Abstract
Purpose of Review
We examined data from the last 5 years describing extracorporeal life support (ECLS) as a bridge to lung transplantation. We assessed predictors of survival to transplantation and post-transplant mortality.
Recent Findings
The number of lung transplants performed worldwide is increasing. This is accompanied by an increase in the type of patients being transplanted, including sicker patients with more advanced disease. Consequently, there is an increase in the need for bridging strategies, with varying success. Several predictors of failure have been identified. Major risk factors include retransplantation, other organ dysfunction, and deconditioning.
Summary
ECLS is a risky strategy but necessary for patients who would otherwise die if not bridged to transplantation. The presence of predictors for failure is not a contraindication for bridging. However, major risk factors should be approached cautiously. Other, more minor risk factors may be considered acceptable. More importantly, the strategy should be individualized for each patient to achieve the best possible outcomes.
Keywords: Lung transplantation, Extracorporeal membrane oxygenation (ECMO), Extracorporeal life support (ECLS), Bridge to transplant
Introduction
Lung transplantation is the definitive treatment for patients with end-stage lung disease [1]. Donor availability remains an impediment in the field of lung transplantation. This discrepancy between organ supply and demand can result in prolonged waitlist times for patients with end-stage lung disease [2]. Due to the shortage of donors, patients who are candidates for lung transplantation may deteriorate or even die while waiting for a suitable organ. Ideally, these individuals could be provided with a form of life support that would allow them to continue rehabilitation, maintain nutrition, and improve strength while awaiting a lung transplant. This clinical scenario has become known as “bridge to transplantation” (BTT) and was conceived to address the problem of waitlist mortality in the critically ill.
Extracorporeal Life Support as a Bridge to Transplant
Current Literature
Since 2009, the use of extracorporeal membrane oxygenation (ECMO) has become more prevalent (https://www.elso.org/Registry/Statistics.aspx, accessed October 7, 2020). This can largely be traced to the two significant events which do not pertain directly to lung transplantation or bridging strategies. The first is the CESAR trial publication, which showed improved outcomes when patients with severe acute respiratory illness are managed at regionalized centers with extracorporeal life support (ECLS) expertise [3]. The second is the emergence of the worldwide H1N1 pandemic [4]. Both the CESAR trial and increased generational experience laid the groundwork for the application of ECLS to bridge patients to transplant.
Critically ill patients who require BTT are challenging patients with significant potential for complications. An important consideration for this group is deconditioning during the bridging period, manifesting as critical illness myopathy (CIM). This is particularly true when high ventilator settings require deep sedation and less chance of physical therapy participation. The ultimate appeal of ECMO is based on the potential to reduce ventilatory and sedation requirements, thus facilitating ambulation and therefore curtailing the usual pattern of deconditioning that afflicts so many critically ill patients. It seems intuitive that patients who are bed-bound would be at higher risk of developing postoperative CIM, have a more difficult recovery, and more complications following transplant. Studies on this topic report that rates of CIM in patients receiving ECMO can range from 30 to 75% [5, 6]. CIM is of interest for centers that utilize bridging strategies because of its negative impact on patient outcomes. Rehder et al. examined the effect of active rehabilitation during ECMO as BTT [7•]. In this study, physical therapy participation during ECMO BTT is associated with shorter post-transplant mechanical ventilation, intensive care unit (ICU), and hospital length of stay. This could be interpreted in many ways; however, one possible explanation is that patients who undergo rehabilitation during the bridging period may avert CIM postoperatively, translating into a shorter time on the ventilator and better outcomes. Indeed, more recent data from multiple centers have shown improvements in successful bridging and improved post-transplant results [8••, 9••, 10, 11]. In those studies, physical therapy participation is associated with improved outcomes, possibly related to reduced CIM and faster recovery following transplantation.
Many studies have recently been published that show the feasibility and safety of ECLS as BTT (Table 1). The studies demonstrate that bridging patients using ECLS can achieve long-term survival comparable with patients who do not require BTT. Compared with non-bridged patients, the most significant difference in mortality appears in the first 6 months after transplant, with flattening of the survival curve subsequently. Additionally, there is an increased incidence of primary graft dysfunction (PGD) in the bridged patients, with rates of PGD grade 3 at 72 h as high as 57% [8••]. While these results are inferior to patients who are not bridged to transplant on ECMO, it is readily understandable why these complex patients would experience higher mortality and PGD in the immediate perioperative period. Using these points, one can make a similar case for mechanical ventilation as a bridging strategy. Indeed, in patients with hypercarbia, mechanical ventilation with a tracheostomy can be achieved with low settings and low sedation, potentially allowing for physical therapy and ambulation. However, in patients with hypoxemia and higher ventilatory settings, sedation requirements are much higher, and physical therapy is less feasible when mechanical ventilation is used alone as BTT. ECMO’s one advantage over mechanical ventilation in this setting is the potential to ambulate more readily, thereby mitigating the effect of deconditioning (Table 2).
Table 1.
Summary of studies describing the use of extracorporeal life support (ECLS) as a bridge to transplant
| Study | N | Median age in years (range) | Median duration of ECLS in days (range) | BTT type | Disease | Survived to transplant | PGD grade 3 at 72 h | Short-term mortality | Long-term survival |
|---|---|---|---|---|---|---|---|---|---|
| Inci et al. (2015) [12] | 30 | 44.5 (14–65) | 21 (1–81) |
VV-ECMO: 33% VA-ECMO: 13% NovaLung: 17% Combination: 23% Unknown: 13% |
COPD: 12% PH: 4% CF: 46% ILD: 38% |
87% | PGD 2/3 at 72 hours: 23% | 12% 30-day mortality |
68% 1-year survival 53% 2-year survival |
| Dellgren et al. (2015) [5] | 20 | 42 (25–59) | 9 (1–229) |
pVV-ECMO: 70% (10% patients converted to pVA-ECMO) pVA-ECMO: 30% |
PH: 10% CF: 15% ILD:60% |
80% | NA | 1% in-hospital mortality |
1-year survival 75% 2-year survival 70% |
| Hayanga et al. (2015) [13] | 119 | 51 (IQR 34–60) | NA | NA |
COPD:15% CF: 17% ILD: 40% |
NA | NA | NA |
1-year survival: 2000-2002: 25% 2003-2005: 77% 2006-2008: 47% 2009-2011: 74% |
| Biscotti et al. (2017) [14] | 72 | 42 (SD ± 15) | 12 |
VV-ECMO: 63% VA-ECMO: 32% VAV-ECMO: 4% PA-LA: 1% |
CF: 38% ILD: 42% |
55.6% | NA | In hospital mortality 7.5% | 2-year survival: 82% |
| Todd et al. (2017) [15] | 12 | 62 (IQR 55–68) | 2 (1–16) |
sVV-ECMO: 75% dVV-ECMO: 17% VA-ECMO: 8% |
CF: 8% ILD: 83.3% |
100% | 33% | 90-day survival 100% | 1 year survival 100% |
| Hayanga et al. (2018) [16] | 49 | Mean: 44.8 (SD ± 13.5) | NA | NA |
COPD: 6% PH: 4% CF: 31% ILD: 59% |
NA | NA |
30-day mortality: 6% 90-day mortality: 10% |
1-year survival: 82% 5-year survival: 66% |
| Hoetzenecker et al. (2018) [8••] | 71 | Mean: 38 (18–62) | 10 (0–95) |
ECCO2R: 6% AV pumpless Novalung: 10% sVV-ECMO: 32% dVV-ECMO: 10% VA-ECMO: 10% PA-LA: 13% Combination: 18% |
COPD: 1% PH: 18% CF: 24% ILD: 37% Re-TXP: 16% |
89% | 57% | NA |
1-year survival: 76% 3-year survival: 68% 5-year survival: 55% |
| Hakim et al. (2018) [10] | 30 | Mean: 46 (SD ± 14) | 8 (0–126) |
sVV-ECMO: 19 dVV-ECMO: 6 VA-ECMO: 5 |
PH: 7% CF: 7% PF: 63% ARDS: 10% Re-TXP: 13% |
87% | 16% |
30-day mortality after cannulation: 13% 30-day post-transplant mortality: 8% |
1-year survival 85% 3-year survival 80% |
| Benazzo et al. (2019) [17] | 120 |
1st era (1998–2004): 35 (IQR 13–56) 2nd era (2005–2010): 31 (IQR 14–66) 3rd era (2010–2017): 36 (IQR 8–68) |
1st era: 2 (1–4) 2nd era: 6 (1–63) 3rd era: 5 (1–80) |
1st era: dVV-ECMO: 10% VA-ECMO: 90% 2nd era: sVV-ECMO: 10% dVV-ECMO: 33% VA-ECMO: 36% PA-LA: 3% Combination: 10% 3rd era: sVV-ECMO: 9% dVV-ECMO: 24% VA-ECMO: 17% PA-LA: 34% Combination: 20% |
1st era CF: 27% Unspecified: 73% 2nd era: COPD: 5% PH: 10% CF: 36% ILD 51% Re-TXP: 18% 3rd era: COPD: 1% PH: 10% CF: 38% ILD: 30% Re-TXP: 13% |
89% |
5.6% (26% no grade) |
1st era 90-day mortality 45% 2nd era 90-day mortality: 27% 3rd era 90-day mortality: 15% |
1-year survival 1st era: 54% 2nd era: 51% 3rd era: 77% 5-year survival 1st era: 22% 2nd era: 42% 3rd era: 65% |
| Tipogarf et al. (2019) [9••] | 121 | 44 (IQR 30–58) | 12 (IQR 6–24) |
VV-ECMO: 52% VA-ECMO: 43% VAV-ECMO: 2.5% RA-LA: 2% PA-LA: 1% |
COPD: 3% PH: 12% CF: 36% ILD: 59% |
59% | In-hospital mortality: 9% |
1-year survival: 88% 3-year survival: 83% |
|
| Langer et al. (2019) [18] | 34 | Mean 42.8 (SD ± 13.5) | 29 (0–129) |
sVV-ECMO: 14% dVV-ECMO: 80% VAV-ECMO: 6% |
COPD: 9% PH: 3% CF: 41% ILD: 35% ReTp: 12% |
85% | In-hospital mortality: 18% |
1-year survival: 79% 3-year survival: 63% |
|
| Kukreja et al. (2020) [19] | 76 | 53 (45.6–61.1) | 9.5 (4–22) |
VV-ECMO: 45% VA-ECMO: 55% |
COPD: 2% PH: 3% CF: 10% ILD: 74% |
68% |
30-day mortality: 2% Survival to discharge: 90% |
1-year survival 86% |
ARDS acute respiratory distress syndrome, AV arterio-venous, BTT bridge to transplantation, CF cystic fibrosis, COPD chronic obstructive pulmonary disease, dVV-ECMO dual-site venovenous extracorporeal membrane oxygenation, ECCO2R extracorporeal carbon dioxide removal, ILD interstitial lung disease, IQR interquartile range, PA-LA pulmonary artery to left atrium bypass with Novalung device, PH pulmonary hypertension, PGD primary graft dysfunction, pVA-ECMO peripheral venoarterial extracorporeal membrane oxygenation, pVV-ECMO peripheral venovenous extracorporeal membrane oxygenation, RA-LA right atrium to left atrium bypass, Re-Txp retransplantation, sVV-ECMO single-site venovenous extracorporeal membrane oxygenation (Avalon cannula), VA venoarterial membrane oxygenation, VAV-ECMO veno-arterial-venous extracorporeal membrane oxygenation, VV-ECMO venovenous extracorporeal membrane oxygenation
Table 2.
A table showing risk factors for ECMO-BTT, including relevant references. The risk factors are separated into major risk factors of substantial risk and less major risk factors which may be mitigated and accepted
| Risk factor | Comment | References |
|---|---|---|
| Major risk factors | ||
| Retransplantation versus first transplantation | Patients bridged to second transplantation have a substantially reduced survival compared with primary transplant recipients. | Hoetzenecker et al. (2018) [8••] |
| Additional organ system dysfunction | Patients with other organ dysfunction, such as liver or kidney dysfunction, carry an increased risk of morbidity and mortality. Certainly, irreversible organ dysfunction should be strongly considered as a contra-indication to BTT |
Tipograf et al. (2019) [9••] Kukreja et al. (2020) [19] |
| Deconditioning | Quantifying the level of deconditioning is difficult. However, the higher the potential for deconditioning during the bridging period, the higher is the incidence of CIM postoperatively. Participating in physical therapy may mitigate the risks associated with deconditioning during BTT |
Rehder et al. (2013) [7•] Hoetzenecker et al. (2018) [8••] Tipograf et al. (2019) [9••] Hakim et al. (2018) [10] Hayes at el. (2016) [11] |
| Minor risk factors | ||
| Type of ECMO-BTT | Venovenous ECMO is associated with improved survival compared with more advanced support. VA-ECMO denotes a more critically-ill patient, and naturally, suggests less success | Kukreja et al. (2020) [19] |
| Center volume/experience | Centers with more experience with lung transplants and ECMO are likely to have greater success using ECMO as BTT. This is true with more complex patients that require more advanced ECLS. |
Hayes et al. (2016) [11] Langer et al. (2019) [18] Hayanga et al. (2016) [20] Halpren et al. (2019 [21]) |
| Length or bridging period | The length of the bridging period is associated with mixed results in the literature. A period of greater than 30 days may be associated with less success. |
Crotti et al. (2013) [6] Langer et al. (2019) [18] |
| Underlying disease process | Patients with cystic fibrosis may have a survival advantage over other diseases. Conversely, patients with interstitial lung disease may have worse outcomes. | Lafarge et al. (2013) [22] |
| Infection | Patients with suppurative lung disease, such as cystic fibrosis, can be safely bridged with ECMO. However, infection with certain organisms, such as Achromonavter, may be challenging to treat with indwelling intravenous cannulae | Biscotti et al. (2015) [23] |
Predictors of Post-transplant Mortality
In addition to the conclusions above, several unique observations have also been noted from individual studies. For example, patients with particular disease processes seem to be more appropriate for ECMO bridging. For instance, Lafarge et al. demonstrated a survival advantage for cystic fibrosis patients compared with idiopathic pulmonary fibrosis (2-year survival 71% vs. 27.3%, respectively) [22]. Of course, these differences in survival may be more attributable to the patients’ age than their disease process as cystic fibrosis patients tend to be younger and, therefore, more capable of tolerating these extreme measures.
Patients undergoing retransplantation have a significantly reduced survival, according to the Toronto group [8••]. In this study, patients bridged to their first transplant had comparable 1-year survival to patients who did not receive BTT (76% in bridged patient versus 86% in non-bridged patients, p = 0.197). However, the only factor associated with survival was the type of transplant. Median survival was 60 months in patients undergoing BTT to their first transplant. In comparison, patients bridged to retransplantation had 15-month median survival (p = 0.041). The survival was even worse in patients bridged to retransplantation and who remained sedated and mechanically ventilated. In this group, the median survival was only 4 months.
The length of time on ECLS before transplantation is associated with mixed results in the literature. There are reports of successful bridging with ECLS for very long periods [24]. However, the study by Crotti et al. showed that the length of time on ECLS before transplantation is predictive of survival after transplant, with longer intervals between ECMO initiation and transplant yielding inferior results. Patients who were bridged for more than 14 days experienced lower survival [6]. Similarly, Langer et al. noted that bridging time of 30 days or longer was associated with worse outcomes [18].
The type of ECMO support needed preoperatively also appears to be predictive of outcomes. Venovenous (VV) ECMO is a more simplistic bridging strategy since it means that their cardiac function is preserved despite the patient’s respiratory failure. Venoarterial (VA) ECMO, however, denotes a more critically ill patient who has developed right heart dysfunction, usually secondary to pulmonary hypertension, on top of their respiratory failure. In addition, VA-ECMO is associated with more complications related to bleeding, limb ischemia, etc. Given these realities, transplant providers must work diligently with their consultants to ensure that critically ill patients who may be candidates for bridging to transplant are referred while VV-ECMO remains a sufficient mode of support. The study by Kukreja et al. demonstrates that right heart dysfunction may be bridged successfully. However, it is associated with worse outcomes [19]. In addition to cardiac dysfunction, the presence of other organ dysfunction, such as renal failure, is a predictor for mortality [9••].
As with many strategies, center volume and experience are associated with improved outcomes. Certainly, patients undergoing ECLS BTT seem to benefit from being at a high-volume center. Many reports showed that low-volume centers had lower post-transplant survival compared with high-volume centers [11, 20, 21]. However, it is unclear whether lung transplant volume or ECLS volume is the determinant of a ‘high-volume center.’ Langer et al. show excellent outcomes from a low-volume transplant center [18]. The authors argue that their experience as a high-volume ECMO center may make up for the lack of transplant volume. This is a reasonable explanation for how a low-volume transplant center can achieve acceptable outcomes for bridging patients to transplant. But the emphasis should be that a center engaged in BTT should have extensive experience in one, but preferably both fields, as the required infrastructure (i.e., critical care expertise, perfusionists) is as important as the technical proficiency of the surgical team.
Application of ECMO as a Bridge to Lung Transplantation
Patient Selection
There are three possible outcomes when ECLS is employed in patients with advanced respiratory failure. These are “bridge to recovery,” “bridge to transplant (BTT),” or “bridge to nowhere/mortality.” Bridge to recovery is only likely for patients with acute respiratory illness, such as aspiration pneumonitis or acute respiratory distress syndrome, without underlying chronic lung disease. In patients with end-stage, irreversible lung disease, it is unlikely that they would transcend into a “bridge to recovery” pathway. Therefore, when considering ECLS as BTT, one must weigh the likelihood of being bridged to lung transplantation versus death. The higher the probability of a bridge to nowhere outcome, the less likely that ECLS strategy should be employed. Patients with pulmonary fibrosis and chronic obstructive pulmonary disease (COPD) will typically be older. They can be challenging to bridge to transplant, particularly if they have any degree of pulmonary hypertension. Conversely, as mentioned previously, cystic fibrosis patients who experience an acute exacerbation are typically more resilient due to their youth.
Absolute contraindications to BTT include multi-organ system failure, severe sepsis, and a neurologic insult with profound deficits. Beyond those contraindications, there are many other relative contraindications, which should be considered. Once an absolute contraindication is ruled out, a decision should be made about the likelihood of this patient’s success with ECLS as BTT.
Bridging patients to transplant who are suffering from septic lung disease, viral, bacterial, or otherwise must be considered individually. For example, infection with multi-resistant species or highly virulent bacteria may be considered a relative contraindication for BTT. An example of this is pulmonary infection with the highly resistant Achromobacter species known for producing a biofilm and is highly resistant to treatment [23]. Indeed, cystic fibrosis patients can be infected with a multitude of organisms. Therefore, consultation with infectious disease specialists is essential to ascertain treatment sensitivities before the deployment of BTT. Blood-stream infections should also be viewed with caution due to the difficulties associated with their treatment in the setting of long-term indwelling cannulas. One consideration is when the infection arises from the lung. In such cases, the lungs remain a source of sepsis without source control. There have been reports of performing bilateral pneumonectomy and bridging with VA-ECMO until a suitable donor is identified [25, 26].
Frailty is relative contraindication for BTT. As discussed above, the risk of deconditioning and developing postoperative CIM can lead to unacceptable morbidity. Hence, ECMO BTT should be avoided in deconditioned and frail patients. Finally, given the association between the length of the bridging period and the failure of BTT, factors associated with prolonged waitlist times should be considered relative contraindications. Such factors include allosensitization and extremes in body size.
Based on the above discussion, the ideal BTT candidate is the young patient with cystic fibrosis, preserved exercise capacity, blood group A, and no allosensitization, who develops rapidly progressive respiratory failure while waiting on the lung transplant list. However, this ideal theoretical patient is more the exception than the norm; thus, each individual and their different clinical characteristics must be considered in isolation.
Choice of ECMO Strategy
Patients who require ECLS BTT can be categorized into three categories: hypercapnic failure, hypoxemic respiratory failure with or without hypercapnia, and patients with cardiac dysfunction with or without respiratory failure. These clinical scenarios must be considered when selecting the patient’s ECMO cannulation strategy.
Hypercapnic Respiratory Failure
Patients with isolated hypercapnic respiratory failure are the least complicated group requiring BTT. They typically have preserved cardiac function; thus, they can be bridged with VV-ECMO. The amount of flow necessary to clear CO2 is less than that needed in hypoxemia or hemodynamic compromise. Because of the need for lower flow rates, these patients are well suited for single-site cannulation using the Avalon Elite dual lumen cannula (Maquet, Rastatt, Germany). A double lumen cannula, in which one lumen serves to drain deoxygenated blood from the patient to the circuit (outflow), and the other lumen function to return blood to the patient from the circuit (inflow). The cannula is traditionally placed through the right internal jugular vein. In recent years, many have also begun to access the left subclavian as it is an ideal site for patient comfort and ease of mobility.
Another option for patients with hypercapnic failure is the use of pumpless extracorporeal lung assist devices, or PECLA (Novalung GmbH, Hechingen, Germany). This device is a low resistance membrane oxygenator used extracorporeally via femoral arterial (outflow) and venous cannulation (inflow). The maintenance of flow through this device is entirely dependent on the patient’s cardiac output. Fischer et al. reported on their experience with PECLA as BTT in 12 patients, with a success rate of 87% [27]. PECLA had the advantage of being a low morbidity option when ECMO was associated with a high complication rate. This device’s disadvantage is that femoral cannulation significantly limits patients’ capacity for ambulation. Recent improvements in ECMO technology and safety in the current era have substantially diminished the role of PECLA. However, it remains a viable option in patients with isolated hypercapnic respiratory failure and preserved hemodynamic function.
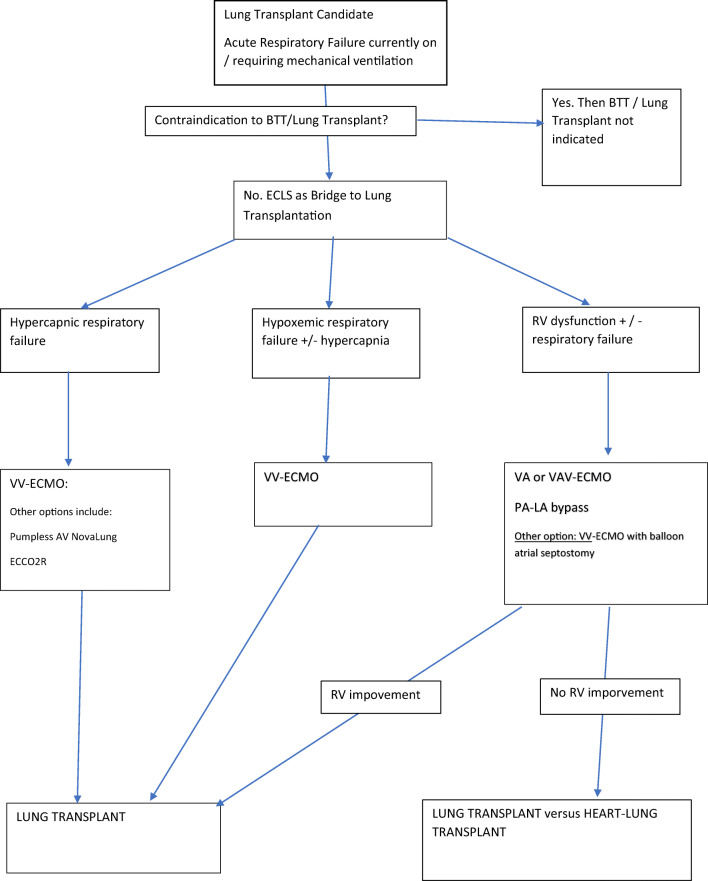
A novel therapy that is increasingly implemented in hypercapnia management is extracorporeal carbon dioxide removal (ECCO2R) (Fig. 1). There are several variations on this strategy, but the concept involves inserting a smaller bore cannula, analogous to a hemofiltration dialysis catheter into the venous system allowing partial venovenous bypass. This provides partial CO2 removal at blood flow rates of 350–550 mL/min. The HemoLung Respiratory Assist System (ALung Technologies, Pittsburgh, PA) allows ECCO2R to be provided through a single 15.5-Fr dual lumen single cannula placed through a peripheral vein, such as the femoral vein or right internal jugular vein. Experience with this technology as BTT is limited to case reports [14]. Still, it has shown promise in managing acute hypercapnic respiratory failure in non-lung transplant patients, such as status asthmaticus or COPD patients [28].
Fig. 1.
Algorithm for the management of patients awaiting lung transplant who require ECMO BTT
Hypoxemic Respiratory Failure
Respiratory failure may manifest as hypoxemia, with or without hypercapnia. Patients with hypoxemia will need higher flow in the range of 3–6 L/min. Consequently, low flow ECLS circuits such as ECCO2R and PECLA are not suitable. The options for treatment include VV-ECMO or VA-ECMO, with multiple variations in drainage and return configurations possible. VV-ECMO is preferred over VA-ECMO for several reasons, such as the lower incidence of bleeding complications and the simplicity of placement and initiation. Furthermore, VA-ECMO will not provide substantial additional oxygen delivery over VV-ECMO in patients with isolated respiratory failure and preserved cardiac function. As has been alluded to previously, the need for VA-ECMO in a patient being considered for lung transplant is indicative of a more critically individual who is likely to have a more complicated perioperative course.
Given the higher flow requirements, larger cannulas are needed to ensure adequate flow. If single-site dual-lumen cannulas are to be used, every attempt should be made to use the largest available size. In certain instances, the right internal jugular or left subclavian veins are not accessible, and dual femoral venous cannulation is a reasonable alternative. In patients that can be managed with flow rates of 4.5 L/min or less and can be liberated from the ventilator and participate in ambulatory rehabilitation, we prefer to use a single-site dual-lumen cannula. With the groins free from cannulas, the patient can participate in ambulatory rehabilitation without any risk of cannula site complications. Patients who can be liberated from the ventilator but require more than 4.5 L/min of flow would be maintained with awake dual-site cannulation. Their rehabilitation would be limited to supine exercises and in-bed active range of motion therapy [7•]. We have avoided ambulation in patients with groin cannulas due to the potential risk of cannula dislodgement.
Right Ventricular Dysfunction
The final group of patients requiring BTT includes patients with right ventricular dysfunction or severe pulmonary hypertension. Whether accompanied by hypoxemia, hypercapnia, or not, the principles of management remain the same. The significant distinction in this group is the need for right ventricular support to correct the physiology. This may be in the form of an arterial inflow from the ECMO circuit, or at a minimum, a means to unload the right ventricle. This requirement makes standard venovenous types of ECLS usually unsuitable. Occasionally, reactive hypoxemic pulmonary vasoconstriction can be mitigated in these patients with just VV-ECMO, but this is the exception, as opposed to the rule, and usually, VA-ECMO is required.
The most prevalent means of support is the use of VA-ECMO, as it provides both hemodynamic support and gas exchange. VA-ECMO may be instituted via peripheral or central cannulation. Most reports in the literature include series where peripheral VA-ECMO was provided via cannulation of the femoral artery and vein. However, a unique scenario arises with this configuration in patients with combined cardiac and respiratory failure. During femoral VA-ECMO, blood is returned to arterial circulation via the femoral artery and flows up the aorta in a retrograde fashion. Concurrently, the native cardiac output pumps blood into the ascending aorta against the ECMO circuit’s flow, resulting in competitive flow within the aorta. The upper body receives blood from the native cardiac output, and the lower body receives most of its perfusion from the ECMO circuit. This is inconsequential when the lungs provide adequate gas exchange, as the native cardiac output will contain oxygenated blood. However, in a situation where there is concomitant pulmonary dysfunction, the blood returning to the left atrium will be poorly oxygenated. Consequently, the native cardiac output, which may provide most perfusion to the upper body, including the coronary vessels and the brain, will contain deoxygenated blood. In this situation, an oxygen saturation probe placed on the right upper extremity will document low saturation, whereas one placed on the lower extremities will document high saturation levels. This pathophysiology is referred to as “upper body hypoxia” or harlequin syndrome.
Several strategies exist to deal with harlequin syndrome. In central VA-ECMO, the ascending aorta is cannulated. Delivering oxygenated blood directly into the ascending aorta eliminates the situation of competitive flow within the aorta. It ensures that the rest of the body receives oxygenated blood from the ECMO circuit. However, it requires access to the chest via either sternotomy or anterior thoracotomy for placement. A second option is to utilize cannulation of the axillary artery to provide arterial inflow. Directing flow into the axillary artery results in a similar flow profile to central cannulation but avoids sternotomy or thoracotomy and may theoretically be less morbid. While this technique provides oxygenated blood to the entire upper body, competitive flow in the ascending aorta may still occur, with the possibility of perfusing the coronary vessels with poorly oxygenated blood, despite having oxygenated blood in the arch vessels. This will not be detected by conventional oximetry and manifest as a worsening cardiac function secondary to ischemia.
The use of central VA-ECMO or peripheral VA-ECMO with axillary arterial inflow are excellent bridging strategies for patients with cardiac dysfunction. Our preferred approach for this patient population has been the use of hybrid veno-arterial-venous ECMO (VAV-ECMO). In this configuration, blood is drained from the patient through a venous outflow cannula and then returned to the patient on both the arterial and venous parts of the circulation. This approach ensures that the whole body receives oxygenated blood regardless of whether it comes from the native cardiac output or the arterial inflow cannula. Several cannulation strategies can be used for the initiation of VAV-ECMO. Our preferred cannulation strategy is using single-site dual-lumen cannulation of the right internal jugular vein or left subclavian vein to provide the VV portion of the circuit and right axillary cannulation for the arterial limb. Several reports with promising results have described the use of this upper-body VAV-ECMO configuration [23, 29]. The femoral artery can be used as inflow since there is no risk of harlequin syndrome with VAV-ECMO. However, the disadvantage of using the femoral artery is the limited ability to ambulate with a groin arterial cannula in place. This complex arrangement, while effective against preventing Harlequin syndrome, requires expertise on the part of the critical care team
Other options for this patient population include using a pulmonary artery to left atrial bypass (PA-LA). In this configuration, the pulmonary artery is cannulated through a median sternotomy. This represents the outflow cannula, which will direct blood to a pumpless oxygenation device, such as the NovaLung lung assist device. The cannula’s placement will establish inflow to the patient into the left atrium via the right superior pulmonary vein. The sternotomy can be closed, and the patients may be liberated from the ventilator and even ambulate while waiting for a suitable organ. However, in the emergent setting, one may need to stabilize the patient with peripheral VA-ECMO before instituting a PA-LA shunt. Once the PA-LA shunt is in place, then the VA-ECMO circuit may be discontinued. Flow through this circuit is provided by the right ventricle, in a similar concept to PECLA. This technique’s main advantage is ambulating with the device in place, allowing for rehabilitation while on the waitlist.
A final option in these patients is conventional VV-ECMO with an atrial septal defect (ASD). While standard VV-ECMO does nothing to support the right ventricle in patients with cardiac dysfunction, an ASD’s addition unloads the right heart to some extent. The ASD can be created in the catheterization laboratory using a balloon atrial septostomy. This level of support requires that left heart function is also intact. This approach’s main advantage is its simplicity of initiation and the lack of sternotomy or thoracotomy. Simply unloading the right ventricle may not be adequate as a means of providing right heart support. Thus, the use of the technique may be limited in patients with significant cardiac dysfunction.
Management of Patient on ECMO:
Patients are managed in intensive care units with daily assessment by a multidisciplinary team that includes a transplant pulmonologist, lung transplant surgeon, intensivist, and active participation from advanced care providers, nutritionists, physical therapists, respiratory therapists, and nurses. The goals of care include using ECMO flows to maintain adequate oxygenation and ventilation. Markers of overall tissue perfusion are monitored, such as serum lactate levels, central venous oxygen saturation, and urine output. If these goals cannot be achieved, one needs to assess whether the current ECMO configuration provides adequate support. This may require increases in ECMO flow rates or sweep gas. Additionally, one may consider escalating the level of support. In the case of single-site VV-ECMO, where flow is limited, we may consider the placement of additional venous cannulas to increase outflow or inflow. The cardiac function is also monitored, and development of new right ventricular dysfunction may prompt switching from VV-ECMO to VAV-ECMO by the placement of an arterial cannula
Most importantly, the daily assessment centers on the patient’s continued progress and suitability as a lung transplant candidate. The development of severe sepsis, additional organ failure, or inability to consistently participate in rehabilitation will prompt the team to question the patient’s appropriateness for BTT. It may even lead to the termination of the bridging strategy and de-listing.
Summary: Our Approach to the Patient Requiring ECMO as a Bride to Transplant
If a patient with end-stage lung disease, a candidate for lung transplantation, develops acute respiratory failure requiring intensive care, they would be considered for BTT. The first step is to rule out a significant contraindication that would make lung transplantation prohibitive, such as substantial neurological insult, septic shock, or zero potential for rehabilitation. If the patient is deemed a candidate for BTT, then we categorize their physiologic disturbance into one of three categories: hypercapnic respiratory failure, hypoxemic respiratory failure with or without hypercapnia, or right ventricular dysfunction. For patients with isolated hypercapnic failure, we utilize VV-ECMO instituted via single-site dual-lumen cannulation through either the right internal jugular vein or left subclavian vein. For patients with hypoxemic failure, we use VV-ECMO as a bridging strategy. However, we start with single-site dual-lumen cannulation. If the patient needs additional flow, then we add other venous cannulae. Finally, for patients with right ventricular dysfunction, we utilize VAV-ECMO or PA-LA shunting. In the emergent setting, the patient may need to be stabilized with peripheral VA-ECMO. Suppose the patient stabilizes to the point that ambulation is possible. In that case, we will switch them to an upper-body configuration VAV-ECMO or PA-LA shunting with a lung assist device.
Conclusion
Given the lack of the donor lungs, ECLS BTT has emerged as a strategy to address waitlist mortality. ECLS BTT can be a risky strategy. However, it may be the only option for patients who face certain death while waiting for a suitable organ. There are many predictors of mortality pre- or post-transplant, including right heart dysfunction and BTT duration. None of these factors individually constitute contraindications for BTT. However, they should be considered in the decision to initiate ECLS. Furthermore, ECLS should be individualized, depending on the patient. When all these factors are addressed, ECLS BTT can be an excellent strategy with good results.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Chambers DC, Cherikh WS, O Harhay M. Hayes D, Hsich E, Khusch K, on behalf of the International Society for Heart and Lung Transplantation et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult lung and heart-lung transplantation Report-2019; Focus theme: Donor and recipient size match. J Heart Lung Transplant. 2019;38(10):1042–1055. doi: 10.1016/j.healun.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gottlieb J. Lung allocation. J Thorac Dis. 2017;9(8):2670–2674. doi: 10.21037/jtd.2017.07.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK, Elbourne D. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 4.Davies A, Jones D, Bailey M, Beca J, Bellomo R, Blackwell N. on behalf of Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA. 2009;302(17):1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 5.Dellgren G, Riise GC, Swärd K, Gilljam M, Rexius H, Liden H, Silverborn M. Extracorporeal membrane oxygenation as a bridge to lung transplantation: a long-term study. Eur J Cardiothorac Surg. 2015;47(1):95–100. doi: 10.1093/ejcts/ezu112. [DOI] [PubMed] [Google Scholar]
- 6.Crotti S, Iotti GA, Lissoni A, Belliato M, Zanierato M, Chierchetti M, et al. Organ allocation waiting time during extracorporeal bridge to lung transplant affects outcomes. Chest. 2013;144:1018–1025. doi: 10.1378/chest.12-1141. [DOI] [PubMed] [Google Scholar]
- 7.•.Rehder KJ, Turner DA, Hartwig MG, Williford WL, Bonadonna D, Walczak RJ, Jr, et al. Active rehabilitation during extracorporeal membrane oxygenation as a bridge to lung transplantation. Respir Care. 2013;58(8):1291–1298. doi: 10.4187/respcare.02155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.••.Hoetzenecker K, Donahoe L, Yeung JC, Azad S, Fan E, Ferguson ND, et al. Extracorporeal life support as a bridge to lung transplantation-experience of a high-volume transplant center. J Thorac Cardiovasc Surg. 2018;155(3):1316–1328. doi: 10.1016/j.jtcvs.2017.09.161. [DOI] [PubMed] [Google Scholar]
- 9.••.Tipograf Y, Salna M, Minko E, Grogan EL, Agerstrand C, Sonett J, et al. Outcomes of extracorporeal membrane oxygenation as a bridge to lung transplantation. Ann Thorac Surg. 2019;107:1456–1463. doi: 10.1016/j.athoracsur.2019.01.032. [DOI] [PubMed] [Google Scholar]
- 10.Hakim HA, Ahmad U, McCurry KR, Johnston DR, Pettersen GB, Budev M, et al. Contemporary outcomes of extracorporeal membrane oxygenation used as bridge to lung transplantation. Ann Thorac Surg. 2018;106:192–198. doi: 10.1016/j.athoracsur.2018.02.036. [DOI] [PubMed] [Google Scholar]
- 11.Hayes D, Jr, Tobias JD, Tumin D. Center volume and extracorporeal membrane oxygenation support at lung transplantation in the lung allocation score era. Am J Respir Crit Care Med. 2016;194(3):317–326. doi: 10.1164/rccm.201511-2222OC. [DOI] [PubMed] [Google Scholar]
- 12.Inci I, Klinzing S, Schneiter D, Schuepbach RA, Kestenholz P, Hillinger S, Benden C, Maggiorini M, Weder W. Outcome of extracorporeal membrane oxygenation as a bridge to lung transplantation: an institutional experience and literature review. Transplantation. 2015;99(8):1667–1671. doi: 10.1097/TP.0000000000000653. [DOI] [PubMed] [Google Scholar]
- 13.Hayanga AJ, Aboagye J, Esper S, Shigemura N, Bermudez CA, D'Cunha J, Bhama JK. Extracorporeal membrane oxygenation as a bridge to lung transplantation in the United States: an evolving strategy in the management of rapidly advancing pulmonary disease. J Thorac Cardiovasc Surg. 2015;149(1):291. doi: 10.1016/j.jtcvs.2014.08.072. [DOI] [PubMed] [Google Scholar]
- 14.Bonin F, Sommerwerck U, Lund LW, Teschler H. Avoidance of intubation during acute exacerbation of chronic obstructive pulmonary disease for a lung transplant candidate using extracorporeal carbon dioxide removal with the Hemolung. J Thorac Cardiovasc Surg. 2013;145(5):e43–e44. doi: 10.1016/j.jtcvs.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 15.Todd EM, Roy SB, Hashimi AS, Serrone R, Panchanathan R, Kang P, et al. Extracorporeal membrane oxygenation as a bridge to lung transplantation: a single-center experience in the present era. J Thorac Cardiovasc Surg. 2017;154:1798–1809. doi: 10.1016/j.jtcvs.2017.06.063. [DOI] [PubMed] [Google Scholar]
- 16.Hayanga AJ, Du AL, Joubert K, Tuft M, Baird R, Pilewski J, et al. Mechanical ventilation and extracorporeal membrane oxygenation as a bridging strategy to lung transplantation: significant gains in survival. American Journal of Transplantation. 2018;18:125–135. doi: 10.1111/ajt.14422. [DOI] [PubMed] [Google Scholar]
- 17.Benazzo A, Schwarz S, Frommlet F, Schweiger T, Jaksch P, Schellongowski P, Staudinger T, Klepetko W, Lang G, Hoetzenecker K, Moser B, Sigueenza JM, Horvath J, Krenn C, Bacher A, Schaden E, Baron DM, Faybik P, Taghavi S. Twenty-year experience with extracorporeal life support as bridge to lung transplantation. J Thorac Cardiovasc Surg. 2019;157:2515–2525. doi: 10.1016/j.jtcvs.2019.02.048. [DOI] [PubMed] [Google Scholar]
- 18.Langer F, Aliyev, Schafers H, Trudzinski FC, Seiler F, Bals R, et al. Improving outcomes in bridge-to-transplant: extended extracorporeal membrane oxygenation support to obtain optimal donor lungs for marginal recipients. ASAIO. 2019;65(5):516–521. doi: 10.1097/MAT.0000000000000843. [DOI] [PubMed] [Google Scholar]
- 19.Kukreja J, Tsou S, Chen J, Trinh BN, Feng C, Golden JA, et al. Risk factors and outcomes of extracorporeal membrane oxygenation as a bridge to lung transplantation. Semin Thorac Cardiovasc Surg. 2020;S1043-0679(20):30133–30137. doi: 10.1053/j.semtcvs.2020.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Hayanga JW, Lira A, Aboagye JK, Hayanga HK, D'Cunha J. Extracorporeal membrane oxygenation as a bridge to lung transplantation: what lessons might we learn from volume and expertise? Interact Cardiovasc Thorac Surg. 2016;22(4):406–410. doi: 10.1093/icvts/ivv379. [DOI] [PubMed] [Google Scholar]
- 21.Halpern AL, Kohtz PD, Helmkamp L, Eldeiry M, Hodges MM, Scott CD, Mitchell JD, Aftab M, Pal JD, Cleveland JC, Reece TB, Meguid RA, Fullerton DA, Weyant MJ. Improved mortality associated with the use of extracorporeal membrane oxygenation. Ann Thorac Surg. 2019;108(2):350–357. doi: 10.1016/j.athoracsur.2019.03.057. [DOI] [PubMed] [Google Scholar]
- 22.Lafarge M, Mordant P, Thabut G, Brouchet L, Falcoz P, Haloun A, et al. Experience of extracorporeal membrane oxygenation as a bridge to lung transplantation in France. J Heart Lung Transplant. 2013;32:905–913. doi: 10.1016/j.healun.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Biscotti M, Sonnett J, Bacchetta M. ECMO as bridge to lung transplant. Thorac Surg Clin. 2015;25:17–25. doi: 10.1016/j.thorsurg.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Kon ZN, Wehman PB, Gibber M, Rabin J, Evans CF, Rajagopal K, Herr D, Iacono A, Garcia JP, Griffith BP. Venovenous extracorporeal membrane oxygenation as a bridge to lung transplantation: successful transplantation after 155 days of support. Ann Thorac Surg. 2015;99:704–707. doi: 10.1016/j.athoracsur.2014.04.097. [DOI] [PubMed] [Google Scholar]
- 25.Barac YD, Bryner B, Bonadonna D, Wolfe C, Reynolds J, Haney JC, Daneshmand MA. Bilateral pneumonectomy with veno-arterial extracorporeal membrane oxygenation as a bridge to lung transplant. J Heart Lung Transplant. 2019;38(11):1231–1232. doi: 10.1016/j.healun.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Cypel M, Waddell T, Singer LG, Del Sorbo L, Fan E, Binnie M, et al. Bilateral pneumonectomy to treat uncontrolled sepsis in a patient awaiting lung transplantation. J Thorac Cardiovasc Surg. 2017;153(4):e67–e69. doi: 10.1016/j.jtcvs.2016.11.031. [DOI] [PubMed] [Google Scholar]
- 27.Fischer S, Simon AR, Welte T, Hoeper MM, Meyer A, Tessmann R, Gohrbandt B, Gottlieb J, Haverich A, Strueber M. Bridge to lung transplantation with the novel pumpless interventional lung assist device NovaLung. J Thorac Cardiovasc Surg. 2006;131(3):719–723. doi: 10.1016/j.jtcvs.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 28.Abrams DC, Brenner K, Burkart KM, Agerstrand CL, Thomashow BM, Bacchetta M, Brodie D. Pilot study of extracorporeal carbon dioxide removal to facilitate extubation and ambulation in exacerbations of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2013;10(4):307–314. doi: 10.1513/AnnalsATS.201301-021OC. [DOI] [PubMed] [Google Scholar]
- 29.Biscotti M, Gannon WD, Agerstrand C, Abrams D, Sonett J, Brodie D, Bacchetta M. Awake extracorporeal membrane oxygenation as bridge to lung transplantation: a 9-year experience. Ann Thorac Surg. 2017;104(2):412–419. doi: 10.1016/j.athoracsur.2016.11.056. [DOI] [PubMed] [Google Scholar]