Abstract
Background
The peri‐outflow tract region could be the origin of ventricular tachycardia (VT) after aortic valve replacement (AVR). However, the clinical characteristics of outflow tract ventricular tachycardias (OTVTs) after AVR are yet to be clarified. This study investigated the incidence, risk factors, and clinical characteristics of patients with OTVTs after AVR.
Methods
We retrospectively analyzed the clinical course of 120 patients who had undergone surgical AVR (SAVR) between April 1980 and October 2018. The patients had no ischemic or diagnosed cardiomyopathies other than primary aortic valve diseases.
Results
Six patients (5.0%) developed OTVTs after SAVR. The average onset was at 10.8 ± 5.7 years after SAVR. All cases of VT arose from the inferior axis and included left and right bundle branch block configuration. Two patients who underwent cardiac magnetic resonance imaging (MRI) had late gadolinium enhancement (LGE) in the midlayer of the left ventricle basal anteroseptal wall. Patients with periaortic VTs had significantly larger left ventricular (LV) diameter at systole, lower LV ejection fraction, higher positive rates of signal‐averaged electrocardiogram (SAECG), and nonsustained VTs on Holter monitoring. On ablation, local fragmented potentials with low voltage zones were observed in accordance with the LGE distribution. Multiple VTs originating from the periaortic region were provoked in the sessions.
Conclusions
Acute OTVT was found in 5% of patients after SAVR. Arrhythmia risk stratification by SAECG, Holter ECG, and cardiac MRI should be considered for a long period in patients after SAVR.
Keywords: aortic valve replacement, arrhythmia, long term, periaortic, ventricular tachycardia
Based on our results, acute onset of OTVT was found in 5% of patients who received SAVR. Therefore, we suggest that arrhythmic risk stratification should be considered for patients who have undergone SAVR for a long period.

1. INTRODUCTION
The incidence of sudden death after cardiac valve replacement surgery is reported to be approximately 0.2%–3.6%. 1 , 2 , 3 The main etiologies of sudden cardiac death include valve thrombosis, anticoagulation‐related hemorrhage, coronary diseases, acute decompensated heart failure, and fatal arrhythmias. 4 , 5 , 6
As one of the cases of arrhythmia after aortic valve replacement (AVR), sustained or incessant nonsustained ventricular tachycardia (VT) arising from the outflow tract (OT), including the periaortic region, has been well described in previous studies. 7 , 8 However, the natural course of such outflow tract ventricular tachycardias (OTVTs) after AVR, including the incidence, time of onset, risk factors, and prognosis, has not been well investigated to date. Therefore, this study aimed to investigate the clinical characteristics of OTVTs after AVR.
2. METHODS
2.1. Study population
For this retrospective cohort study, we analyzed the medical database of patients admitted at Ome Municipal General Hospital, Tokyo, Japan. In total, the data of 188 patients, who had undergone surgical AVR (SAVR) between April 1980 and October 2018, were extracted based on the disease name registration “aortic valve replacement.” Among them, 143 (76.1%) and 45 (23.9%) had undergone SAVR at our or at another hospital, respectively.
Thirty‐nine patients who had followed up for <1 year were excluded. All patients had undergone transthoracic echocardiography (TTE) and coronary angiography, as part of screening before SAVR. Moreover, we excluded patients with ischemic heart diseases and other diagnosed cardiomyopathies, except for cases of patients with primary aortic valve diseases. Subsequently, we excluded 29 patients with a history of ischemic heart disease (such as old myocardial infarction) or a history of percutaneous coronary intervention or coronary artery bypass grafting. Finally, the data of 120 patients who had undergone SAVR without ischemic or diagnosed cardiomyopathies other than primary aortic valve diseases were analyzed retrospectively (Figure 1).
FIGURE 1.
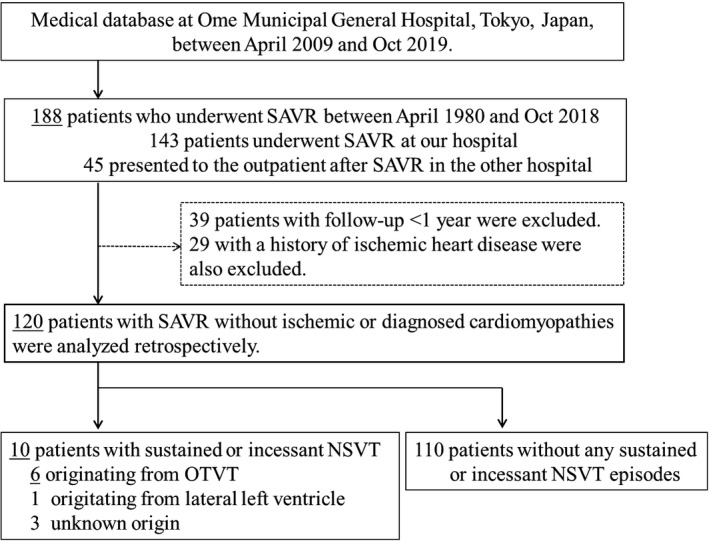
Study population. In this study, 120 patients who underwent SAVR without ischemic or diagnosed cardiomyopathies were analyzed retrospectively. In total, six patients had sustained or incessant nonsustained VT episodes originating from the OT. AVR, aortic valve replacement; NSVT, nonsustained ventricular tachycardia; OTVT outflow tract ventricular tachycardia; SAVR, surgical aortic valve replacement; VT, ventricular tachycardia; OT, outflow tract
2.2. Ethics and informed consent
Informed consent was obtained using an opt‐out form. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in a priori approval by our institutional review boards (approval number, 31; approval date, October 7, 2019).
2.3. Medications during the follow‐up periods
Warfarin was used for anticoagulation in patients with mechanical valves at doses adjusted to achieve target international normalized ratio values of 1.6‐2.6 and 2.0‐3.0 in older (≥70 years) and younger patients (<70 years), respectively. Antiplatelet drugs were used in patients with biological valves based on the Japanese guidelines for anticoagulation and antiplatelet administration for aortic prosthetic valves. 9 The use of other medications, including beta‐blockers, angiotensin‐converting enzyme inhibitors (ACEIs), angiotensin‐II receptor blockers (ARBs), and other anti‐arrhythmia drugs, was at the discretion of the primary outpatient physicians.
2.4. Outpatient follow‐up tests
All 120 patients underwent TTE and laboratory investigations at least once a year, as a routine check. Signal‐averaged electrocardiogram (SAECG) and Holter ECG were performed in patients with possible clinical symptoms of arrhythmias and in those who consented to undergo the tests to stratify the arrhythmia risk. SAECG was considered positive when at least two of the following criteria were met: filtered QRS duration >114 ms; terminal (last 40 ms) QRS root means square voltage <20 μV; and low amplitude (<40 μV) signal duration > 38 ms
2.5. Definition of outflow tract ventricular tachycardia
The endpoint was the occurrence of OTVT, which was defined as a sustained or incessant nonsustained VT (NSVT) originating from the OT detected with 12 lead ECG and not associated with ischemic heart disease.
2.6. Cardiac magnetic resonance imaging (MRI) with gadolinium (Gd) enhancement
Cardiac MRI with Gd enhancement (1.5T scanner, Intera Nova dual, Philips, The Netherlands) was performed for some cases of periaortic VT to detect the scars.
2.7. Electrophysiological study, mapping, and ablation
Electrophysiological study, mapping (Carto 3, Biosense Webster, Irvine, CA, USA), and ablation were also performed for some cases of sustained OTVT that could not be suppressed by the medication control. Ablation was performed using a nonirrigated 4.0 mm tip catheter (CARTO Navistar Catheter, Biosense Webster), which is a safe procedure to access the OT and is used for OTVT ablation in our institute with a power of 30‐40 W and a temperature limit of 50°C. The ablation was deemed successful when there was an immediate suppression and when there were no sustained VTs after repeating the induction protocol.
2.8. Statistical analysis
Continuous variables are expressed as means ± standard deviations. Categorical variables are expressed as numbers (percentages). Continuous and categorical variables were compared using Student's t test, Fisher's exact test, univariate Cox regression analysis, and Kaplan‐Meier curve analysis. All data were analyzed using JMP v12.0.0 (SAS Institute Inc; Cary, NC, USA). P‐values < .05 were considered statistically significant.
3. RESULTS
3.1. Baseline patient characteristics
Of the 120 patients [sex, 74 men (61.7%) and 46 women (38.3%); mean age, 73.4 ± 13.5 years], 68 (56.7%) and 52 (43.3%) had mechanical and biological valves, respectively. The patients had the following primary aortic diseases: aortic stenosis (AS) (n = 70; 58.3%), aortic regurgitation (AR) (n = 30; 25.0%), infective endocarditis (IE) (n = 19; 15.8%), and rupture of sinus of Valsalva aneurysm (n = 1; 0.9%). The mean duration since AVR was 11.2 ± 8.6 years (Table 1).
TABLE 1.
Baseline characteristics of patients who underwent surgical AVR without other structural heart diseases (n = 120)
| Age (y) | 73.4 ± 13.5 |
| Male sex | 74 (62) |
| BMI (kg/cm2) | 22.3 ± 4.1 |
| Aortic valve type | |
| Mechanical valve | 68 (57) |
| Biological valve | 52 (43) |
| Mitral valve operation | 19 (16) |
| Primary aortic valve diseases | |
| AS | 70 (58) |
| AR | 30 (25) |
| IE | 19 (16) |
| Others | 1 (1) |
| Period after AVR (years) | 11.3 ± 8.6 |
| HT | 72 (60) |
| DL | 58 (48) |
| DM | 27 (23) |
| CKD | 58 (48) |
| Hemodialysis | 5 (4) |
| Old cerebral infarction | 12 (10) |
| Paroxysmal atrial fibrillation | 10 (8) |
| Persistent atrial fibrillation | 19 (16) |
| Atrial tachycardia | 1 (1) |
| Medication | |
| Beta blocker | 67 (56) |
| ACEI/ARB | 45 (38) |
| Aldosterone antagonist | 10 (8) |
| Amiodarone | 7 (7) |
| Antiarrhythmic drug group 1 | 4 (3) |
Abbreviations: ACEI/ARB, angiotensin‐converting enzyme inhibitors/angiotensin II receptor blockers; AR, Aortic regurgitation; AS, Aortic stenosis; AVR, aortic valve replacement; BMI, body mass index; CKD, Chronic kidney disease;CRT‐D, Cardiac resynchronization therapy‐defibrillator; DL, Dyslipidemia; DM, Diabetes mellitus; HT, Hypertension; ICD, implantable cardioverter defibrillator; IE, Infective endocarditis; Others, Rupture of sinus of Valsalva aneurysm.
3.2. Patients with acute onset of OTVTs
Episodes of sustained or incessant VTs were observed in 10 patients (8.3%) after SAVR during the follow‐up period. Especially, six patients (5.0%) had episodes of the VT originating from the OT that was detected using 12 lead ECG and were not associated with ischemic heart disease (Figure 2). Additionally, one (0.9%) and three patients (2.5%) had sustained episodes of VT originating from the lateral LV portion and from an unknown origin, respectively. Furthermore, five out of six patients with OTVTs developed acute onset of chest discomfort and brain ischemic symptoms due to hypotension related to the sustained or incessant NSVTs. The median and mean durations between AVR and the onset of periaortic VTs were 9.0 and 10.8 ± 5.7 years, respectively (Figure 3). Morphologically, all VTs had an inferior axis, and four and two patients had right and left bundle branch block patterns, respectively. The mean cycle length of VTs was 306.7 ± 37.8 ms (Table 2).
FIGURE 2.
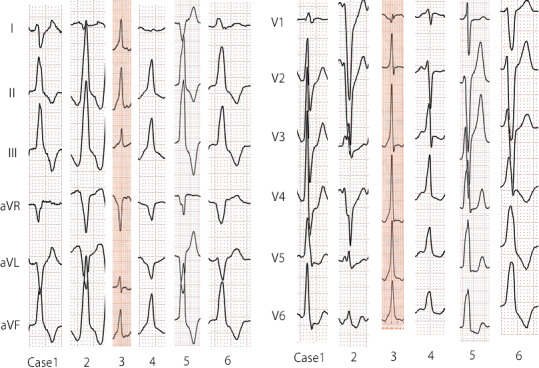
Comparison of 12 ECG findings of OTVTs long after SAVR. Morphologically, all OTVTs had an inferior axis. Four and two patients had RBBB and LBBB patterns, respectively. ECG, electrocardiogram; LBBB, left bundle branch block; OTVT, outflow tract ventricular tachycardia; RBBB, right bundle branch block; VT, ventricular tachycardia
FIGURE 3.
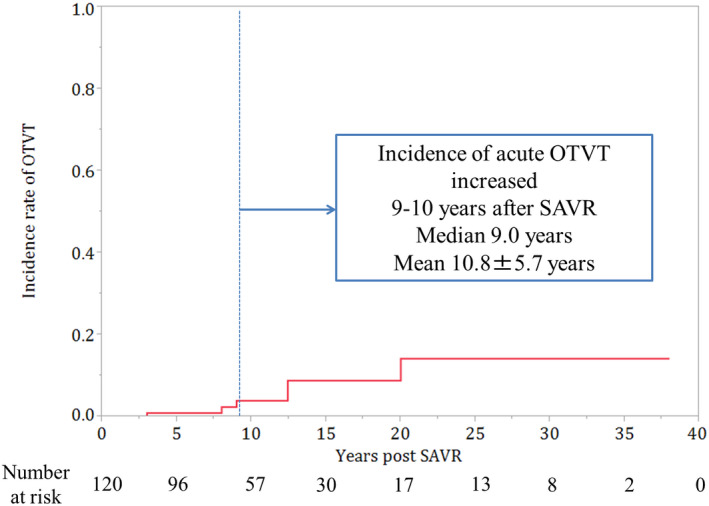
Incidence rate of OTVT after SAVR (Graphical abstract). Incidence of OTVT increased at 10 years after SAVR. AVR, aortic valve replacement; SAVR, surgical aortic valve replacement; VT, ventricular tachycardia
TABLE 2.
Comparison of echocardiography findings between patients with OTVTs and controls
|
OTVT group (n = 6) |
Control group (n = 106) |
P‐value | |
|---|---|---|---|
| AV Vmax (m/s) | 2.75 ± 0.54 | 2.72 ± 0.70 | .92 |
| AVA (cm2) | 1.71 ± 0.22 | 1.64 ± 0.41 | .69 |
| LVEF (%) | 56.7 ± 10.2 | 66.0 ± 7.8 | <.01 |
| LVDd (mm) | 47.8 ± 11.1 | 44.1 ± 6.5 | .19 |
| LVDs (mm) | 33.8 ± 9.8 | 27.9 ± 5.8 | .02 |
| IVSd (mm) | 13.2 ± 3.0 | 11.9 ± 1.9 | .13 |
| LVPWd (mm) | 11.9 ± 1.4 | 11.3 ± 1.5 | .32 |
| Paravalvular leak | 1 (17) | 7 (7) | .37 |
| Univariate Cox regression analysis for OTVT | |||
|---|---|---|---|
| Characteristics | Hazard ratio (%/mm) | 95% CI | P‐value |
| LVEF (%) | 1.10 | 1.02‐1.20 | .02 |
| LVDs (mm) | 0.87 | 0.77‐0.99 | .03 |
Abbreviations: AV, aortic valve; AVA, aortic valve area; CI, confidence Interval; IVSd, interventricular septum; LVDd, left ventricular diastolic dimension; LVDs, left ventricular end‐systolic dimension; OTVT, outflow tract ventricular tachycardia.
3.3. Comparison of patients with OTVTs and those without any VTs
We compared the clinical background data of the patients with OTVT (OTVT group; n = 6) with the corresponding of those without any VT episodes (control group). We additionally excluded four (3.3%) patients with sudden cardiac death without documented OTVTs from the control group. Thus, 106 patients were included in the control group.
The type of valve (mechanical valve: 66.7% vs 54.7%, respectively; P = .69), primary aortic valve disease (AS: 50.0% vs 60.4%, P = .68; AR: 33.3% vs 25.5%, P = .65; and IE: 16.7% vs 13.2%, P = .59, respectively), and the time since AVR (10.8 ± 5.7 vs 11.4 ± 8.8 years, respectively; P = .88) were not significantly different between the groups. Similarly, we did not find significant differences between the groups in the other clinical factors (ie, age, male sex, body mass index, hypertension, dyslipidemia, diabetes mellitus, chronic kidney disease, and atrial fibrillation) and in the use of beta‐blockers, ACEIs/ARBs, amiodarone, and anti‐aldosterone drugs group 1 (Table S1).
Echocardiography revealed that the peak aortic jet velocity (AV Vmax), aortic valve area, and the rate of paravalvular leakage (moderate‐to‐severe) were not different between the groups. However, TTE revealed that the participants in the OTVT group had significantly lower left ventricular ejection fraction (LVEF) and larger left ventricular internal dimension in systole (LVDs) than those in the control group (LVEF: 56.7 ± 10.2 vs 66.0 ± 7.8%, P < .01; LVDs: 33.8 ± 9.8 vs 27.9 ± 5.8 mm, P = .02). Univariate Cox regression analysis revealed that the LVEF and the LVDs were prognostic factors for OTVT [hazard ratio (HR): 1.10 (95% confidence interval (CI): 1.02‐1.20), P = .02; and HR: 0.87 (95% CI: 0.77‐0.99), P = .03; respectively] (Table 2).
3.4. Signal averaged ECG and Holter results
Overall, 37 patients, including six patients with periaortic VTs, underwent SAECG. Positive results were obtained from five (83.3%) out of six patients in the periaortic VT group and from seven (23.3%) of 31 patients in the control group. We also performed Holter monitoring in 48 patients, including six patients with periaortic VTs. Sporadic NSVTs were observed in all six patients with OTVT and in five (11.9%) of 42 patients in the control group. The positive rate of SAECG abnormality and NSVT in Holter was significantly higher in the periaortic VT than in the control group (P < .01 and P < .01, respectively). Moreover, the cycle length of NSVT between the OTVT and the control group was not significantly different (Table 3).
TABLE 3.
SAECG/Holter comparison between patients with OTVT and controls
| OTVT group (n = 6) | Control group (n = 31) | P‐value | |
|---|---|---|---|
| SAECG positive | 5/6 (83) | 7/31 (23) | <.01 |
| fQRS | 4/6 (67) | 10/31 (32) | .17 |
| RMS | 5/6 (83) | 6/31 (19) | <.01 |
| LAS | 3/6 (50) | 6/31 (19) | .14 |
| OTVT group (n = 6) | Control group (n = 42) | P‐value | |
|---|---|---|---|
| NSVT in Holter | 6/6 (100) | 5/42 (12) | <.01 |
| Cycle length of NSVT (ms) | 394.8 ± 42.2 | 415.4 ± 62.2 | .54 |
Abbreviations: fQRS, filtered QRS duration >114 ms; LAS, low amplitude (<40 μV) signal (LAS) duration >38 ms; NSVT, nonsustained ventricular tachycardia; OTVT, outflow tract ventricular tachycardia; RMS, terminal (last 40 ms) QRS root means square voltage <20 μV; SAECG; signal averaged ECG; VT, ventricular tachycardia.
3.5. Cardiac MRI of patients with OTVT
Two of six patients with periaortic VTs underwent cardiac MRI with Gd enhancement (patients 2 and 3). The imaging revealed late Gd enhancement (LGE) mainly in the basal midlayer of the anteroseptal wall to aorta‐mitral continuity (AMC) just below the prosthetic aortic valves in both cases (Figure 4). We also performed cardiac MRI for two patients in the control group who had positive SAECG and NSVT in Holter. Nevertheless, LGE was not observed in them. Especially, the prevalence of positive LGE in the control group was 0%, but the number of cardiac MRI performances was too small to make a comparison.
FIGURE 4.
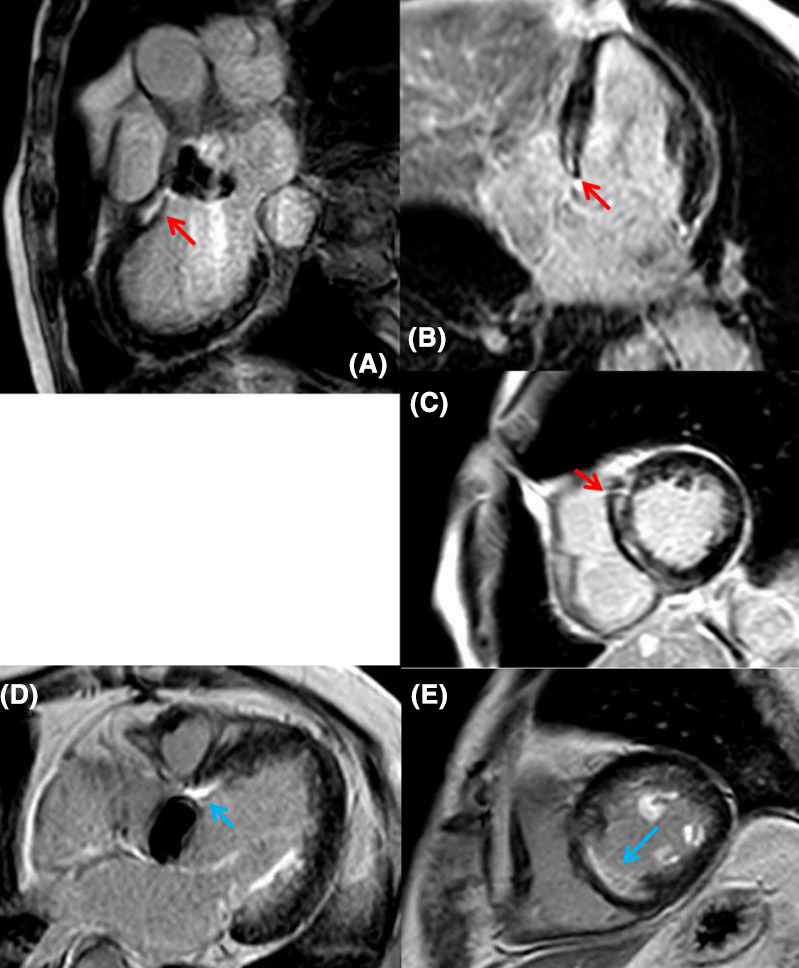
LGE in a patient with an OTVT. A: sagittal view; B: horizontal view, C: oblique section [Case 2]. D: horizontal view; E: oblique section [Case 3]. LGE was observed in the midlayer of basal RV/LV antero‐septum just below the aortic valve (A‐C: Red allow; D, E: Blue allow). LGE, late gadolinium enhancement; VT, ventricular tachycardia; OTVT, outflow tract ventricular tachycardia; RV, right ventricular; LV, left ventricular
3.6. Electrophysical mapping and ablation for OTVTs
Three of six patients with periaortic VTs underwent catheter ablation (patients 2, 3, and 6).
The mechanisms of the periaortic VTs were diagnosed with reentry due to reproducible induction by programmed stimuli; however, the precise circuits could not be analyzed because of their hemodynamic instability. Multiple VTs originating from periaortic lesions were provoked in each case. Especially, in Case 2, two types of VTs were provoked, which originated from the septal side of the right ventricular outflow tract (RVOT) and AMC, respectively. Similarly, in Case 3, three types of VTs were provoked, which originated from the septal side of the RVOT, the endocardium side of AMC, and the left coronary cusp (LCC). Finally, in Case 6, three types of VTs were provoked, which originated from the septal side of RVOT, the endocardium side of AMC, and the LCC, respectively. Interestingly, these VTs were originated from periaortic lesions. Radiofrequency ablation was performed based on pace mapping for each VT, and all patients were noninducible at the end of ablation. In patients 2 and 3, good pace map with pacing delay was observed; moreover, during sinus rhythm in these cases, local fragmented potentials with low voltage zones were detected in the RVOT septal, in accordance with the distribution of LGE on cardiac MRI (Figure 4A‐C, Figure S1).
3.7. Prognosis of patients with OTVT after SAVR
Three patients who did not undergo VT ablation were prescribed amiodarone to suppress the periaortic VT. Subcutaneous (S‐ICD) and intravenous implantable cardioverter defibrillators (TV‐ICD) were implanted in two patients with periaortic VTs, as secondary prevention (patients 1 and 2). Periaortic VT recurrence was documented in only one patient who had undergone amiodarone and S‐ICD treatment at 6 months after the first occurrence. The other patients did not present any recurrence of periaortic VT under each treatment. However, patient 3, who underwent VT ABL and AMD treatments without ICD, suddenly died due to a cardiac episode at 1.5 years after the ABL without documented VT occurrence (Table 4).
TABLE 4.
Clinical characteristics of patients with OTVT
| Patient No. | Age | Primary aortic diseases |
Period after AVR (years) |
Valve type | VT ECG configuration | VT axis | VT cycle length (ms) |
Treatment for OTVT/ Recurrence period after treatment/ Prognosis after OTVT occurrence |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 81 | IE | 20.3 |
Mechanical (DVR) |
RBBB | Inferior | 350 | AMD | ‐‐‐ | ICD |
Recur (0.5y)/ Alive (2.2y) |
| Patient 2 | 79 | AR | 9.1 | Biological | LBBB | Inferior | 330 | ‐‐‐ | ABL | ICD | Recur(‐)/NCD (0.9y) |
| Patient 3 | 74 | AS | 8.0 | Mechanical | RBBB | Inferior | 280 | AMD | ABL | ‐‐‐ | Recur(‐)/Alive (3.6y) |
| Patient 4 | 73 | AS | 12.4 | Mechanical | LBBB | Inferior | 320 | AMD | ‐‐‐ | ‐‐‐ | Recur(‐)/Alive (0.5y) |
| Patient 5 | 78 | AS | 12.4 | Biological | LBBB | Inferior | 240 | AMD | ‐‐‐ | ‐‐‐ | Recur(‐)/NCD (1.0y) |
| Patient 6 | 56 | AR | 3.0 | Mechanical | LBBB | Inferior | 300 | AMD | ABL | ‐‐‐ | Recur(‐)/SCD (1.6y) |
| Mean | 73.5±9.0 | 10.8±5.7 | 306.7±37.8 | ||||||||
Reccur/Alive/NCD/SCD (‐) corresponds to the periods between events and the first occurrence of the periaortic VTs.
Abbreviations: ABL, Ablation; AMD, Amiodarone; AR, Aortic regurgitation; AS, Aortic stenosis; AVR, aortic valve replacement; DVR, double valve replacement; ECG, electrocardiogram; ICD, intracardiac defibrillator; IE, infective endocarditis; LBBB, left bundle branch block; RBBB, right bundle branch block; NCD; noncardiac death; SCD, sudden cardiac death; VT, ventricular tachycardia; y, years.
Additionally, among 120 patients after SAVR, four (3.3%) patients without documented periaortic VTs were transported to the emergency department with sudden cardiopulmonary arrest and could not be resuscitated.
4. DISCUSSION
One of the main findings of this study was that 5.0% of the patients developed acute onset of sustained or incessant nonsustained OTVTs after SAVR. Previous studies have reported the electrophysiological characteristics of VTs after AVR; however, the incidence rate of OTVTs after AVR remains unclear 7 , 8 and, in general, we do not consider AVR as an important risk for arrhythmia. Although one of the study's limitations was that we could not distinguish between the OTVTs related to AVR and an idiopathic outflow/summit VT, which shows outflow/septal LGE unrelated to the aortic valve, 10 the incidence of idiopathic VTs in the general population was reported to be approximately 15.8 cases per 100,000 patients (0.0158%). 11 Therefore, the incidence of OTVTs after SAVR (5.0%) in this retrospective cohort was apparently high.
Regarding the timing of the VT incidences after AVR, a bimodal pattern has been reported, indicating that such incidences occur immediately after operations or years later. 7 , 8 , 12 , 13 The OTVTs in this study occurred at 10.8 ± 5.7 years after SAVR, in line with the results of previous studies. 12 The high incidence of OTVTs at more than 10 years after SAVR raises a concern of causality.
The periaortic region could be an arrhythmic substrate for scar‐related VT, even with and without structural heart diseases. 9 , 14 , 15 In addition, Piers et al found typical scar patterns, including basal anteroseptal scars below the atrioventricular annulus in patients with nonischemic cardiomyopathy, using contrast‐enhanced MRI. 15 Although small in number, the basal anteroseptal wall scars below the prosthetic valve were detected in our study using cardiac MRI in patients with periaortic VTs. Especially, we performed ablation for OTVTs originating from multiple sites around the periaortic regions, including the anteroseptal scars. Based on the observed high incidence of OTVTs after SAVR and the spatial distribution of the periaortic scars, they appear to be influenced by chronic mechanical stimulation to the arrhythmic sensitive basal anteroseptal left ventricle and the periaortic region by the artificial valve over a long time. Therefore, we estimated that when the periaortic scars occur in the septum after SAVR, they could lead to injury of the conduction system and could possibly result in a bundle block reentry VT incident, which was previously reported long after SAVR. 8
Another possible explanation for the periaortic scars may include the presence of hidden cardiomyopathies unrelated to SAVR. While all patients underwent screening tests before SAVR, hidden cardiomyopathies cannot be sufficiently ruled out completely. Actually, Castano et al reported that 16% of patients with severe AS had hidden transthyretin cardiac amyloidosis. 16 The presence of more clinical data on echocardiography, cardiac MRI, SAECG, and Holter earlier in the time course, including the time before AVR performance, would have been useful in proving the development of peri‐outflow scars.
Moreover, we examined how many patients without OTVTs have an abnormal substrate in the peri‐outflow region. SAECG positive [7/30 (23%)] and sporadic NSVTs were observed in five of 42 patients (12%) without any documented VT incidences. They could be potential candidates for OTVTs, and more precise risk stratification methods should be considered. Although the incidence of NSVT in the control group was reported to be similar to that in patients with left ventricular hypertrophy, 17 in this study, four patients in the control group had a sudden cardiac death. We also analyzed the prognostic factor of the sudden death in the control group, as a sub‐analysis, but the significant prognostic factor was not clear in patients’ characteristics and echocardiography data. SAECG and Holter monitoring were performed for only one of four patients who had a sudden death, because the other three patients in the control group did not have any symptoms before their sudden death.
Regarding the VT prognosis after AVR, Liang et al demonstrated the safety and good outcomes of catheter ablation. 7 In contrast, Nishimura et al recently reported that 65% of reentrant circuits of periaortic VT with and without AVR had endocardial activation gaps within the tachycardia cycle length (three‐dimensional circuitry), which were associated with higher recurrence rates compared to the two‐dimensional complete circuits at 1 year after ablation (73% vs 37%). In our study, we could not analyze the precise circuits of the OTVTs, which were estimated to originate from the periaortic region after AVR; this is partly attributed to the hemodynamic instability of the VT and the difficulty to access the periaortic region after mechanical valve replacement with endocardial approach.
4.1. Limitations
This study included a small number of clinical events and patients for statistical analysis. Moreover, the study had a retrospective design and conducted at a single center. We could not distinguish between cases of OTVT related to AVR and idiopathic OTVT precisely. More detailed analyses and comparisons between cardiac MRI and high‐density endo‐ and epi‐mapping are required to understand the precise circuits of periaortic VTs after SAVR performance. Large‐scale, prospective studies are needed to validate our findings.
5. CONCLUSIONS
Based on our results, acute onset of OTVT was found in 5% of patients who received SAVR. Therefore, we suggest that arrhythmic risk stratification should be considered for patients who have undergone SAVR for a long period. Based on our findings, lower LVEF, larger LVDs, NSVTs on Holter monitoring, positive SAECG, and cardiac MRI could be considered as prognostic factors for OTVTs after SAVR.
CONFLICTS OF INTEREST
Authors declare no conflict of interests for this article.
Supporting information
Fig S1
Table S1
ACKNOWLEDGMENTS
None.
Goto K, Ono Y, Osaka Y, et al. Incidence of outflow tract ventricular tachycardia long after surgical aortic valve replacement. J Arrhythmia. 2021;37:418–425. 10.1002/joa3.12502
REFERENCES
- 1. Torka MC, Salefsky BE, Hacker RW. Intermediate clinical results after aortic valve replacement with the Carpentier‐Edwards pericardial bioprosthesis. Ann Thorac Surg. 1995;60(Suppl 2):S311–S315. [DOI] [PubMed] [Google Scholar]
- 2. Fernandez J, Laub GW, Adkins MS, Anderson WA, Chen C, Bailey BM, et al. Early and late‐phase events after valve replacement with the St. Jude Medical prosthesis in 1200 patients. J Thorac Cardiovasc Surg. 1994;107(2):394–406. [PubMed] [Google Scholar]
- 3. Taniguchi T, Morimoto T, Shiomi H, Ando K, Kanamori N, Murata K, et al. Initial surgical versus conservative strategies in patients with asymptomatic severe aortic stenosis. J Am Coll Cardiol. 2015;66(25):2827–38. [DOI] [PubMed] [Google Scholar]
- 4. Rooney SJ, Moreno de la Santa P, Lewis PA, Butchart EG. Sudden death in a large prosthetic valve series based on a single prosthesis: experience with the medtronic Hall valve. J Heart Valve Dis. 1994;3(1):5–9. [PubMed] [Google Scholar]
- 5. Konishi Y, Matsuda K, Nishiwaki N, Shimada I, Kitao Y, Ban T, et al. Ventricular arrhythmias late after aortic and/or mitral valve replacement. Jpn Circ J. 1985;49(6):576–83. [DOI] [PubMed] [Google Scholar]
- 6. Burke AP, Farb A, Sessums L, Virmani R. Causes of sudden cardiac death in patients with replacement valves: an autopsy study. J Heart Valve Dis. 1994;3(1):10–6. [PubMed] [Google Scholar]
- 7. Liang JJ, Castro SA, Muser D, Briceno DF, Shirai Y, Enriquez A, et al. Electrophysiologic substrate, safety, procedural approaches and outcomes of catheter ablation for ventricular tachycardia in patients after aortic valve replacement. JACC Clin Electrophysiol. 2019;5(1):28–38. [DOI] [PubMed] [Google Scholar]
- 8. Eckart RE, Hruczkowski TW, Tedrow UB, Koplan BA, Epstein LM, Stevenson WG. Sustained ventricular tachycardia associated with corrective valve surgery. Circulation. 2007;116(18):2005–11. [DOI] [PubMed] [Google Scholar]
- 9. Murasaki K. Guidelines for management of anticoagulant and antiplatelet therapy in cardiovascular disease (JCS 2009). Nihon Rinshon. 2011;69(Suppl 9):567–71. [PubMed] [Google Scholar]
- 10. Nagashima K, Tedrow UB, Koplan BA, Michaud GF, John RM, Epstein LM, et al. Reentrant ventricular tachycardia originating from the periaortic region in the absence of overt structural heart disease. Circ Arrhythm Electrophysiol. 2014;7(1):99–106. [DOI] [PubMed] [Google Scholar]
- 11. Sirichand S, Killu AM, Padmanabhan D, Hodge DO, Chamberlain AM, Brady PA, et al. Incidence of idiopathic ventricular arrhythmias: a population‐based study. Circ Arrhythm Electrophysiol. 2017;10(2):e004662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mohammed N, Dandekar U, Osman F. Ventricular tachycardia following aortic valve replacement. Heart. 2015;101(10):793. [DOI] [PubMed] [Google Scholar]
- 13. Zaker‐Shahrak R, Altmann D, Sommer P, Gaspar T, Schönbauer R, Arya A. Sustained monomorphic left ventricular outflow tract tachycardia early after aortic valve replacement. Cardiol J. 2012;19(3):320–2. [DOI] [PubMed] [Google Scholar]
- 14. Nishimura T, Beaser AD, Aziz ZA, Upadhyay GA, Ozcan C, Raiman M, et al. Peri‐aortic ventricular tachycardia in structural heart disease: evidence of localized reentrant mechanisms. Heart Rhythm. 2020;17(8):1271–9. [DOI] [PubMed] [Google Scholar]
- 15. Piers SRD, Tao Q, van Carine FB, van Taxis H, Schalij MJ, van der Geest RJ, et al. Contrast‐enhanced MRI‐derived scar patterns and associated ventricular tachycardias in nonischemic cardiomyopathy: implications for the ablation strategy. Circ Arrhythm Electrophysiol. 2013;6(5):875–83. [DOI] [PubMed] [Google Scholar]
- 16. Castaño A, Narotsky DL, Hamid N, Khalique OK, Morgenstern R, DeLuca A, et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J. 2017;38(38):2879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McLenachan JM, Henderson E, Morris KI, Dargie HJ. Ventricular arrhythmias in patients with hypertensive left ventricular hypertrophy. N Engl J Med. 1987;317(13):787–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1


