Abstract
Sepsis is a life-threatening condition with dysregulated host response to infection. It is a major determinant of mortality in the intensive care unit (ICU). Procalcitonin (PCT) is widely investigated for prognosis in patients with sepsis. Most of the studies have cited that elevated PCT concentrations and PCT non-clearance are associated with poor outcomes in patients with sepsis and some studies have cited as having no additional benefit. Most of the studies have evaluated single PCT measurement and correlated with prognosis and outcome in septic patients. Limited literature is there about serial PCT levels and its impact on the outcome of patients with sepsis. We searched literature through PubMed, Embase, Web of Knowledge, and the Cochrane Library from 2007 to 2017 and present a systematic review and meta-analysis of studies evaluating the utility of serial measurement of PCT for prognosis in critically ill patients. Articles that assessed PCT non-clearance as a marker of mortality data were included. The primary objective of this meta-analysis was to pool the results of all the available studies on serial PCT non-clearance as a mortality predictor and formulate overall area under receiver operating curve (AUROC). To find out the overall proportion of mortality in PCT non-clearance was our secondary objective. To detect the mortality using PCT non-clearance, ROC curve analysis was done. Area under curve (AUC) of the studies was varying between 0.52 and 0.86. Overall AUC was observed 0.711 (95% confidence interval (CI): 0.662–0.760) under fixed effect model and 0.708 (95% CI: 0.648–0.769) under random effect model. There was moderate variation among the studies, i.e., I2 50.80% (95% CI: 0.00–80.42%). The overall proportion of mortality was 37.54% with much heterogeneity (I2 88.24%) among the studies. PCT non-clearance is a fair predictor of mortality. Further studies are needed to define optimal cut off point for PCT non-clearance in ICU patients with sepsis.
Keywords: Procalcitonin, sepsis, septic shock
Introduction
Sepsis is a life-threatening condition that arises when a host responds to an infection and damages its organs.[1] It is a major determinant of mortality in an intensive care unit (ICU). Early identification of septic patients with poor clinical outcome is a constant clinical challenge for intensivists. Severity scores like Acute Physiology and Chronic Health Evaluation (APACHE II) and Sequential Organ Failure Assessment (SOFA) score have been validated for risk stratification.[2,3] Studies have identified that blood biomarkers may provide additional information for disease progression in sepsis.[4,5,6,7,8] No single biomarker in sepsis has been proven as a gold standard.
Procalcitonin (PCT) has been widely investigated in infectious diseases. PCT is a 116 amino acid prohormone of calcium metabolism regulator calcitonin. This precursor of calcitonin is produced by the C-cells of thyroid under the control of calcitonin gene-related peptide 1 (CALC-1) gene. Normally, the expression of this gene is found in neuroendocrine cells of the thyroid.[9] During microbial infection, there is overexpression of the CALC-1 gene in various extrathyroidal cells and tissues including kidney, liver, pancreas, leukocytes, and adipose tissue.[10] Therefore, unlike calcitonin, this prohormone is synthesized in numerous extrathyroidal tissues in response to bacterial lipopolysaccharide, endotoxins, and cytokines induced by a bacterial infection like IL-1 and TNF-α.[11,12] Plasma PCT levels rise within 3 hours, peak between 6 and 24 hours of sepsis onset, and decrease with the control of infection. Multiple studies have evaluated the diagnostic role of PCT in sepsis. Limited studies are there about the prognostic role of PCT in patients with sepsis. Regarding the prognostic value of PCT in sepsis, most of the studies have evaluated the effect of single PCT concentration on mortality in septic patients.[13,14,15,16,17,18,19,20,21,22,23,24,25,26,27] Limited number of studies have evaluated serial PCT levels and the effect of its kinetics on prognosis and outcome in ICU patients with sepsis.[28,29,30,31,32,33,34,35,36,37] In this systematic review and meta-analysis, we pooled results of all the available studies where serial monitoring of PCT was done and PCT non-clearance was used for mortality prediction in ICU patients with sepsis and septic shock. The primary objective of this meta-analysis was to pool the results of all the available studies on serial PCT non-clearance as a mortality predictor and formulate overall area under receiver operating curve (AUROC). To find out the overall proportion of mortality in PCT non-clearance was our secondary objective.
Methodology
We systematically searched studies using PubMed, Embase, Web of Knowledge, and the Cochrane Library. The search terms were: (procalcitonin or PCT or “PCT clearance” or “PCT-c” or “PCT decrease” or “PCT kinetics”) and (sepsis or septicemia or septic shock) and (mortality or prognosis). We searched articles over 10 years, i.e, from 2007 to 2017. We included articles written in English only. We further reviewed the reference list of the selected articles to obtain potentially relevant articles. For the study, articles in which PCT non-clearance was used as a prognostic marker were taken into account. Only original articles were included. PCT non-clearance was noted in percentage. Articles that assessed PCT non-clearance as a marker of mortality data were included. The primary objective of this meta-analysis was to pool the results of all the available studies on serial PCT non-clearance as a mortality predictor and formulate overall AUROC. To find out the overall proportion of mortality in PCT non-clearance was our secondary objective. PCT non-clearance and its effect on mortality or non-survival were assessed with sensitivity and specificity. Reviews, letters, commentaries, correspondences, case reports, conference abstracts, expert opinions, editorials, and animal experiments were excluded. Articles involving pediatric patients (≤18 years) were also excluded.
The summary of the above studies is being presented in Table 1.
Table 1.
Characteristic of studies showing sensitivity and specificity of PCT non-clearance as a mortality predictor in ICU patients with sepsis and its severity
| Author | Year | Place | Study design | Clinical setting | Follow up | PCT assay | PCT non-clearance | Sample size | Overall mortality n (n%) | Severity of sepsis | SEN % | SPE % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Karlsson[28] | 2010 | Finland | Prospective | ICU | In hospital mortality | Cobas PCT | PCT ↓ <50% in 72 h | 242 | 62 (25.6) | Severe sepsis | 88.7 | 27.8 |
| Guan[29] | 2011 | China | Prospective | ICU | 28 day mortality | Lumitest PCT | PCT ↓ <25% in 5 days | 37 | 12 (32.4) | Sepsis, sev sepsis, septic shock | 100 | 100 |
| Tschaikowsky[30] | 2011 | Germany | Prospective | SICU | 28 day mortality | KRYPTOR-PCT | PCT ↓ <50% in 7 days | 64 | 21 (32.8) | Severe sepsi, septic shock | 35.3 | 97.1 |
| Ruiz-Rodriguez[31] | 2012 | Spain | Prospective | ICU | ICU mortality | Lumitest PCT | PCT ↓ <50% in 48 h | 27 | 18 (66.7) | Septic shock | 89 | 72 |
| Suberviola[32] | 2012 | Spain | Prospective | ICU | In hospital mortality | KRYPTOR-PCT | PCT ↓ <70% in 72 h | 88 | 21 (23.9) | Septic shock | 52.6 | 94.2 |
| Schuetz (Derivation cohort)[33a] | 2013 | USA | Prospective | ICU | ICU mortality | VIDAS | PCT ↓ <60% in 72 h | 154 | 45 (29.2) | Severe sepsis, septic shock | 60 | 67 |
| Schuetz (Validation cohort)[33b] | 2013 | USA | Prospective | ICU | ICU mortality | VIDAS | PCT ↓ <60% in 72 h | 102 | 18 (17.6) | Severe sepsis, septic shock | 78 | 61 |
| Mat Nor[34] | 2014 | Malaysia | Prospective | ICU | In hospital mortality | KRYPTOR-PCT | PCT ↓ <30% in 48 h | 67 | 27 (40.3) | Severe sepsis | 74.1 | 55 |
| Gracia de Guadiana-Romualdo[35] | 2015 | Spain | Prospective | ICU | In hospital mortality | Cobas PCT | PCT ↓ <40% in 48 h | 100 | 28 (28) | Severe sepsis and septic shock | 64.3 | 62.5 |
| Poddar[36] | 2015 | India | Prospective | ICU | 28 day mortality | Cobas PCT | PCT ↓ <50% in 4 days | 171 | 80 (46.7) | Severe sepsis, septic shock | 68 | 64 |
| Schuetz (Intention to diagnose population)[37a] | 2017 | USA | Prospective | ICU | 28 day mortality | KRYPTOR-PCT | PCT ↓ <80% in 4 days | 646 | 107 (16.5) | Severe sepsis, septic shock | 77.3 | 38.8 |
| Schuetz (Subpopulation with ICU care on day 4)[37b] | 2017 | USA | Prospective | ICU | 28 day mortality | KRYPTOR-PCT | PCT ↓ <80% in 4 days | 276 | 73 (26.4) | Severe sepsis, septic shock | 79.2 | 32.1 |
PCT: Procalcitonin, ICU: Intensive Care Unit, SICU: Surgical intensive care unit, CI: Confidence Interval, SEN: Sensitivity, SPE: Specificity
Statistical analysis
Mortality of the PCT non-clearance patients and AUROC of the PCT non-clearance was assessed for the individual study as well as a pooled measure was estimated according to fixed-effects model or random-effects model as appropriate. Q-test and I2 indexes were calculated to assess inter-study heterogeneity. Values of 25%, 50%, and 75% for the I2 test considered as low, medium, and high heterogeneity, respectively. I2 values of less than 50% were considered as acceptable between-study heterogeneity, and the fixed-effects model was selected. Otherwise, the random-effects model was selected. AUC of the individual study, as well as pooled AUC, was obtained and represented using Forest plot. Similarly, Funnel plot was used to present the publication bias in terms of standard error with corresponding measures. The same exercise was done and the forest plot as well as funnel plot was presented for the proportion of the mortality of the non-clearance patients. Statistical analyses were performed using the software MedCalc Version 17.2. A P value of less than 0.05 was considered statistically significant.
Results
After inclusion of all relevant articles, 10 studies with a total sample size of 1974 patients were included in this systematic review.[28,29,30,31,32,33,34,35,36,37] Cut off for PCT non-clearance was different in all studies. Overall mortality, sensitivity, and specificity of PCT non-clearance as a predictor of mortality in individual studies are represented in Table 1.
Five studies had done a ROC curve of the ability of PCT non-clearance to predict mortality.[28,31,33,34,36] To detect the mortality using PCT non-clearance, ROC curve analysis was done. Forest plot analysis was used to evaluate AUC of the studies where PCT non-clearance was used as a mortality predictor [Figure 1]. AUCs of the studies were varying between 0.52 and 0.86. Overall AUC observed was 0.711 (95% CI = 0.662–0.760) under fixed effect model and 0.708 (95% CI = 0.648–0.769) under random effect model. Moderate heterogeneity was observed among the studies [Q = 10.17, d.f = 5, P = 0.071, I2 (inconsistency) = 50.8%, 95% CI = 0%–80.4%]. Similarly, the Funnel plot was drawn to show the standard error of the studies corresponding to their observed AUC and is shown in Figure 2.
Figure 1.

Forest plot showing the area under curve (95% CI) of the studies to detect procalcitonin non-clearance as a predictor of mortality
Figure 2.
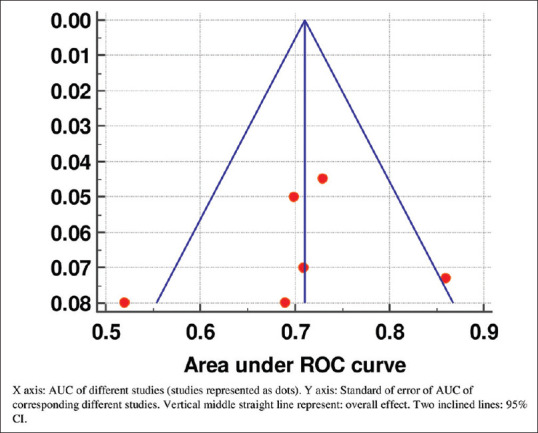
Funnel plot showing the area under curve of the different studies with their corresponding standard error
Similarly, the proportion of the mortality in the PCT non-clearance group in the studies was observed using forest plot analysis [Figure 3]. Six studies assessed the proportion of mortality in patients with PCT non-clearance.[28,29,30,32,33,37] The proportion of the mortality was varying among the studies between 20.09% to 100%. Overall mortality was observed as 37.54% (95% CI = 25.6–50.24) under the random effect model. Much heterogeneity was observed among the studies [Q = 59.53, d.f. = 7, P < 0.001, I2 (inconsistency) = 88.24%, 95% CI = 79.12%–93.38%]. Similarly, funnel plot graph showing the standard error of the mortality of the different studies is shown in Figure 4.
Figure 3.
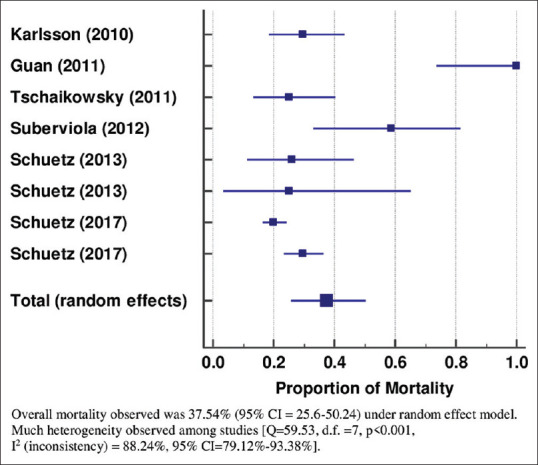
Forest plot showing proportion of mortality in patients with procalcitonin non-clearance
Figure 4.
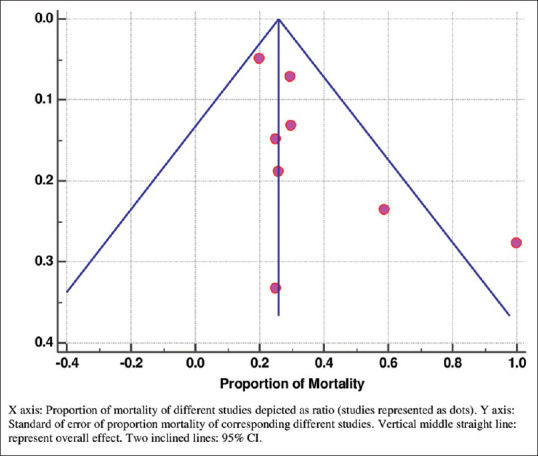
Funnel plot showing proportion of mortality in PCT non-clearance patients of different studies with their corresponding standard error
Discussion
Sepsis is life-threatening organ dysfunction with the dysregulated host response. Complex pathophysiology plays role in sepsis. Not a single biomarker can predict mortality in sepsis. PCT is a widely studied biomarker. Literature on PCT as a diagnostic marker of sepsis is well established. However, there are limited studies on PCT as a prognostic marker. Mortality is a marker of outcome in ICU. Most of the studies have evaluated single PCT as a mortality predictor in patients with sepsis.[13,14,15,16,17,18,19,20,21,22,23,24,25,26,27] PCT non-clearance as a predictor of mortality has also been assessed in a few studies.
In a prospective study by Karlson et al.,[28] in 2010, the predictive value of PCT for survival in 242 adult patients with severe sepsis and septic shock was investigated. Patients with septic shock had higher PCT concentrations (P = 0.02). Though PCT concentrations did not differ between hospital survivors and non survivors, mortality was lower in patients whose PCT concentration decreased by >50% by 72 hours compared to those with less than 50% decrease (12.2% vs. 29.8%, P = 0.007) in 72 hours. It was concluded that PCT concentrations were higher in more severe forms of sepsis, but a substantial concentration decrease was more important for survival than the absolute values.
In a prospective cohort study in 2011, Guan et al.[29] enrolled 37 septic shock patients of ICU with PCT >10 ng/ml. PCT was measured at enrollment and 5 days thereafter. All survivors had a decrease in PCT concentration (median decrease of 9.73 ng/ml) and an increase was seen in non-survivors (median increase of 5.95 ng/ml). A significant decrease in PCT concentration (>25%) was observed in 25 survivors but none in 12 non-survivors. Kinetics of PCT and SOFA correlated with each other. The authors concluded that a significant decrease in PCT concentration rather than PCT concentration itself is a useful indicator of survival in septic shock patients when PCT concentration is greater than 10 ng/ml.
In a prospective study, Tschaikowsky et al.[30] evaluated the performance of PCT, as a percentage of baseline (POB) in predicting hospital survival in 64 postoperative patients with severe sepsis. Plasma PCT was serially measured from day 1 to day 14 in parallel with clinical data until day 28. In survivors, PCT significantly decreased from days 1 to 14. The authors suggested that a decrease in PCT-POB on day 7 in combination with C-Reactive Protein (CRP-POB) may serve to monitor efficacy and guide duration of therapy in critically ill patients.
In a prospective, observational study by Ruiz-Rodriguez et al.,[31] in ICU patients with septic shock and multiorgan dysfunction, serum concentrations of PCT were determined within 12 h of onset of septic shock and serial levels were measured after 24, 48, and 72 h. PCT clearance (PCT-c) was calculated as initial PCT minus PCT at subsequent hours and then multiplied by 100. It was found that PCT clearance was higher in survivors than that in non-survivors, with significant differences at 24 and 48 hr. (p < 0.05). The AUROC was 0.74 for PCT clearance at 24 h, and 0.86 at 48 h.
In 88 patients with septic shock admitted to an ICU of a tertiary care teaching hospital in Spain, Suberviola et al.[32] found that PCT level at admission was comparable between survivors and nonsurvivor (P = 0.6). The PCT level was recorded on admission to ICU and again after 72 hours. Those patients with increasing PCT levels showed higher hospital mortality than those with decreasing levels (58.8% vs 15.4%, p < 0.01). The best AUC for prognosis (0.79) was found for PCT clearance [(initial PCT- final PCT) ÷ (initial PCT)] × 100. A PCT clearance of 70% or higher offered a sensitivity and specificity of 94.7% and 53%, respectively for survival. Serial PCT measurements were more predictive of the prognosis of septic shock patients than single measurements.
In a retrospective analysis by Schuetz et al.,[33] in 2013 in adult patients with sepsis from critical care units in two independent institutions in the USA, cohorts were used for derivation or validation to study the association between PCT change over the first 72 critical care hours and mortality. The authors found that PCT change was strongly associated with ICU and in-hospital mortality independent of clinical risk scores. When PCT decreased by at least 80%, the negative predictive value for ICU/in-hospital mortality was 90%/90% in the derivation cohort and 91%/79% in the validation cohort. It was concluded that PCT kinetics over the first 72 hours of critical care provides prognostic information about ICU and in-hospital mortality in patients with confirmed or likely sepsis independent of clinical severity scores.
Mat Nor et al.[34] in 2014 evaluated the prognostic value of dynamic changes of PCT in sepsis patients by conducting a prospective observational study in adult ICU. Daily PCT was measured for 3 days. A 48-h PCT clearance (PCTc-48) was defined as POB PCT minus 48 h PCT over baseline PCT. Day 1-PCT was associated with the diagnosis of sepsis but was not predictive of mortality. In sepsis patients, PCTc-48 was associated with the prediction of survival (AUC 0.69 (95% CI: 0.53 to 0.84)). Patients with PCTc-48 >30% were independently associated with survival (Hazard Ratio (HR) 2.90 (95% CI: 1.22–6.90)).
In a single-center prospective observational study in 2015 by Gracia de Guadiana-Romualdo et al.,[35] in ICU of a university hospital, 100 severe sepsis and septic shock patients were included to assess the prognostic value of serial measurements of PCT in relation to hospital mortality and to determine whether their addition to severity scores (APACHE II and SOFA) was able to improve prognostic accuracy. Measurements were done on admission and after 48 hours. The best AUC for predicting in-hospital mortality corresponded to APACHE II on admission and SOFA after 48 hours (AUC ROC: 0.75 for both). PCT clearance was higher among survivors. (AUC ROC: 0.66). A combination of severity scores and PCT clearance did not result in superior AUCs. They concluded that PCT levels on admission showed no prognostic value but PCT clearance was predictive of in-hospital mortality.
In a prospective observational study by Poddar et al.,[36] in the ICU of a tertiary care Institute in India in 2015, PCT was measured at admission (D0) and after 72–96 h (D4) in 171 adult patients admitted with severe sepsis or septic shock. Change in PCT values from D0 to D4 was correlated with the primary outcome, 28 days mortality. The C-statistic of the percentage change in PCT from D0 to D4 to predict survival was 0.73 (95% CI: 0.65–0.82) when compared to 0.78 (95% CI: 0.71–0.86) for change of SOFA score. A 75% fall in PCT value yielded 47% sensitivity and 93% specificity to predict survival at 28 days. A 50% fall predicted 28 days survival with 68% sensitivity and 64% specificity. Among patients in whom absolute fall in PCT was >1 ng/ml, a 70% fall predicted survival with 75% sensitivity and 64% specificity. It was concluded that in critically ill patients with severe sepsis/septic, shock change (fall) in PCT was associated with good outcome and non-clearance with the worst outcome.
Schuetz et al.[37] in 2017 in a prospective multicenter observational clinical trial in a large sepsis patient population recruited across the United States, validated that inability to decrease PCT levels by more than 80% between baseline and day 4 was associated with increased 28-day all-cause mortality. PCT was measured daily over the first 5 days. The 28-day all-cause mortality was two-fold higher when PCT did not show a decrease of more than 80% from baseline to day 4 (20% vs 10%; P = 0.001).
In this systematic review and meta-analysis, we highlighted those above studies.[28,29,30,31,32,33,34,35,36,37] We evaluated only the prognostic utility of serial PCT clearance. In this review, PCT non-clearance has emerged as a mortality predictor. The overall AUROC curve observed was 0.711 (95% CI = 0.662–0.760) under fixed effect model and 0.708 (95% CI = 0.648–0.769) under random effect model. There was moderate variation among the studies, i.e., 50.80% (95% CI = 0.00–80.42%), which is acceptable. As there was moderate variation among the studies (50.80%), we focused on a random effect model and got overall AUROC curve 0.708 (95% CI = 0.648–0.769), which is acceptable. We also evaluated the proportion of mortality among different studies. Although huge variability was observed in the proportion of mortality in different studies (20.09% to 100%), it is quite expected with a different sample size of different studies. Overall mortality of almost 40% (37.54%) in the PCT non-clearance group and has its prognostic implications as highlighted above by various literature.
Our review has certain limitations. Only patients of ICU were included, thereby could not be applied to emergency department patients. Different studies that were cited had different cutoffs. Subgroup analysis was not possible due to a limited number of studies. Different confounding variables for mortality were not adjusted in this study. Optimal cutoff of PCT non-clearance could not be detected. Only articles published in English were included.
Despite being an important diagnostic marker of sepsis, PCT has its prognostic implications. It correlates with sepsis and its severity and predicts mortality in critically ill ICU patients. US Food and Drug Administration (FDA) has recently approved the use of PCT testing to guide antibiotic use in the content of acute respiratory illness and sepsis. PCT is the cornerstone of antibiotic stewardship program. In their recent consensus document, Sheutz et al. refined the established PCT algorithm by incorporating the severity of illness.[38] Due to the use of different cut off to discontinue antibiotic use in the emergency department, ward, and ICU patients, Sheutz et al. simplified the approach to discontinue antibiotic use in that document. They proposed an algorithm for PCT use in three broad categories, i.e., patient with mild illness outside ICU, patient with moderate illness outside ICU, and patient with severe illness in the ICU. Further prospective studies can be undertaken to define optimal cut off point for PCT non-clearance with respect to its various other prognostic implications. Correlation of PCT non-clearance along with clinical parameters and organ failures can guide therapeutic interventions.
Conclusion
PCT non-clearance is a predictor of mortality in patients with sepsis. This systematic review and meta-analysis suggest that serial measurements of PCT are a valuable tool for prognosis in ICU patients. PCT non-clearance is an important predictor of mortality in critically ill ICU patients with sepsis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiguchi T, Nakamori Y, Yamakawa K, Kitayama J, Wada D, Ogawa Y, et al. Maximal chemiluminescent intensity in response to lipopolysaccharide assessed by endotoxin activity assay on admission day predicts mortality in patients with sepsis. Crit Care Med. 2013;43:1443–9. doi: 10.1097/CCM.0b013e31827ca960. [DOI] [PubMed] [Google Scholar]
- 3.Gründler K, Angstwurm M, Hilge R, Baumann P, Annecke T, Crispin A, et al. Platelet mitochondrial membrane depolarization reflects disease severity in patients with sepsis and correlates with clinical outcome? Crit Care. 2014;18:R31. doi: 10.1186/cc13724. doi: 10.1186/cc13724 PMID: 24521521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pierrakos C, Vincent JL. Sepsis biomarkers: A review. Crit Care. 2010;14:R15. doi: 10.1186/cc8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vincent J-L. The clinical challenge of sepsis identification and monitoring. PLoS Med. 2016;13:e1002022. doi: 10.1371/journal.pmed.1002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behnes M, Bertsch T, Lepiorz D, Lang S, Trinkmann F, Brueckmann M, et al. Diagnostic and prognostic utility of soluble CD 14 subtype (presepsin) for severe sepsis and septic shock during the first week of intensive care treatment. Crit Care. 2014;18:507. doi: 10.1186/s13054-014-0507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akpinar S, Rollas K, Alagöz A, Seğmen F, Sipit T. Performance evaluation of MR-proadrenomedullin and other scoring systems in severe sepsis with pneumonia. J Thorac Dis. 2014;6:921–9. doi: 10.3978/j.issn.2072-1439.2014.06.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vassiliou AG, Mastora Z, Orfanos SE, Jahaj E, Maniatis NA, Koutsoukou A, et al. Elevated biomarkers of endothelial dysfunction/activation at ICU admission are associated with sepsis development. Cytokine. 2014;69:240–7. doi: 10.1016/j.cyto.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Le Moullec JM, Julienne A, Chenais J, Lasmoles F, Guiliana JM, Milhaud J, et al. The complete sequence of human procalcitonin. FEBS Lett. 1984;167:93–7. doi: 10.1016/0014-5793(84)80839-x. [DOI] [PubMed] [Google Scholar]
- 10.Becker K, Miller R, Nylen E, Cohen R, Siliva O, Snider R. Calcitonin gene family of peptides. In: Becker K, editor. Principles and Practice of Endocrinology and Metabolism. Philadelphia: Lippincott; 2001. pp. 520–34. [Google Scholar]
- 11.Muller B, White JC, Nylen E, Snider R, Becker K, Hebner JF. Ubiquitous expression of the calcitonin-1 gene in multiple tissues in response to sepsis. J Clin Endocrinol Metab. 2001;86:396–404. doi: 10.1210/jcem.86.1.7089. [DOI] [PubMed] [Google Scholar]
- 12.Linscheid P, Seboek D, Schaer DJ, Zulewski H, Keller U, Muller B. Expression and secretion of procalcitonin and calcitonin gene related peptide by adherent monocytes and by macrophage-activated adipocytes. Crit Care Med. 2004;32:1715–21. doi: 10.1097/01.ccm.0000134404.63292.71. [DOI] [PubMed] [Google Scholar]
- 13.Adamik B, Kübler-Kielb J, Golebiowska B, Gamian A, Kübler A. Effect of sepsis and cardiac surgery with cardiopulmonary bypass on plasma level of nitric oxide metabolites, neopterin, and procalcitonin: Correlation with mortality and postoperative complications. Intensive Care Med. 2000;26:1259–67. doi: 10.1007/s001340000610. PMID: 11089751. [DOI] [PubMed] [Google Scholar]
- 14.Meng FS, Su L, Tang YQ, Wen Q, Liu YS, Liu ZF, et al. Serum procalcitonin at the time of admission to the ICU as a predictor of short-term mortality. Clin Biochem. 2009;42:1025–31. doi: 10.1016/j.clinbiochem.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Yin Q, Liu B, Chen Y, Zhao Y, Li C. The role of soluble thrombomodulin in the risk stratification and prognosis evaluation of septic patients in the emergency department. Thromb Res. 2013;132:471–6. doi: 10.1016/j.thromres.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Suberviola B, Castellanos-Ortega A, Ruiz Ruiz A, Lopez-Hoyos M, Santibañez M. Hospital mortality prognostication in sepsis using the new biomarkers suPAR and proADM in a single determination on ICU admission. Intensive Care Med. 2013;39:1945–52. doi: 10.1007/s00134-013-3056-z. [DOI] [PubMed] [Google Scholar]
- 17.Clec'h C, Fosse JP, Karoubi P, Vincent F, Chouahi I, Hamza L, et al. Differential diagnostic value of procalcitonin in surgical and medical patients with septic shock. Crit Care Med. 2006;34:102–7. doi: 10.1097/01.ccm.0000195012.54682.f3. [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Wang H, Liu J, Chen B, Li G. Serum soluble triggering receptor expressed on myeloid cells-1 and procalcitonin can reflect sepsis severity and predict prognosis: A prospective cohort study. Mediators Inflamm. 2014;2014:641039. doi: 10.1155/2014/641039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masson S, Caironi P, Spanuth E, Thomae R, Panigada M, Sangiorgi G, et al. Presepsin (soluble CD14 subtype) and procalcitonin levels for mortality prediction in sepsis: Data from the Albumin Italian Outcome Sepsis trial? Crit Care. 2014:R6. doi: 10.1186/cc13183. doi: 10.1186/cc13183 PMID: 24393424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yaroustovsky M, Plyushch M, Popov D, Samsonova N, Abramyan M, Popok Z, et al. Prognostic value of endotoxin activity assay in patients with severe sepsis after cardiac surgery. J Inflamm (Lond) 2014;10:8. doi: 10.1186/1476-9255-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng L, Zhou X, Su LX, Feng D, Jia YH, Xie LX. Clinical significance of soluble hemoglobin scavenger receptor CD163 (sCD163) in sepsis, a prospective study. PLoS One. 2012;7:e38400. doi: 10.1371/journal.pone.0038400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain S, Sinha S, Sharma SK, Samantaray JC, Aggrawal P, Vikram NK, et al. Procalcitonin as a prognostic marker for sepsis: A prospective observational study. BMC Res Notes. 2014;7:458–458. doi: 10.1186/1756-0500-7-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dahaba AA, Hagara B, Fall A, Rehak PH, List WF, Metzler H, et al. Procalcitonin for early prediction ofsurvival outcome in postoperative critically ill patients with severe sepsis. Br J Anaesth. 2006;97:503–8. doi: 10.1093/bja/ael181. [DOI] [PubMed] [Google Scholar]
- 24.Magrini L, Travaglino F, Marino R, Ferri E, De Berardinis B, Cardelli P, et al. Procalcitonin variations after Emergency Department admission are highly predictive of hospital mortality in patients with acute infectious diseases. Eur Rev Med Pharmacol Sci. 2013;17:133–42. [PubMed] [Google Scholar]
- 25.Savva A, Raftogiannis M, Baziaka F, Routsi C, Antonopoulou A, Koutoukas P, et al. Soluble urokinase plasminogen activator receptor (suPAR) for assessment of disease severity in ventilator-associated pneumonia and sepsis. J Infect. 2011;63:344–50. doi: 10.1016/j.jinf.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 26.Kenzaka T, Okayama M, Kuroki S, Fukui M, Yahata S, Hayashi H, et al. Use of a semiquantitative procalcitonin kit for evaluating severity and predicting mortality in patients with sepsis. Int J Gen Med. 2012;5:483–8. doi: 10.2147/IJGM.S32758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giamarellos-Bourboulis EJ, Tsangaris I, Kanni T, Mouktaroudi M, Pantelidou I, Adamis G, et al. Procalcitonin as an early indicator of outcome in sepsis: A prospective observational study. J Hosp Infect. 2011;77:58–63. doi: 10.1016/j.jhin.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 28.Karlsson S, Heikkinen M, Pettilä V, Alila S, Väisänen S, Pulkki K, et al. Predictive value of procalcitonin decrease in patients with severe sepsis: A prospective observational study. Crit Care. 2010;14:R205. doi: 10.1186/cc9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guan J, Lin Z, Lue H. Dynamic change of procalcitonin, rather than concentration itself, is predictive of survival in septic shock patients when beyond 10 ng/mL. Shock. 2011;36:570–4. doi: 10.1097/SHK.0b013e31823533f9. [DOI] [PubMed] [Google Scholar]
- 30.Tschaikowsky K, Hedwig-Geissing M, Braun GG, Radespiel-Troeger M. Predictive value of procalcitonin, interleukin-6, and C-reactive protein for survival in postoperative patients with severe sepsis. J Crit Care. 2011;26:54–64. doi: 10.1016/j.jcrc.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Ruiz-Rodríguez JC, Caballero J, Ruiz-Sanmartin A, Ribas VJ, Pérez M, Bóveda JL, et al. Usefulness of procalcitonin clearance as a prognostic biomarker in septic shock. A prospective pilot study. Med Intensiva. 2012;36:475–80. doi: 10.1016/j.medin.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 32.Suberviola B, Castellanos-Ortega A, González-Castro A, García-Astudillo LA, Fernández-Miret B. Prognostic value of procalcitonin, C-reactive protein and leukocytes in septic shock. Med Intensiva. 2012;36:177–84. doi: 10.1016/j.medin.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Schuetz P, Maurer P, Punjabi V, Desai A, Amin DN, Gluck E. Procalcitonin decrease over 72 hours in US critical care units predicts fatal outcome in sepsis patients. Crit Care. 2013;17:R115. doi: 10.1186/cc12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mat Nor MB, MdRalib A. Procalcitonin clearance for early prediction of survival in critically ill patients with severe sepsis. Crit Care Res Pract. 2014;2014:819034. doi: 10.1155/2014/819034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.García de Guadiana-Romualdo LM, Rebollo-Acebes S, Esteban-Torrella P, Jiménez-Sánchez R, Hernando-Holgado A, Ortín-Freire A, et al. Prognostic value of lipopolysaccharide binding protein and procalcitoninin patients with severe sepsis and septic shock admitted to intensive care. Med Intensiva. 2015;39:207–12. doi: 10.1016/j.medin.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Poddar B, Gurjar M, Singh S, Aggarwal A, Singh R, Azim A, et al. Procalcitonin kinetics as a prognostic marker in severe sepsis/septic shock. Indian J Crit Care Med. 2015;19:140–6. doi: 10.4103/0972-5229.152755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuetz P, Birkhahn R, Sherwin R, Jones AE, Singer A, Kline JA, et al. Serial procalcitonin predicts mortality in severe sepsis patients: Results from the multicenter procalcitonin monitoring sepsis (MOSES) study. Crit Care Med. 2017;45:781–9. doi: 10.1097/CCM.0000000000002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schuetz P, Beishuizen A, Broyles M, Ferrer R, Gavazzi G, Gluck EH, et al. Procalcitonin (PCT) guided antibiotic stewardship: An international experts consensus on optimized clinical use. Clin Chem Lab Med. 2019;57:1308–18. doi: 10.1515/cclm-2018-1181. [DOI] [PubMed] [Google Scholar]


