Abstract
Background
the incidence of novel coronavirus disease (COVID19) is elevated in areas with heightened socioeconomic vulnerability. Early reports from US hospitals also implicated social disadvantage and chronic disease history as COVID19 mortality risk factors. However, the relationship between race and COVID19 mortality remains unclear.
Methods
we examined in-hospital COVID19 mortality risk factors in a multi-hospital tertiary health care system that serves greater Detroit, Michigan, a predominantly African American city with high rates of poverty and chronic disease. Consecutive adult patients who presented to emergency departments and tested positive for COVID19 from 3/11/2020 through 4/18/2020 were included. Using log-binomial regression, we assessed the relationship between in-hospital mortality and residence in census tracts that were flagged for extreme socioeconomic vulnerability, patient-level demographics, and clinical comorbidities.
Findings
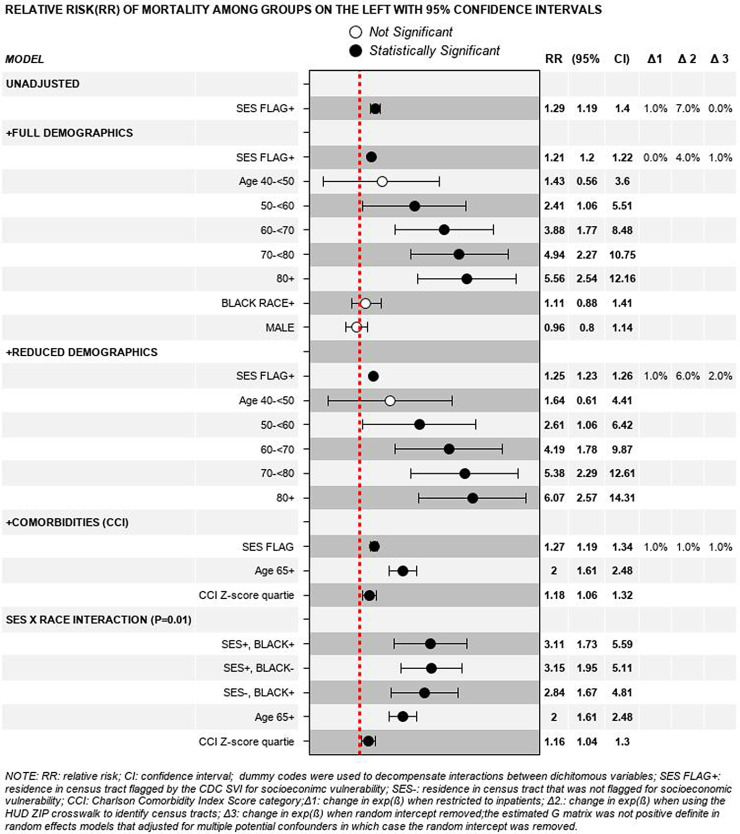
a total of 1,015 adults tested positive for COVID19 during the study period; 80% identified as Black people, 52% were male and 53% were ≥ 65 years of age. The median body mass index was 30•4 and the median Charlson Comorbidity Index score was 4. Patients from census tracts that were flagged for vulnerability related to socioeconomic status had a higher mortality rate than their peers who resided in less vulnerable census tracts (β 0.26, standard error (SE) 0.11, degrees of freedom (df) 378, t-value (t) 2.27, exp(β) 1.29, p-value 0.02). Adjustment for age category, Black race, sex and/or the Charlson Comorbidity Index score category reduced the magnitude of association by less than 10% [exp(β) 1.29 vs. 1.21]. Black race [p = 0.38] and sex [p = 0.62] were not associated with mortality in this sample.
Interpretation
people who lived in areas flagged for extreme socioeconomic vulnerability had elevated mortality risk in our predominantly African-American cohort of COVID19 patients who were able to seek hospital care during the so-called ‘first wave’ of the pandemic. By contrast, Black race was not associated with mortality in our sample.
Research in context.
Evidence before this study
Elevated mortality rates were reported during previous infectious disease outbreaks and pandemics in areas with high poverty rates, racial segregation and low education attainment. US reports have implicated chronic disease history and poverty as risk factors for COVID19 related mortality, yet there is a dearth of information about predominantly Black populations in densely populated urban cities.
Added value for this study
This study uniquely examined relationships between census tract social vulnerability characteristics and COVID19 in-hospital mortality in a large urban health system that predominantly serves African Americans. Mortality risk was elevated among patients from census tracts that were flagged by the Centers for Disease Control and Prevention Social Vulnerability Index for socioeconomic vulnerability (i.e., poverty, unemployment, low per capita income and/or low high school diploma rates ≥ 90th percentile vulnerability). By contrast, there was no relationship between COVID19 mortality and race.
Implication of all the available evidence
Our findings prompt us to infer that Black people in large urban centers who access hospital care and test positive for COVID19 are not inherently at elevated risk of COVID19 mortality. Rather, our results support the possibility that socioeconomic vulnerability, not race per se, explain the elevated mortality risk in our sample.
Alt-text: Unlabelled box
1. Introduction
The incidence of novel coronavirus disease (COVID19) is elevated in US counties and ZIP codes with an increased prevalence of social vulnerability related to socioeconomic status [[1], [2], [3], [4], [5]]. Early reports from US hospitals also implicated social disadvantage and chronic disease history as risk factors for elevated COVID19 mortality risk [6,7]. However, the relationship between race and COVID19 related mortality is not fully clear.
Multiple studies of people able to access hospital care reported no Black-White people difference in COVID19 mortality risk after adjusting for sociodemographic factors and clinical comorbidities [[8], [9], [10], [11]]. Yet, not all studies agree. At least two peer-reviewed articles reported increased COVID19 mortality risk in Black patients in adjusted models [7,12].
We examined the antecedents of in-hospital COVID19 mortality in a multi-hospital tertiary health care system that serves greater Detroit, Michigan, a predominantly Black or African American city with high rates of poverty, obesity, hypertension, diabetes, asthma and other factors that might heighten vulnerability [13]. Detroit emerged as an early hot spot for COVID19 related mortality during the so-called ‘first wave’ of the pandemic in the US. By the 50th day of the virus in Michigan, Detroit reported more than one thousand COVID19 related deaths; a per capita mortality rate [149 per 100,000 residents] that briefly exceeded New York city, then the global epicenter.
We chose to focus on the first several weeks of the so-called ‘first wave’, prior to the emergence of COVID19 variants in metropolitan Detroit, based on the premise that a homogenous novel exposure within a population will identify markers of susceptibility [14]. The case-mix at our hospitals provided us an opportunity to test for disparities in a sample that is enriched with vulnerability related to both chronic comorbidities and socioeconomic status. We collected information about basic demographic and clinical factors, but firstly we tested for differences between patients who lived in census tracts with increased social vulnerability and their peers who lived in less vulnerable census tracts. Residential characteristics were included in our a priori study design based on our interests in identifying modifiable risk factors.
2. Methods
2.1. Study design and participants
This study included consecutive adult COVID19 patients who were admitted to any of five Detroit Medical Center (DMC) hospitals and tested positive for SARS-CoV-2 from 3/11/20 – 4/18/20. Trained data abstractors collected demographic data, social and medical histories, and discharge disposition information from medical records. Patient addresses were collected in order to obtain census tract information about social vulnerability. Geocoding was performed using SAS v9.4. When address information was insufficient to identify the census tract and ZIP codes were available, we used the HUD-USPS ZIP code crosswalk to select the census tract with the maximum estimated fraction of residents corresponding to the patients’ ZIP code [15]. The Detroit Medical Center Clinical Research Office and the Wayne State University Institutional Review Boards approved this study. Informed patient consent was not required due to the retrospective nature of the study.
2.2. Social vulnerability index
Information about census tracts was obtained from the 2018 Centers for Disease Control and Prevention Social Vulnerability Index (SVI) [16]. The SVI ranks census tracts on fifteen social factors (e.g., unemployment, minority status, and disability) that are subclassified under four themes: Socioeconomic, Household Composition and Disability, Minority Status and Language, and Housing Type and Transportation. We focused on socioeconomic vulnerability in this study. Because of our interest in extreme vulnerability, we used a flag variable that was developed by the CDC to classify extreme vulnerability. We compared patients who lived in census tracts that were flagged for any of the following versus patients who lived in census tracts that were not flagged: persons below poverty estimate ≥ 90th percentile; civilian (age 16+) unemployed estimate ≥ 90th percentile vulnerability; per capita income vulnerability ≥ 90th percentile; persons (age 25+) with no high school diploma ≥ 90th percentile.
2.3. Comorbidities
Clinical risk factors were summarized using the Charlson Comorbidity Index (CCI) [17]; patient groups were created based on quartiles of the frequency distribution in this cohort (0–1, bottom quartile; 2–4, middle quartiles; and 5+, top quartile).
2.4. COVID19 diagnosis
The gold-standard diagnosis in this study was based on the real-time polymerase chain reaction (RT-PCR) testing of a nasopharyngeal swab or an oropharyngeal swab collected in viral transport media. Restrictions on Sars-CoV-2 testing were widened to allow clinician discretion in Michigan in late February 2020. The DMC began testing in March, when the first two cases in the state were reported in Metropolitan Detroit. Testing was initially performed at the State Bureau of Laboratories using kits provided by the CDC. Next, commercial laboratories were used to increase testing capacity (Quest Diagnostics and Eurofins Viracor, Inc). In-house rapid testing for Sars-CoV-2 began at the DMC on April 3rd, 2020.
2.5. Statistical analysis
Differences in distributions were examined using the Chi-square or Fisher's exact test, or the Man Whitney U-Test, as appropriate. Using log-binomial regression, we assessed the relationship between in-hospital mortality and residence in census tracts that were flagged for extreme socioeconomic vulnerability, patient-level demographics, and clinical comorbidities.
First, we tested whether people who lived in census tracts that were flagged for socioeconomic vulnerability had higher in-hospital mortality rates than people who lived in areas that were not flagged. Next, we adjusted for basic demographic factors (age category, sex and dichotomous race Black vs. non-Black). After removing fixed effects that were not associated with mortality, a fixed effect for the Charlson Comorbidity Index score category was added. Because of collinearity, we used age-standardized Charlson Comorbidity Index z-scores in models that included a fixed effect for advanced age. Based on known associations between race and socioeconomic status, we also fit an interaction model to test whether the association between Black race and COVID19 mortality differed according to residence in census tracts that were flagged for socioeconomic vulnerability.
We used random effects models that included a random intercept for hospitals nested within census tracts to estimate patient specific risks, but we also used marginal models that did not include random intercepts to model population averages. Confounders were defined a priori as variables that were associated with census tract socioeconomic vulnerability and in-hospital mortality whose adjustment changed estimated relative risks by >10%. Multivariable adjustment was performed when there were at least ten outcome events per independent variable [18]. Estimates with 95% confidence intervals (CI) that did not cross the null value (i.e., ‘1•0′) were considered statistically significant.
2.6. Role of the funding source
There was no funding source for this project. AS SK, JP, PL, RS, TC had full access to all of the data in the study and the corresponding authors had the responsibility to submit the manuscript.
3. Results
3.1. Cohort characteristics
A total of 1015 adults tested positive for COVID19 during the study period; 80% identified as Black, 52% were male and 53% were ≥ 65 years of age. The median body mass index was 30•4 (interquartile rang-IQR, 26–37) and the median Charlson Comorbidity Index score was 4 (IQR, 2–5). Diagnoses were based on tests performed by the State laboratory (14%), commercial labs (72% Labcorp and 2% Viracor) and using in-house rapid tests (12%).
Six percent of the cohort was discharged home from the ED, 75% was admitted to the hospital, 13% was admitted to the ICU, 1% was transferred to another facility and 3•6% died in the ED. The median ED length of stay was 5•6 h (IQR 2•9–18•4). Of the hospitalized patients, 51% was discharged home, 5% was discharged to long term acute care, 5% was discharged to another facility, and 38% expired. The median inpatient hospital length of stay was seven days (IQR 4–13).
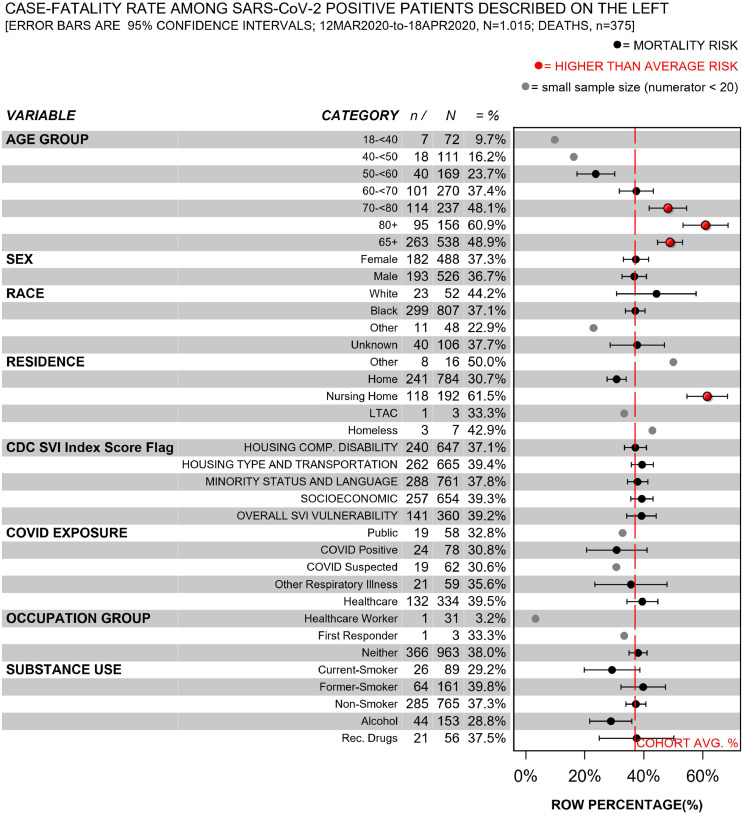
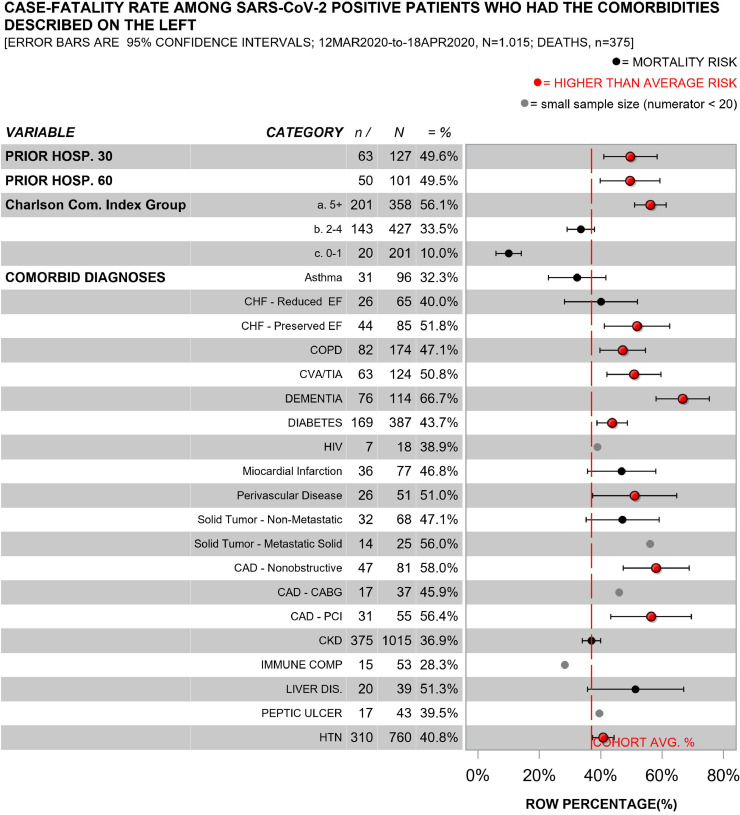
The characteristics of COVID19 survivors (n = 640) and non-survivors (n = 375) are described in Figs. 1–2; (eFigs. 1–2 describe inpatients only). People who died had advanced age, multiple comorbidities (e.g., hypertension, diabetes, dementia, chronic obstructive pulmonary disorder, coronary artery disease), and they were identified as residing in nursing homes prior to admission more frequently than survivors.
Fig. 1.
Case-fatality rate among all patients who tested positive for SARS-cov-2 by demographic and social factors.
Fig. 2.
Case-fatality rate among all patients who tested positive for SARS-cov-2 by clinical comorbidities.
3.1.1. Census tract characteristics and in-hospital mortality risk
We were able to identify 76% of the census tracts for patient home addresses directly without using the HUD ZIP crosswalk; the CDC SVI flagged 61% of these 271 distinct census tracts for socioeconomic vulnerability. The remaining census tracts were identified using the HUD ZIP crosswalk. There were no differences in the frequency distributions of mortality (p = 0.39), advanced age (65+, p = 0.49), Black race (p = 0.10), sex (p = 0.71) or the Charlson Comorbidity Index score category (p = 0.66) by crosswalk usage. By contrast, patients whose home address was matched to a census tract using the HUD ZIP crosswalk lived in areas that were flagged for extreme socioeconomic vulnerability less frequently than their peers whose addresses were directly matched to census tracts (54vs. 68%, p = 0.0001). Consequently, we used information from patients whose census tract was identified by their ZIP code only to conduct sensitivity analyses.
3.1.2. Unadjusted model and adjustment for basic demographics
Patients from census tracts that were flagged for vulnerability related to socioeconomic status had a 20%-to-30% higher mortality rate than their peers who resided in less vulnerable census tracts (Fig. 3). Adjustment for age category, Black race and sex changed the estimated magnitude of association by less than 10% [exp(β) 1.29 vs. 1.21].
Fig. 3.
Unadjusted and adjusted relative risks of mortality.
3.1.3. Adjustment for basic demographics ± clinical comorbidities
After removing fixed effects that were not associated with elevated mortality risk (Black race [p = 0.38] and sex [p = 0.62]), we entered a fixed effect for the age adjusted Charlson Comorbidity Index z-score. Adjustment for comorbidities changed the estimated magnitude of association between socioeconomic vulnerability and mortality by less than 5% [exp(β) 1.29 vs. 1.27].
3.1.4. Interaction between black race and residential social vulnerability
Based on known associations between race and socioeconomic status, and because in our sample patients who identified as Black lived in areas flagged for extreme vulnerability more often than their peers who did not (67 vs. 57%, respectively, p = 0.01), we tested whether the relationship between race and COVID19 mortality differed by residence in census tracts with socioeconomic vulnerability.
Adjusting for advanced age and the Charlson Comorbidity Index z-score, the relationship between race and mortality depended on residence in a vulnerable census tract (p = 0.01). Mortality risk was increased by more than threefold in Black and non-Black patients alike when both groups lived in census tracts that were flagged for SES vulnerability (exp(β) 3.1, p = 0.005 and 3.2, p = 0.006, respectively).
Black patients had elevated mortality risk compared to non-Black patients in our sample only when both groups did not live in census tracts that were flagged for SES vulnerability [(exp(β) 2.8, p = 0.01]. Importantly, post hoc analysis of this subcohort provided evidence that residual confounding biased the association between race and mortality away from the null hypothesis of no association (i.e., a false positive). Namely, threefold more non-Black than Black patients lived in census tracts that had socioeconomic vulnerability percentile rankings below the 30th percentile (i.e., 30% vs. 8%; median (IQR) percentile rankings 0.70 (0.30–0.82) vs. 0.79 (0.67–0.84), respectively).
3.1.5. Sensitivity analyses
The results were consistent in models that did not include random intercepts and thus estimated population averages rather than patient-specific risks. Restricting the analysis to patients who were admitted to the hospital or excluding those identified by rapid test did not substantively alter our findings; nor did inclusion of information from the 24% of patients whose addresses were matched to census tracts by the HUD ZIP crosswalk [i.e., estimated relative risks changed by < 10% in all scenarios].
4. Discussion
This study uniquely examined the antecedents of in-hospital COVID19 mortality during the so-called ‘first wave’ of the pandemic, prior to the emergence of known variants, in an urban, multi-hospital, tertiary health care system that serves a predominantly African American population that is enriched both for socioeconomic vulnerability and chronic disease. We have two main findings.
First, COVID19 mortality risk was increased by 20 to 30% in patients who resided in census tracts that were flagged for socioeconomic vulnerability, irrespective of basic adjustment for potentially confounding factors. The magnitude of this association is impressive when balanced against the prevalence of exposure in our sample. Hypothetically, if our (marginal model) findings were validated and proven to be causal, then socioeconomic vulnerability would contribute to nearly one of four COVID19 related in-hospital deaths in our sample, and by extension in similarly vulnerable populations (i.e., population attributable risk percent = 24%).
Second, there was no difference in COVID19 mortality risk between Black and non-Black patients in our sample, neither in unadjusted nor adjusted models. Mortality risk appeared to be higher in Black than non-Black patients only when both groups lived in census tracts that were not flagged for socioeconomic vulnerability. This relationship should be interpreted with caution, however, because post hoc analysis provided evidence that residual confounding biased the association away from the null hypothesis of no association (i.e., a Type I Error or False Positive).
Elevated mortality rates were reported during previous infectious disease epidemics and pandemics in areas with high poverty rates, racial segregation, low education attainment and low SES [[19], [20], [21], [22], [23]]. Recent geographic-level analyses similarly associated elevated COVID19 mortality risk (or risk of severe infection) with the proportion of residents identified as Black or African American (or other racial/ethnic minorities) [24], [25], [26], [27], household overcrowding or population density [28], and socioeconomic vulnerability [3,4,29,30]. Yet, not all findings are consistent; e.g., there was no racial disparity in COVID19 mortality rates among ZIP codes grouped by the proportion of Black residents in a nearby (affluent) county[31]. Clinical studies have also provided evidence that African Americans who seek hospital care are at increased risk of COVID19 mortality; however, the racial disparities appear to be explained by social factors or differences in comorbidities in most [[8], [9], [10], [11]], but not all [7,12], reports.
We found that residence in census tracts with extreme socioeconomic vulnerability was over-represented among Black patients, and COVID19 mortality risk was significantly elevated among people who lived in vulnerable areas irrespective of race. Consequently, we infer that socioeconomic vulnerability (and its correlates) including upstream factors (e.g., systemic racism) and what might be related downstream factors (i.e., chronic diseases [32]), not race per se, explain the elevated mortality risk in our sample. Thus, we agree that race is more useful in medical research if viewed as a social construct rather than a biologic factor (particularly in the US) [33,34]. This view is strongly supported by epidemiologic studies [35].
The absence of racial disparity in COVID19 mortality in our study is consistent with adjusted models in recent reports from health systems that care for more affluent patient populations in Louisiana and Michigan [7,11,36,37].
In contrast to the above studies, we detected an association between mortality and socioeconomic vulnerability that was not materially altered by adjustment for clinical comorbidities. The discrepancy is probably due to differences in study design and participants. We studied extreme socioeconomic vulnerability defined as living in census tracts that had unemployment, income, or education vulnerability ≥ the 90th percentile estimated nationally by the CDC, whereas previous reports focused on low (or linear) income [7,37], and population density [8]. We also used log-binomial regression instead of logistic regression because the outcome was common in our sample, and we uniquely performed a multilevel analysis that accounted for correlated errors among patients nested in different census tracts. Most important is that the burden of comorbidities (and socioeconomic vulnerability) in our cohort was substantially greater than in prior studies (e.g., the mean Charlson Comorbidity Index score was 3.8 in our study and ranged from 1to2.6 in previous cohorts). Consequently, our findings may be specific to similarly vulnerable groups.
Our results are additionally consistent with previous reports that patients with advanced age [38] and chronic diseases [[39], [40], [41], [42], [43], [44], [45]] are at elevated risk of COVID19 related mortality. Patients from nursing homes were particularly susceptible in our sample (and elsewhere [46]). Indeed, COVID19 has revealed elderly care as a blind spot in pandemic prepardeness.
There are four main strengths of our study. First, our sample is enriched for socioeconomic vulnerability and chronic comorbidities, which provided sufficient power to test our hypotheses. Second, we used data from the first several weeks of the pandemic in the US, prior to the emergence of SARS-CoV-2 variants in metropolitan Detroit in 2021. Third, medical record abstractors were trained. Fourth, we performed a careful, multilevel, analysis, and we examined whether estimated population average risks were consistent with estimated patient-specific risks.
While there are benefits to studying a sample enriched for vulnerability related to socioeconomic vulnerability and chronic comorbidities, this also narrows generalizability. On the other hand, we aimed to generalize to large tertiary health care systems in urban centers that serve similarly vulnerable populations and we did just that. Nevertheless bias is possible, particularly if appearance at our centers was affected by socioeconomic vulnerability and by COVID19 (i.e., Berkson's bias [47]). To offset this possibly, we modeled patient-specific risks (random effects model) as well as population average risks (marginal model) and our findings were consistent.
We were unable to link all patient addresses to census tracts. To minimize the potential impact, we used a ZIP code to census tract crosswalk and selected the location with the highest estimated fraction of residents to enable sensitivity analyses. Our findings were consistent when using information from the 24% of participants whose census tracts were identified using the HUD ZIP crosswalk. Still, it is possible that some patients were allocated to an incorrect census tract.
We did not have sufficient information to examine additional parameters (e.g., ethnicity, body mass index and patient-level socioeconomic vulnerability indicators, post-discharge mortality). It is possible that some patients had COVID19 but died prior to testing during the study period, and that some who died may have had false negative tests, which may have influenced our findings. Lastly, like all observational studies, we are unable to distinguish between causation and association.
In summation, people who lived in areas flagged for extreme socioeconomic vulnerability had elevated mortality risk in our predominantly African American cohort of COVID19 patients who sought emergency care in a large US city during the so-called ‘first wave’ of the pandemic. Race was not associated with mortality in this sample. Collectively, our findings prompt us to infer that Black patients who test positive for COVID19 are not inherently at elevated risk of mortality. Rather, our results support the possibility that socioeconomic vulnerability and its correlates including upstream contributors (e.g., systemic racism) and what might be related downstream factors (i.e., chronic diseases), not race per se, are responsible for the heightened mortality rate we observed. Systemic racism is implicated because we feel it shapes the circumstances that are responsible for the inequitable distribution of resources across communities of color. The findings from this study underscore the importance to better understand how policies and systematic social or political constructs might contribute to discordant population-level health outcomes. Interventions to improve health equity in areas with extreme socioeconomic vulnerability are warranted.
Declaration of Competing Interest
Authors in the manuscript declare no competing interests.
Acknowledgments
Contributors
AS SK, JP, PL, RS, TC had the idea and designed the study and had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of data analysis. SK designed the analytic plan and performed the analysis. AS, SK, PL, TC drafted the paper. All authors critically revised the manuscript for important intellectual content and gave final approval for the version to be submitted. AS, JP, NS, JM, HP, SR, AO also helped with data collection. All authors agree to be accountable for all aspects of the work.
Funding
None.
Data sharing statement
All relevant data are provided in the online supplement; for additional information please contact the corresponding authors.
Acknowledgments
We are grateful to Valerie Mika for the effort in developing the REDCap Database used to conduct this study, and to the Data Abstractors: Alison Bradley BS; Samia Mazunder BS; Mia Ma BS; Pei Chan Ma BA; Taylor Pruis BS; Aditya Agrawal BS; Fiona Clowney BS; Ally Flessel BS; Tyler Willford BS; Kelly Hilk BS; Erin Spencer BS; Keerteshwrya Mishra BS; Suha Syed BS; Oliva Rizzo BS; Lily Lu MS; Karim Dirani BS; Farhan Ayaz MBBS; Jessica Shuck BS, Lauren Buck BS; Ashley Griffin BS; Abe Lovelace BS.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.100814.
Contributor Information
Steven J. Korzeniewski, Email: sKorzeni@med.wayne.edu.
Teena Chopra, Email: tchopra@medwayne.edu.
Appendix. Supplementary materials
References
- 1.Dasgupta S., Bowen V.B., Leidner A. Association between social vulnerability and a country's risk for becoming a COVID-19 hotspot—United States. Morb Mortal Wkly Rep. 2020;69(42):1535. doi: 10.15585/mmwr.mm6942a3. June 1–July 25, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clouston S.A.P., Natale G., Link B.G. Socioeconomic inequalities in the spread of coronavirus-19 in the United States: a examination of the emergence of social inequalities. Soc Sci Med. 2021;268 doi: 10.1016/j.socscimed.2020.113554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hawkins R.B., Charles E.J., Mehaffey J.H. Socio-economic status and COVID-19-related cases and fatalities. Public Health. 2020;189:129–134. doi: 10.1016/j.puhe.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khazanchi R., Beiter E.R., Gondi S., Beckman A.L., Bilinski A., Ganguli I. County-level association of social vulnerability with COVID-19 cases and deaths in the USA. J Gen Intern Med. 2020;35(9):2784–2787. doi: 10.1007/s11606-020-05882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karmakar M., Lantz P.M., Tipirneni R. Association of social and demographic factors With COVID-19 incidence and death rates in the US. JAMA Netw Open. 2021;4(1) doi: 10.1001/jamanetworkopen.2020.36462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wadhera R.K., Wadhera P., Gaba P. Variation in COVID-19 hospitalizations and deaths across New York City boroughs. JAMA. 2020;323(21):2192–2195. doi: 10.1001/jama.2020.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price-Haywood E.G., Burton J., Fort D., Seoane L. Hospitalization and mortality among black patients and white patients with COVID-19. N Engl J Med. 2020;382(26):2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu T., Mack J.A., Salvatore M. Characteristics Associated with racial/ethnic disparities in COVID-19 outcomes in an academic health care system. JAMA Netw Open. 2020;3(10) doi: 10.1001/jamanetworkopen.2020.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z., Zheutlin A., Kao Y.H. Hospitalized COVID-19 patients of the Mount Sinai Health System: a retrospective observational study using the electronic medical records. BMJ Open. 2020;10(10) doi: 10.1136/bmjopen-2020-040441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egede L.E., Walker R.J., Garacci E., Raymond J.R. Racial/ethnic differences in COVID-19 screening, hospitalization, and mortality in Southeast Wisconsin. Health Aff. 2020;39(11):1926–1934. doi: 10.1377/hlthaff.2020.01081. (Millwood) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price-Haywood E.G., Burton J., Fort D., Seoane L. Hospitalization and mortality among black patients and white patients with COVID-19. N Eng J Med. 2020 doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raisi-Estabragh Z., McCracken C., Bethell M.S. Greater risk of severe COVID-19 in black, Asian and minority ethnic populations is not explained by cardiometabolic, socioeconomic or behavioral factors, or by 25(OH)-vitamin D status: study of 1326 cases from the UK biobank. J Public Health. 2020;42(3):451–460. doi: 10.1093/pubmed/fdaa095. (Oxf) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prevention CoDCa . Center of Disease Control and Prevention; 2019. CDC: 500 Cities Local Data for Better Health. December 5ed. Online: [Google Scholar]
- 14.Rose G. Sick individuals and sick populations. Int J Epidemiol. 2001;30(3):427–432. doi: 10.1093/ije/30.3.427. discussion 33-4. [DOI] [PubMed] [Google Scholar]
- 15.Wilson R., Din A. Understanding and enhancing the U.S. Department of housing and urban development's ZIP code crosswalk files. Cityscape: US Department of Housing and Urban Development; 2018. p. 277–94.
- 16.Flanagan B., Gregory E., Hallisey E., Heitgerd J., Lewis B. A social vulnerability index for disaster management. J Homel Secur Emerg Manag. 2011;8(1):1–22. [Google Scholar]
- 17.Charlson M., Peterson J., Szatrowski T.P., MacKenzie R., Gold J. Long-term prognosis after peri-operative cardiac complications. J Clin Epidemiol. 1994;47(12):1389–1400. doi: 10.1016/0895-4356(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 18.Steyerberg E.W., Eijkemans M.J.C., Harrell Jr F.E., Habbema J.D.F. Prognostic modeling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Stat Med. 2000;19(8):1059–1079. doi: 10.1002/(sici)1097-0258(20000430)19:8<1059::aid-sim412>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 19.Reich N.G., Lessler J., Cummings D.A., Brookmeyer R. Estimating absolute and relative case fatality ratios from infectious disease surveillance data. Biometrics. 2012;68(2):598–606. doi: 10.1111/j.1541-0420.2011.01709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pini A., Stenbeck M., Galanis I. Socioeconomic disparities associated with 29 common infectious diseases in Sweden, 2005–14: an individually matched case-control study. Lancet Infect Dis. 2019;19(2):165–176. doi: 10.1016/S1473-3099(18)30485-7. [DOI] [PubMed] [Google Scholar]
- 21.Younus M., Hartwick E., Siddiqi A.A. The role of neighborhood level socioeconomic characteristics in Salmonella infections in Michigan (1997–2007): assessment using geographic information system. Int J Health Geogr. 2007;6(1):56. doi: 10.1186/1476-072X-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed F., Ne Ahmed, Pissarides C., Stiglitz J. Why inequality could spread COVID-19. Lancet Public Health. 2020;5(5):e240. doi: 10.1016/S2468-2667(20)30085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lancet The. Redefining vulnerability in the era of COVID-19. Lancet. 2020;395(10230):1089. doi: 10.1016/S0140-6736(20)30757-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tirupathi R., Muradova V., Shekhar R., Salim S.A., Al-Tawfiq J.A., Palabindala V. COVID-19 disparity among racial and ethnic minorities in the US: a cross sectional analysis. Travel Med Infect Dis. 2020;38 doi: 10.1016/j.tmaid.2020.101904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ojinnaka C.O., Adepoju O.E., Burgess A.V., Woodard L. Factors associated with COVID-related mortality: the case of Texas. J Racial Ethn Health Dispar. 2020;1(6):1–11. doi: 10.1007/s40615-020-00913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackey K., Ayers C.K., Kondo K.K. Racial and Ethnic disparities in COVID-19-related infections, hospitalizations, and deaths: a systematic review. Ann Intern Med. 2020;174(3):368–373. doi: 10.7326/M20-6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bassett M.T., Chen J.T., Krieger N. Variation in racial/ethnic disparities in COVID-19 mortality by age in the United States: a cross-sectional study. PLoS Med. 2020;17(10) doi: 10.1371/journal.pmed.1003402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong D.W.S., Li Y. Spreading of COVID-19: density matters. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0242398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sese L., Nguyen Y., Giroux Leprieur E. Impact of socioeconomic status in patients hospitalized for COVID-19 in the Greater Paris area. Eur Respir J. 2020;56(6) doi: 10.1183/13993003.02364-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanson A.E., Hains D.S., Schwaderer A.L., Starr M.C. Variation in COVID-19 diagnosis by zip code and race and ethnicity in Indiana. Front Public Health. 2020;8 doi: 10.3389/fpubh.2020.593861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akanbi M.O., Rivera A.S., Akanbi F.O., Shoyinka A. An Ecologic study of disparities in COVID-19 incidence and case fatality in Oakland Country, MI, USA, during a state-mandated shutdown. J Racial Ethn Health Dispar. 2020;1(8) doi: 10.1007/s40615-020-00909-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maness S.B., Merrell L., Thompson E.L., Griner S.B., Kline N., Wheldon C. Social determinants of health and health disparities: COVID-19 exposures and mortality among African American people in the United States. Public Health Rep. 2021;136(1):18–22. doi: 10.1177/0033354920969169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper R.S. Race in biological and biomedical research. Cold Spring Harb Perspect Med. 2013;3(11) doi: 10.1101/cshperspect.a008573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooper R.S. Race in biological and biomedical research. Cold Spring Harb Perspect Med. 2013;3(11) doi: 10.1101/cshperspect.a008573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaufman J.S., Dolman L., Rushani D., Cooper R.S. The contribution of genomic research to explaining racial disparities in cardiovascular disease: a systematic review. Am J Epidemiol. 2015;181(7):464–472. doi: 10.1093/aje/kwu319. [DOI] [PubMed] [Google Scholar]
- 36.Yehia B.R., Winegar A., Fogel R. Association of Race with mortality among patients hospitalized with coronavirus disease 2019 (COVID-19) at 92 US hospitals. JAMA Network Open. 2020;3(8) doi: 10.1001/jamanetworkopen.2020.18039. e2018039-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quan D., Luna Wong L., Shallal A. Impact of race and socioeconomic status on outcomes in patients hospitalized with cOVID-19. J Gen Intern Med. 2021 doi: 10.1007/s11606-020-06527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 39.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Du R.H., Liang L.R., Yang C.Q. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55(5) doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet North Am Ed. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;323(20):1239–1242. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang J., Zheng Y., Gou X. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goyal P., Choi J.J., Pinheiro L.C. Clinical characteristics of covid-19 in New York city. N Engl J Med. 2020:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McMichael T.M., Currie D.W., Clark S. Epidemiology of COVID-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382(21):2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berkson J. Limitations of the application of fourfold table analysis to hospital data. Biometrics. 1946;2(3):47–53. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.