Abstract
Background and Aims:
Catheter-related bladder discomfort (CRBD) is a major cause of postoperative morbidity following urological procedures. The aim of this study was to compare the effect of caudal bupivacaine alone and with adjuvant fentanyl and nalbuphine to minimize the severity of CRBD after tubeless percutaneous nephrolithotomy (PCNL).
Material and Methods:
A randomized prospective study was conducted on one hundred thirty-two (American society of Anaesthesiologist physical status I to II) patients who presented for tubeless PCNL under general anesthesia. Patients were randomly divided into four groups control (C), bupivacaine (B), bupivacaine-fentanyl (BF), and bupivacaine-nalbuphine (BN) by using computer-generated codes. All patients received local infiltration at the procedure site while Groups B, BF, and BN received caudal epidural block (CEB) under ultrasound guidance after conclusion of the procedure. Groups B, BF, and BN received bupivacaine alone, bupivacaine-fentanyl, and bupivacaine-nalbuphine, respectively, for CEB. Patients were monitored 24 h for CRBD scale, visual analogue score (VAS), and duration of analgesia at 30 min, 1, 2, 4, 6, 12, 18, and 24 h intervals. The analgesics were supplemented if the CRBD score was >2 and VAS was ≥4. Student t-test, analysis of variance, and Chi-square test were applied for quantitative, within group occurrence, and qualitative analysis respectively.
Results:
The CRBD scores were considerably lower in the Groups BF and BN as compared to Groups C and B during the first four hours. The duration of analgesia was significantly prolonged in Group BN (475 ± 47 min) versus BF (320 ± 68 min) versus B (104 ± 40 min) versus C (26 ± 14 min).
Conclusions:
The severity of CRBD can be reduced with CEB. The effect of CEB can be prolonged with the addition of opioid.
Keywords: Bupivacaine, catheter-related bladder discomfort, caudal epidural block, fentanyl, nalbuphine
Introduction
The urinary catheter is required for few days after urological surgical procedure. It is responsible for catheter-related bladder discomfort (CRBD).[1] The severity may vary with urological procedures and patient thresholds. CRBD is associated with burning sensation with or without urge incontinence, discomfort in the suprapubic region, and urinary urgency.[2,3] The reported incidence of CRBD varies from 50% to 90% after elective urological surgery.[4] The severe CRBD can lead to an increase in postoperative morbidity like bleeding, arrhythmia, and increased severity of coronary artery disease.[4] These may bring exacerbated postoperative pain and increased hospital stay.[4]
The symptoms of CRBD occur due to involuntary detrusor smooth muscle contraction.[5] The available treatment options are antimuscarinic agents, nonsteroidal antiinflammatory drugs (NSAID), opioids, anesthetics, and antiepileptic agents.[4] All treatment options are associated with risk of mild-to-moderate side effects with variable response. Therefore, efficient treatment is important for preventing CRBD. The caudal epidural block (CEB) is commonly performed in pediatric anesthesia. This block is more popular in infant and toddler because of easily palpable sacral landmarks in pediatric patients. Recently, the studies were also available for the role of CEB for postoperative analgesia in adults.[6,7,8,9] The role of CEB to minimize the CRBD was not studied previously. A randomized control trial (RCT) was conducted at our institution. The primary objective was to compare the incidence and severity of CRBD in control group versus patients receiving bupivacaine, bupivacaine-fentanyl, or bupivacaine-nalbuphine in caudal epidural space. The secondary objectives were to compare the duration of analgesia, total number of dosages of analgesics requirement in 24 h, and patient comfort in four groups.
Material and Methods
The study was started after approval from the institutional ethical committee (ref. no.EC/348/2016, dated 5th April 2016). One hundred sixty-eight patients were evaluated for eligibility and one hundred thirty-two patients were enrolled for the study. All patients were preoperatively evaluated and investigated with complete blood count, renal function test, electrocardiography, chest X-ray, and other routine investigation for general anesthesia. The written and informed consent was taken from the patient. They were posted for tubeless percutaneous nephrolithotomy under general anesthesia (GA).[Figure 1] The inclusion criteria were the age ≥18 years and American Society of Anaesthesiologist physical status (ASA) I to II. The exclusion criteria were the patient allergic to study drugs, obesity (BMI >30), long-term opioid use, coagulopathy, skin infection at local site, bladder outflow obstruction, overactive bladder (frequency more than eight times in a one day or more than three times during night hours), and benign prostatic hyperplasia. A single-blinded randomized prospective study was conducted from April 2016 to March 2017. The computer-generated randomized codes were used for sealed envelopes. A research staff prepared these envelops. The anesthesia technician opened these envelops and prepared the study drugs.
Figure 1.
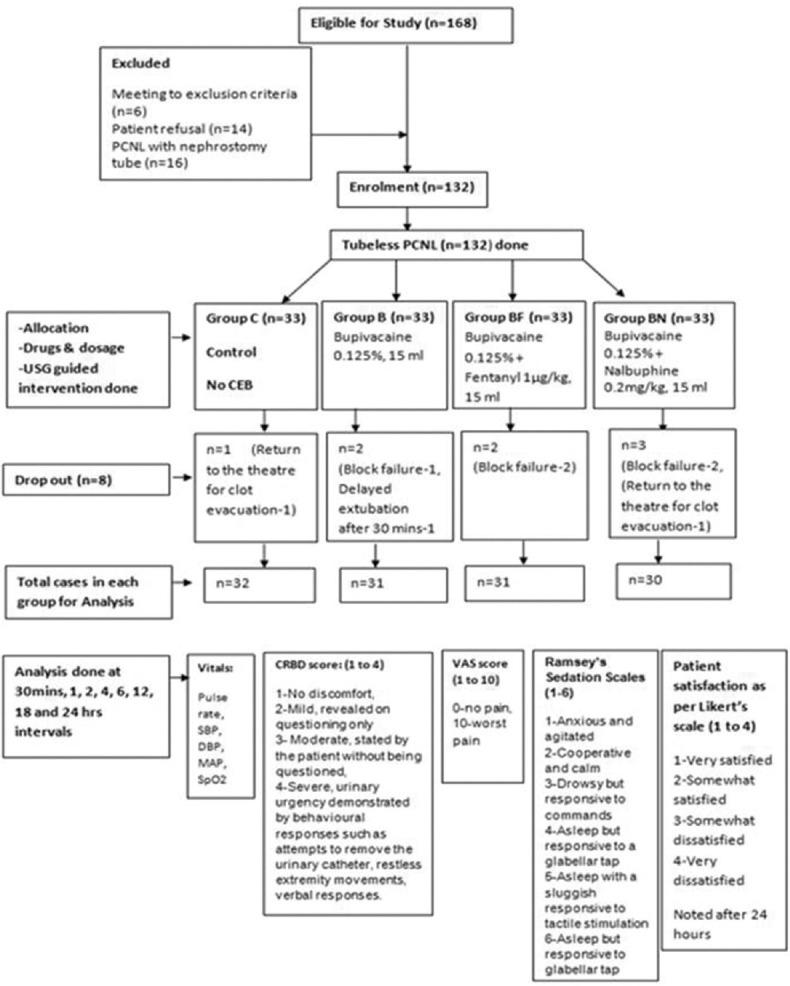
Consort flow diagram
The patients were randomly divided into four equal groups; C (Control, n = 33), B (bupivancaine, n = 33), BF (bupivancaine-fentanyl, n = 33), and BN (bupivancaine-nalbuphine, n = 33). All patients have received 15 ml of drugs in caudal epidural space at the end of surgery except Group C. The prepared study drug was kept on sterile epidural tray. Group B received 0.125% bupivacaine only, Group BF received 0.125% bupivacaine with fentanyl 1μg/kg, and Group BN received 0.125% bupivacaine with nalbuphine 0.2mg/kg.[Figure 1] The CEB was given by the trained anesthesiologist under ultrasound guidance. Intraoperatively, the anesthesiologist administering the drug was not blinded. Postoperative assessment and data recording were done by the same anesthesiologist.
The detail preoperative evaluation and the procedure-related counselling were done. 18 or 20 gauge intravenous catheter was placed in ward and intravenous fluid was started. The premedication glycopyrrolate 0.004mg/kg and metaclopramide 0.15mg/kg intravenously were given in preoperative ward. In operation theater, the routine monitors were applied like, electrocardiogram (ECG), pulse oximetry (SpO2), noninvasive blood pressure (NIBP), and end-tidal carbon dioxide (EtCO2). All patients received 0.03–0.05mg/kg midazolam and 1–2μg/kg fentanyl. The preoxygenation was started with 100% oxygen for 1–3 min. The anesthesia induction was done with 1–2mg/kg propofol and intubation was facilitated with 0.5–1mg/kg succinyl choline or 0.4 to 0.6 mg/kg atracurium intravenously. After intubation, bilateral air entry was checked and endotracheal tube was fixed and kept on the ventilator. Patient was placed in lithotomy position for retrograde placement of ureteric catheter and 16 French foley's urinary catheter. Patient was turned to prone position to perform the tubeless PCNL. All the precautions were taken care to avoid endotracheal tube migration and to prevent injury to patient. At the end of procedure, local infiltration was done with 10 ml bupivancaine 0.25% at percutaneous tract.
The CEB was given in prone position under ultrasound guidance at the end of the procedure to minimize the failure rate. The CEB was given with 22G one and half inches hypodermic needle under strict aseptic precautions. The B K medical ultrasound machine was used with 12–15 MHz linear probe. The linear probe was kept on sacral hiatus in such a way that the needle entry was done with out-of-plane view until it pierced the sacrococcygeal membrane. Then, the probe position was rotated to a long axis view to visualize the needle tip position. The 15ml volume of drug was injected and spread of drug in epidural space was confirmed. A small antiseptic dressing was applied on skin after CEB. The patient was turned supine. The reversal was given and extubated. Patient was shifted in postoperative recovery room. The dropout was considered when there was delayed extubation after 30 min, CEB failure or patient return to operation theater. The block failure was considered when there was a complaint for CRBD score more than two in first 30 min after CEB or not able to locate the caudal epidural space on ultrasound. The motor blockage was assessed postoperatively after one hour of CEB. Patient's age, sex, foley's catheter size, vitals, duration of surgery, CRBD score, visual analogue scores (VAS) for pain, sedation score, and duration of analgesia (measured by endpoint when patient needs rescue analgesia) were recorded postoperatively at 30 min, 1, 2, 4, 6, 12, 18, and 24h intervals. [Figure 1] CRBD was evaluated with the 4-point (1: no discomfort, 2: mild, revealed on questioning only, 3: moderate, stated by the patient without being questioned, 4: severe, urinary urgency demonstrated by behavioral responses such as attempts to remove the urinary catheter, restlessness extremity movement, verbal responses).[10] Postoperative pain level was evaluated by visual analogue score (0–10) due to percutaneous intervention. If the VAS ≥4 or CRBD >2, the analgesic was administered intravenously depending on the patient's severity of pain and medical conditions. The different analgesics were given as per necessary likes: intravenous paracetamol 1g, tramadol 50mg, and diclofenac 75mg. Sedation levels were recorded according to Ramsey's Sedation Scales (1–6). The Bromage scale (1–4) was used to assess the motor blockage (1 = complete motor block, unable to move feet or knees; 2 = almost complete, able to move feet only; 3 = partial, just able to move knees; and 4 = full flexion of knees and feet).[11] The documentation of adverse events such as bradycardia (Heart rate <60/min), hypotension (systolic blood pressure <90mmHg), respiratory depression (SpO2<90%), nausea, vomiting, pruritus, and deep sedation were done. Patient satisfaction was assessed with a 4-point Likert's scale at 24h after the surgery.
The sample size was estimated based on a preliminary experiment according to the incidence of CRBD is about of 50% among four groups and assuming that it can be reduced up to 40% and as per PS- power and sample calculation version 3.0 Jan 2009, the sample size within each group comes around 30. A dropout rate was considered around 10%. The sample size comes around 33.
The statistical analysis was done by SPSS (Statistical Package for the Social Science) Version 15.0. With level of significance, α = 0.05 or 5% and Power of test, 1-β= 0.80 or 80% with the target of applying the Student's t-test for testing the significance among the means of the parameters understudy. ANOVA (Analysis of Variance) was carried for within group analysis. The Chi-square test was used for testing the significance of the occurrence of an event.
Results
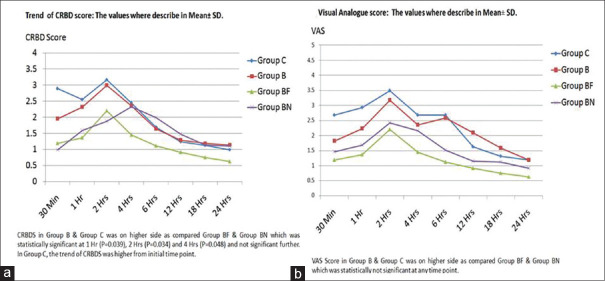
Eight patients were dropped out from one hundred thirty-two enrolled patients. In dropped out cases, five cases were block failure; two cases were returned to the operation theater for clot evacuation; one case was delayed extubation [Figure 1]. The distributions of patients in all four groups were comparable with respect to demographic data, physical status, and duration of surgery [Table 1]. All patients were evaluated at eight different time points post surgery. The CRBD scores (CRBDS) were significantly lower in the Groups BF and BN as compared to Groups C and B during the first four hours [Figure 2]. There were no differences noticed in CRBDS after 6 h. The level of comfort was improved over a period of time. At 24 h, 100% patients in all groups were found to be comfortable. In Group C, fifteen (46.9%) patients and Group B, six (19.4%) patients till 6 h CRBDS was >2, while in case of Group BN CRBDS >2 was in two (6.7%) patients andGroup BF CRBDS >2 was in two patients (6.5%) (P = 0.006*, using Chi-square test) [Figure 3]. VAS score in Groups B and C was on a higher side as compared Groups BF and BN, which was statistically not significant at any time point [Figure 2]. The sedation scale was observed not significant statistically in all the groups. Duration of analgesia was higher in Group BN (475 ± 47 min), followed by in Group BF (320 ± 68 min) as compared to other Groups B (104 ± 40 min) and Group C (26 ± 14 min). P value (P = 0.0001**) was highly significant, which was calculated using One-way ANOVA [Table 2]. Patient satisfaction score was worst in Group C (3.02 ± 0.32) and Group B (2.8 ± 0.42) as compared to Group BN (1.2 ± 0.56) and Group BF (1 ± 0.32). P = 0.032* using one-way ANOVA [Table 2].
Table 1.
Distribution of Demographic Data
| Group C (n=32) | Group B (n=31) | Group BF (n=31) | Group BN (n=30) | P | |
|---|---|---|---|---|---|
| AGE (years) | 43±31 | 45.2±12.9 | 46.7±14.2 | 47.7±11.9 | 0.78 |
| SEX (M:F) | 18:14 | 20:11 | 22:9 | 17:13 | 0.68 |
| HT (cm) | 162±6.75 | 164±7.32 | 167±5.63 | 162±6.75 | 0.81 |
| WT (kg) | 73±10.14 | 69±9.15 | 71±8.18 | 73±10.14 | 0.79 |
| ASA (I/II) | 19/13 | 22/9 | 23/8 | 18/12 | 0.58 |
| Duration of Surgery (Min) | 62±18.9 | 59±21.1 | 63±19.4 | 62±18.9 | 0.72 |
The values of AGE, HT (height), WT (weight) and Duration of surgery shown as Mean±SD, P is calculated using One Way ANOVA (Analysis of Variance). For Gender and ASA (American Society of Anaethesiology physical status), P-value was calculated using Chi-square test.
Figure 2.
Graphs for the trend of (a) catheter-related bladder discomforts score (1–4) and (b) visual analogue score (0–10)
Figure 3.
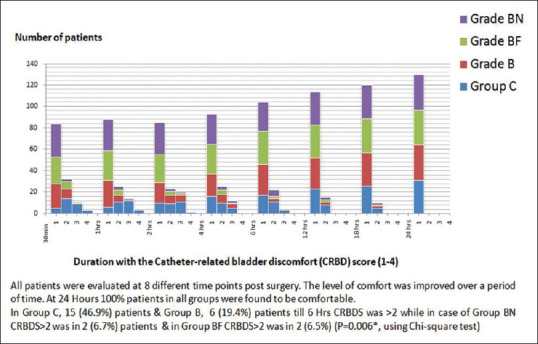
Number of patients with the catheter-related bladder discomforts (CRBD) score (1–4)
Table 2.
Comparison of Duration of analgesia (min) (Mean±SE) and Patient satisfaction score (1-4) after 24 h (Mean±SD) in all groups
| Group C n=32 | Group B n=31 | Group BF n=31 | Group BN n=30 | P | |
|---|---|---|---|---|---|
| Duration of analgesia (min) | 26±14 | 104±40 | 320±68 | 475±47 | P=0.0001** |
| Patient satisfaction score (1-4) after 24 h | 3.02±0.32 | 2.84±0.42 | 1.03±0.32 | 1.21±0.56 | P=0.032* |
SE; Standard Error, SD; Standard Deviation
There was no motor weakness reported postoperatively in all groups. There was no adverse event found during this study in all groups except pruritus seen in only Group BF in four patients. The requirement of rescue analgesic was high in Group C followed by Group B, Group BF, and Group BN. In the case of Groups C and B, it was almost two dosages per patient and in Groups BF and BN, it was half (0.5) dosages.(P = 0.03*, using Chi-square test) [Figure 4].
Figure 4.
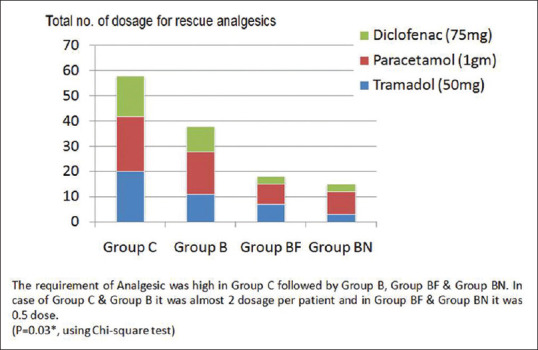
Total no. of dosage for analgesics requirement in 24 h
Discussion
CRBD is a major source of dissatisfaction to the patient after the urological procedure. The urinary catheter discomfort is the first complaint after the patient recovers from the anesthesia.[12] Sometimes patient may become irritable, extremely restless, and may try to tug on the catheter. There are many modalities of treatment available to minimize the severity of CRBD like: oxybutynin, tolterodine,[13,14] and butylscopolamine,[15] nonsteriodal antiinflammatory drugs (NSAID), tramadol, ketamine,[16] paracetamol,[10] pregabaline, and gabapentin.[17] All can achieve a better improvement of CRBD as compared to placebo.[4] But, these are associated with dosage-related systemic side effects.
The understanding of mechanism and pathophysiology of the CRBD is required for better management and decreased the morbidity. The mechanism of the CRBD can be explained by understanding the neural innervations of the bladder. The parasympathetic efferent fibers carried by S2, S3, S4, are motor to detrusor muscle. The sympathetic efferent fibers carried by T11, T12, L1, L2 are inhibitory to detrusor muscle and motor to the preprostatic sphincter. The sphinctter urethrae is supplied by the pudendal nerves (S2, S3, S4), which are voluntary. The spasm of the bladder wall leads to generation of pain sensations, which are carried mainly by parasympathetic nerves (S2-S4) and partly by sympathetic nerves (T11-L2). The lateral spinothalamic tract carries the sensations of bladder distension and pain via posterior columns.[18] The catheterization can stimulate the sensory nerves of the bladder which can lead to acetylcholine release and brings the muscarinic or parasympathetic mediated involuntary contraction of the detrusor muscle. Based on this mechanism, different anticholinergic, opioid, and prostaglandin inhibitors are available with varying degrees of success for the CRBD management.[19,20] CEB can be used for management of CRBD to minimize the side effect related to systemic injection.
CEB is mainly used for the pediatrics lower abdominal and urological procedures for perioperative analgesia. The CEB is not popular for adult patients. There are difficult to palpate the landmark and high chance of failure rate in adult. However, the success rate of CEB can be increased with the help of imaging modality like ultrasonography and fluoroscopy.[21,22] Presently, this space is exclusively used by the interventional pain physician to treat the chronic low back pain.
The caudal epidural space is an extension of lumber epidural to the sacrum until the sacrococcygeal membrane (SCM). The SCM forms due to the failed fusion of the laminae of the 5th sacral vertebra. The distance from SCM to dural sac is around 3 to 4 cm. The volume for caudal epidural space is varied from10 to 30ml. In an adult, the suggested dosages are 15–20 ml to block the lower limb and perineum.[23]15ml volume was selected for our study. Sah et al. observed that bupivacaine 0.125% in the lumber epidural did not lead to motor weakness during labor analgesia.[11] The result is comparable to our study, and it does not affect the postoperative outcome. Our idea behind the study was to prolong the effect of local anesthetic agent (bupivacaine) by the help of adjuvant. It was studied that bupivacaine with opioid has the synergistic effect to prolong the duration of the analgesia.
Nalbuphine is the synthetic opioid agonist-antagonist analgesic. It has agonistic action at kappa and antagonist activity at μ opioid receptor.[24] It was observed that it has fewer side effects, better drug profile, and it improves the quality of postoperative analgesia.[25] Verma et al. found that the addition of Nalbuphine with heavy bupivacaine for subarachnoid block was significantly improved the duration of the postoperative analgesia for lower limb orthopedic surgeries.[26] In a study conducted by Chatrath et al. for orthopedic surgeries, they used a combination of epidural bupivacaine with nalbuphine for postoperative analgesia. The duration of analgesia was last for 380 ± 11.49 min. While in our study, it was last for 475 ± 47 min for Group BN. The study noticed that the quality of postoperative analgesia and patient's satisfaction was better as compared to tramadol as an adjuvant.[27] In the present study, the patient's satisfaction was better with Group BN as compared to GroupsC and B. Fentanyl is a synthetic opioid agonist. It binds mainly with the mu receptors within the spinal cord. Fentanyl easily crosses the lumbar dura and enters into the spinal cords.[28] Ahuja et al. did the study in 60 children undergoing infra umbilical surgery for CEB for postoperative analgesia. They noticed that the mean durations of analgesia in bupivacaine and fentanyl groups were 4.10 ± 0.5 h and 5.95 ± 0.63h, respectively. The similar results were found in the present study. It suggested that there was a prolongation of the duration of analgesia with the addition of fentanyl with bupivacaine.[29]
Postoperative analgesia usage in our study may have skewed the results to some extent. It may reduce the severity of the CRBD. The severity of pain was less at the operation site in tubeless as compared to standard PCNL.[30] Hence, we have selected only tubeless PCNL. Another limitation of our study is that the severity of the CRBD may vary with person to person depending upon the individual pain threshold, previous exposure to urinary catheter, and size of foley's catheter.
Conclusion
CEB is an alternative to minimize the severity of the CRBD. There is an advantage of using the regional block for postoperative pain management over the systemic administration of analgesic drugs. Our study suggests that successfully given CEB with opioid adjuvant is the more efficient preventive and treatment option for the CRBD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We are thankful to all departments and postoperative staffs.
References
- 1.Hu B, Li C, Pan M, Zhong M, Cao Y, Zhang N, et al. Strategies for the prevention of catheter-related bladder discomfort: A PRISMA-compliant systematic review and meta-analysis of randomized controlled trials. Medicine. 2016;95:e4859. doi: 10.1097/MD.0000000000004859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Binhas M, Motamed C, Hawajri N, Yiou R, Marty J. Predictors of catheter-related bladder discomfort in the post-anaesthesia care unit. Ann Fr Anesth Reanim. 2011;30:122–5. doi: 10.1016/j.annfar.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal A, Yadav G, Gupta D, Singh PK, Singh U. Evaluation of intra-operative tramadol for prevention of catheter-related bladder discomfort: A prospective, randomized, double-blind study. Br J Anaesth. 2008;101:506–10. doi: 10.1093/bja/aen217. [DOI] [PubMed] [Google Scholar]
- 4.Bai Y, Wang X, Li X, Pu C, Yuan H, Tang Y, et al. Management of catheter-related bladder discomfort in patients who underwent elective surgery. J Endourol. 2015;29:640–9. doi: 10.1089/end.2014.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Z, Cao Z, Xu C, Wang H, Zhang C, Pan A, et al. Solifenacin is able to improve the irritative symptoms after transurethral resection of bladder tumors. Urology. 2014;84:117–21. doi: 10.1016/j.urology.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 6.Kiribayashi M, Inagaki Y, Nishimura Y, Yamasaki K, Takahashi S, Ueda K. Caudal blockade shortens the time to walking exercise in elderly patients following low back surgery. J Anesth. 2010;24:192–6. doi: 10.1007/s00540-009-0840-6. [DOI] [PubMed] [Google Scholar]
- 7.Kita T, Maki N, Song YS, Arai F, Nakai T. Caudal epidural anesthesia administered intraoperatively provides for effective postoperative analgesia after total hip arthroplasty. J Clin Anesth. 2007;19:204–8. doi: 10.1016/j.jclinane.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Wong SY, Li JY, Chen C, Tseng CH, Liou SC, Tsai SC, et al. Caudal epidural block for minor gynecologic procedures in outpatient surgery. Chang Gung Med J. 2004;27:116–21. [PubMed] [Google Scholar]
- 9.Ikuerowo SO, Popoola AA, Olapade-Olaopa EO, Okeke LI, Shittu OB, Adebayo SA, et al. Caudal block anesthesia for transrectal prostate biopsy. Int Urol Nephrol. 2010;42:19–22. doi: 10.1007/s11255-006-9095-4. [DOI] [PubMed] [Google Scholar]
- 10.Ergenoglu P, Akin S, Yalcin Cok O, Eker E, Kuzgunbay B, Turunc T, et al. Effect of intraoperative paracetamol on catheter-related bladder discomfort: A prospective, randomized, double-blind study. Curr Ther Res Clin Exp. 2012;73:186–94. doi: 10.1016/j.curtheres.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sah N, Vallejo M, Phelps A, Finegold H, Mandell G, Ramanathan S, et al. Efficacy of ropivacaine, bupivacaine, and levobupivacaine for labor epidural analgesia. J Clin Anesth. 2007;19:214–7. doi: 10.1016/j.jclinane.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal A, Dhiraaj S, Pawar S, Kapoor R, Gupta D, Singh PK. An evaluation of the efficacy of gabapentin for prevention of catheter-related bladder discomfort: A prospective, randomized, placebo-controlled, double-blind study. Anesth Analg. 2007;105:1454–7. doi: 10.1213/01.ane.0000281154.03887.2b. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal A, Dhiraaj S, Singhal V, Kapoor R, Tandon M. Comparison of efficacy of oxybutynin and tolterodine for prevention of catheter related bladder discomfort: A prospective, randomized, placebo-controlled, double-blind study. Br J Anaesthesia. 2006;96:377–80. doi: 10.1093/bja/ael003. [DOI] [PubMed] [Google Scholar]
- 14.Tauzin-Fin P, Sesay M, Svartz L, Krol-Houdek MC, Maurette P. Sublingual oxybutynin reduces postoperative pain related to indwelling bladder catheter after radical retropubic prostatectomy. Br J Anaesth. 2007;99:572–5. doi: 10.1093/bja/aem232. [DOI] [PubMed] [Google Scholar]
- 15.Ryu JH, Hwang JW, Lee JW, Seo JH, Park HP, Oh AY, et al. Efficacy of butylscopolamine for the treatment of catheter-related bladder discomfort: A prospective, randomized, placebo-controlled, double-blind study. Br J Anaesth. 2013;111:932–7. doi: 10.1093/bja/aet249. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal A, Gupta D, Kumar M, Dhiraaj S, Tandon M, Singh PK. Ketamine for treatment of catheter related bladder discomfort: A prospective, randomized, placebo controlled and double blind study. Br J Anaesth. 2006;96:587–9. doi: 10.1093/bja/ael048. [DOI] [PubMed] [Google Scholar]
- 17.Srivastava VK, Agrawal S, Kadiyala VN, Ahmed M, Sharma S, Kumar R. The efficacy of pregabalin for prevention of catheter-related bladder discomfort: A prospective, randomized, placebo-controlled double-blind study. J Anesth. 2015;29:212–6. doi: 10.1007/s00540-014-1911-x. [DOI] [PubMed] [Google Scholar]
- 18.Chaurasia BD. Urinary bladder and the urethra. In: Garg K, editor. Human Anatomy. 5th ed. New Delhi: CBS Publisers; 2010. pp. 375–84. [Google Scholar]
- 19.Caulfield MP, Birdsall NJ. International union of pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279–90. [PubMed] [Google Scholar]
- 20.Yamanishi T, Chapple CR, Chess-Williams R. Which muscarinic receptor is important in the bladder? World J Urol. 2001;19:299–306. doi: 10.1007/s003450100226. [DOI] [PubMed] [Google Scholar]
- 21.Yoon JS, Sim KH, Kim SJ, Kim WS, Koh SB, Kim BJ. The feasibility of color Doppler ultrasonography for caudal epidural steroid injection. Pain. 2005;118:210–4. doi: 10.1016/j.pain.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Park Y, Lee JH, Park KD, Ahn JK, Park J, Jee H. Ultrasound-guided vs. fluoroscopy-guided caudal epidural steroid injection for the treatment of unilateral lower lumbar radicular pain: A prospective, randomized, single-blind clinical study. Am J Phys Med Rehabil. 2013;92:575–86. doi: 10.1097/PHM.0b013e318292356b. [DOI] [PubMed] [Google Scholar]
- 23.Brown DL. Caudal block. In: Ehab Farag, Loran Mounir-Soliman., editors. Brown's Atlas of Regional Anesthesia. 5th ed. Philadelphia: Elsevier; 2017. pp. 291–8. [Google Scholar]
- 24.Schmauss C, Doherty C, Yaksh TL. The analgetic effects of an intrathecally administered partial opiate agonist, nalbuphine hydrochloride. Eur J Pharmacol. 1982;86:1–7. doi: 10.1016/0014-2999(82)90389-2. [DOI] [PubMed] [Google Scholar]
- 25.Tiwari AK, Tomar GS, Agrawal J. Intrathecal bupivacaine in comparison with a combination of nalbuphine and bupivacaine for subarachnoid block: A randomized prospective double-blind clinical study. Am J Ther. 2013;20:592–5. doi: 10.1097/MJT.0b013e31822048db. [DOI] [PubMed] [Google Scholar]
- 26.Verma D, Naithani U, Jain DC, Singh A. Post operative analgesic efficacy of intrathecal tramadol versus nalbuphine added to bupivacaine in spinal anaesthesia for lower limb orthopaedic surgery. J Evol Med Dent Sci. 2013;2:6196–206. [Google Scholar]
- 27.Chatrath V, Attri JP, Bala A, Khetarpal R, Ahuja D, Kaur S. Epidural nalbuphine for postoperative analgesia in orthopedic surgery. Anesth Essays Res. 2015;9:326–30. doi: 10.4103/0259-1162.158004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campbell FA, Yentis SM, Fear DW, Bissonnette B. Analgesic efficacy and safety of a caudal bupivacaine-fentanyl mixture in children. Can J Anaesth. 1992;39:661–4. doi: 10.1007/BF03008226. [DOI] [PubMed] [Google Scholar]
- 29.Ahuja S, Yadav S, Joshi N, Chaudhary S, Madhu S. Efficacy of caudal fentanyl and ketamine on post-operative pain and neuroendocrine stress response in children undergoing infraumbilical and perineal surgery: A pilot study. J Anaesthesiol Clin Pharmacol. 2015;31:104–9. doi: 10.4103/0970-9185.150558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agrawal MS, Agarwal M. Percutaneous nephrolithotomy: Large tube, small tube, tubeless, or totally tubeless? Indian J Urol. 2013;29:219–24. doi: 10.4103/0970-1591.117285. [DOI] [PMC free article] [PubMed] [Google Scholar]