealed that only one isolate was positive for the blaNDM. This isolate, N7, was taxonomically identified as E. coli by 16S rDNA gene sequencing. MLST revealed that E. coli strain N7 belonged to ST746.
Phenotypic Antimicrobial Resistance
Escherichia coli strain N7 showed resistance to ampicillin (MIC, 1,024 mg/L), cefotaxime (MIC, 256 mg/L), ceftazidime (MIC, 512 mg/L), ciprofloxacin (MIC, 1,024 mg/L), colistin (MIC, 8 mg/L), doripenem (MIC, 512 mg/L), fosfomycin (MIC, 1,024 mg/L), gentamycin (MIC, 512 mg/L), imipenem (MIC, 256 mg/L) levofloxacin (MIC, 256 mg/L), meropenem (MIC, 256 mg/L), netilmicin (MIC, 256 mg/L), piperacillin (MIC, 1,024 mg/L), tetracycline (MIC, 512 mg/L), tobramycin (MIC, 256 mg/L), and trimethoprim-sulfamethoxazole (MIC, 4/76 mg/L), but was susceptible to chloramphenicol (Table 1). Compared to the CLSI clinical breakpoint, E. coli strain N7 exhibited high level of resistance to eight classes of the antibiotics tested, except for trimethoprim-sulfamethoxazole. Regarding the extent of the antibiotic resistance up to 15 of 16 antibiotics tested, strain N7 is likely to be an XDR bacterium.
TABLE 1.
MICs of antimicrobials tested for E. coli strain N7 compared with other E. coli strains.
| No. | Antibiotics | MICs (mg/L) of E. coli strains | CLSI clinical breakpoint (mg/L) | |||
| N7 | QD28 (Rahman et al., 2014) | QD29 (Rahman et al., 2014) | EC405 (Zhu et al., 2016) | |||
| 1 | GEN | 512 | 32 | ≥256 | – | 16 |
| 2 | CIP | 1024 | 6 | ≥32 | – | 4 |
| 3 | MEM | 256 | ≥32 | ≥32 | ≥32 | 4 |
| 4 | SXT | >4/76 | – | – | – | 4/76 |
| 5 | CTX | 256 | ≥256 | ≥256 | ≥256 | 4 |
| 6 | CAZ | 512 | ≥256 | ≥256 | ≥256 | 16 |
| 7 | AMP | 1024 | – | – | – | 32 |
| 8 | PIP | 1024 | – | – | – | 128 |
| 9 | TET | 512 | 16 | |||
| 10 | FOF | 1024 | 2 | ≥1024 | – | 256 |
| 11 | NET | 256 | – | – | – | 32 |
| 12 | DOR | 512 | – | – | – | 4 |
| 13 | LVX | 256 | – | – | – | 8 |
| 14 | TOB | 256 | 10 | ≥256 | – | 16 |
| 15 | CHL | S | – | – | – | 32 |
| 16 | CST | 8 | 0.38 | 0.5 | – | 2 |
| 17 | IMP | 256 | – | – | – | 4 |
GEN: gentamicin; CIP: ciprofloxacin, MEM, meropenem; SXT, trimethoprim-sulfamethoxazole; CTX, cefotaxime; CAZ, ceftazidime; AMP, ampicillin; PIP, piperacillin; TET, tetracycline; FOF, fosfomycin; NET, netilmicin; DOR, doripenem; LVX, levofloxacin; TOB, tobramycin; CHL, chloramphenicol; CST, colistin; IMP, imipenem S, susceptible.
Determinants for Antimicrobial Resistance and Pathogenicity
WGS data showed that 23 ARGs were present in E. coli strain N7 and 21 ARGs were found on two incompatible plasmids and contigs, except for the blaCTX–64 and mdf(A) genes, which were located on the chromosome (Table 2). We found two plasmids in E. coli strain N7, identified as an IncX3 plasmid (pKJNI-5), and a hybrid plasmid consisting of IncHI2 and N-type (pKJNI-2).
TABLE 2.
Genome features of E. coli N7 and its antimicrobial resistance genes.
| Sequence type | Replicon | Origin of replication/plasmid incompatibility | Length (bp) | GC (%) | Resistance genes(n = 23) | Class of antimicrobials |
| ST746 | Chromosome | oriC | 4,614,699 | 50.84 | blaCTX–64 | β-Lactam |
| mdf(A) | Macrolide | |||||
| Plasmid pKJNI-2 | IncHI2, IncN | 255,628 | 46.75 | blaCTX–14 | β-Lactam | |
| aac(3)-IV, aadA1, aadA2, aph(3′)-Ia, aph(4)-Ia | Aminoglycoside | |||||
| sul1, sul2, sul3 | Sulfonamide | |||||
| dfrA12 | Trimethoprim | |||||
| fosA3 | Fosfomycin | |||||
| oqxA, oqxB | Quinolone | |||||
| mph(A) | Macrolide | |||||
| floR, cmlA1 | Phenicol | |||||
| Plasmid pKJNI-5 | IncX3 | 71,870 | 47.54 | blaNDM–5 | β-Lactam | |
| Contig 3 | 19,713 | 52.99 | tet(A) | Tetracycline | ||
| Contig 5 | 8,333 | 57.82 | erm(B) | Macrolide | ||
| tet(M) | Tetracycline | |||||
| aadA22 | Aminoglycoside |
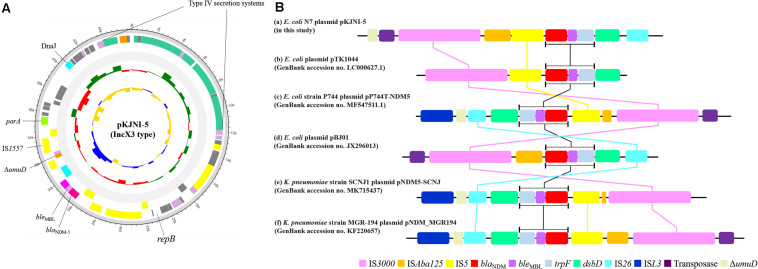
Figure 1A shows the structure of the IncX3 type plasmid pKJNI-5 containing the blaNDM–5 gene. As shown in the figure, the blaNDM–5 gene was always followed by a gene cluster composed of bleomycin resistance gene (bleMBL), phosphoribosyl anthranilate isomerase (trpF), and protein-disulfide reductase (dsbD), as previously reported (Nakano et al., 2014; Zhu et al., 2016; Ho et al., 2018; Yuan et al., 2019). The gene cluster of blaNDM–5-bleMBL-trpF-dsbD was also bracketed by IS3000-ISAba125-IS5 in the upstream region and IS26 in the downstream region (Figure 1B), which was also well conserved among diverse bacteria with the blaNDM-5 and blaNDM-1 genes (Nakano et al., 2014; Zhu et al., 2016; Ho et al., 2018; Yuan et al., 2019). It should be noted that IS3000 was always found upstream of the gene cluster of blaNDM–5-bleMBL-trpF-dsbC/D regions among diverse bacteria. In addition, except in E. coil pTK1044, IS26 is always located downstream of the gene cluster. Figure 1A shows the presence of a type IV secretion system (virD2-virB1-virB4-virB5-virB6-virB8-virB9-virB10-virB11-virD4) at a site opposite that of blaNDM–5 on the plasmid pKJNI-5.
FIGURE 1.
Structure of pKJNI-5 plasmid (IncX3 type) of E. coli N7 harboring blaNDM–5 (A) and comparative sequence analysis of regions of blaNDM–5 of E. coli N7 with other previously reported genetic structures of blaNDM (B). (A) From outermost to innermost ring of plasmid, forward and reverse CDS, track for rRNA and tRNA, GC Skew and GC Ratio was drawn. (B) The genetic environment of blaNDM–5 of (a) pKJNI-5 isolated in this study, (b) pBJ01, (c) pTK1044, (d) pNDM-MGR194, (e) pP744-T-NDM-5, (f) pNDM5-SCNJ were compared. Indication of each color was described below the genetic structures.
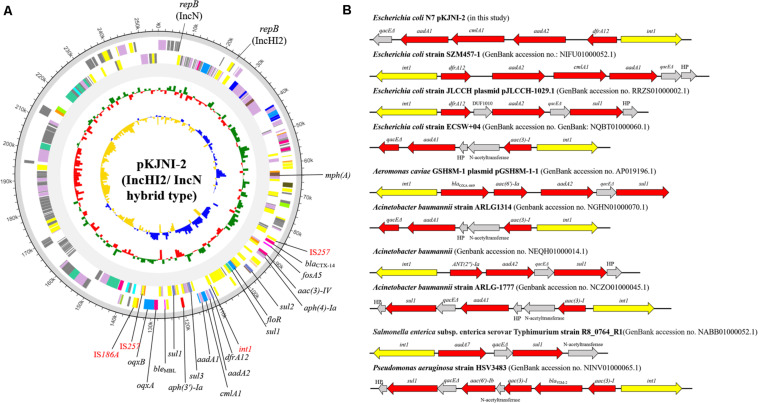
Figure 2A shows the IncHI2/N hybrid-type plasmid pKJNI-2, which carries 16 ARGs {aminoglycosides [aac(3)-IV, aadA1, aadA2, aph(3′)-Ia, and aph(4)-Ia], β-lactams (blaCTX–14), fosfomycin (fosA3), macrolide [mph(A)], phenicols (floR and cmlA1), quinolones (oqxA and oqxB), sulfonamide (sul1, sul2, and sul3), and trimethoprim (dfrA12)}. Even in the presence of the phenicol resistance gene on the IncHI2/N hybrid plasmid, E. coli strain N7 was susceptible to chloramphenicol. IS257 brackets 15 ARGs except for macrolide [mph(A)], and contains a class 1 integron, which carries resistance genes to aminoglycoside (aadA1 and aadA2), chloramphenicol (cmlA1), and trimethoprim (dfrA12) (Figure 2B). The gene cassette associated with class 1 integron of E. coli strain N7 was compared with that of previously submitted genomic data of other bacterial strains including Aeromonas caviae, A. baumannii, Salmonella Typhimurium, P. aeruginosa (Figure 2B). It shows similar patterns of carrying a narrow range of resistance genes to aminoglycoside, β-lactam, chloramphenicol, sulfonamide, and trimethoprim.
FIGURE 2.
Circular map and comparative sequence analysis of pKJNI-2 plasmid (IncHI2/IncN hybrid type) and comparison of class 1 integron structures. (A) From outermost to innermost ring of plasmid, forward and reverse CDS, track for rRNA and tRNA, GC Skew and GC Ratio was drawn. ARGs and mobile genetic elements were indicated in black and red colors, respectively. (B) Class 1 integron structure of pKJNI-2 was compared with those previously reported. The int1 gene and ARGs were colored in yellow and red colors, respectively.
In addition, E. coli strain N7 carries the following eight virulence factors: adhesion-associated molecules (fimH, flu, ecpABCDE, and sfmA), and toxins-encoding genes (hlyE and gadA). E. coli strain N7 belongs to H37 but O-serotype was not determined.
Discussion
In the present study, we report on the emergence of XDR E. coli strain N7 which is positive for blaNDM–5 and characterization of the genetic context of ARGs, including blaNDM–5. Since the discovery of NDM in a Swedish patient who traveled to India, its variants have grown to 28 different types from diverse bacteria, mostly isolated from clinical samples. In South Korea, NDM-5 producing Enterobacteriaceae have been reported only in clinical environments (Park et al., 2016, 2019; Kim et al., 2020), and NDM-9 producing K. variicola were found in river (Di et al., 2017).
Escherichia coli strain N7 belonging to ST746 isolated from the urban influent of JN WWTP shows a variant of the NDM, NDM-5 type. From the WGS, we identified two plasmids such as a narrow host range plasmid IncX3 (Johnson et al., 2012) and a hybrid IncHI2/N. The narrow host range plasmid IncX3 carries a cluster structure of 5′-IS3000-DISAba125-IS5-blaNDM–5-bleMBL-trpF-dsbD-IS26-3′ containing the blaNDM–5 gene. Figure 1A shows the composition of ISs, which cassettes structural genes of 5′-blaNDM–5-bleMBL-trpF-dsbD-3′ (Liu et al., 2013; Nakano et al., 2014; Zhu et al., 2016; Yuan et al., 2019) with a minor change in the presence and absence of IS5 and the extent of truncated ISAba125 among the analyzed E. coli and K. pneumoniae strains. The question is still remained why the structural genes of 5′-bleMBL-trpF-dsbD-3′ with blaNDM–5 are always clustered together. In addition, the IncX3 type plasmid in E. coli strain N7 also contains a type IV secretion system (virD2-virB1-virB4-virB5-virB6-virB8-virB9-virB10-virB11-virD4) located at a site opposite that of blaNDM–5. It should be noted that the type IV secretion system has also been hypothesized to be involved in horizontal gene transfer between other bacteria (Juhas et al., 2008). Taken together, E. coli strain N7 is likely to have a system to transfer recently emerged blaNDM–5 gene to other bacteria due to multiple ISs and type IV secretion system, although it contains the narrow host range vector system (Liakopoul et al., 2018).
It is known that E. coli ST746 carries extended-spectrum β-lactamase (ESBL) genes from fishes (Sellera et al., 2018) and human patients (Wu et al., 2018). In this study, E. coli strain N7 harbored ESBL and eight virulence factors. Surprisingly, E. coli strain N7, which showed MIC of meropenem at 256 mg/L, was also resistant to several antibiotics with very high MIC values for the tested antimicrobials (Table 1), compared to other NDM-5 producing E. coli strains (Hornsey et al., 2011; Rahman et al., 2014; Zhu et al., 2016; Jhang et al., 2018). This XDR pattern can be explained by the presence of several resistance genes located on the broad host range plasmid (Figure 2; Zhao et al., 2018). Therefore, the presence of XDR E. coli, isolated from the influent of WWTP located in a city, along with the carbapenem-resistance gene raises public health concerns due to the possible dissemination of ARGs to other pathogenic bacteria, and difficulty in treatment of infections. Indeed, XDR pathogenic E. coli strains have been reported from human patients (harboring blaKPC–2) (Jeong et al., 2018) and from chickens (co-producing blaNDM and mcr-1) (Lv et al., 2018), increasing the likelihood of infectious disease outbreaks. The characteristics of XDR E. coli strain N7 can be attributed to the presence of corresponding resistance genes located on two plasmids of an IncX3 and a hybrid IncHI2/N. The occurrence of the IncHI2 plasmid has been frequently reported in Salmonella strains with multiple ARGs (Chen et al., 2016). In our experiments, most of the resistance genes were found on the hybrid plasmid IncHI2/N of E. coli strain N7, containing diverse resistance determinants, including aminoglycoside [aac(3)-IV, aadA1, aadA2, aph(3′)-Ia, and aph(4)-Ia], β-lactam (blaCTX–64, blaCTX–14, and blaNDM–5), fosfomycin (fosA5), macrolide [mdf(A) and mph(A)], phenicol (floR and cmlA1), quinolone (oqxA and oqxB), sulfonamide (sul1, sul2, and sul3), and trimethoprim (dfrA12).
Conclusion
In conclusion, NDM-5 producing E. coli strain N7, which shows a high level of carbapenem resistance and an XDR pattern, was found in the megacity influent of Jungnang WWTP, Seoul, South Korea. Our findings suggest that pathogenic XDR E. coli originating from urban activities may be disseminated into the river from WWTP and is a potential carrier or spreader of ARGs, including emerging carbapenemase genes. Thus, we need to focus on the continuous surveillance of carbapenemase-producing bacteria in diverse environments.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author Contributions
HS: experiment, data analysis, and manuscript writing. YK: methodology. DH: revision of manuscript. H-GH: overall revision, methodology, and data analysis. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding. This work was supported by the Korea Ministry of Environment (MOE) as “the Environmental Health Action Program” (2016001350006).
References
- Bharadwaj R. (2012). Prevalence of New Delhi metallo-β-lactamase (NDM-1)-positive bacteria in a tertiary care centre in Pune, India. Int. J. Antimicrob. Agents 39 2011–2012. 10.1016/j.ijantimicag.2011.10.007 [DOI] [PubMed] [Google Scholar]
- Bush K. (2010). Alarming β-lactamase-mediated resistance in multidrug-resistant Enterobacteriaceae. Curr. Opin. Microbiol. 13 558–564. 10.1016/j.mib.2010.09.006 [DOI] [PubMed] [Google Scholar]
- Carattoli A., Zankari E., Garciá-Fernández A., Larsen M. V., Lund O., Villa L., et al. (2014). In silico detection and typing of plasmids using plasmidfinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 58 3895–3903. 10.1128/AAC.02412-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Fang T., Zhou X., Zhang D., Shi X., Shi C. (2016). IncHI2 plasmids are predominant in antibiotic-resistant Salmonella isolates. Front. Microbiol. 7:1566. 10.3389/fmicb.2016.01566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z., Thomas P. W., Ju L., Bergstrom A., Mason K., Clayton D., et al. (2018). Evolution of New Delhi metallo-β-lactamase (NDM) in the clinic: effects of NDM mutations on stability, zinc affinity, and mono-zinc activity. J. Biol. Chem. 293 12606–12618. 10.1074/jbc.RA118.003835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clermont O., Bonacorsi S., Bingen E. (2000). Rapid and simple determination of the Escherichia Coli phylogenetic group. Appl. Environ. Microbiol. 66 4555–4558. 10.1128/AEM.66.10.4555-4558.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical Laboratory Standars and Institue (2016). Clinical and Laboratory Standards Institute: Performance Standards for Antimicrobial Susceptibility Testing Supplement M100S. Wayne, PA: Clinical and Laboratory Standards Institute. [Google Scholar]
- Di D. Y. W., Jang J., Unno T., Hur H. G. (2017). Emergence of klebsiella variicola positive for NDM-9, a variant of New Delhi metallo-β-lactamase, in an urban river in South Korea. J. Antimicrob. Chemother. 72 1063–1067. 10.1093/jac/dkw547 [DOI] [PubMed] [Google Scholar]
- Doi Y., Paterson D. L. (2015). Carbapenemase-producing Enterobacteriaceae. Semin. Respir. Crit. Care Med. 36 74–84. 10.1055/s-0035-1544208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmann C. (2003). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 9 9–15. 10.1046/j.1469-0691.2003.00790 [DOI] [PubMed] [Google Scholar]
- Ho P. L., Wang Y., Liu M. C. J., Lai E. L. Y., Law P. Y. T., Cao H., et al. (2018). IncX3 epidemic plasmid carrying blaNDM-5 in Escherichia coli from swine in multiple geographic areas in China. Antimicrob. Agents Chemother. 62:e02295–17. 10.1128/AAC.02295-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornsey M., Phee L., Wareham D. W. (2011). A novel variant, NDM-5, of the New Delhi metallo-β-lactamase in a multidrug-resistant Escherichia Coli ST648 isolate recovered from a patient in the United Kingdom. Antimicrob. Agents Chemother. 55 5952–5954. 10.1128/AAC.05108-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S., Kim J. O., Yoon E. J., I, Bae K., Lee W., Lee H., et al. (2018). Extensively drug-resistant Escherichia Coli sequence Type 1642 carrying an IncX3 plasmid containing the blaKPC-2 gene associated with transposon Tn4401a. Ann. Lab. Med. 38 17–22. 10.3343/alm.2018.38.1.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhang J., Wang H. Y., Yoo G., Hwang G. Y., Uh Y., Yoon K. J. (2018). NDM-5 and OXA-48 co-producing uropathogenic Escherichia coli isolate: first case in Korea. Ann. Lab. Med. 38 277–279. 10.3343/alm.2018.38.3.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joensen K. G., Tetzschner A. M. M., Iguchi A., Aarestrup F. M. (2015). Rapid and easy in silico serotyping of Escherichia coli isolates by use of whole-genome sequencing data. J. Clin. Microbiol. 53 2410–2426. 10.1128/JCM.00008-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T. J., Bielak E. M., Fortini D., Hestbjerg L., Debroy C., Nolan L. K., et al. (2012). Plasmid expansion of the IncX plasmid family for improved identification and typing of novel plasmids in drug-resistant Enterobacteriaceae. Plasmid. 68 43–50. 10.1016/j.plasmid.2012.03.001 [DOI] [PubMed] [Google Scholar]
- Juhas M., Crook D. W., Hood D. W. (2008). Type IV secretion systems: tools of bacterial horizontal gene transfer and virulence. Cell. Microbiol. 10 2377–2386. 10.1111/j.1462-5822.2008.01187.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J., Kim J. I., Yi G. (2019). Genome analysis GeneCo: a visualized comparative genomic method to analyze multiple genome structures. Bioinformatics 35 5303–5305. 10.1093/bioinformatics/btz596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkman A., Do T. T., Walsh F., Virta M. P. J. (2018). Antibiotic-resistance genes in waste water. Trends Microbiol. 26 220–228. 10.1016/j.tim.2017.09.005 [DOI] [PubMed] [Google Scholar]
- Liakopoul A., Van Der Goot J., Bossers A., Betts J., Brouwer M. S. M., Kant A., et al. (2018). Genomic and functional characterisation of IncX3 plasmids encoding blaSHV-12 in Escherichia coli from human and animal origin. Sci. Rep. 8:7674. 10.1038/s41598-018-26073-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Li W., Wang J., Pan J., Sun S., Yu Y., et al. (2013). Identification and characterization of the first Escherichia coli strain carrying NDM-1 gene in China. PLoS One 8:e66666. 10.1371/journal.pone.0066666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Yang F., Mathieu J., Mao D., Wang Q., Alvarez P. J. J. (2013). Proliferation of multidrug-resistant New Delhi metallo-β-lactamase genes in municipal wastewater treatment plants in Northern China. Environ. Sci. Technol. Lett. 1 26–30. 10.1021/ez400152e [DOI] [Google Scholar]
- Lv L., Zeng Z., Song Q., Cao Y., Wang J., Li W., et al. (2018). Emergence of XDR Escherichia coli carrying both blaNDM and mcr-1 genes in chickens at slaughter and the characterization of two novel blaNDM-bearing plasmids. J. Antimicrob. Chemother. 73 2261–2263. 10.1093/jac/dky176 [DOI] [PubMed] [Google Scholar]
- Nakano R., Nakano A., Hikosaka K., Kawakami S., Matsunaga N., Asahara M., et al. (2014). First report of metallo-β-lactamase NDM-5-producing Escherichia coli in Japan. Antimicrob. Agents Chemother. 58 7611–7612. 10.1128/AAC.04265-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. Y., Seo J., Shin J., Chung Y. J., I, Jeon Y., Yun S. J., et al. (2020). Clonal spreading of NDM-5 carbapenemase-producing Escherichia coli isolates in a hospital in South Korea. Diagn. Microbiol. Infect. Dis. 97 2016–2018. 10.1016/j.diagmicrobio.2020.115027 [DOI] [PubMed] [Google Scholar]
- Poirel L., Walsh T. R., Cuvillier V., Nordmann P. (2011). Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis 70 119–123. 10.1016/j.diagmicrobio.2010.12.002 [DOI] [PubMed] [Google Scholar]
- Rahman M., Shukla S. K., Prasad K. N., Ovejero C. M., Pati B. K., Tripathi A., et al. (2014). Prevalence and molecular characterisation of New Delhi metallo-β-lactamases NDM-1, NDM-5, NDM-6 and NDM-7 in multidrug-resistant Enterobacteriaceae from India. Int. J. Antimicrob. Agents 44 30–37. 10.1016/j.ijantimicag.2014.03.003 [DOI] [PubMed] [Google Scholar]
- Sellera F. P., Fernandes M. R., Moura Q., Carvalho M. P. N., Lincopan N. (2018). Extended-spectrum-β-lactamase (CTX-M)-producing Escherichia coli in wild fishes from a polluted area in the atlantic coast of South America. Mar. Pollut. Bull. 135 183–186. 10.1016/j.marpolbul.2018.07.012 [DOI] [PubMed] [Google Scholar]
- Tacconelli E., Magrini N., Kahlmeter G., Singh N. (2017). Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Geneva: World Health Organization, 1–7. [Google Scholar]
- Park M., Park S. D., Lee M. H., Kim S. H., Lim K., Lee G., et al. (2016). The first report of NDM-5-producing uropathogenic Escherichia coli isolates in South Korea. Diagn. Microbiol. Infect. Dis. 85 198–199. 10.1016/j.diagmicrobio.2016.02.006 [DOI] [PubMed] [Google Scholar]
- Park Y., Choi Q., Kwon G. C., Koo S. H. (2019). Emergence and transmission of New Delhi metallo−β−lactamase−5−producing Escherichia coli sequence Type 361 in a tertiary hospital in South Korea. J. Clin. Lab. Anal. 34:e23041. 10.1002/jcla.23041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh T. R., Weeks J., Livermore D. M., Toleman M. A. (2011). Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect. Dis. 11 355–362. 10.1016/S1473-3099(11)70059-7 [DOI] [PubMed] [Google Scholar]
- Wu L., Chen J., Wang L., Wu Z. (2018). Whole genome sequence of an MCR-1-carrying, extended-spectrum β-lactamase (ESBL)-producing Escherichia coli ST746 isolate recovered from a community-acquired urinary tract infection. J. Glob. Antimicrob. Resist. 13 171–173. 10.1016/j.jgar.2018.03.014 [DOI] [PubMed] [Google Scholar]
- Yong D., Toleman M. A., Giske C. G., Cho H. S., Sundman K., Lee K. (2009). Characterization of a new metallo-β-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence Type 14 from India. Antimicrob. Agents Chemother. 53 5046–5054. 10.1128/AAC.00774-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S. H., Ha S. M., Kwon S., Lim J., Kim Y., Seo H., et al. (2017). Introducing EzBioCloud: a taxonomically united database of 16S RRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 67 1613–1617. 10.1099/ijsem.0.001755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y., Li Y., Wang G., Li C., Chang Y. F., Chen W., et al. (2019). blaNDM-5 carried by a hypervirulent Klebsiella pneumoniae with sequence Type 29. Antimicrob. Resist. Infect. Control 8:140. 10.1186/s13756-019-0596-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zankari E., Hasman H., Cosentino S., Vestergaard M., Rasmussen S., Lund O., et al. (2012). Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 67 2640–2644. 10.1093/jac/dks261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Chen W., Xu X., Zhou X., Shi C. (2018). Transmissible ST3-IncHI2 plasmids are predominant carriers of diverse complex IS 26-Class 1 integron arrangements in multidrug-resistant Salmonella. Front. Microbiol. 9:2492. 10.3389/fmicb.2018.02492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. Q., Zhao J. Y., Xu C., Zhao H., Jia N., Li Y. N. (2016). Identification of an NDM-5-producing Escherichia coli sequence Type 167 in a neonatal patient in China. Sci. Rep. 6:29934. 10.1038/srep29934 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.