Abstract
A 33‐year‐old male who underwent surgery for Tetralogy of Fallot presented with atrial flutter. Electrophysiology study revealed concealed entrainment along the mid lateral right atrium with postpacing interval shorter than the tachycardia cycle length. Ablation at this site terminated the tachycardia. The presence of shorter PPI than TCL was due to a large virtual electrode leading to downstream capture of far field tissue. This case demonstrates that sites showing PPI shorter than TCL are in a slow conducting narrow critical isthmus and hence constitute good ablation targets.
Keywords: ablation, atrial flutter, entrainment, narrow isthmus, PPI‐TCL
1. CARDIAC ARRHYTHMIA SPOT LIGHT
A 33‐year‐old gentleman who underwent surgery for Tetralogy of Fallot at 5 years of age presented with recurrent episodes of palpitations. Electrocardiogram [ECG] taken during the tachycardia showed atypical atrial flutter with right bundle branch aberrancy. His baseline ECG showed sinus rhythm with the same aberrancy. Therapy with a combination of beta‐blockers and amiodarone was ineffective.
After stopping amiodarone for 4 weeks, he was taken up for a cardiac electrophysiological study (EPS) with the intent to proceed with 3‐D mapping guided Radiofrequency ablation using an Abbott Ensite NAVX Navigation system. Three diagnostic Electrophysiology (EP) catheters were placed for EPS – (a) a quadripolar catheter (2‐5‐2 spacing) at the right ventricular (RV) apex, (b) a decapolar catheter (2‐5‐2 spacing) in the coronary sinus (CS), and (c) a duo‐decapolar catheter (2‐10‐2 spacing)—around the tricuspid annulus (TA) with its tip near the os (Os refers to the mouth or orifice of a given structure) of CS and the proximal poles at the atrial septum. On atrial burst pacing, atrial flutter identical to the clinical tachycardia (positive flutter waves in leads II, III, aVF and equiphasic in V1 with an isoelectric interval between flutter waves) was easily inducible. The tachycardia cycle length was 275 ms. The activation pattern in the duo‐decapolar showed the earliest signal in the mid poles 9‐10 [which was along the mid lateral right atrium (LRA)].
Entrainment mapping revealed manifest fusion on pacing from the proximal and distal CS at 5 milliamperes [mA]. Pacing from the mid Duo‐decapolar electrodes 9‐10 failed to capture at the same output. Pacing from here at 12 mA revealed concealed fusion with difference between postpacing interval [PPI] and tachycardia cycle length [TCL] (dPPI) of—11 ms as depicted in Figure 1.
FIGURE 1.
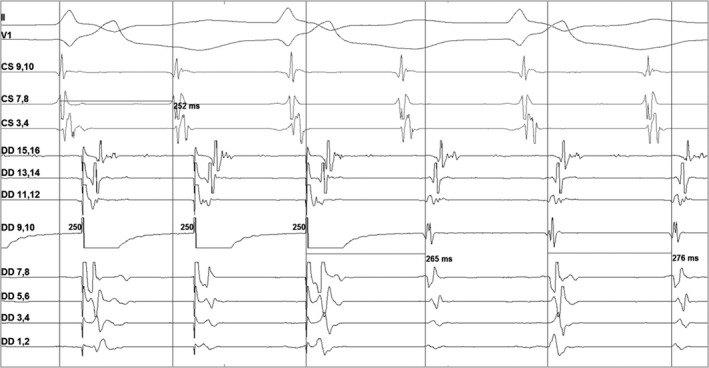
Intracardiac electrogram showing entrainment from midpoles (9‐10) of duo‐decapolar catheter. From top to bottom, II and V1 represent lead II and lead V1 of 12 lead ECG. CS 9‐10 to 1‐2 (not in picture) represent proximal to distal poles of decapolar catheter in coronary sinus. DD1‐2 represent distal poles and DD19‐20 (which are not shown here) represent proximal poles of the duo‐decapolar catheter
What is the mechanism for PPI shorter than the TCL at this site?
2. DISCUSSION
Based on the entrainment mapping, we could surmise that the distal and the proximal CS electrodes were away from the tachycardia reentrant circuit, while the duo‐decapolar 9‐10 electrodes were either close to the circuit or near the exit zone of the critical isthmus region.
At this point, a high‐density activation map of the right atrium was created using AdvisorTM HD Grid Mapping Catheter (Abbott Laboratories). At the site of interest (SOI)—the mid lateral right atrium, there were highly fragmented signals which spanned 66% of the tachycardia cycle length (Figure 2). These areas of slow conduction had voltages as low as 0.2 to 0.3 mV. Tachycardia was ‘bump’—terminated while mapping this probable isthmus. The width of this critical isthmus was 4mm and the conduction velocity here was 0.12 m/s.
FIGURE 2.
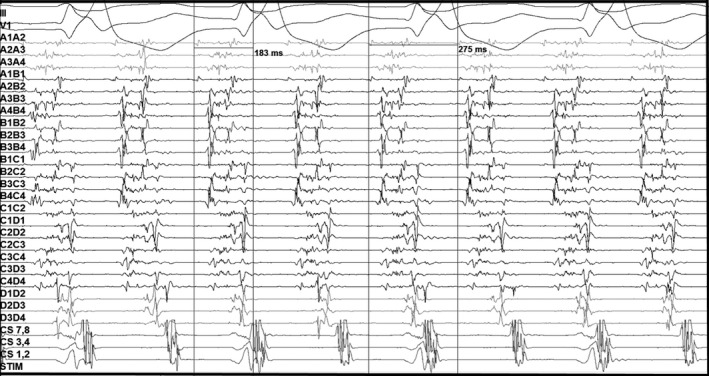
High‐density mapping with multipolar catheter showing highly fragmented signals in the mid lateral right atrium covering 66% of TCL
The common reasons for a negative dPPI are pitfalls related to performing and interpreting the entrainment manoeuver. Causes for a pseudoshort PPI are intermittent capture, spontaneous TCL variability, transient acceleration of the tachycardia, and incorrect annotation of the return cycle. The latter can result from erroneously measuring the PPI to a large far field signal as can occur while mapping close to intracardiac structures like the papillary muscle. The inability to distinguish between a near and far field electrogram can also happen when the substrate is markedly diseased with low amplitude fractionated signals. In our case, all these pseudoshort PPI causes were first excluded.
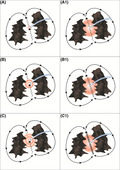
We were then left with the possibility of downstream capture, because of high‐output pacing resulting in a large virtual electrode (Figure 3 left‐side panels A, B, C), being the mechanism behind the negative dPPI at that site. Additional contributing factors could have been the chance orientation of the pacing electrodes that confronted a zone of anisotropy (involving the narrow critical isthmus) which was overcome by high‐output pacing. The capture of adjoining transitioning tissue with better conduction velocity further downstream in the circuit by the leading edge of the depolarization wave front, aids in foreshortening of the return cycle duration. With a smaller pacing virtual electrode, or lower pacing output during the tachycardia, these factors would have prolonged the overall conduction velocity within the circuit and the PPI duration. Further, the size of the virtual electrode showing the truncation of the PPI‐TCL duration may not have been sufficient to result in manifest fusion with the given catheter position and its resolution characteristics.
FIGURE 3.
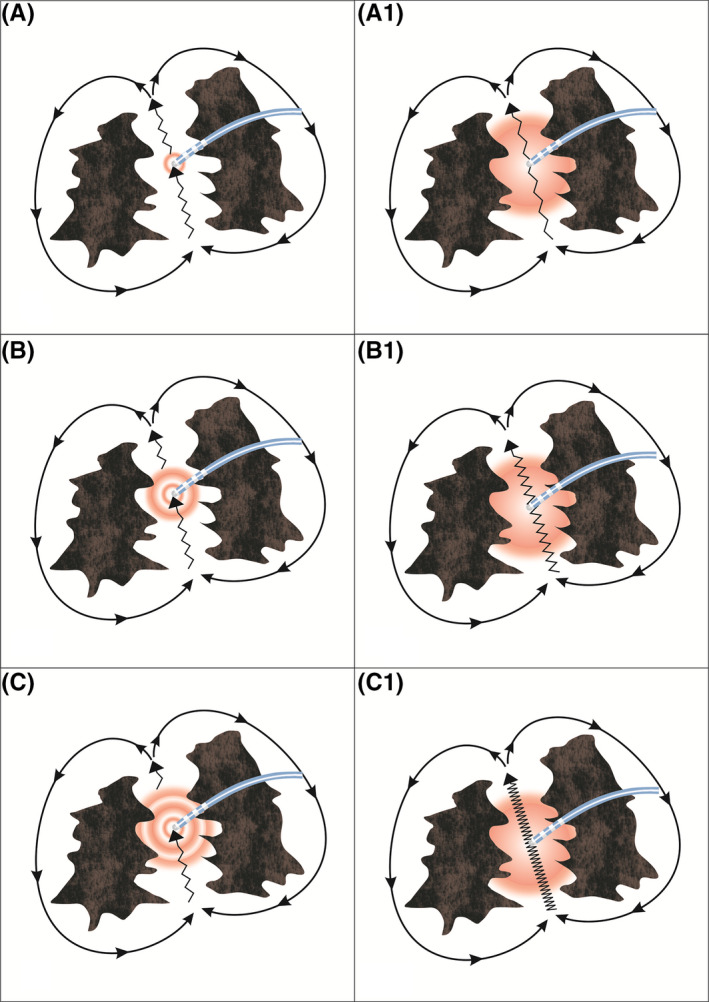
The left side panels A, B and C show that by increasing the pacing output from A to C, the dPPI becomes more negative because of increase in size of the virtual electrode. The right side panels A1, B1 and C1 show that the slower the conduction velocity in the isthmus (conduction velocity in C1 is slower than that in A1) the more negative is the dPPI with a fixed output
The sites showing shorter PPI than TCL were commonly located in the region of narrow critical isthmus whose width is <25 mm and exhibit a slow conduction velocity 0.49 + 0.43 m/s and low voltages 0.48 ± 0.79 mV. Since, the SOI (mid lateral right atrium) was in such an area, pacing from here yielded a negative dPPI. It can be deduced that the tachycardia was because of localized reentry in the region of mid lateral right atrium as we demonstrated; (a) reentry with atrial overdrive pacing, (b) simultaneous recording of fragmented signals spanning 66% of the TCL using the HD grid catheter and (c) the entire circuit was confined to the lateral RA wall (Figure 4). Radiofrequency energy application here was immediately successful in terminating the tachycardia. Ablation was carried out around this isthmus to eliminate all fragmented potentials. Post ablation there was no tachycardia inducible despite aggressive atrial burst and programmed pacing in the presence of isoprenaline.
FIGURE 4.
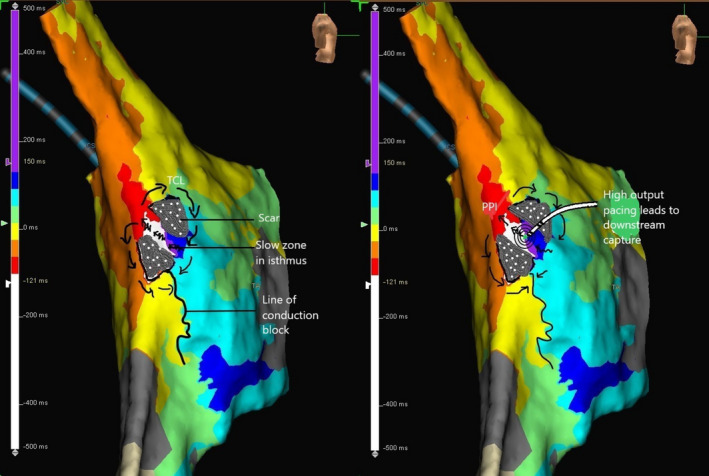
Activation map of the atrial flutter showing nearly the entire cycle length of the flutter circuit confined to the lateral right atrium with early (red color coded) meeting the late (purple color coded). Arrows indicate the probable reentry circuit of the atypical atrial flutter and the squiggly arrows represent the zone of slow conduction in the critical isthmus. The right half of the figure demonstrates high‐output pacing leading to a large virtual electrode and downstream capture of tissue. PPI, postpacing interval; TCL, tachycardia cycle length
dPPI is a function of the distance and conduction velocity between the entrained site and the reentrant circuit. It is also dependent on the electroanatomical properties of the entrained site. The slower the conduction velocity in the entrained area the greater will be the gain in time off the TCL should downstream capture occur (Figure 3 right‐side panels A1, B1, C1). This is further facilitated by high output bipolar stimulation resulting in a large virtual electrode. The demonstration of a shorter PPI than TCL in the midlateral right atrium region aroused our suspicion and prompted extensive mapping in that region; eventually facilitating a successful ablation.
3. CONCLUSION
The sites demonstrating shorter PPI than TCL are usually markers of slow conducting narrow critical isthmus and hence constitute good ablation targets. This is applicable in stable tachycardia with constant capture during entrainment where errors in measurement are ruled out.
ETHICS STATEMENT
As this was a case report ethics clearance was not obtained. Patient consent was obtained prior to the procedure for publication of the patient clinical materials if found suitable in a medical journal. Hence, we request you to waive off the ethics committee clearance.
CONFLICT OF INTEREST
The authors declare no conflict of interests for this article.
Manickavasagam A, Nair K, Patloori SCS, Chase D, Roshan J. Postpacing interval is shorter than tachycardia cycle length—What's the mechanism?. J Arrhythmia. 2021;37:455–457. 10.1002/joa3.12514


