Abstract
Background
Cryoballoon ablation is a commonly used approach to treat patients with atrial fibrillation (AF).
Objectives
Report on the safety and efficacy of cryoballoon ablation for the treatment of AF in the largest global cohort of cryoablated patients prospectively studied within a single registry.
Methods
The Cryo AF Global Registry is a prospective, multi‐center registry. Patients with paroxysmal AF (PAF) or persistent AF (PsAF) were treated with the cryoballoon catheter according to routine practices at 93 sites across 36 countries. Primary efficacy endpoints included freedom from AF and freedom from AF/atrial flutter (AFL)/atrial tachycardia (AT) ≥30 seconds. The primary safety endpoint was serious device‐ or procedure‐related adverse events over 12 month follow‐up.
Results
During this evaluation window, 2922 subjects completed an index cryoballoon procedure, and 1440 completed 12 month follow‐up. The cohort was 61 ± 12 years of age, 36.3% female, and 78.7% PAF. Serious device‐ and procedure‐related adverse event rates were 1.5% and 3.4%, respectively. Freedom from AF/AFL/AT after the 90 day blanking period was 86.4% (95% CI: 84.3%‐88.3%) in patients with PAF and 70.9% (95% CI: 64.6%‐76.4%) in patients with PsAF. Freedom from AF/AFL/AT in first‐line PAF and PsAF was 90.0% (95% CI: 86.4%‐92.7%) and 72.9% (95% CI: 58.6%‐83.0%) at 12 months, respectively.
Conclusions
The Cryo Global AF Registry is the largest evaluation to demonstrate cryoablation is an efficient, safe, and effective treatment for patients with AF worldwide. Cryoablation was commonly used to treat patients prior to an AAD failure and may facilitate earlier therapy for patients on the AF disease continuum.
Keywords: antiarrhythmic drug, atrial fibrillation, catheter ablation, cryoballoon, pulmonary vein isolation
Cryoballoon ablation was an efficient, safe, and effective treatment for 2,922 patients with paroxysmal and persistent atrial fibrillation treated according to standard‐of‐care techniques at 93 unique sites in 36 countries around the world.

Abbreviations
- AAD
antiarrhythmic drug
- AEF
atrioesophageal fistula
- AF
atrial fibrillation
- AFL
atrial flutter
- AT
atrial tachycardia
- LA
left atrium
- PAF
paroxysmal atrial fibrillation
- PNI
phrenic nerve injury
- PsAF
persistent atrial fibrillation
- PV
pulmonary vein
- PVI
pulmonary vein isolation
1. INTRODUCTION
The Arctic Front™ Cardiac Cryoablation System has been widely adopted for the treatment of patients with atrial fibrillation (AF), and local standards of care with the cryoballoon catheter have been established at hospitals worldwide. Well‐controlled clinical trials evaluating cryoballoon ablation have demonstrated the safety and efficacy of pulmonary vein isolation (PVI) when treating patients with either paroxysmal (PAF) or persistent AF (PsAF) who are refractory to antiarrhythmic drugs (AADs). 1 , 2 , 3 , 4 Recent reports from controlled clinical trials have also demonstrated the safety and efficacy of cryoballoon ablation for treatment of patients prior to AAD failure. 5 , 6 The characteristics that define patients selected for, and outcomes of, cryoballoon ablation for the treatment of AF in real‐world practice globally is unknown. The Cryo AF Global Registry was designed to assess the procedural characteristics, safety, and efficacy of cryoablation for treatment of patients with AF according to real‐world practice in a broad spectrum of global care centers.
2. METHODS
2.1. Study design
The Cryo AF Global Registry (ClinicalTrials.gov registration: NCT02752737) is a prospective, multi‐center, observational, postmarket registry with ongoing data collection. In this evaluation window, data were collected on procedures performed by 239 unique operators at 93 sites across 36 countries in Africa, Europe, Asia, North America, and South America (Table S1). The objectives of this analysis were to assess the acute procedural characteristics, safety, and efficacy when using a cryoballoon ablation catheter (Medtronic, Inc) to treat patients with AF. The study was conducted according to Good Clinical Practices, in compliance with local laws, regulations and standards, and in accordance with the principles outlined in the Declaration of Helsinki. Each site received approval by an independent ethics/institutional review board and obtained written informed patient consent for all subjects prior to enrollment. This registry was sponsored by Medtronic, Inc. A seven‐member international physician steering committee was utilized to advise and oversee data collection methods, data quality, analyses, and publication cadence based on data collection milestones.
2.2. Patient population
Subjects were required to be ≥18 years old (or minimum age determined by local regulations) and have a planned procedure using the Medtronic cryoablation system to be included in the registry. Patients were excluded if they were participating in a concurrent, unapproved trial or were unable to participate according to local laws. Additionally, subject data were excluded from this current analysis if patients were diagnosed with long‐standing PsAF (continuous AF > 12 months), had a prior cardiac ablation for the treatment of an atrial arrhythmia(s), had incomplete baseline data reported, or had not completed an index ablation procedure at the time of the database freeze. Within the dataset, subjects with zero failed AADs prior to enrollment in the trial were considered non‐drug refractory. “First‐line” patients were denoted as patients who were: (a) non‐drug refractory and (b) not taking an AAD at baseline. Subjects were classified as having PAF if they had an episode(s) of AF that terminated spontaneously or with intervention within 7 days of onset. Patients were classified as having PsAF if they had a sustained episode(s) of AF that lasted longer than 7 days but less than 12 months, including episodes ≥7 days that were terminated by cardioversion.
2.3. Cryoballoon ablation procedure
The cryoballoon ablation procedure was conducted according to standard of care at each participating site. This procedure has been described in detail and typical utilization has been published. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 In brief, transseptal puncture provided access to the left atrium (LA). A dedicated, 15‐F OD steerable sheath (FlexCath or FlexCath Advance Steerable Sheath; Medtronic, Inc) was used to introduce a 23 or 28 mm cryoballoon ablation catheter (Arctic Front; Arctic Front Advance; Arctic Front Advance – ST; Arctic Front Advance Pro; Medtronic, Inc) into the LA. The cryoballoon catheter and sheath were delivered to the targeted pulmonary vein (PV) with either a J‐tip guidewire or a dedicated inner‐lumen octopolar/decapolar circular mapping catheter (Achieve or Achieve Advance, Medtronic, Inc). Upon antral occlusion of the targeted PV, the cryoapplication was initiated. The number and duration of cryoapplications per PV were determined by the physician.
Sites were recommended to monitor phrenic nerve function during right‐sided PVI by pacing with a diagnostic catheter at the level of the right subclavian vein and adjunctive monitoring for diaphragmatic function. All cryoapplications were terminated upon detection of an attenuated diaphragmatic response. Adjunctive imaging (eg, intracardiac echocardiography, three‐dimensional electroanatomical mapping), intraprocedural esophageal temperature monitoring, ablation tools (eg, focal cryoablation, radiofrequency catheter ablation), and/or adjunctive lesions applied to each patient were physician determined and documented. PVI was demonstrated by entrance and/or exit block following the ablation. Further, postablation testing (eg, testing acute PVI with adenosine or isoproterenol), periprocedural anticoagulation, and AAD initiation or continuation was left to the discretion of the physician. Patients were discharged from the hospital using local standard‐of‐care policies.
2.4. Patient follow‐up
This analysis examined patients enrolled and treated in the registry with either complete or ongoing follow‐up between May 2016 and January 2020. Patients were followed according to center standard‐of‐care protocols with a postprocedure visit either in person or by phone required at 12 months post the index procedure. Arrhythmia recurrence was monitored by any of the following methods: electrocardiogram, Holter monitor, trans‐telephonic monitor, insertable cardiac monitor, pacemaker, and/or implantable cardioverter defibrillator. Additional information collected included cardiovascular medications, adverse events, and quality of life assessments as measured by the EQ‐5D‐3L questionnaire. 9
2.5. Endpoints
The primary efficacy objectives were the 12 month freedom from a ≥30 second recurrence of AF and the 12 month freedom from a ≥30 second recurrence of combined AF, atrial flutter (AFL) and/or, atrial tachycardia (AT) following a 90 day blanking period. During the 90 day blanking period patients were managed per standard of care. Thereafter, during the efficacy follow‐up assessment, a patient was monitored for atrial arrhythmia recurrence with or without the usage of AADs. Freedom from recurrence of AF/AFL/AT was also analyzed in sub‐cohorts of first‐line subjects with PAF and PsAF. The primary safety objective was the serious device‐ or procedure‐related adverse event rates. Investigators classified adverse events by seriousness (according to international standards [ISO 14 155:2001]) and relatedness to the cryoablation system and/or ablation procedure. Serious adverse events included all events that led to death, or to a serious deterioration in health that resulted in either (a) a life‐threatening illness or injury, (b) a permanent impairment in body structure or function, (c) in‐patient or prolonged hospitalization, or (d) medical intervention to prevent life‐threatening illness or injury. All adverse events were followed until the event resolved, the event was unresolved with no further actions, or the subject exited the study. Ancillary objectives were examined in this dataset, including: patient baseline demographics, characterization of the cryoablation procedure, and quality‐of‐life post cryoballoon ablation. Changes in quality of life over the study period were measured by the EQ‐5D‐3L questionnaire (score of 1 represents maximal quality of life) at baseline and 12 month follow‐up.
2.6. Statistical analysis
Baseline characteristics and clinical data were summarized using the appropriate summary statistics; continuous variables are summarized as mean and standard deviation, and categorical variables are summarized as counts and percentages. Differences in baseline characteristics between the cohort of subjects enrolling with PAF vs subjects enrolling with PsAF were tested with a two‐sample t‐test for continuous variables and Fisher's exact test for categorical variables. Kaplan‐Meier methods were used to estimate 12 month freedom from arrhythmia recurrence. Standard error was approximated with Greenwood's formula. Differences in arrhythmia recurrence rates were compared between PAF and PsAF cohorts with a log‐rank test. Differences in recurrence rates were compared between first‐line and drug‐refractory patients with a Cox regression model after accounting for differences in baseline AF classification (PAF or PsAF). In the regression model, arrhythmia recurrence was the dependent variable while AAD status (first‐line or drug‐refractory) and baseline AF type were included as covariates. Differences in safety event rates between PsAF and PAF patients were assessed with a Fisher's exact test. Changes in quality of life from baseline to 12 months were assessed with a t‐test. Values of P < .05 were considered significant. Statistical analyses were conducted using SAS software version 9.4 (SAS Institute).
3. RESULTS
3.1. Patient follow‐up and characteristics
Of the 3276 subjects enrolled in the Cryo AF Global Registry between May 2016 and January 2020, 2922 eligible subjects completed a cryoballoon ablation index procedure (denoted the Procedure Analysis Cohort). Within this timeframe, 1440 subjects from 26 of 36 countries had been followed for 12 months post ablation (denoted the Efficacy Analysis Cohort; Figure 1). Of the Efficacy Analysis Cohort, 1320 (91.7%) patients had at least one follow‐up visit with an average of 3.0 visits per patient over 12 month follow‐up. While rhythm monitoring was not protocol required at follow‐up visits, 1151 (79.9%) patients underwent arrhythmia monitoring at least once in the 12 months after the cryoablation, and 62.6% of patients were monitored for atrial arrhythmia recurrence more than once during follow‐up per standard of care. Over the follow‐up period, 1140 (79.2%) patients were monitored by ECG and Holter monitors, 11 (0.8%) patients were monitored remotely (eg transtelephonic monitoring), and 62 (4.3%) patients had implanted cardiac devices from which rhythm data were reviewed by a clinician. The frequency, timing, and type of arrhythmia monitoring utilized is provided in Figure 2. During follow‐up, 81 of the 1440 (5.6%) subjects exited early for the following reasons: 2 (0.1%) withdrawn by the investigator, 55 (3.8%) lost to follow‐up, 22 (1.5%) subjects requested withdrawal, and 2 (0.1%) for other reasons.
FIGURE 1.
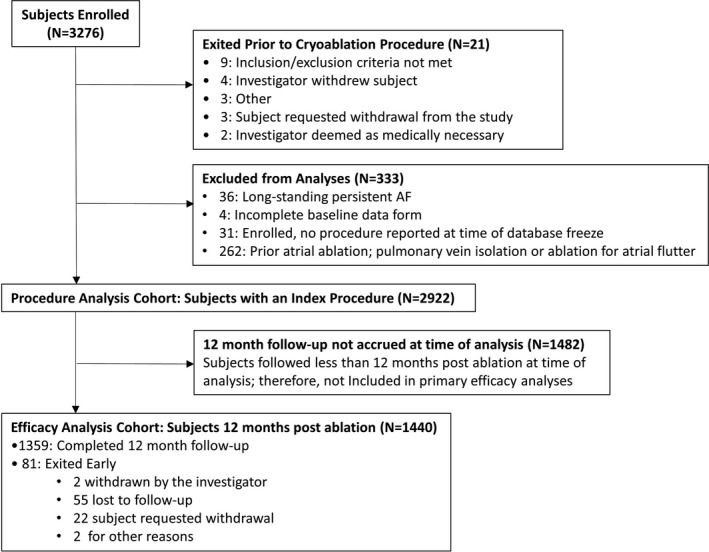
Patient Flow. Subject enrollment and criteria for the Procedure Analysis Cohort and Efficacy Analysis Cohort. Efficacy analyses were completed on the subset of subjects who were a minimum of 12 months post cryoablation at the time of the analysis
FIGURE 2.
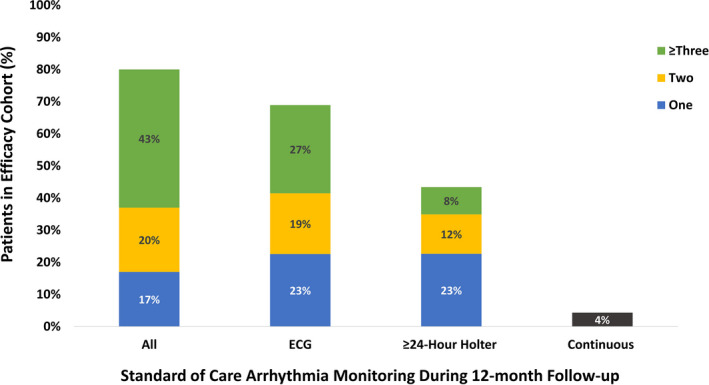
Monitoring Methods for Atrial Arrhythmia Recurrence. The proportion of the Efficacy Analysis Cohort to be monitored for atrial arrhythmia recurrence during the 12 month follow‐up period with one (blue), two (yellow), or three or more (green) ECGs, ≥24 hour Holter Monitors, and all combined methods for arrhythmia monitoring is depicted. Patients who were evaluated with continuous monitoring methods (black) are also presented. In total, 79.9% of patients underwent monitoring for atrial arrhythmia recurrence at least once during the follow‐up period
Baseline patient characteristics are detailed in Table 1. On average, the cohort was 61 ± 12 years of age, 36.3% female, had a CHA2DS2‐VASc score of 2.1 ± 1.6, and was diagnosed with AF for a mean of 3.1 ± 4.6 years prior to cryoablation. In total, 41.5% of subjects had not failed an AAD prior to the cryoablation procedure and were deemed non‐drug refractory. For 904 (30.9%) subjects, the cryoablation was considered first‐line therapy as they were non‐drug refractory and were not on an AAD at the time of study enrollment. Subjects with PAF comprised 78.7% of the total cohort. The PsAF sub‐group had a significantly larger mean LA diameter, lower left ventricular ejection fraction, higher body mass index, higher CHA2DS2‐VASc scores, was older, was more often male, and had a higher rate of co‐morbidities (P < .01).
TABLE 1.
Baseline patient characteristics
| Subject characteristics |
Total cohort (N = 2922) |
Paroxysmal AF (N = 2301) |
Persistent AF (N = 621) |
P‐value f |
|---|---|---|---|---|
| Female sex (N [%]) | 1062 (36.3%) | 875 (38.0%) | 187 (30.1%) | <.01 |
| Age in years (mean ± STD) | 61 ± 12 | 60 ± 12 | 62 ± 11 | <.01 |
| Body mass index in kg/m2 (mean ± STD) a | 27 ± 5 | 27 ± 5 | 29 ± 5 | <.01 |
| CHA2DS2‐VASc Score (mean ± SD) | 2.1 ± 1.6 | 2.1 ± 1.6 | 2.3 ± 1.6 | <.01 |
| Years diagnosed with AF (mean ± STD) b | 3.1 ± 4.6 | 3.2 ± 4.7 | 3.0 ± 4.3 | .38 |
| History of atrial flutter (N [%]) | 185 (6.3%) | 140 (6.1%) | 45 (7.2%) | .31 |
| History of atrial tachycardia (N [%]) | 42 (1.4%) | 37 (1.6%) | 5 (0.8%) | .18 |
| Left atrial diameter in mm (mean ± STD) c | 41 ± 7 | 40 ± 7 | 45 ± 8 | <.01 |
| Left ventricular ejection fraction in % (mean ± STD) d | 60 ± 9 | 61 ± 8 | 56 ± 10 | <.01 |
| Number of failed AADs (mean ± STD) | 0.7 ± 0.7 | 0.7 ± 0.7 | 0.7 ± 0.7 | .61 |
| 0 previously failed AADs (N [%]) | 1214 (41.5%) | 953 (41.4%) | 261 (42.0%) | |
|
310 (10.6%) | 205 (8.9%) | 105 (16.9%) | |
|
904 (30.9%) | 748 (32.5%) | 156 (25.1%) | |
| 1 prior AAD failure | 1281 (43.8%) | 1008 (43.8%) | 273 (44.0%) | |
| 2 prior AAD failures | 318 (10.9%) | 242 (10.5%) | 76 (12.2%) | |
| 3 or more prior AAD failures | 44 (1.5%) | 35 (1.5%) | 9 (1.4%) | |
| Not reported | 65 (2.2%) | 63 (2.7%) | 2 (0.3%) | |
| Hypertension (N [%]) | 1609 (55.1%) | 1221 (53.1%) | 388 (62.5%) | <.01 |
| Prior cardiac device implant e (N [%]) | 132 (4.5%) | 97 (4.2%) | 35 (5.6%) | .13 |
| History of congestive heart failure (N [%]) | 153 (5.2%) | 86 (3.7%) | 67 (10.8%) | <.01 |
| NYHA classification | <.01 g | |||
| Subject does not have heart failure (N [%]) | 2006 (68.7%) | 1611 (70.0%) | 395 (63.6%) | |
| Class I | 350 (12.0%) | 281 (12.2%) | 69 (11.1%) | |
| Class II | 233 (8.0%) | 153 (6.6%) | 80 (12.9%) | |
| Class III | 78 (2.7%) | 36 (1.6%) | 42 (6.8%) | |
| Class IV | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| NYHA status not reported (N [%]) | 255 (8.7%) | 220 (9.6%) | 35 (5.6%) | |
| Prior myocardial infarction (N [%]) | 79 (2.7%) | 65 (2.8%) | 14 (2.3%) | .49 |
| Prior stroke/transient ischemic attack (N [%]) | 182 (6.2%) | 131 (5.7%) | 51 (8.2%) | .03 |
| Coronary artery disease (N [%]) | 295 (10.1%) | 240 (10.4%) | 55 (8.9%) | .26 |
| Diabetes (N [%]) | 389 (13.3%) | 305 (13.3%) | 84 (13.5%) | .84 |
| Sleep apnea (N [%]) | 123 (4.2%) | 97 (4.2%) | 26 (4.2%) | 1.00 |
Abbreviations: AF, atrial fibrillation; STD, standard deviation
2915 subjects with BMI reported; 2295 PAF, 620 PsAF subjects
2739 subjects with AF diagnosis date reported; 2151 PAF, 588 PsAF subjects
1939 subjects with left atrial diameter reported; 1526 PAF, 413 PsAF
2469 subjects with left ventricular ejection fraction reported; 1945 PAF, 524 PsAF subjects
Prior cardiac device includes implantable pulse generator (IPG), implantable cardioverter defibrillator (ICD), cardiac resynchronization therapy pacemaker (CRT‐P), cardiac resynchronization therapy defibrillator (CRT‐D), and insertable cardiac monitor (ICM).
Statistical tests comparing paroxysmal AF cohort vs persistent AF cohort. Continuous variables compared with t‐test, binary variables compared with exact test
Wilcoxon rank‐sum test comparing distribution of severity of NYHA (from no heart failure through Class IV) between PAF and PsAF.
3.2. Procedural characteristics
Procedure‐related data are detailed in Table 2. Non‐general anesthesia was utilized in 62.6% of procedures. Subjects were predominantly treated with the Arctic Front Advance (second‐generation) cryoballoon (92.8%), and a 28 mm cryoballoon was used in 99.3% of procedures. Pre‐procedural imaging with MRI or CT was performed in 21.3% of subjects, and intraprocedural 3D electroanatomical mapping was performed in 13.3% of procedures. Phrenic nerve function was monitored during ablation in 99.1% of procedures with a pace and palpate technique employed in 87.9% of cases. The mean total procedure duration (time from first venous access to last catheter removal) was 82 ± 34 minutes, mean LA dwell time was 55 ± 25 minutes, and average fluoroscopy time was 18 ± 18 minutes.
TABLE 2.
Index procedure characteristics
| Procedural characteristics |
Total cohort (N = 2922) |
Paroxysmal AF (N = 2301) |
Persistent AF (N = 621) |
|---|---|---|---|
| Ablation tools a | |||
| 23 mm Cryoballoon (N [%]) | 34 (1.2%) | 26 (1.1%) | 8 (1.3%) |
| 28 mm Cryoballoon (N [%]) | 2903 b (99.3%) | 2285 (99.3%) | 618 (99.5%) |
| Arctic Front Advance (N [%]) | 2711 (92.8%) | 2149 (93.4%) | 562 (90.5%) |
| Arctic Front Advance Pro (N [%]) | 202 (6.9%) | 143 (6.2%) | 59 (9.5%) |
| Achieve Mapping Catheter (N [%]) | 2624 (89.8%) | 2075 (90.2%) | 549 (88.4%) |
| Total lab occupancy time in minutes c (mean ± STD) | 138 ± 53 | 138 ± 54 | 137 ± 48 |
| Total procedure time in minutes d (mean ± STD) | 82 ± 34 | 81 ± 34 | 85 ± 34 |
| Left atrial dwell time in minutes e (mean ± STD) | 55 ± 25 | 54 ± 25 | 59 ± 27 |
| Total fluoroscopy time in minutes f (mean ± STD) | 18 ± 18 | 18 ± 18 | 17 ± 20 |
| Total cryoapplication duration in minutes g (mean ± STD) | 18.9 ± 6.6 | 18.7 ± 6.4 | 19.8 ± 7.4 |
| Sedation method (N [%]) | |||
| General anesthesia | 1093 (37.4%) | 863 (37.5%) | 230 (37.0%) |
| Conscious sedation | 1828 (62.6%) | 1437 (62.5%) | 391 (63.0%) |
| Pre‐procedural imaging (CT and/or MRI) | 621 (21.3%) | 464 (20.2%) | 157 (25.3%) |
| Intra‐procedural 3D electroanatomical mapping | 389 (13.3%) | 365 (15.9%) | 24 (3.9%) |
| Intracardiac echocardiography | 769 (26.3%) | 594 (25.8%) | 175 (28.2%) |
| Esophageal temperature monitoring (N [%]) | 1090 (37.3%) | 866 (37.6%) | 224 (36.1%) |
| Pulmonary vein venography | 2774 (94.9%) | 2189 (95.1%) | 585 (94.2%) |
| Phrenic nerve monitoring | 2896 (99.1%) | 2282 (99.2%) | 614 (98.9%) |
| Pacing / palpate | 2568 (87.9%) | 2008 (87.3%) | 560 (90.2%) |
| Diaphragm stimulation | 1050 (35.9%) | 772 (33.6%) | 278 (44.8%) |
| Compound motor action potential | 890 (30.5%) | 708 (30.8%) | 182 (29.3%) |
| Other | 277 (9.5%) | 210 (9.1%) | 67 (10.8%) |
| Pulmonary vein ablation acute success h (N [%]) | 2775 (95.0%) | 2174 (94.5%) | 601 (96.8%) |
| PVI touch‐up with focal cryo catheter (N [%]) | 4 (0.1%) | 4 (0.2%) | 0 (0.0%) |
| PVI touch‐up with focal RF catheter (N [%]) | 40 (1.4%) | 31 (1.3%) | 9 (1.4%) |
| Isoproterenol and/or adenosine to assess PVI (N [%]) | 377 (12.9%) | 280 (12.2%) | 97 (15.6%) |
| Additional ablation lesions | |||
| Cavo‐tricuspid isthmus line (N [%]) | 286 (9.8%) | 256 (11.1%) | 30 (4.8%) |
| Other non‐PVI ablation (N [%]) | 142 (4.9%) | 129 (5.6%) | 13 (2.1%) |
| Cryoballoon applications | |||
| PV electrical potentials monitored (N [%]) | 2396 (82.0%) | 1886 (82.0%) | 510 (82.1%) |
| Number of applications per vein (mean ± STD) | |||
| (mean ± STD) | 1.5 ± 0.9 | 1.5 ± 0.9 | 1.5 ± 1.0 |
| (median [IQR]) | 1 [1, 2] | 1 [1, 2] | 1 [1, 2] |
| Number of veins | 11517 | 9042 | 2475 |
| Duration of cryoapplication in seconds | |||
| ALL PVs | |||
| (mean ± STD) | 185 ± 53 | 183 ± 53 | 193 ± 54 |
| (median [IQR]) | 180 [179, 240] | 180 [172, 240] | 180 [180, 240] |
| Number of applications | 17829 | 14011 | 3818 |
| RIPV | 184 ± 54 | 181 ± 53 | 191 ± 56 |
| RSPV | 178 ± 55 | 176 ± 54 | 184 ± 56 |
| LIPV | 190 ± 52 | 187 ± 52 | 202 ± 52 |
| LSPV | 189 ± 51 | 187 ± 51 | 196 ± 50 |
| LCPV | 193 ± 51 | 193 ± 49 | 192 ± 57 |
| Cryoballoon nadir temperature (°C) | |||
| ALL PVs | |||
| (mean ± STD) | ‐48 ± 7 | ‐48 ± 7 | ‐48 ± 7 |
| (median [IQR]) | ‐48 [‐53, ‐43] | ‐47 [‐53, ‐43] | ‐48 [‐54, ‐43] |
| Number of veins i | 11498 | 9026 | 2472 |
| RIPV | ‐47 ± 7 | ‐47 ± 7 | ‐48 ± 7 |
| RSPV | ‐51 ± 6 | ‐51 ± 6 | ‐51 ± 7 |
| LIPV | ‐45 ± 6 | ‐45 ± 6 | ‐45 ± 6 |
| LSPV | ‐49 ± 6 | ‐49 ± 6 | ‐50 ± 6 |
| LCPV | ‐49 ± 7 | ‐50 ± 7 | ‐47 ± 7 |
Abbreviations: IQR, interquartile range; PV, pulmonary vein; PVI, pulmonary vein isolation; STD, standard deviation.
Of the 2922 procedures, cryoballoon device model was reported in 2913
24 patients were treated with both a 23 mm and 28 mm; 19 PAF and 5 PsAF subjects
2913 subjects reported total lab occupancy time; 2294 PAF and 619 PsAF
2912 subjects reported total procedure time; 2291 PAF and 621 PsAF
2911 subjects reported left atrial dwell time; 2290 PAF and 621 PsAF
2808 subjects reported total fluoroscopy time; 2204 PAF and 604 PsAF
2913 subjects reported total cryoapplication duration; 2292 PAF and 621 PsAF subjects
All targeted pulmonary veins isolated after cryoballoon ablation and focal touch‐up
Of the 11517 pulmonary veins treated, nadir temperature was reported in 11498 veins
Overall, 95.0% of patients had all targeted PVs acutely isolated during the index procedure. Of those, PVI was completed in 0.1% of subjects with focal cryoablation, and with focal radiofrequency ablation in 1.4% of subjects. Cavo‐tricuspid isthmus line ablation was performed in 9.8% of patients, and other non‐PV ablation adjunctive to PVI was performed in 4.9% of subjects during the index procedure. The average number of freezes per PV was 1.5 ± 0.9 for a mean duration of 185 ± 53 seconds. The mean balloon nadir temperature of the freezes was −48 ± 7°C, and PV potentials were monitored during 82.0% of cryoapplications.
3.3. Safety
Of the 2922 subjects in the Procedure Analysis Cohort, 110 serious procedure‐related events in 100 (3.4%) subjects occurred. Among those events, 47 were physician classified as serious cryoballoon device‐related adverse events that occurred in 44 (1.5%) subjects. There were no differences in the rate of serious device‐related adverse events (1.3% vs 1.8%; P = .57) or serious procedure‐related adverse events (3.4% vs 3.5%; P = .80) among subjects with PAF or PsAF, respectively (Figure 3). Overall, the most frequent serious adverse events were supraventricular arrhythmia recurrences (25 subjects). These events were deemed serious as they required hospitalization, cardioversion, or repeat ablation. Adverse events related to the puncture site for catheter access were the second most frequent event with 21 (0.7%) serious procedure‐related events (nine of which were related to the study device). Phrenic nerve injury (PNI) unresolved at discharge was observed in 15 subjects (0.5%). Of the 15 PNIs, nine (0.3%) resolved in a timeframe between 2 days and 6 months after the procedure, three (0.1%) resolved by 12 months (prior to study exit), and three (0.1%) asymptomatic events remained unresolved at 12 months. Procedure‐related cardiac tamponade/pericardial effusion occurred in 11 subjects (0.4%), of those nine were treated with pericardiocentesis and the remaining two were treated without surgical intervention. There were five (0.2%) procedure‐related stroke or transient ischemic attack events. A full list of serious adverse events is provided in Table 3.
FIGURE 3.
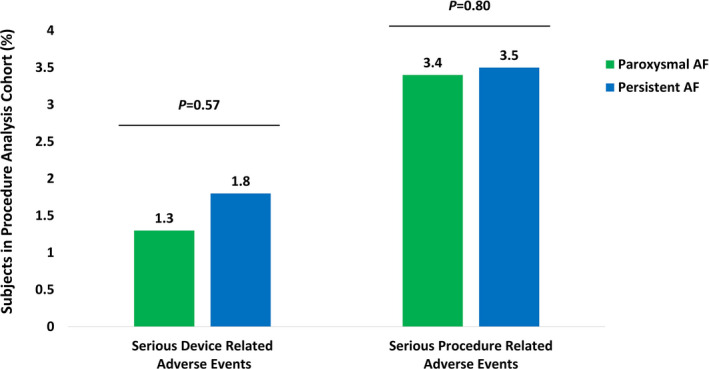
Rate of Serious Adverse Events. Serious device‐related and serious procedure‐related adverse events rates for subjects with paroxysmal (green) and persistent AF (blue). Fisher's exact tests identified no difference in the serious device‐related adverse event rates (1.3% vs 1.8%; P = .57) or the serious procedure‐related adverse event rates (3.4% vs 3.5%; P = .80) between paroxysmal and persistent AF cohorts, respectively
TABLE 3.
Primary safety events related to the index cryoballoon ablation procedure
|
Number of Events a (Number, % Subjects) |
||
|---|---|---|
| Adverse events | Serious device related | Serious procedure related |
| Total | 47 (44, 1.5%) | 110 (100, 3.4%) |
| Supraventricular arrhythmia recurrences b | 9 (9, 0.3%) | 25 (25, 0.9%) |
| Atrial fibrillation | 5 (5, 0.2%) | 16 (16, 0.5%) |
| Atrial flutter or atrial tachycardia | 3 (3, 0.1%) | 7 (7, 0.2%) |
| Groin‐site complication c | 9 (9, 0.3%) | 21 (20, 0.7%) |
| Phrenic nerve injury | 15 (15, 0.5%) | 15 (15, 0.5%) |
| Cardiac tamponade or pericardial effusion | 6 (6, 0.2%) | 11 (11, 0.4%) |
| Pulmonary or bronchial complication d | 1 (1, 0.0%) | 9 (9, 0.3%) |
| Myocardial infarction or ischemic cardiac event e | 2 (2, 0.1%) | 5 (5, 0.2%) |
| Pericarditis | 1 (1, 0.0%) | 5 (5, 0.2%) |
| Stroke or TIA f | 1 (1, 0.0%) | 5 (5, 0.2%) |
| Postoperative hypotension | 0 (0, 0.0%) | 4 (4, 0.1%) |
| Presyncope | 1 (1, 0.0%) | 2 (2, 0.1%) |
| Cardiac failure | 0 (0, 0.0%) | 1 (1, 0.0%) |
| Erosive esophagitis | 1 (1, 0.0%) | 1 (1, 0.0%) |
| Face injury g | 0 (0, 0.0%) | 1 (1, 0.0%) |
| Fluid overload | 0 (0, 0.0%) | 1 (1, 0.0%) |
| Headache | 0 (0, 0.0%) | 1 (1, 0.0%) |
| Sepsis | 0 (0, 0.0%) | 1 (1, 0.0%) |
| Stress cardiomyopathy | 1 (1, 0.0%) | 1 (1, 0.0%) |
| Urinary retention | 0 (0, 0.0%) | 1 (1, 0.0%) |
Procedure Analysis Cohort: Total Subjects with an index procedure (N = 2922)
Atrial fibrillation, atrial flutter, atrial tachycardia, nodal arrhythmia, sinus bradycardia, supraventricular tachycardia
Arteriovenous fistula, arteriovenous fistula aneurysm, arteriovenous fistula site hematoma, femoral artery dissection, hematoma, incision site hematoma, puncture site hematoma, vascular access site hemorrhage, vascular pseudoaneurysm, vascular pseudoaneurysm ruptured, vessel puncture site discharge, vessel puncture site hematoma
Hematemesis, hemoptysis, hypercapnia, pneumothorax, pulmonary embolism, pneumonia, pleurisy
Angina pectoris, coronary arteriospasm, myocardial infarction
Cerebral infarction, cerebrovascular accident, ischemic stroke, lacunar stroke
Due to a post‐ablation fall
Death occurred in 12 (0.4%) subjects during the data collection period. Three of the deaths occurred within 30 days of the procedure; the remaining deaths occurred between day 104 and 315 after the index procedure and were unrelated to the AF ablation procedure. The cause of death as reported by the investigator for the three subjects who died within 30 days of the procedure were as follows: (a) a cerebrovascular accident 24 days after the index procedure that was related to the AF ablation procedure; (b) pneumonia/chronic obstructive pulmonary disease unrelated to the AF ablation 14 days after the procedure; and (c) a non‐ST elevated myocardial infarction with cardiogenic shock unrelated to the AF ablation that occurred 3 days after the procedure.
3.4. Efficacy
Of the 218 patients who reported an atrial arrhythmia recurrence during follow‐up, 79.8% recurred with AF, 10.6% with AFL/AT, and 9.6% recurred with both AF and AFL/AT. The 12 month Kaplan‐Meier estimate of freedom from a ≥30 second recurrence of AF after the 90 day blanking period was significantly higher for patients with PAF (87.9% [95% CI: 85.8%‐89.7%]) compared to the estimate of freedom from AF for patients with PsAF at the 12 month timepoint (73.1% [95% CI: 66.8%‐78.3%]; P < .01). Likewise, the 12 month Kaplan‐Meier estimate of freedom from ≥30 second recurrence of AF/AFL/AT for subjects with PAF was greater than subjects with PsAF at 86.4% (95% CI: 84.3%‐88.3%) and 70.9% (95% CI: 64.6%‐76.4%), respectively (P < .01; Figure 4A). Overall, the rate of freedom from AT/AFL/AT recurrence was significantly higher in first‐line patients compared to AAD refractory patients (P = .02; Figure 4B). The 12 month Kaplan‐Meier estimate of freedom from AF/AFL/AT in first‐line PAF compared to drug‐refractory PAF was 90.0% (95% CI: 86.4%‐92.7%) vs 84.4% (95% CI: 81.5%‐86.8%; P = .01), respectively. First‐line PsAF subjects had a 12 month Kaplan‐Meier estimate of freedom from AF/AFL/AT of 72.9% (95% CI: 58.6%‐83.0%) compared to 70.2% (95% CI: 62.9%‐76.4%; P = .67) of subjects with drug‐refractory PsAF. Irrespective of arrhythmia recurrence, the number of patients on AADs reduced from 49% at index procedure discharge to 23% at the 12 month follow‐up.
FIGURE 4.

Freedom from Atrial Arrhythmia Recurrence. (A) Kaplan‐Meier 12 month estimate of freedom from ≥30 second recurrences of AF/AFL/AT in paroxysmal (green) and persistent AF (blue) after a 90 day blanking period. The 12 month freedom from arrhythmia recurrence was significantly higher in the paroxysmal AF cohort (86.4% [95% CI: 84.3%‐88.3%]) than the persistent AF cohort (70.9% (95% CI: 64.6%‐76.4%); P < .01) (B) Kaplan‐Meier estimate of freedom from ≥30 second recurrences of AF/AFL/AT at 12 months after a 90 day blanking period in patients with paroxysmal AF (green lines) and persistent AF (blue lines) who were drug refractory (solid lines) or treated with first‐line cryoballoon ablation (dashed lines). Cox regression model identified that patients who were drug refractory prior to cryoballoon ablation had lower rates of freedom from atrial arrhythmia recurrence than first‐line cryoablation patients (P = .02). *A total of 46 patients were missing baseline AAD information; therefore, 1394/1440 patients were included in the drug refractory vs first‐line Kaplan‐Meier estimates of freedom from atrial arrhythmia recurrence.
3.5. Quality of Life
A significant increase in patient reported quality of life as measured by the EQ‐5D‐3L questionnaire was observed across the full study cohort at 1 year (Table 4). The average EQ‐5D‐3L score increased from 0.89 ± 0.14 at baseline to 0.92 ± 0.12 (difference of 0.03 ± 0.14, P < .01) at 1 year. This increase in quality of life was observed in both the patients with PAF (increase of 0.03 ± 0.14, P < .01) and patients with PsAF (increase of 0.03 ± 0.14, P < .01).
TABLE 4.
Quality of life as measured by EQ‐5D‐3L
| Baseline | 12 months | Difference | P‐value b | |
|---|---|---|---|---|
| All Subjects a | 0.89 ± 0.14 | 0.92 ± 0.12 | 0.03 ± 0.14 | <.01 |
| Paroxysmal AF | 0.90 ± 0.14 | 0.93 ± 0.11 | 0.03 ± 0.14 | <.01 |
| Persistent AF | 0.87 ± 0.15 | 0.90 ± 0.14 | 0.03 ± 0.14 | <.01 |
Of the 1440 subjects in the Efficacy Analysis Cohort, 1303 completed a 12 month visit, of which 1213 completed an EQ‐5D questionnaire at both baseline and 12 months
t‐test
4. DISCUSSION
To our knowledge, the Cryo AF Global Registry is the first and largest assessment of a global patient population who underwent cryoballoon ablation for the treatment of AF examined over a 12 month follow‐up period. Cryoablation in this diverse cohort of patients with PAF and PsAF was completed safely with a 3.4% procedure‐related serious adverse event rate (1.5% device‐related serious adverse event rate) and efficiently with a mean 82 ± 34 minute procedure time. Freedom from AF/AT/AFL at 12 months was 86.4% (95% CI: 84.3%‐88.3%) for PAF and 70.9% (95% CI: 64.6%‐76.4%) for PsAF cohorts, and both sets of patients reported significant improvements in EQ‐5D‐3L measures of quality of life (P < .01). Overall, freedom from arrhythmia recurrence was higher in first‐line subjects compared to AAD‐refractory subjects (P = .02). These observations demonstrate that cryoballoon ablation is safe and effective for the treatment of patients with AF when delivered according to varying world‐wide standard‐of‐care policies. These data also demonstrate the benefit of earlier catheter ablation on reducing atrial arrhythmia recurrence.
4.1. Global standard of care for cryoballoon ablation
Similar to controlled clinical trials, patients with PsAF had baseline characteristics indicative of more advanced AF disease progression and a higher rate of comorbidities than enrolled patients with PAF. 3 , 4 Regardless of AF classification, standard‐of‐care approaches to treat patients with AF resulted in a consistent and efficient procedure with an average total procedure time of 82 minutes. Procedural techniques may have enabled these efficiencies. Specifically, a PVI‐only approach was commonly employed (86%) for patients with both PAF and PsAF, corroborating the essential role of PVI for the treatment of all forms of AF. 10 The frequent use of conscious sedation (63%) in the registry may also have contributed to the observed procedural efficiency as conscious sedation has been reported to confer shorter procedure times compared to general anesthesia with similar safety, efficacy, and patient reported satisfaction. 11 , 12 , 13 Additionally, few procedures were conducted with adjunctive equipment or additional PVI testing methods, including: pre‐procedural imaging (21%), electroanatomical mapping (13%), or isoproterenol/adenosine testing to assess acute PVI (13%). The ability to perform an effective cryoballoon ablation procedure with few adjunctive tools worldwide corroborates prior regional reports. 14 , 15 These data support the utility of this cryoablation system to address the growing population of patients with AF in a resource constrained environment.
4.2. Real‐World safety of cryoballoon ablation
World‐wide safety of AF ablation has not been published since 2010, in which outcomes after AF ablation performed by radiofrequency ablation catheters were estimated via a voluntary survey. 16 Here, the world‐wide safety profile of cryoballoon ablation procedures conducted according to standard practice at 93 unique global centers (3.4% serious procedure‐ and 1.5% serious device‐related adverse event rate) aligns with smaller, controlled cryoballoon ablation trials and region‐specific observational registries. 2 , 3 , 4 , 17 , 18 , 19 , 20 Despite larger LA diameters and generally higher rates of comorbidities in the PsAF cohort compared to the PAF cohort, adverse event rates were similar for both groups treated in this registry. Specifically, the rates of PNI at discharge (0.5%), cardiac tamponade/pericardial effusion (0.4%), and neurological events (0.2%) were low. Phrenic nerve monitoring to aid in early detection of diaphragmatic injury was conducted in 99% of procedures and likely contributed to the low rate of PNI, which is in alignment with contemporary reports. 17 , 18 , 19 Previous studies have also reported low rates of cardiac tamponade/pericardial effusion following cryoballoon ablation, 17 , 18 , 19 , 20 and the Cryo AF Global Registry confirms the low risk of this event after cryoballoon ablation in typical practice. Finally, one procedure‐related death was reported in this registry (0.03%), which indicates the improved safety of AF ablation since the Cappato et al 2010 survey, which reported 0.15% AF procedure‐related deaths. 16 This dataset of nearly 3000 patients treated according to standard of care by 239 different operators with varying experience across 36 countries, confirms the safety of cryoballoon ablation for the treatment of patients with AF in a real‐world setting.
4.3. Clinically relevant efficacy of cryoballoon ablation
Freedom from arrhythmia recurrence in PAF (86%) and PsAF (71%) observed in this world‐wide registry is similar to other real‐world reports of success after cryoablation ranging between 73% and 87% in PAF and 56%‐64% in PsAF populations. 17 , 18 , 19 , 20 , 21 , 22 Despite relatively similar baseline patient characteristics, the real‐world success of cryoballoon ablation in patients with PsAF in this registry was numerically higher than the recently reported STOP Persistent AF clinical trial. 4 The STOP Persistent AF trial included weekly trans‐telephonic monitoring and may have influenced this difference, but both studies demonstrated improvement in indicators of AF disease burden including significant improvements in measures of quality of life. As monitoring methods were aligned with standard clinical practice, 23 the success observed in this registry may better reflect the incidence of clinically relevant atrial arrhythmia recurrences following cryoballoon ablation of PAF and PsAF.
Notably, 42% of all treated patients had not failed an AAD prior to cryoballoon ablation in this registry, which often pre‐empts enrollment in a clinical trial. This observation indicates that cryoablation is used to treat patients early in the disease process, and it may be employed as an alternative to prescription of an AAD in real‐world clinical practice. Early rhythm control was recently reported to reduce the risk of cardiovascular morbidities in patients with AF. 24 Further, ablation for the treatment of AF earlier (after diagnosis of AF) has been demonstrated to improve success of catheter ablation and reduce the risk of AF disease progression. 25 , 26 , 27 Indeed, in this study first‐line cryoballoon ablation resulted in significantly higher rates of success compared to drug refractory patients; 90% of PAF and 73% of PsAF first‐line patients maintained freedom from arrhythmia recurrence at 12 months. Initial results from the CRYO FIRST and STOP First trials (presented online at DGK 2020 and EHRA, respectively) demonstrate that first‐line cryoballoon catheter ablation improves efficacy compared to AADs with a similar risk for serious adverse events. 5 , 6 The observations in this registry indicate that cryoballoon ablation in non‐drug‐refractory patients with PAF and PsAF is performed as standard‐of‐care therapy safely and effectively and may contribute to earlier and improved management of AF in real‐world practice.
4.4. Study limitations
This study was designed to evaluate the real‐world use of cryoballoon ablation for the treatment of AF according to standard‐of‐care policies across the globe; therefore, it was a non‐randomized, observational study design. Patients were not excluded from the trial for pre‐existing baseline characteristics, and investigators were trained to approach all eligible patients for participation in the registry; however, the results may be influenced by patient selection bias. Comparisons between sub‐cohorts (eg, PAF, PsAF, No‐AAD, first‐line, and drug refractory) may be influenced by baseline characteristics. Patients were monitored for atrial arrhythmia recurrence according to site‐specific standard practice, and a uniform approach was not protocol required across centers. As follow‐up was physician and patient determined, and 20% of patients did not have atrial arrhythmia monitoring performed during follow‐up, it is possible that asymptomatic and other atrial arrhythmias were not detected; however, the results reflect the real‐world clinical experience of patients and providers. As the registry is ongoing, 12 month follow‐up was not available for all patients who were treated with cryoballoon ablation during the study window, and not all geographies in the study had 12 month follow‐up data included in this report. Therefore, procedural outcomes were evaluated among all 2922 eligible patients who underwent an index procedure (Procedure Analysis Cohort) and efficacy was assessed in the subset of 1440 patients with 12 month follow‐up available during this study window (Efficacy Analysis Cohort).
5. CONCLUSIONS
The Arctic Front Cardiac Cryoablation System has been adopted globally, and the results from this Cryo AF Global Registry demonstrate robust safety, efficiency, and efficacy across a broad range of patients with AF when cryoballoon ablation is performed according to local standards of care. Cryoballoon ablation was frequently performed with few adjunctive tools, which may contribute to procedural efficiencies. Additionally, the high rates of freedom from arrhythmia recurrence that were observed in this real‐world experience are congruent with the significant improvements in quality of life measured between baseline and the 12 month timepoint. A large percentage of non‐drug‐refractory and first‐line patients were treated within this registry and suggests that cryoballoon ablation can be used safely and effectively as a first‐line therapy.
Disclosure
This study was sponsored by Medtronic, Inc, Minneapolis, MN. FJ Kueffer and KM Braegelmann are employees of Medtronic. Dr Földesi has received compensation for teaching and proctoring from Medtronic, The Johnson & Johnson Co., Abbott Laboratories, and Biotronik SE & Co. The remaining authors declare no conflict of interests for this article.
Supporting information
Table S1
ACKNOWLEDGEMENTS
The authors thank the Cryo AF Global Registry sites and staff for their vital contributions to the trial, and the authors are grateful to Scott A. Sarazin, Valentine Obidigbo, Hae Lim, and Bob Hokanson from Medtronic for their support of the trial and generation of the manuscript.
Chun KRJ, Okumura K, Scazzuso F, et al Safety and efficacy of cryoballoon ablation for the treatment of paroxysmal and persistent AF in a real‐world global setting: Results from the Cryo AF Global Registry. J Arrhythmia. 2021;37:356–367. 10.1002/joa3.12504
Funding information
This study was sponsored by Medtronic, Inc.
REFERENCES
- 1. Packer DL, Kowal RC, Wheelan KR, et al. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol. 2013;61(16):1713–23. [DOI] [PubMed] [Google Scholar]
- 2. Kuck K‐H, Brugada J, Fürnkranz A, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374(23):2235–45. [DOI] [PubMed] [Google Scholar]
- 3. Boveda S, Metzner A, Nguyen DQ, et al. Single‐procedure outcomes and quality‐of‐life improvement 12 months post‐cryoballoon ablation in persistent atrial fibrillation: results from the multicenter CRYO4PERSISTENT AF trial. JACC Clin Electrophysiol. 2018;4(11):1440–7. [DOI] [PubMed] [Google Scholar]
- 4. Su WW, Reddy VY, Bhasin K, Champagne J, Sangrigoli RM, Braegelmann KM, et al. Cryoballoon ablation of pulmonary veins for persistent atrial fibrillation: results from the Multicenter STOP persistent AF trial. Heart Rhythm. 2020;17(11):1841–7. 10.1016/j.hrthm.2020.06.020 [DOI] [PubMed] [Google Scholar]
- 5. Kuniss M, Pavlovic N, Velagic V, et al.Cryoballoon catheter ablation versus antiarrhythmic drugs as a first‐line therapy for patients with paroxysmal atrial fibrillation: the Cryo FIRST trial. DGK Conference; Mannheim Germany. 2020. [DOI] [PubMed]
- 6. Wazni O, Dandamudi G, Sood N, et al. Safety and efficacy of cryoballoon catheter ablation as a first line treatment for patients with paroxysmal atrial fibrillation: primary results of the randomized STOP AF First Study. European Society Cardiology Conference; Virtual. 2020.
- 7. Su W, Kowal R, Kowalski M, et al. Best practice guide for cryoballoon ablation in atrial fibrillation: the compilation experience of more than 3000 procedures. Heart Rhythm. 2015;12(7):1658–66. [DOI] [PubMed] [Google Scholar]
- 8. Su W, Aryana A, Passman R, et al. Cryoballoon Best Practices II: Practical guide to procedural monitoring and dosing during atrial fibrillation ablation from the perspective of experienced users. Heart Rhythm. 2018;15(9):1348–55. [DOI] [PubMed] [Google Scholar]
- 9. Rabin R, de Charro F, Szende A, Oppe M, Devlin N, Parkin D. EQ‐5D value sets: inventory, comparative review and user guide [Full Book]. Springer; 2003. [Google Scholar]
- 10. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275–e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wasserlauf J, Knight BP, Li Z, et al. Moderate sedation reduces lab time compared to general anesthesia during cryoballoon ablation for AF without compromising safety or long‐term efficacy. Pacing Clin Electrophysiol. 2016;39(12):1359–65. 10.1111/pace.12961 [DOI] [PubMed] [Google Scholar]
- 12. Miśkowiec D, Kasprzak JD, Wejner‐Mik P, et al. Conscious sedation during cryoballoon ablation of atrial fibrillation: a feasibility and safety study. Minerva Cardioangiol. 2018;66(2):143–51. 10.23736/S0026-4725.17.04505-4 [DOI] [PubMed] [Google Scholar]
- 13. Wasserlauf J, Kaplan RM, Walega DR, Arora R, Chicos AB, Kim SS, et al. Patient‐reported outcomes after cryoballoon ablation are equivalent between moderate sedation and general anesthesia. J Cardiovasc Electrophysiol. 2020;31(7):1579–84. 10.1111/jce.14547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sagone A, Iacopino S, Pieragnoli P, et al. Cryoballoon ablation of atrial fibrillation is effectively feasible without previous imaging of pulmonary vein anatomy: insights from the 1STOP project. J Interv Card Electrophysiol. 2019;55(3):267–75. 10.1007/s10840-018-0500-6 [DOI] [PubMed] [Google Scholar]
- 15. Stabile G, Tondo C, Curnis A, et al. Efficacy of cryoballoon ablation in patients with paroxysmal atrial fibrillation without time to pulmonary vein isolation assessment. Int J Cardiol. 2018;272:118–22. 10.1016/j.ijcard.2018.07.070 [DOI] [PubMed] [Google Scholar]
- 16. Cappato R, Calkins H, Chen S‐A, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3(1):32–8. [DOI] [PubMed] [Google Scholar]
- 17. Knight BP, Novak PG, Sangrigoli R, et al. STOP AF PAS investigators. Long‐Term outcomes after ablation for paroxysmal atrial fibrillation using the second‐generation cryoballoon: final results from STOP AF post‐approval study. JACC Clin Electrophysiol. 2019;5(3):306–14. [DOI] [PubMed] [Google Scholar]
- 18. Mörtsell D, Arbelo E, Dagres N, et al. Cryoballoon vs. radiofrequency ablation for atrial fibrillation: a study of outcome and safety based on the ESC‐EHRA atrial fibrillation ablation long‐term registry and the Swedish catheter ablation registry. Europace. 2019;21(4):581–9. [DOI] [PubMed] [Google Scholar]
- 19. Su W, Orme GJ, Hoyt R, et al. Retrospective review of arctic front advance cryoballoon ablation: a multicenter examination of second‐generation cryoballoon (RADICOOL trial). J Interv Card Electrophysiol. 2018;51(3):199–204. [DOI] [PubMed] [Google Scholar]
- 20. Chun KRJ, Perrotta L, Bordignon S, et al. Complications in catheter ablation of atrial fibrillation in 3,000 consecutive procedures: balloon versus radiofrequency current ablation. JACC Clin Electrophysiol. 2017;3(2):154–61. [DOI] [PubMed] [Google Scholar]
- 21. Tondo C, Iacopino S, Pieragnoli P, et al. Pulmonary vein isolation cryoablation for patients with persistent and long‐standing persistent atrial fibrillation: Clinical outcomes from the real‐world multicenter observational project. Heart Rhythm. 2018;15(3):363–8. 10.1016/j.hrthm.2017.10.0381 [DOI] [PubMed] [Google Scholar]
- 22. Sawhney V, Schilling RJ, Providencia R, et al. Cryoablation for persistent and longstanding persistent atrial fibrillation: results from a multicentre European registry. Europace. 2020;22(3):375–81. 10.1093/europace/euz313 [DOI] [PubMed] [Google Scholar]
- 23. Arbelo E, Brugada J, Hindricks G, et al. Atrial fibrillation ablation pilot study investigators. The atrial fibrillation ablation pilot study: a European Survey on Methodology and results of catheter ablation for atrial fibrillation conducted by the European Heart Rhythm Association. Eur Heart J. 2014;35(22):1466–78. 10.1093/eurheartj/ehu001 [DOI] [PubMed] [Google Scholar]
- 24. Kirchhof P, Camm AJ, Goette A, et al. Early rhythm‐control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305–16. 10.1056/NEJMoa2019422 [DOI] [PubMed] [Google Scholar]
- 25. Lunati M, Arena G, Iacopino S, et al. Is the time between first diagnosis of paroxysmal atrial fibrillation and cryoballoon ablation a predictor of efficacy? J Cardiovasc Med (Hagerstown). 2018;19(8):446–52. [DOI] [PubMed] [Google Scholar]
- 26. Chew DS, Black‐Maier E, Loring Z, Noseworthy PA, Packer DL, Exner DV, et al. Diagnosis‐to‐ablation time and recurrence of atrial fibrillation following catheter ablation: a systematic review and meta‐analysis of observational. Circ Arrhythm Electrophysiol. 2020;13(4):e008128. 10.1161/CIRCEP.119.008128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuck KH, Lebedev D, Mikhaylov E, et al. Catheter ablation can delay progression from paroxysmal to persistent atrial fibrillation: the Attest trial. ESC Congress; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


