Abstract
Background
Coronavirus disease 19 (COVID-19) is spreading globally and treatment options remain limited. A formulation of niclosamide, a potent anti-SARS-CoV-2 agent and a broad-spectrum antiviral treatment candidate, optimized for inhalation and intranasal administration (UNI91104) was developed.
Methods
We conducted a randomized, placebo-controlled, double-blind, single-centre, dose-ascending Phase 1 trial to assess the safety of UNI91104 in Denmark (NCT04576312). Healthy volunteers were randomly assigned to a ascending single dose in cohort 1–4 and five doses over 2.5 days in cohort 5. Inclusion criteria included a minimum 80% of predicted lung function. Exclusion criteria included severe, clinically significant allergies and current acute or chronic condition especially airway diseases. Safety was evaluated through adverse events (AEs) and pulmonary function tests including forced expiratory volume in one second (FEV1) and fractional exhaled nitric oxide (FeNO) tests. The primary endpoints were defined as the frequency of reported AEs and the change of safety variables relative to pre-dose. Data from all enroled healthy volunteers receiving any amount of IMP was included in the primary analyses. The pharmacokinetics of UNI91104 was determined.
Findings
The trial was conducted between 29 June 2020 and 08 August 2020. Thirty-four healthy volunteers received UNI91104 and ten placebo. No serious AEs or discontinuation were reported. Mild irritation in the upper respiratory tract following inhalation of UNI91104 was reported as most frequent AE (45 events in 26 healthy volunteers, 59% of all healthy volunteers). Nasal application was well-tolerated. There was no evidence of difference in the change of mean levels of pulmonary function tests between active and placebo group across all cohorts. Five healthy volunteers (11.4%) (1 on placebo) had signs of increased transient FeNO and 4 on active (9.1%) experienced asymptomatic drops in FEV1, which resolved spontaneously or were reversible with a β2-agonist. Niclosamide exhibited dose-proportional pharmacokinetics following inhalation and intranasal administration.
Interpretation
UNI91104, a promising candidate for inhalation and intranasal therapy against COVID-19 and other viral respiratory tract infections is well-tolerated in healthy volunteers and warrants further testing in patient trials.
Funding
The study was funded by Innovationsfonden Denmark and UNION therapeutics.
Research in Context.
Evidence before this study
Niclosamide has broad spectrum antiviral properties and is a potent inhibitor of SARS-CoV-2. Due to its low oral availability, administration via inhalation and intranasal is favored to target the primary site of viral replication. Clinical experience with inhaled or intranasally applied niclosamide has not been reported hitherto.
Added value of the study
We conducted a single-centre, double-blinded, dose-ascending Phase 1 trial to assess the safety of five doses of UNI91104 (concentrated solution of niclosamide for inhalation and intranasal application) in healthy volunteers. We showed that UNI91104 is well-tolerated in all dose groups tested, with mild and transient irritation in the upper respiratory tract being the most frequent AE. Nasal application did not result in any AE findings.
Implications of all available evidence
Data from this study supports the testing of UNI91104, a promising candidate for viral respiratory infections such as COVID19, in patient trials.
Alt-text: Unlabelled box
1. Introduction
Since the World Health Organization (WHO) declared the outbreak of the novel coronavirus as a public health emergency of international concern, coronavirus disease 2019 (COVID-19), caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread globally at an immense pace [1]. More than 67 million cases and 1.5 million deaths have been reported globally as of December 7th, 2020 (Johns Hopkins University). While vaccines have been rapidly developed for COVID-19, it is unlikely that vaccination alone will curb COVID-19 globally. Accordingly, additional therapeutic and prophylactic approaches are needed. Despite tremendous research efforts with numerous treatment candidates being tested in clinical trials, treatment options are limited [2]. Intravenously administrated remdesivir, dexamethasone and neutralizing antibodies remain the primary treatment options for COVID-19 patients [3,4]. Similar to these three compounds, most drugs under investigation are systemically administered although 1) the primary site of viral infection occurs along the respiratory tract warranting high local exposure and 2) in other airway diseases, such as asthma and chronic obstructive pulmonary disorder (COPD), inhaled medications are favored over systemic therapies, for both acute and maintenance treatment, due to higher local exposure and a reduced risk of side effects [5,6]. Accordingly, there is an unmet need for novel effective and safe therapies for COVID-19 that achieve high local concentrations along the respiratory tract to act at the primary site of infection.
Niclosamide has been identified as a potent agent against SARS-CoV-2 with an IC50 of 0.28 µM and against other respiratory viruses in vitro, such as influenza, respiratory synctial virus and in vivo, such as MERS-CoV and SARS-CoV-2 7, 8, 9, 10, 11, 12, 13. Brunaugh et al. tested an optimized formulation of niclosamide powder by incorporating human lysozyme as carrier molecule (NIC-hLYS) in a murine SARS-CoV-2 and MERS-CoV infection model. They found that intranasal treatment of SARS-CoV-2- and MERS-CoV-infected transgenic mice with 240 µg/kg NIC-hLYS resulted in a significant reduction of viral titers in lung and brain, decreased levels of interstitial pneumonia and reduced inflammation on day 14 post-infection. Moreover, treatment with niclosamide resulted in 30% and 43% survival on day 10 post-infection compared to 0% survival in non-treated SARS-CoV-2 and MERS-CoV mice, respectively [12].
This data supports the approach of treating viral respiratory infection with niclosamide via inhalation/intranasally as it was shown to limit viral replication in the lungs but also reduces viral load extrapulmonary. Due to its low oral bioavailability, we developed a formulation of niclosamide optimized for inhalation and intranasal application called hereafter UNI91104 (concentrated niclosamide solution for inhalation and intranasal application) designed to deliver a high drug exposure at the primary site of SARS-CoV-2 replication 14, 15, 16. Herein, we report the findings of a randomized, placebo-controlled, double-blind Phase 1 trial to assess the safety of ascending doses of UNI91104 in healthy volunteers, in preparation for therapeutic evaluation in patients with COVID-19.
2. Methods
2.1. Study design
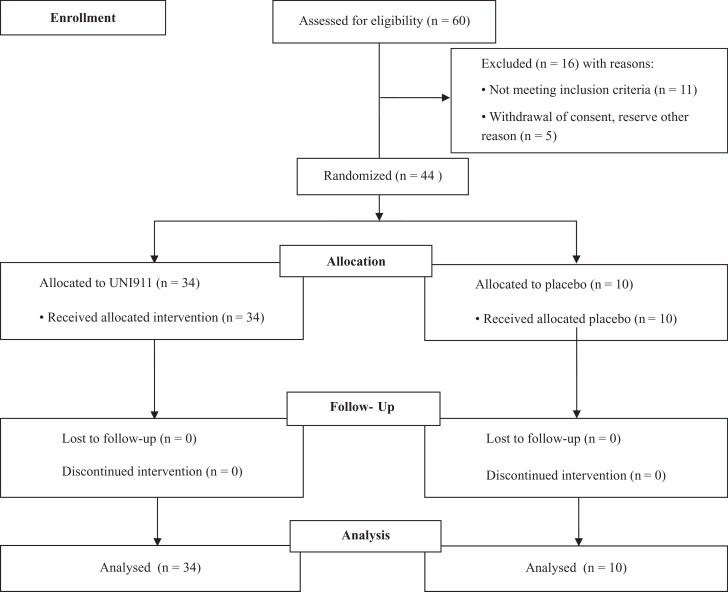
This was a single-centre, interventional, double-blinded, placebo-controlled, Phase 1 study to assess the safety and pharmacokinetics of UNI91104 in healthy volunteers (Trial registry: EudraCT 2020-002049-40 and ClinicalTrials.gov NCT04576312). The study was approved by the Danish Medicines Agency and the relevant regional ethics committee. The study was conducted at Dantrials, Bispebjerg and Frederiksberg Hospital Copenhagen, Denmark. Extended lung function measurement at screening and follow-up was performed at Centre for Physical Activity Research (CFAS), Rigshospitalet, Capital Region, Copenhagen. Forty-four eligible healthy volunteers were enroled in 5 sequential cohorts that were initiated sequentially after evaluation by the Safety Monitoring Committee. The study has been reported in accordance with the CONSORT guidelines (Table S1). The flow of participants is outlined in Fig. 1.
Fig. 1.
Trial design.
2.2. Participants
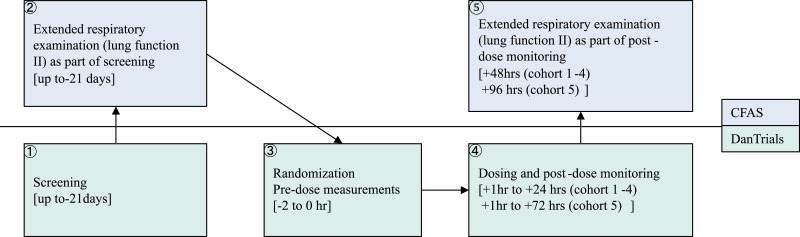
Eligibility was assessed during screening at Dantrials followed by an extended respiratory examination at Centre for Physical Activity Research up to 21 days before dosing (Fig. 2). Eligible were male or nonpregnant and nonlactating female who were abstinent or agreed to use effective contraceptive methods throughout the course of the study, who had an electrocardiogram (ECG) without clinically significant abnormalities (including QTcF < 450 ms), had a chest X-ray without clinically significant abnormalities, who were ≥ 18 and < 65 years and were normally active and in good health with no current chronic diseases and normal physical examination, who had a minimum 80% of predicted lung function, including forced expiratory volume in one second (FEV1) after β2-agonist, total lung capacity (TLC), diffusion capacity for carbon monoxide (DCO), and cardiopulmonary exercise test (CPET) with pulse oximetry (operationalized as a VO2max of > 20 mL/min/kg in females and > 25 mL/min/kg in males, who had less than 5% change in oxygen saturation during CPET, and no cardiac arrythmia as judged by the investigator).
Fig. 2.
Flow chart of study procedures related to dosing
The flow chart describes the flow of a healthy volunteer in the study within the Centre for Physical Activity Research (CFAS) and DanTrials. Timepoints of each measurement is shown in hours (hrs) relative to first dose administered.
Healthy volunteers who had severe, clinically significant allergies, current acute or chronic condition especially airway diseases, renal impairment, underlying condition that might interfere with inhalation of the investigational product, and consumed alcohol in the 24 h before dosing were excluded. All healthy volunteers gave their oral and written (signed informed consent form) consent to participate in the study and they have been informed that the data will be published and they have not objected to this. The full list of inclusion and exclusion criteria can be found in Table S2.
2.3. Randomization and masking
The study was partly conducted in an open-label design with the first healthy volunteer in cohort 1–4 being a sentinel healthy volunteer receiving active drug followed by eight double-blinded healthy volunteers in each cohort (6 assigned to active and 2 to placebo (in total n = 9)), whereas all healthy volunteers in cohort 5 were double-blinded (6 assigned to active and 2 to placebo (n = 8)). Screening and enrolment was done sequentially for one cohort after the other. The randomization list was created manually by the DanTrials’ unblinded study staff. A randomisation list was created using an online randomisation list generator from Tuft's University (http://randomization.com). The randomisation list was created specifying a block of 4 with a 3:1 assignment to either “active” or “placebo”. A total of 10 blocks were produced. A randomization number was assigned in ascending order to each eligible subject at Day 0 according to the randomization list by cohort. The randomization list was secured in a locked cabinet and/or an electronic file with restricted access to only designated unblinded study staff. The label with treatment label was kept in a sealed envelope. The participants were enroled and assigned to intervention by the principal investigator or sub-principal investigator of the study. The vehicle solution (excipients without active pharmaceutical ingredient) was used as placebo solution in this study. As the IMP and placebo solution differed in colour and taste the following precautions were taken to mitigate the risk that participants and investigators identify the assignment: the IMP was stored without access for blinded study staff; the nebulizer and the nasal device was filled with the appropriate solution and volume according to a randomization list by unblinded staff at the clinical site; IMP was placed in a non-see through container during drug administration to retain masking; blinded study staff did not have direct contact with the IMP; participants were instructed by the study staff not to discuss any characteristics (e.g., the appearance and taste) of the study drug with blinded study staff or other research subjects; during waiting, treatment, and assessment periods, reasonable efforts were made to isolate subjects from each other. Conversations between subjects, which can be a source of undesired unblinding were thereby prevented.
2.4. Procedures
For cohort 1–4, treatment was single ascending doses of UNI91104 or placebo in 9 healthy volunteers in each cohort. For cohort 5, 8 healthy volunteers were treated with 5 doses of UNI91104 or placebo. The doses of the different cohorts are displayed in Table S3. Day 0 was the first day of dosing.
Throughout the study, both the investigational drug and placebo were administered by qualified study staff.
The general physical examination, serum chemistry and hematology sampling as well as urinalysis were performed at screening, and 24, and 48 h after the last dose. AEs were collected from the time of start of the dose/first dose and throughout the study period. In terms of respiratory function, three safety focus points were used for the safety assessment – 1) development of airway obstruction or inflammation, 2) development of restrictive airway illness and 3) drug-induced airway impairment resulting in reduced levels in the physical activity test with low oxygen uptake. Details of the schedule of procedures for each cohort and safety variables, including the respiratory function tests, are summarized in Table S4. The detailed description of performed respiratory function tests can be found in the supplementary material. Abnormal and clinically relevant changes in the respiratory function tests following UNI91104, when asymptomatic, led to an adverse event reporting with the AE classified in the “Investigations” System Organ Class Term.
Abnormal and clinical relevant changes were recorded and in this study defined as an increase in FeNO of > 10 ppb between screening and +48 h post-dose/last dose, a reduction of FEV1 > 12% and > 200 mL between two measurements, development of reversibility defined as increase in FEV1 of at least 12% and 200 mL from pre-β2 agonist administration, change in maximal oxygen consumption (VO2max) of > 20%, change in oxygen saturation of > 5% or a reduction in DCO and/or TLC of > 20%.
2.5. Outcomes
The primary objective of this study was to assess safety of UNI91104 in healthy volunteers through the following parameters: AEs, general physical examination, general safety assessments, vital signs, clinical laboratory analysis including urinalysis, hematology, and serum chemistry, forced vital capacity (FVC), TLC, DCO, FEV1 including reversibility to β2 agonist, fractional exhaled nitric oxide (FeNO), resting pulse oximetry and CPET with ECG and pulse oximetry. The primary endpoints were defined as the AE frequency in each cohort and treatment group, the change from baseline for all measured safety variables, and frequency of out of range values.
2.6. Pharmacokinetic analysis
The pharmacokinetics (PK) following inhalation administration of UNI91104 was evaluated using a validated Phoenix WinNonlin® v8.1 (Certara, Princeton, NJ, USA), and a variety of plasma PK parameters including maximum plasma concentration of niclosamide (Cmax), time to maximum plasma concentration (Tmax), area under the concentration-time curve (AUC), the apparent plasma elimination half-life (T½), apparent oral drug clearance CL/F), apparent volume of distribution (Vz/F) were reported using standard noncompartmental (NCA) methods. Blood samples for PK analysis were collected pre-dose, +½ h, +1 h, +1½ h, +2 h, +3 h, +6 h, +12 h, and +24 h after single dose administration in cohorts 1–4 in K2EDTA tubes. In cohort 5, samples were collected in the morning on Day 0 at pre-dose and +1 h, +3 h, + 6 h after dosing and in the evening pre-dose, 1 and 3 h after BID dosing up to 5 consecutive doses. No PK sampling was performed on Day 1. On Day 2, blood samples for PK analysis were collected pre-dose, +½ h, +1 h, +1½ h, +2 h, +3 h, +6 h, +12 h, +24 h, +48 h post-dose. Plasma was separated from whole blood for subsequent analysis. Niclosamide concentrations in plasma was measured using validated LC-MS/MS.
2.7. Statistical analyses
The study was not powered for inferential statistics. The sample size was calculated to obtain the power needed to detect AEs at least once and was considered sufficient to meet the study objectives and to assess treatment safety, but was not powered for statistical inference. Two sets of populations for analysis were distinguished, the Safety Set and the Pharmacokinetics Set. The Safety Analysis Set includes data from all enroled healthy volunteers receiving any amount of investigational medicinal product (IMP). Significance of differences was tested in an exploratory fashion using the Mann–Whitney Test. No imputation for missing data was made. Data from patients receiving placebo were combined across cohorts for adverse events but not for the safety variables. For all analyses, the statistical software Stata® (version 16) was used in the most recent sub-version available at database lock.
The PK Analysis Set includes data from healthy volunteers who were treated with active and have no missing data affecting the PK assessment. Healthy volunteers with at least one quantifiable drug concentration were included in the PK analysis. No imputation for missing data was made. All pharmacokinetic parameters were calculated using non-compartmental analysis (NCA) with a validated installation of the software Phoenix® WinNonlin® version 8.1. The PK parameters were reported as geometric mean,%Coefficient of variation, geometric CV%, minimum and maximum, median, arithmetic mean and standard deviation.
A non-linear power model was used to assess dose proportionality using PK data from cohort 1–4, accumulation ratio was calculated for Cmax and AUC between Day 2 and Day 0.
2.8. Role of funding sources
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
The study was performed from 29 June 2020 to 08 August 2020. The trial profile is displayed in Fig. 1. Forty-four healthy volunteers were randomized of which 34 were assigned to treatment and 10 to placebo. The demographics and clinical characteristics at baseline are summarized in Table 1. For all baseline characteristics, no significant differences were found between treatment groups and cohort - except ECG which showed a significant difference between cohorts which was caused by a comparatively high number of HVs with abnormal but not clinically relevant ECG assessments in cohort 5.
Table 1.
Demographics and clinical characteristics of patients at baseline.
| Characteristics | Statistic |
UNI91104 0.1%, Cohort 1 (N = 7) |
UNI91104 1.0%, Cohort 2 (N = 7) |
UNI91104 1.0%, Cohort 3 (N = 7) |
UNI91104 1.0%, Cohort 4 (N = 7) |
UNI91104 1.0%, Cohort 5 (N = 6) |
Placebo All cohorts (N = 10) |
Overall Study (N = 44) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Mean ± SD (Range) | 25.14 ± 3.72 (18 - 29) |
24.29 ± 3.04 (19 - 29) |
27.14 ± 7.47 (20 - 42) |
25.57 ± 6.95 (19 - 38) |
24.33 ± 3.93 (19 - 29) |
26.9 ± 7.75 (18 - 45) |
25.68 ± 5.78 (18 - 45) |
||||
| Sex | Male: n (%) | 6 (85.7%) | 5 (71.4%) | 4 (57.1%) | 5 (71.4%) | 5 (83.3%) | 6 (60%) | 31 (70.5%) | ||||
| Female: n (%) | 1 (14.3%) | 2 (28.6%) | 3 (42.9%) | 2 (28.6%) | 1 (16.7%) | 4 (40%) | 13 (29.5%) | |||||
| Sex Ratio [M/F] | 6 | 2.5 | 1.33 | 2.5 | 5 | 1.5 | 2.38 | |||||
| Body Weight [kg] | Mean ± SD (Range) | 72.57 ± 8.9 (59 - 87) |
69.71 ± 10.66 (53 - 88) |
76.71 ± 19.71 (47 - 104) |
81.86 ± 18.81 (61 - 113) | 77.67 ± 11.59 (59 - 89) | 83 ± 15.1 (55 - 112) |
77.32± 14.76 (47 - 113) |
||||
| BMI [kg/m²] | Mean ± SD (Range) | 22.47 ± 1.22 (20.9 - 23.94) |
22.48 ± 1.58 (20.45 - 24.9) |
24.49 ± 4.61 (18.59 - 31.05) |
24.63 ± 5 (18.83 - 33.02) |
23.65 ± 2.3 (20.66 - 26.2) |
25.74 ± 5.84 (18.59 - 38.75) |
24.04 ± 4.04 (18.59 - 38.75) |
||||
| ECG | Normal: n (%) | 6 (85.7%) | 7 (100%) | 7 (100%) | 6 (85.7%) | 2 (33.3%) | 10 (100%) | 38 (86.4%) | ||||
| Abnormal and NCR: n (%) | 1 (14.3%) | 0 (0%) | 0 (0%) | 1 (14.3%) | 4 (66.7%) | 0 (0%) | 6 (13.6%) | |||||
| Abnormal and CR: n (%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |||||
| FEV1 [L] | Mean ± SD (Range) | 4.33 ± 0.79 (2.78 - 5.32) |
4.15 ± 0.81 (3.02 - 5.5) |
4 ± 0.8 (3.04 - 5.22) |
4.34 ± 0.93 (3.02 - 6.05) |
4.42 ± 0.51 (3.85 - 5.11) |
3.96 ± 0.69 (3.14 - 5.2) |
4.18 ± 0.74 (2.78 - 6.05) |
||||
| FVC [L] | Mean ± SD (Range) | 5.27 ± 0.99 (3.39 - 6.39) |
4.71 ± 1.09 (3.41 - 6.8) |
5.01 ± 1.33 (3.71 - 7.12) |
5.51 ± 1.41 (3.97 - 8.31) |
5.66 ± 0.88 (4.76 - 7.16) |
5.09 ± 0.87 (3.73 - 6.34) |
5.19 ± 1.08 (3.39 - 8.31) |
||||
| DCOc [mmol/(min×kPa)] | Mean ± SD (Range) | 11.64 ± 2.04 (8.35 - 14.99) |
10.08 ± 1.9 (7.34 - 13.48) |
10.28 ± 2.42 (6.8 - 13.35) |
10.93 ± 1.86 (8.66 - 14.6) |
12.54 ± 1.73 (9.94 - 15.15) |
10.79 ± 1.99 (7.34 - 13.22) |
10.99 ± 2.05 (6.8 - 15.15) |
||||
| FeNO [ppb] | Mean ± SD (Range) | 18.79 ± 20.41 (6.5 - 64.5) |
16.07 ± 7.55 (5 - 25) |
14.21 ± 7.02 (8.5 - 29.5) |
20.57 ± 11.75 (5 - 36.5) |
20 ± 17.85 (5 - 55) |
15.45 ± 10.81 (6.5 - 42) |
17.32 ± 12.63 (5 - 64.5) |
||||
Abbreviations: N = number of healthy volunteers, n = number of healthy volunteers with specific feature;% = calculated using the number of patients in the safety population as the denominator (n/N x 100). BMI = Body Mass Index; ECG = electrocardiography; FEV1 = volume that has been exhaled at the end of the first second of forced expiration; FVC = forced vital capacity; DCOc = corrected carbon monoxide diffusion capacity; FeNO = fractional exhaled nitric oxide; SD = standard deviation; CR = clinically relevant; NCR = clinically not relevant.
Note: All healthy volunteers were not hispanic and not latino. In the placebo group, there 1/10 were Asian and 9/10 White/Caucasian. In the UNI91104 group, there were 2/34 Black/African American, 1/34 Black/African American + White/Caucasian and 31/34 White/Caucasian.
No serious AEs or early discontinuation were reported. In total, 32 healthy volunteers (73%; 5 receiving placebo and 27 UNI91104) experienced one or more AEs during the study, of which 27 healthy volunteers (61%; 2 receiving placebo and 25 UNI91104) presenting at least one AE that was categorized by the investigator as definitely, probably, or possibly related to the study drug or procedure (Table S5). All of the AEs were classified as being mild in intensity and most of them were categorized under “respiratory, thoracic and mediastinal disorders” with “upper respiratory tract irritation” being the most frequent AE descriptor (45 events in 26 healthy volunteers, 59% of all healthy volunteers). The incidence and duration of the upper respiratory tract irritation was dose-dependent for the active drug (Table 2). Nine out of 34 healthy volunteers treated with UNI91104 had a clinically relevant change in safety variables that led to AE reporting. The nasal applications did not result in any clinically relevant findings with regard to local tolerability.
Table 2.
Adverse events by treatment, system organ class and preferred term (safety population).
| Characteristics | Statistic |
UNI91104 0.1%, Cohort 1 (N = 7) |
UNI91104 1.0%, Cohort 2 (N = 7) |
UNI91104 1.0%, Cohort 3 (N = 7) |
UNI91104 1.0%, Cohort 4 (N = 7) |
UNI91104 1.0%, Cohort 5 (N = 6) |
Placebo All cohorts (N = 10) |
Overall Study (N = 44) |
|---|---|---|---|---|---|---|---|---|
| Eye disorders | n (%) E | 0 (0%) 0 | 0 (0%) 0 | 1 (14.3%) 1 | 0 (0%) 0 | 1 (16.7%) 2 | 0 (0%) 0 | 2 (4.5%) 3 |
| Eye irritation | n (%) E | 0 (0%) 0 | 0 (0%) 0 | 1 (14.3%) 1 | 0 (0%) 0 | 1 (16.7%) 2 | 0 (0%) 0 | 2 (4.5%) 3 |
| Gastrointestinal disorders | n (%) E | 1 (14.3%) 1 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 2 (33.3%) 5 | 0 (0%) 0 | 3 (6.8%) 6 |
| Abdominal pain upper | n (%) E | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (16.7%) 1 | 0 (0%) 0 | 1 (2.3%) 1 |
| Diarrhoea | n (%) E | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (16.7%) 1 | 0 (0%) 0 | 1 (2.3%) 1 |
| Gastrooesophageal reflux disease | n (%) E | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (16.7%) 2 | 0 (0%) 0 | 1 (2.3%) 2 |
| Nausea | n (%) E | 1 (14.3%) 1 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (2.3%) 1 |
| Vomiting | n (%) E | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (16.7%) 1 | 0 (0%) 0 | 1 (2.3%) 1 |
| Infections and infestations | n (%) E | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (16.7%) 1 | 1 (10%) 1 | 2 (4.5%) 2 |
| Nasopharyngitis | n (%) E | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (16.7%) 1 | 1 (10%) 1 | 2 (4.5%) 2 |
| Investigations | n (%) E | 0 (0%) 0 | 2 (28.6%) 2 | 0 (0%) 0 | 3 (42.9%) 3 | 4 (66.7%) 10 | 0 (0%) 0 | 9 (20.5%) 15 |
| Blood bilirubin increased | n (%) E | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (14.3%) 1 | 0 (0%) 0 | 0 (0%) 0 | 1 (2.3%) 1 |
| Forced expiratory volume decreased | n (%) E | 0 (0%) 0 | 1 (14.3%) 1 | 0 (0%) 0 | 1 (14.3%) 1 | 2 (33.3%) 6 | 0 (0%) 0 | 4 (9.1%) 8 |
| Forced expiratory volume increased (Reversibility) | n (%) E | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (16.7%) 1 | 0 (0%) 0 | 1 (2.3%) 1 |
| Fractional exhaled nitric oxide increased | n (%) E | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (14.3%) 1 | 3 (50%) 3 | 0 (0%) 0 | 4 (9.1%) 4 |
| Lung diffusion test decreased | n (%) E | 0 (0%) 0 | 1 (14.3%) 1 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (2.3%) 1 |
| Musculoskeletal and connective tissue disorders | n (%) E | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (10%) 1 | 1 (2.3%) 1 |
| Swollen joint | n (%) E | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (10%) 1 | 1 (2.3%) 1 |
| Nervous system disorders | n (%) E | 2 (28.6%) 2 | 1 (14.3%) 1 | 1 (14.3%) 1 | 2 (28.6%) 2 | 1 (16.7%) 2 | 3 (30%) 3 | 10 (22.7%) 11 |
| Dizziness | n (%) E | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (16.7%) 1 | 0 (0%) 0 | 1 (2.3%) 1 |
| Headache | n (%) E | 1 (14.3%) 1 | 1 (14.3%) 1 | 1 (14.3%) 1 | 2 (28.6%) 2 | 1 (16.7%) 1 | 3 (30%) 3 | 9 (20.5%) 9 |
| Syncope | n (%) E | 1 (14.3%) 1 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (2.3%) 1 |
| Respiratory, thoracic and mediastinal disorders | n (%) E | 1 (14.3%) 1 | 4 (57.1%) 4 | 6 (85.7%) 6 | 7 (100%) 7 | 6 (100%) 25 | 2 (20%) 4 | 26 (59.1%) 47 |
| Cough | n (%) E | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (16.7%) 1 | 0 (0%) 0 | 1 (2.3%) 1 |
| Oropharyngeal pain | n (%) E | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (10%) 1 | 1 (2.3%) 1 |
| Upper respiratory tract irritation | n (%) E | 1 (14.3%) 1 | 4 (57.1%) 4 | 6 (85.7%) 6 | 7 (100%) 7 | 6 (100%) 24 | 2 (20%) 3 | 26 (59.1%) 45 |
All events occurring before IMP application are incl. as medical history; E: number of events; N: number of patients within the population dosed with the respective treatment in the safety population; n: number of patients with adverse event;%: Calculated using the number of patients in the safety population as the denominator (n/N x 100). All adverse events are coded using MedDRA version 22.0.
All AEs (Table 2) reported resolved completely, most within 1–2 h of administration. In the multiple administration group, most healthy volunteers reported that symptoms decreased over time with repeated dosing.
Following a single-dose application of UNI91104 at volumes up to 6 mL (cohort 1–4) and repeat-dose application of five doses of 6 mL UNI91104 over 2.5 days (cohort 5), there was no evidence of a difference in change of mean levels of lung function (FEV1 including change pre vs. post β2-agonist, FVC, TLC, DCO, FeNO), resting pulse oximetry, CPET or ECG at post-dose (up to 96 h) relative to placebo (Table S6).
In two healthy volunteers in cohort 2, two healthy volunteers in cohort 4, four healthy volunteers in cohort 5 receiving UNI91104 and one healthy volunteer receiving placebo in cohort 5, abnormal and clinically relevant changes in lung function tests were observed (Table S7). The individual findings included five cases of asymptomatic increases in FeNO (range: 11–37 ppb; 1 on placebo and 4 on active) of which two receiving active were likely associated with undiagnosed asthma. Asymptomatic airway obstruction, defined as a drop in FEV1 with more than 200 mL and 12%, was observed in 4 healthy volunteers: one occurred in cohort 2, 24 h post dosing, one in cohort four, at 3 h post dose and two occurred in cohort 5. In both the subjects in cohort 5 the drop was repeated (in one subject after dose 3 and 5 and in the second subject after dose 4 and 5) and took place majorly 1 hour after the dosing (Fig. S1). These events were resolved either spontaneously without treatment or following treatment with an inhaled short acting β2-agonist and normalized to the pre-dose value in 2/3 healthy volunteers (Fig. S1). Two of the healthy volunteers with a drop in FEV1 also had an increase in FeNO. There was no relationship between an increase in respiratory rate or decrease in oxygen saturation and drops in FEV1, and in none of the healthy volunteers did auscultation of the lungs reveal any pathological findings. In conclusion, the adverse events and changes in safety variables observed in cohort 1–5 were not considered to be of clinical importance nor otherwise concerning by the Safety Monitoring Committee.
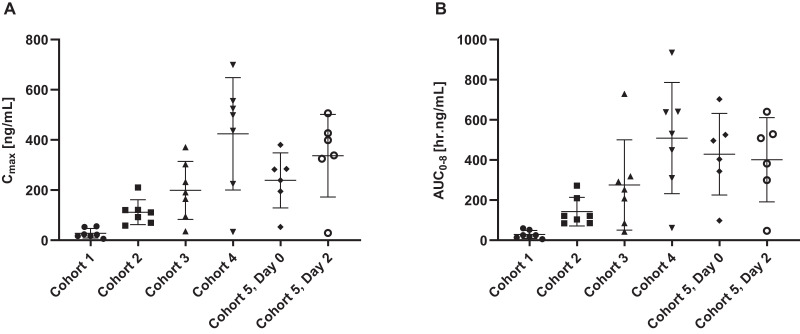
UNI91104 exhibited dose-proportional pharmacokinetics with Cmax, AUC0–8, AUC0-inf increasing in a dose proportional manner. The mean Cmax and AUC0–8 levels following a single dose application of 6 mL UNI91104 in cohort 4 were 424 ng/mL and 509 h.ng/mL, respectively (Fig. 3). Following repeat dosing of 6 mL UNI91104 in cohort 5, Cmax and AUC0–8 levels were 337 ng/mL and 401 h.ng/mL, respectively. The mean accumulation ratio (CV%) of AUC0-inf was 1.05 (10.5), which is close to unity, suggesting no accumulation after repeat dosing of UNI911. The half-life ranged from 0.8 to 3.5 h. A summary of estimated pharmacokinetic parameters is displayed in Table S8.
Fig. 3.
Pharmacokinetic profile (arithmetic mean ± SD) of niclosamide in plasma following UNI91104 administration per cohort.
Doses of each cohort are displayed in Table S3. Abbreviations: AUC = Area under the curve; Cmax = maximum concentration.
4. Discussion
The primary safety assessment indicates that UNI91104 has an acceptable safety profile and is generally well-tolerated in healthy volunteers when administered by inhalation at doses up to 50.4 mg niclosamide (6 mL 1% niclosamide solution) and intranasal administration of up to 2.5 mg niclosamide. All adverse events were mild and the majority was related to irritation in the upper respiratory tract, which were transient and resolved within a few hours.
We observed that combined nebulised and intranasal administration of 52.90 mg niclosamide resulted in similar systemic concentrations as an oral dose of 2 g (niclosamide tablets, Yomesan) [14,15,17]. Thus, potential systemic side effects that may occur after administration of UNI91104 are expected to mimic that of Yomesan, which is generally considered safe and on the WHO list of essential medicines. Of note, the reported treatment-related side effects in this study were acute, local effects and, thus, not systemically derived. This is most likely due to the lower systemic exposure following UNI91104 administration. With respect to the assessment of lung function, three safety focus points were used: airway obstruction or inflammation, restrictive airway impairment, and drug-induced impairment of physical capacity with reduced VO2max. Most of the participants showed no or modest responsiveness to inhalation of UNI91104 with almost unchanged parameters pertaining to lung function and airway inflammation.
Some of the few healthy volunteers experiencing asymptomatic decreases in FEV1 or an increase in FeNO, both acutely and at the follow up measurement (at 48 h post-dose), had childhood asthma but did not have current asthma symptoms nor current anti-asthma treatment. Those with high FeNO at pre-dose who showed signs of bronchoconstriction during drug administration could have been excluded if FeNO had been part of the exclusion criteria, but FeNO was only included as a safety monitoring tool to assess airway damage. However, the changes in both FEV1 and FeNO were asymptomatic and of minor clinical importance, and in clinical situations these patients would not have been precluded for any nebulised treatment.
Although some variation was seen in the level of lung function, we observed no reduction in exercise capacity in any healthy volunteer, even though this measurement was performed following several tiring procedures. Thus, despite some changes in lung function were demonstrated, there were no changes in pulmonary and cardiac fitness (and no desaturation).
Limitations of this study include the small sample size, the short treatment duration, lack of ethnic diversity, and the exclusion of patients with diagnosed respiratory comorbidities, e.g. COPD and asthma.
Asthma patients, who are well-treated with anti-asthma therapy including inhaled steroids according to the Global Initiative for Asthma guidelines, would probably not have airway hyperresponsiveness, not even in a period with respiratory infection [6,18]. Therefore, such patients may not react to inhalation of potential irritating compounds. This is also the case for patients with COPD in which airway hyperresponsiveness is not common. Therefore, in a COVID-19 patient cohort having subjects with both asthma and COPD, primarily patients with unknown or untreated respiratory disease might experience some asymptomatic airway limitation, which would however be of minor clinical importance.
Inhaled niclosamide therapy is expected to induce a higher local exposure in the oropharynx and lungs than oral ingestion of niclosamide. Indeed, a considerable proportion of inhaled drugs is deposited and subsequently absorbed via the oropharynx and lungs, while the remainder is usually swallowed and absorbed via the gastrointestinal tract. Thus, in treatment of respiratory disease, the inhaled route of administration is favored because a larger proportion of the active substance reaches the desired target while reducing the risk of systemic side effects [6]. In case of COVID-19 infection, the primary location of SARS-CoV-2 infection would be the upper and lower airways, and nebulised administration of an anti-COVID-19 drug is beneficial as a high concentration would be achieved where the viral burden is greatest.
The nasal cavity also represents an important area in the establishment of infection with SARS-CoV-2 and from which dissemination to the lower parts of the respiratory tree is likely to occur [19]. If the virus is not eradicated from the nasopharynx it may remain a potential source for re-dissemination to the lungs. Because only a small fraction of inhaled drug is likely to reach the nasopharynx/nasal cavity, inhalation is complemented by administration of niclosamide via a nasal spray.
Moreover, intranasal administration of niclosamide alone could be an effective pre- or postexposure prophylaxis for COVID-19 in addition to the use of the combined inhalation and intranasal administration for treatment of COVID-19. Indeed, a trial examining the prophylactic potential of intranasally applied niclosamide has been initiated already (2020-004144-28). Furthermore, we are also planning to explore the impact of repeated administration of UNI91104 on FeNO and FEV1 with lower doses in subsequent Phase 1 trials and patient trials with mild COVID-19.
Collectively, our findings suggest that UNI91104 is safe and well-tolerated in healthy volunteers with no systemic side effects and mild reversible local side effects. Hence, UNI91104 may be a promising candidate for inhalation and intranasal therapy against COVID-19 and other viral respiratory tract infections but warrants further testing in patient trials.
Author Contributions
VB, MOAS, and AW wrote the manuscript. VB, MOAS, MJ and US conceived the study. VB, TB, JS, VB, DS, HKJ, MH, were involved in the conductance of the clinical trial and interpretation of the data. MOAS and VB accessed and verified the data. All authors reviewed and approved the manuscript.
Declaration of Interests
Dr. Sommer, Ms. Weiss and Mr Jellingsø are shareholders or benefit from an employee incentive scheme in UNION therapeutics. All other authors have nothing to disclose.
Acknowledgments
Data sharing
Individual participant data of this study will be not made available. All relevant data is part of the main text or supplemental material.
Acknowledgment
We would like to thank Wanja Nyoro for the great support in the conduct of the clinical study and Morten Boesen and Rasmus Toft-Kehler for securing funding for this study. We are also grateful for the support from The Novo Nordisk Foundation under NFF grant number: NNF20CC0035580. We are thankful for the hard work, expertise and dedication of Amalie Vedsted-Jakobsen, BaSc and Malene Halgreen Mikkelsen, BaM in the conduct of this study. This trial received funding support from Innovationsfonden Denmark (grant number: 0208-00081 and 0153-00209) and UNION therapeutics.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanepe.2021.100084.
Contributor Information
Vibeke Backer, Email: backer@dadlnet.dk.
Morten Otto Alexander Sommer, Email: morten.sommer@uniontherapeutics.com, msom@bio.dtu.dk.
Appendix. Supplementary materials
References
- 1.World Health Organization . WHO; 2020. Novel coronavirus (COVID-19) situation. [Google Scholar]
- 2.Thorlund K., Dron L., Park J., Hsu G., Forrest J.I., Mills E.J. A real-time dashboard of clinical trials for COVID-19. Lancet Digit Health. 2020:e286–e287. doi: 10.1016/S2589-7500(20)30086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NIH. COVID-19 Treatment Guidelines Panel . National Institutes of Health (NIH); 2020. Coronavirus disease 2019 (COVID-19) treatment guidelines. [PubMed] [Google Scholar]
- 4.DeFrancesco L. COVID-19 antibodies on trial. Nat Biotechnol. 2020:1–11. doi: 10.1038/s41587-020-0732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mason R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J. 2020 doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Global Initiative for Asthma Global initiative for asthma: global strategy for asthma management and prevention (Updated 2020) Rev Fr d'Allergol d'Immunol Clin. 2020;36(6):685–704. [Google Scholar]
- 7.Jeon S., Ko M., Lee J., Choi I., Byun S.Y., Park S. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrob Agents Chemother. 2020 doi: 10.1128/AAC.00819-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gassen N.C., Niemeyer D., Muth D., Corman V.M., Martinelli S., Gassen A. SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-coronavirus infection. Nat Commun. 2019 doi: 10.1038/s41467-019-13659-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gassen Nils C., Papies J., Bajaj T., Dethloff F., Emanuel J., Weckmann K. Analysis of SARS-CoV-2-controlled autophagy reveals spermidine, MK-2206, and niclosamide as putative antiviral therapeutics. bioRxiv. 2020 [Google Scholar]
- 10.Jurgeit A., McDowell R., Moese S., Meldrum E., Schwendener R., Greber U.F. Niclosamide is a proton carrier and targets acidic endosomes with broad antiviral effects. PLoS Pathog. 2012;8(10) doi: 10.1371/journal.ppat.1002976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu J., Shi P.Y., Li H., Zhou J. Broad spectrum antiviral agent niclosamide and its therapeutic potential. ACS Infect Dis. 2020;6(5):909–915. doi: 10.1021/acsinfecdis.0c00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunaugh A.D., Seo H., Warnken Z., Ding L., Seo S.H., Smyth H.D.C. Broad-spectrum, patient-adaptable inhaled niclosamide-lysozyme particles are efficacious against coronaviruses in lethal murine infection models. bioRxiv. 2020 [Google Scholar]
- 13.Niyomdecha N., Suptawiwat O., Boonarkart C., Thitithanyanont A., Auewarakul P. Repurposing of antiparasitic niclosamide to inhibit respiratory syncytial virus (RSV) replication. Virus Res. 2021 doi: 10.1016/j.virusres.2020.198277. [DOI] [PubMed] [Google Scholar]
- 14.Burock S., Daum S., Tröger H., Kim T.D., Krüger S., Rieke D.T. Niclosamide a new chemotherapy agent? Pharmacokinetics of the potential anticancer drug in a patient cohort of the NIKOLO trial. J Clin Oncol. 2018 e14536-e14536. [Google Scholar]
- 15.Schweizer M.T., Haugk K., McKiernan J.S., Gulati R., Cheng H.H., Maes J.L. A phase I study of niclosamide in combination with enzalutamide in men with castration-resistant prostate cancer. PLoS ONE. 2018;13(6) doi: 10.1371/journal.pone.0198389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang Y.W., Yeh T.K., Lin K.T., Chen W.C., Yao H.T., Lan S.J. Pharmacokinetics of anti-SARS-CoV agent niclosamide and its analogs in rats. J Food Drug Anal. 2006;14(4) [Google Scholar]
- 17.Andrews P., Thyssen J., Lorke D. The biology and toxicology of mollucidides, Bayluscide®. Pharmacol Ther. 1983;19(2):245–295. doi: 10.1016/0163-7258(82)90064-x. [DOI] [PubMed] [Google Scholar]
- 18.Reddel H.K., FitzGerald J.M., Bateman E.D., Bacharier L.B., Becker A., Brusselle G. GINA 2019: a fundamental change in asthma management. Eur Respir J. 2019 doi: 10.1183/13993003.01046-2019. [DOI] [PubMed] [Google Scholar]
- 19.Hou Y.J., Okuda K., Edwards C.E., Martinez D.R., Asakura T., Dinnon K.H. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020 doi: 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.