Abstract
The episodic outbreak of COVID-19 due to SARS-CoV-2 is severely affecting the economy, and the global count of infected patients is increasing. The actual number of patients had been underestimated due to limited facilities for testing as well as asymptomatic nature of the expression of COVID-19 on individual basis. Tragically, for emerging economies with high population density, the situation has been more complex due to insufficient testing facilities for diagnosis of the disease. However, the recent reports about persistent shedding of viral RNA of SARS-CoV-2 in the human feces have created a possibility to track the prevalence and trends of the disease in communities, known as wastewater-based epidemiology (WBE). In this article, we highlight the current limitations and future prospects for WBE to manage pandemics.
Keywords: COVID-19, SARS-CoV-2, Prevalence, Wastewater surveillance, Monitoring, Developing Countries
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been identified as the cause of ongoing pandemic of severe pneumonia known as COVID-19. The first COVID-19 cases were reported from China in December 2019, followed by a rapid spread to other countries in a matter of a few weeks. The World Health Organization (WHO) declared the status of this outbreak from epidemic to pandemic on March 11, 2020 [1]. The first confirmed clinically diagnosed case of COVID-19 in the EU was announced on January 24, 2020, and the corona cases have been increasing dramatically in countries across all continents [2].
The currently reported figures are primarily based on patients who have been clinically tested. The asymptomatic nature of COVID-19 among several population cohorts leads to considerable uncertainty in estimation of the actual number of patients and the true situation of COVID-19 spread [1, 3, 4••, 5•]. It is therefore difficult to quickly determine the extent of COVID-19 spread within a population. In emerging economies with high population density such as India, Bangladesh, Pakistan, Brazil, Sri Lanka, and others, the situation is complex, as a significant number of SARS-CoV-2 affected patients are likely to be asymptomatic and oligosymptomatic, which are generally not clinically tested and therefore create uncertainty in the estimation of the disease burden of COVID-19 [6, 7]. First confirmed cases of SARS-CoV-2 RNA in municipal wastewater were reported from Netherlands [8••], Australia [9•], Japan [10•], India [11•], Italy [12•], Spain [13•], Sweden [14•], Bangladesh [15••], and the USA [16, 17•]. These findings were also concomitant to the first officially confirmed clinically diagnosed cases in these countries. These findings could act as a proven concept for potential, although hidden, information on the prevalence of SARS-CoV-2 infections and offer a cost-effective alternative to screen a large number of random individuals in the population. Researchers from Spain identified the genetic material of SARS-CoV-2 in preserved wastewater samples from March 2019 while it was not clinically diagnosed [18•]. Several studies have indicated a close relationship between the fluxes of human viruses in wastewater as well as surface water with the health status of communities [19•, 20•]. The concentration of viruses in both wastewater and surface water bodies could serve as a proxy for monitoring of the circulation of human viruses among the population [7, 16, 18•, 19•, 20•, 21]. Recently, some reports have identified that infection with SARS-CoV-2 is accompanied by persistent shedding of viral RNA in the human feces in 27 to 89% of COVID-19 infected patients at densities from 0.8 to 7.5 log10 gene copies per gram [18•, 22]. In contrast to a long-way process of laboratory testing of individual patients, the presence of SARS-CoV-2 RNA in feces enriches to sewage water. This creates a promising opportunity to investigate the genetic constituents of SARS-CoV-2 in wastewater [23••]. This approach is referred as the wastewater-based epidemiology (WBE) and could be used for surveillance of the enteric viruses in environmental matrices [5•, 8••, 9•, 10•, 12•, 13•, 14•, 15••, 16, 17•, 23, 24••, 25–28].
Risk From Wastewater Spillage in Developing Countries
Around 20% people are still open defecating in the Central and Southern Asia and and Sub-Saharan Africa regions, and on the other hand, in the Sub-Saharan Africa region, only 20% of the total population have improved hygienic sanitation facilities [29–31]. In the Central and Southern Asia region, only 40% of the urban population have improved hygienic facilities whereas the hygienic sanitation facilities are almost absent. As a result, the transfer of human feces enriched wastewater from non-sewered sources to unplanned discharge places, in most cases, pose a potential threat for contamination of the surface water sources, [24••, 32••, 33•]. It is important to mention that in several developing countries, a significant proportion of population are dependent on groundwater sources for drinking purpose without adequate purification and thus in a potentail rsk of exposure to different types of contaminants. In this particular context, it is imperative to look at the possible of the presence of any virus as indicated in different literature in the past concerning the presence of viruses in groundwater [34, 35], including the genetic materials of SARS-CoV-2 virus in the groundwater system [32••].
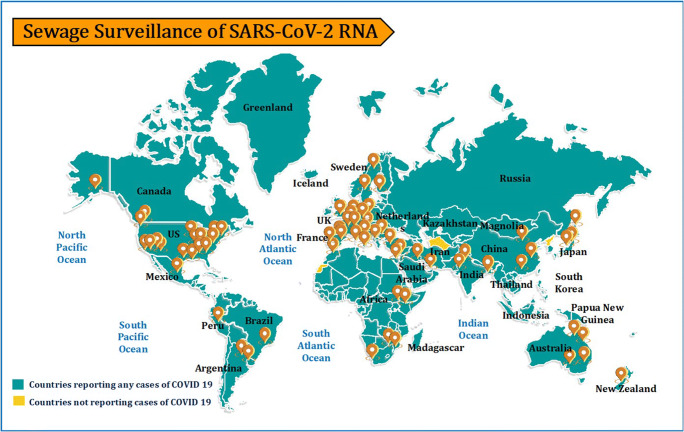
The detection of the SARS-CoV-2 virus via RNA in environmental waters has so far not been proven as a potential pathway transmission of COVID-19, and still, there is a need for international and national efforts for assessing the risk from SARS CoV-2 being spread through water [34, 35]. Studies carried out over the past decades have indicated that viruses exhibit very different survivability characteristics, and the surrogates may not accurately predict the environmental fate of the pathogenic virus. Quantitative risk assessments should be conducted for highly pathogenic enveloped viruses in wastewater, recreational waters, and drinking waters, and also in the groundwater. In order to develop both capacity and proper institutional setup in different countries worldwide, national sewage and wastewater authorities should be encouraged to develop the capacity as well as tools to monitor wastewater (Fig. 1). Despite the growing volume of literature on WBE, little is yet known about how COVID-19 spreads or travels through groundwater resources, not to mention its potential repercussions for groundwater management [33•, 36]. Considering the current challenges for using wastewater as a tool for the surveillance of the SARS-CoV-2 are to a major extent due to analytical methods, and more specifically related to:
RNA isolation step: the most limited and less known step is the isolation of nucleic acid. Therefore, this step should be improved resulting in more cost-effective, and coverage of the method should be calculated with standards [14, 37, 38].
Recovery efficiency during isolation of RNA: there are different approaches to concentrate viruses before RNA isolation step; however, their recovery efficiency needs to be investigated [14, 37, 38].
The estimation tool linking the population data and sewage data; modeling for an outbreak and sewage estimation should be linked.
The microbiology of the built environment: improving our understanding of viral communities in the built environment is needed, specifically which viruses are present and their sources, spatial and temporal dynamics, and interactions with the bacterial population [39].
Fig. 1.
Location of institutions in the world, including the emerging economies, conducting sewage surveillance for early detection of SARS-CoV-2 outbreak (adopted from CDC—https://www.cdc.gov/coronavirus/2019-ncov/global-covid-19/world-map.html)
What Are the Potential Role and Limitations of WBE Applications?
WBE application provides an opportunity to monitor the trend of SARS-CoV-2 spread in communities especially in areas with non-sewered sanitation, through developing a systematic approach, which includes identification of appropriate locations, sampling procedures, preservations from the sites to the laboratory, isolation of the SARS-CoV-2 genetic materials, and recovery. In order to achieve an optimal result from WBE applications, it is important to detect, quantify, and analyze the trends in the spatial and temporal abundance and variability of the genetic materials of the SARS-CoV-2 in environmental water. Some important factors which need to be considered are:
Developing standard protocol for sample collection and robust methods for extraction of the viral RNA and analysis of the target genetic materials
Physiochemical characteristics of the water such as Chemical Oxygen Demand (COD), Total Kjelldahl Nitrogen (TKN), Total Dissolved Solids (TDS), and Total Suspended Solids (TSS) used to calculate the served population which is strictly connected to human feces which could improve the accurate estimation; and
Demographic details and actual data of the clinically diagnosed COVID-19 patients in a particular area or locality necessary to analyze the consistency of the results for the communities.
WBE surveillance in sewage complement the current clinical surveillance, on patients with SARS-CoV-2, demonstrated that the increase of the average viral load of SARS-CoV-2 in wastewater samples with respect to time precisely followed the increase in the number of fatal cases of SARS-CoV-2. WBE can be successfully applied only by data sharing and coordination of methodologies among different country and society.
The presence of SARS-CoV-2 in wastewater is detected before the beginning of the exponential growth of the epidemic [3]. Same aspects have been observed experimentally with the circulation of SARS-CoV-2 in wastewater even before the first cases were reported by authorities [13•]. Thus, a simple theoretical approach of WBE methodology starts from the concentration of SARS-CoV-2 (converted into gene copies/m3) measured in municipal wastewater taken along the network or at the inlet of a WWTP, but at a point that represents a known urbanized area drained by the sewer system. The daily viral load in wastewater (expressed in copies/d) is then calculated. Unfortunately, analytical data later confirmed that the load of SARS-CoV-2 virus in feces is highly variable. Despite of all these challenges, detection and monitoring of genetic material of SARS-CoV-2 in wastewater could be deployed as a system for surveillance of the prevalence of the disease burden in the communities [40••].
In summary, WBE could be a promising tool for SARS-CoV-2 surveillance in wastewater, but extensive and highly coordinated studies are required, including the quantification of individual virus load in feces and during the disease, because this information is very uncertain at the moment but is fundamental for accurate estimations. It is also important to mention that the origin of many natural resource management problems, particularly water and sanitation challenges in developing countries, are related to the governance of the service delivery provisions, which also pose risk for increased vulnerability to COVID-19, lacking adequate mechanisms for governing the COVID-19 crisis [41, 42].
Way Forward
Several scientific pieces of literature on the occurrence of contaminants originating from pit latrines and the factors affecting the transport of these contaminants conclude with the note that they may negatively affect human health. Sewage can directly enter natural water systems due to lack of wastewater treatment plant, overflow, or complete lack of the sewer network providing a pathway for groundwater contamination. Hydrogeological conditions for any particular area play a key role in setting the safe distance from drinking water sources like tube well to pit latrine, and accordingly, the horizontal distances and depth vary from site to site [4••, 41, 43, 44, 45••]. Therefore, WBE tool can provide a new horizon of learning about the characteristics of novel viruses like SARS-CoV-2 and its presence of the genetic materials in groundwater in the developing countries while implementing the provision of safe drinking water service delivery, from groundwater sources without any prior treatment.
Development of an international platform for research collaboration can bring quick and positive result where every individual community or country would be encouraged to contribute depending on their ability and more importantly by sharing each other’s knowledge and expertise. At the same time, considering the urgency and the nature of the spread of the SARS-CoV-2 and other similar types of viruses, it is imperative to develop a rapid and economic WBE tool for monitoring the status and trends of COVID-19 mass infection level despite the current challenges faced by the pioneering countries in this particular context. Some of these challenges include a statistically representative sampling of sewage, size of the population, and the volume of sewage water. Considering the existing situation and the availability of technical knowledge and financial limitation, the global community needs to develop a WBE tool to detect and monitor the spread of COVID-19 without further delay.
Acknowledgements
We acknowledge the SciLifeLab and the WaterCenter@KTH and Unicef for providing the support to initiate the action research on the tracing the SARS-CoV-2 in sewage and environmental water systems.
Funding
Open access funding provided by Royal Institute of Technology.
Declarations
Conflict of Interest
Prosun Bhattacharya, Manish Kumar, M. Tahmidul Islam, Rehnuma Haque, Sudip Chakraborty, Arslan Ahmad, Nabeel Khan Niazi, Zeynep Cetecioglu, David Nilsson, Julian Ijumulana, Tom van der Voorn, Md. Jakariya, Maqsud Hossain, Firoz Ahmed, Mahbubur Rahman, Nargis Akter, Dara Johnston, and Kazi Matin Ahmed declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Prosun Bhattacharya and Manish Kumar share first authorship.
This article is part of the Topical Collection on Water Pollution
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prosun Bhattacharya and Manish Kumar contributed equally to this work.
Contributor Information
Prosun Bhattacharya, Email: prosun@kth.se.
Manish Kumar, Email: manish.kumar@iitgn.ac.in.
Md. Tahmidul Islam, Email: mtisl@kth.se, Email: tahmidulislam@wateraid.org.
Rehnuma Haque, Email: rehnuma.haque@icddrb.org.
Sudip Chakraborty, Email: sudip.chakraborty@unical.it.
Arslan Ahmad, Email: Arslan.Ahmad@wur.nl, Email: arslan.ahmad@sibelco.com.
Nabeel Khan Niazi, Email: nabeelkniazi@gmail.com, Email: NabeelKhan.Niazi@usq.edu.au.
Zeynep Cetecioglu, Email: zeynepcg@kth.se.
David Nilsson, Email: david.nilsson@abe.kth.se.
Julian Ijumulana, Email: julianij@kth.se.
Tom van der Voorn, Email: tom.van.der.voorn@uni-osnabrueck.de.
Md. Jakariya, Email: md.jakariya@northsouth.edu
Maqsud Hossain, Email: muhammad.maqsud@northsouth.edu.
Firoz Ahmed, Email: firoz19701016@gmail.com.
Mahbubur Rahman, Email: mahbubr@icddrb.org.
Nargis Akter, Email: nakter@unicef.org.
Dara Johnston, Email: djohnston@unicef.org.
Kazi Matin Ahmed, Email: kmahmed@du.ac.bd.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.WHO. Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases2020. Report No.: WHO/COVID-19/laboratory/2020.4.
- 2.Lescure F-X, Bouadma L, Nguyen D, Parisey M, Wicky P-H, Behillil S, Gaymard A, Bouscambert-Duchamp M, Donati F, le Hingrat Q, Enouf V, Houhou-Fidouh N, Valette M, Mailles A, Lucet JC, Mentre F, Duval X, Descamps D, Malvy D, Timsit JF, Lina B, van-der-Werf S, Yazdanpanah Y. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. The Lancet Infectious diseases. 2020;20(6):697–706. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wurtzer S, Marechal V, Mouchel JM, Maday Y, Teyssou R, Richard E, et al. Evaluation of lockdown impact on SARS-CoV-2 dynamics through viral genome quantification in Paris wastewaters. medRxiv. 2020. 10.1101/2020.04.12.20062679. [DOI] [PMC free article] [PubMed]
- 4••.Wu Y, Guo C, Tang L, Hong Z, Zhou J, Dong X, et al. Prolonged presence of SARSCoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5•.Kumar M, Mohapatra S, Mazumder P, Singh A, Honda R, Lin C, Kumari R, Goswami R, Jha PK, Vithanage M, Kuroda K. Making waves perspectives of modeling and monitoring of SARS-CoV-2 in aquatic environment for COVID-19 pandemic. Curr Pollution Rep. 2020;6:468–479. doi: 10.1007/s40726-020-00161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, Hoelscher M, Bleicker T, Brünink S, Schneider J, Ehmann R, Zwirglmaier K, Drosten C, Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang W, Du RH, Li B, Zheng XS, Yang XL, Hu B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9(1):386–389. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8••.Medema G, Heijnen L, Elsinga G, Italiaander R, Brouwer A. Presence of SARS-coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ Sci Technol Lett. 2020:7. 10.1021/acs.estlett.0c00357This article is one of the premier publications that reports the detection of SARS-CoV-2 virus RNA in sewage and correlation with reported prevalence of COVID-19 cases that demonstrates wastewater surveillance as a sensitive tool to monitor the circulation of the virus in the society. [DOI] [PubMed]
- 9•.Ahmed W, Angel N, Edson J, Bibby K, Bivins A, O'Brien JW, et al. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci Total Environ. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Haramoto E, Malla B, Thakali O, Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci Total Environ. 2020;737:140405. doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11•.Kumar M, Patel AK, Shah AV, Raval J, Rajpara N, Joshi M, Joshi CG. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Sci Total Environ. 2020;746:141326. doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12•.La Rosa G, Iaconelli M, Mancini P, Bonanno Ferraro G, Veneri C, Bonadonna L, et al. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci Total Environ. 2020;736:139652. doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13•.Randazzo W, Truchado P, Cuevas-Ferrando E, Simón P, Allende A, Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181:115942. doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14•.Jafferali MH, Khatami K, Atasoy M, Birgersson M, Williams C, Cetecioglu Z. Benchmarking virus concentration methods for quantification of SARS-CoV-2 in raw wastewater. Sci Total Environ. 2020;755:142939. doi: 10.1016/j.scitotenv.2020.142939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15••.Ahmed F, Islam MA, Kumar M, Hossain M, Bhattacharya P, Islam MT, et al. First detection of SARS-CoV-2 genetic material in the vicinity of COVID-19 isolation centre in Bangladesh: variation along the sewer network. Sci Total Environ. 2021:145724. 10.1016/j.scitotenv.2021.145724This article highlights the first detection of SARS-CoV-2 genetic material along with the sewer network as well as the decay/accumulation processe in primary to tertiary drains from developing country perspectives. [DOI] [PMC free article] [PubMed]
- 16.Wu FQ, Xiao A, Zhang JB, Gu XQ, Lee WL, Kauffman K, et al. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. medRxiv. 2020. 10.1101/2020.04.05.20051540. [DOI] [PMC free article] [PubMed]
- 17•.Sherchan SP, Shahin S, Ward LM, Tandukar S, Aw TG, Schmitz B, et al. First detection of SARS-CoV-2 RNA in wastewater in North America: a study in Louisiana, USA. Sci Total Environ. 2020;743:140621. doi: 10.1016/j.scitotenv.2020.140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Rimoldi SG, Stefani F, Gigantiello A, Polesello S, Comandatore F, Mileto D, et al. Presence and vitality of SARS-CoV-2 virus in wastewaters and rivers. medRxiv. 2020. 10.1101/2020.05.01.20086009This study carried out in Milano Metroplitan area indicates the gradual decrease of SARS-CoV-2 viral RNA in raw wastewater and river water samples after 8 days and natural decay of viral pathogenicity in time from emission.
- 19•.Prevost B, Lucas FS, Goncalves A, Richard F, Moulin L, Wurtzer S. Large scale survey of enteric viruses in river and waste water underlines the health status of the local population. Environ Int. 2015;79:42–50. doi: 10.1016/j.envint.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 20•.Hellmér M, Paxéus N, Magnius L, Enache L, Arnholm B, Johansson A, et al. Detection of pathogenic viruses in sewage provided early warnings of hepatitis A virus and norovirus outbreaks. Appl Environ Microbiol. 2014;80(21):6771–6781. doi: 10.1128/aem.01981-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lodder W, Rutjes S, Takumi K, de Roda Husman AM. Aichi virus in sewage and surface water, the Netherlands. Emerg Infect Dis. 2013;19:1222–1230. doi: 10.3201/eid1908.130312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng S, Fan J, Yu F, Feng B, Lou B, Zou Q, Xie G, Lin S, Wang R, Yang X, Chen W, Wang Q, Zhang D, Liu Y, Gong R, Ma Z, Lu S, Xiao Y, Gu Y, Zhang J, Yao H, Xu K, Lu X, Wei G, Zhou J, Fang Q, Cai H, Qiu Y, Sheng J, Chen Y, Liang T. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. Bmj. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23••.Bivins A, North D, Ahmad A, Ahmed W, Alm E, Been F, Bhattacharya P, et al. Wastewater-based epidemiology: global collaborative to maximize contributions in the fight against COVID-19. Environ Sci Technol. 2020;54(13):7754–7757. doi: 10.1021/acs.est.0c02388. [DOI] [PubMed] [Google Scholar]
- 24••.Kitajima M, Ahmed W, Bibby K, Carducci A, Gerba CP, Hamilton KA, et al. SARS-CoV-2 in wastewater: State of the knowledge and research needs. Sci Total Environ. 2020;739:139076. doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar M, Mazumder P, Mohapatra S, Thakur AK, Dhangar K, Taki K, Mukherjee S, Patel AK, Bhattacharya P, Mohapatra P, Rinklebe J, Kitajima M, Hai FI, Khursheed A, Furumai H, Sonne C, Kuroda K. A chronicle of SARS-CoV-2: Seasonality, environmental fate, transport, inactivation, and antiviral drug resistance. J Hazardous Mater. 2021;405:124043. doi: 10.1016/j.jhazmat.2020.124043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mccall C, Wu H, Miyani B, Xagoraraki I. Identification of multiple potential viral diseases in a large urban center using wastewater surveillance. Water Res. 2020;184:116160. doi: 10.1016/j.watres.2020.116160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xagoraraki I, O’Brien E. Wastewater-based epidemiology for early detection of viral outbreaks. In: O’Bannon DJ, editor. Women in water quality: investigations by prominent female engineers. Cham: Springer International Publishing; 2020. pp. 75–97. [Google Scholar]
- 28.Tang A, Tong ZD, Wang HL, Dai YX, Li KF, Liu JN, Wu WJ, Yuan C, Yu ML, Li P, Yan JB. Detection of novel coronavirus by RT-PCR in stool specimen from asymptomatic child, China. Emerg Infect Dis. 2020;26(6):1337–1339. doi: 10.3201/eid2606.200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.JMP . Joint Monitoring Programme for Water Supply || Sanitation and hygiene (JMP) 2019. [Google Scholar]
- 30.UNICEF. Safer sanitation and hygiene - Quality, equitable access and sustainability [database on the Internet] 2020; Available from: https://www.unicef.org/bangladesh/en/better-access-safe-drinking-water/safer-sanitation-and-hygiene). Accessed: 29 Aug 2020
- 31.UNICEF/WHO. Progress on household drinking water, sanitation and hygiene 2000-2017: special focus on inequalities. World Health Organization. 2019.
- 32••.Tran HN, Le GT, Nguyen DT, Juang R-S, Rinklebe J, Bhatnagar A, et al. SARS-CoV-2 coronavirus in water and wastewater: a critical review about presence and concern. Environmental Research. 2021;193:110265. doi: 10.1016/j.envres.2020.110265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33•.Kumar M, Thakur AK, Mazumder P, Kuroda K, Mohapatra S, Rinklebe J, et al. Frontier review on the propensity and repercussion of SARS-CoV-2 migration to aquatic environment. J Hazard Mater Lett. 2020:1. 10.1016/j.hazl.2020.100001This paper discusses about the survival and fate of the enveloped SARS-CoV-2 virus and compared with other highly persistent fecally transmitted viruses are in the aquatic environment. [DOI] [PMC free article] [PubMed]
- 34.Abbaszadegan M, Lechevallier M, Gerba C. Occurrence of viruses in US groundwaters. Journal AWWA. 2003;95(9):107–120. doi: 10.1002/j.1551-8833.2003.tb10458.x. [DOI] [Google Scholar]
- 35.Fout GS, Martinson BC, Moyer MW, Dahling DR. A multiplex reverse transcription-PCR method for detection of human enteric viruses in groundwater. Appl Environ Microbiol. 2003;69(6):3158–3164. doi: 10.1128/aem.69.6.3158-3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.US/EPA. Virus attachment, release, and inactivation during groundwater transport, Research Project Database 2013.
- 37.Fong TT, Lipp EK. Enteric viruses of humans and animals in aquatic environments: health risks, detection, and potential water quality assessment tools. Microbiol Mol Biol Rev. 2005;69(2):357–371. doi: 10.1128/mmbr.69.2.357-371.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmed W, Bertsch PM, Bivins A, Bibby K, Farkas K, Gathercole A, Haramoto E, Gyawali P, Korajkic A, McMinn BR, Mueller JF, Simpson SL, Smith WJM, Symonds EM, Thomas KV, Verhagen R, Kitajima M. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci Total Environ. 2020;739:139960. doi: 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prussin AJ, 2nd, Belser JA, Bischoff W, Kelley ST, Lin K, Lindsley WG, et al. Viruses in the Built Environment (VIBE) meeting report. Microbiome. 2020;8(1):1. doi: 10.1186/s40168-019-0777-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40••.Nghiem LD, Morgan B, Donner E, Short MD. The COVID-19 pandemic: considerations for the waste and wastewater services sector. Case Stud Chem Environ Eng. 2020;1:100006. doi: 10.1016/j.cscee.2020.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Islam MS, Mahmud ZH, Islam MS, Saha GC, Zahid A, Ali AZ, et al. Safe distances between groundwater-based water wells and pit latrines at different hydrogeological conditions in the Ganges Atrai floodplains of Bangladesh. J Health Popul Nutr. 2016;35:26. doi: 10.1186/s41043-016-0063-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhattacharjee S, Saha B, Saha B, Uddin MS, Panna CH, Bhattacharya P, Saha R. Groundwater governance in Bangladesh: established practices and recent trends. Groundwater for Sustainable Development. 2019;8:69–81. doi: 10.1016/j.gsd.2018.02.006. [DOI] [Google Scholar]
- 43.Patel AK, Das N, Kumar M. Multilayer arsenic mobilization and multimetal co-enrichment in the alluvium (Brahmaputra) plains of India: a tale of redox domination along the depth. Chemosphere. 2019;224:140–150. doi: 10.1016/j.chemosphere.2019.02.097. [DOI] [PubMed] [Google Scholar]
- 44.Hossain M, Rahman SN, Bhattacharya P, Jacks G, Saha R, Rahman M. Sustainability of arsenic mitigation interventions – an evaluation of different alternative safe drinking water options provided in Matlab, an arsenic hot spot in Bangladesh. Front Environ Sci. 2015;3:30. doi: 10.3389/fenvs.2015.00030. [DOI] [Google Scholar]
- 45.Saha R, Dey NC, Rahman M, Bhattacharya P, Rabbani GH. Geogenic arsenic and microbial contamination in drinking water sources: exposure risks to the coastal population in Bangladesh. Front Environ Sci. 2019;7:57. doi: 10.3389/fenvs.2019.00057. [DOI] [Google Scholar]