Abstract
Purpose
To provide information about the relationship between follow‐up period and follicular development in patients with infertility due to premature ovarian insufficiency (POI) who are undergoing hormone replacement therapy (HRT). It is necessary to detect follicle development for artificial insemination or in vitro fertilization.
Methods
This retrospective cohort study was conducted at a university hospital in Tokyo, Japan, from April 2014 to February 2019 in 20 patients [follicular development group, 11 women (55%); non‐follicular development group, 9 women (45%)] with POI; their follicular development was followed up weekly. Background characteristics, including age, follicle‐stimulating hormone (FSH) and anti‐Mullerian hormone levels (AMH), the period from the last spontaneous menstruation to hormone replacement therapy initiation, and follow‐up period during HRT were investigated. The period without follicular development was tabulated, and the subsequent cumulative follicular development detection rate was calculated.
Results
At least 1‐year follow‐up, the cumulative follicular development rate was 70%; follicular development was observed with a probability of 49.1% at 3 months, 33.4% at 6 months, and 8.3% at 12 months in the follow‐up period.
Conclusions
The results show that the longer the non‐follicle development period, the lower the probability of subsequent follicular development in patients with POI during HRT.
Keywords: amenorrhea, hormone replacement therapy, infertility, menopause, retrospective study
Cumulative follicular development rate during follow‐up during HRT. At 1‐year follow‐up, the cumulative follicular development rate was 70%; follicular development was observed with a probability of 49.1% at 3 months, 33.4% at 6 months, and 8.3% at 12 months in the follow‐up period.

1. INTRODUCTION
Premature ovarian insufficiency (POI) implies an early depletion of primordial follicles and is observed in patients aged <40 years, those with amenorrhea for ≥4 months, and those with follicle‐stimulating hormone (FSH) levels at menopause level, 1 with a prevalence of 0.1% in women aged <30 years and 1% in those aged <40 years. 2 Due to long‐term estrogen deficiency, POI is known to increase the risk of cardiovascular disorders, stroke, fractures, and cognitive dysfunction. 3 , 4 , 5 , 6 , 7 , 8
It has been recommended that hormone replacement therapy (HRT) can be performed until the age of 50 years. 9 , 10 Even at menopause, patients still have at least 1000 primordial follicles in their ovaries. 11 , 12 Patient with POI rarely has a natural recovery of ovarian function. 13 , 14 The natural pregnancy rate in these patients has been reported to be 2%‐14%. 15 Although the number of remaining primordial follicles is extremely less, the quality of the egg is age dependent. 16 In order to perform artificial insemination or in vitro fertilization, it is necessary to detect very rare follicle development.
It has been reported that supplementation with 0.05 mg of ethinylestradiol from the cycle before the initiating follicle stimulation increased the frequency of detecting follicle development using gonadotropin stimulation, 17 and patients were followed up at our hospital while HRT was continued during the period of withdrawal bleeding. It is difficult to inform outpatient the probability of detecting follicular development because the following information is not available; the length of the follow‐up period required for patients with long amenorrhea period and how much follicular development may be observed during HRT. There are no studies on the course and background factors of follicular development in POI patients undergoing HRT, and it is difficult to provide information regarding follow‐up to patients.
In our hospital, HRT is performed for POI patients, and among them, for those who wish to become pregnant, regular outpatient consultation is performed to detect follicular development, which is very rare and randomly detected.
The purpose of this study was to provide information on the follow‐up period and follicular development in POI patients undergoing HRT.
2. MATERIALS AND METHOD
2.1. Study design and population
This retrospective cohort study included 21 patients with POI who desired to have a baby and whose weekly follicular development was followed up between April 2014 and February 2019. This study was conducted after obtaining approval from the institutional review board of Jikei University School of Medicine (IRB number 30‐160‐9181). All patients met the diagnostic criteria for POI, including amenorrhea for ≥4 months, age <40 years, and FSH level of ≥25 mIU/mL. 1 Those who were unable to visit the outpatient clinic weekly during the follicular phase or who discontinued HRT were excluded. The cause of POI and follow‐up period with HRT was described. Patients were divided into two groups based on the presence or absence of follicular development, and their background characteristics such as age at the last spontaneous menstruation (LSM) and first‐visit FSH and AMH levels, LSM to the HRT, and the duration of follow‐up period during HRT were compared. The period without follicular development was tabulated, and the subsequent cumulative follicular development detection rate was calculated. In the follicle development group, FSH levels during menstruation of each cycle were compared between a cycle in which follicle development was observed and a cycle in which follicle development was not observed.
Background factors were recorded for pregnant women.
2.2. Study protocol
For HRT, a steady dose of estrogen agents (estradiol [E2], Julina tablets® 0.5 mg every day or ESTRANA Tapes® 0.72 mg every 2 days; conjugated estrogens, PREMARIN TABLETS® 0.625 mg every day) was administered continuously during the period of withdrawal bleeding. The reason for the continuous use of estrogen agents during the period of withdrawal bleeding was to prevent the levels of FSH and luteinizing hormone (LH) from increasing during menstruation (Figure 1). Blood samples were analyzed for serum FSH, LH, E2, and AMH levels at the first visit. In patients in whom more than 1 month had elapsed since the last menstrual period, norgestrel‐ethinylestradiol agents (PLANOVAR®) were administered for 10 days to induce withdrawal bleeding. After the induction of withdrawal bleeding, the patient was ordered for weekly visits after 3‐5 days of the menstrual cycle. At each outpatient visit, serum FSH, LH, E2, and progesterone levels in blood samples were analyzed to monitor follicular development. Because FSH and LH levels suppressed by norgestrel‐ethinylestradiol agents (PLANOVAR®) are increased after withdrawal bleeding, the presence of follicles that develop during the menstrual cycle can be inferred by confirming the increase in E2 level. When the E2 level was ≥80‐100 pg/mL, transvaginal ultrasonography was performed to confirm follicular development. In this method, follicular development was defined using transvaginal ultrasonography with a diameter of 10 mm or greater, and follicle stimulation was performed on follicles that grew naturally up to 10 mm. Therefore, the E2 level of 80‐100 pg/mL was set as the threshold for transvaginal ultrasonography. After confirming follicular development, ovarian stimulation was performed using 150 or 225 units of a gonadotropin agent (Follitropin alfa; Gonalef®, purified human menopausal gonadotropin; GONAPURE INJECTION®, or human menopausal gonadotropin; HMG INJECTION TEIZO®) and a gonadotropin‐releasing hormone antagonist (cetrorelix acetate; Cetrotide®). Timed intercourse (TI), artificial insemination of husband semen (AIH), and in vitro fertilization (IVF) were performed. When follicular development was not observed in the follow‐up for approximately 3 weeks from the start of menstruation, norgestrel‐ethinylestradiol agents (PLANOVAR®) were administered for 10‐14 days to induce withdrawal bleeding. Analyses were performed using an electrochemiluminescence immunoassay (commercial kits; Elecsys®, Roche Diagnostics K.K.).
FIGURE 1.
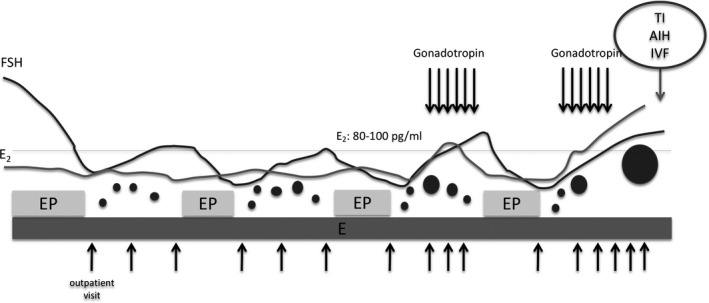
Our protocol of hormone replacement therapy. A steady dose of estrogen agents was administered continuously during the period of withdrawal bleeding. Blood levels of serum FSH, LH, E2, and P4 were analyzed at each visit, and when the E2 level was 80‐100 pg/mL or higher, transvaginal ultrasonography was performed. After confirming follicular development, ovarian stimulation was initiated using FSH agents and TI, AIH, and IVF were performed. When follicular development was not observed for 3 wk, norgestrel‐ethinylestradiol agent (PLANOVAR®) was administered for 10‐14 d to induce withdrawal bleeding. AIH, artificial insemination of husband's semen; E, estrogen agent; E2, estradiol; EP, norgestrel‐ethinylestradiol agent; FSH, follicle‐stimulating hormone; IVF, in vitro fertilization; LH, luteinizing hormone; P4, progesterone; TI, timed intercourse
2.3. Statistical analysis
Descriptive statistics such as mean ± SD or median [interquartile interval] were used to represent quantitative variables and numbers (percentages) were used for qualitative variables. Student's t test and Mann‐Whitney U test were used for univariate analysis. All tests were two‐sided, with a P value of <.05 being defined as significantly different. Statistical analysis was performed using Prism 8 software for macOS Version 8.4.2 (464) (GraphPad Software, LLC).
3. RESULTS
3.1. Background characteristics
One case was excluded because she could not be followed up regularly on an outpatient basis. In total, 20 patients underwent HRT during the study period and were followed up regularly. Background characteristics of all patients are shown in Table 1. In 14 patients (74%), AMH level was below the detection sensitivity. Of the 20 patients, 11 showed follicular development, and 33 follicular development cycles were monitored.
TABLE 1.
Background characteristics of all patients
| Cases | Age at menarche | Age at first visit | Cause of POI | AMH (ng/mL) | FSH level at first visit (mIU/mL) | Estradiol level at first visit (pg/mL) | Parity | Period of LSM to HRT | Estrogen type | Follow‐up period (d) | Time to first follicle development (d) | Follicular development (times) | Fertility treatment | Atresia (times) | Pregnancy | Ovulation method | FSH level at follicular development mIU/mL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 15 | 32 | Chemotherapy | 0.01 > | 122.0 | 39.3 | 0 | 2057 | Estradiol | 1143 | — | 0 | — | — | − | — | — |
| 2 | 11 | 31 | Endometriosis, ovarian surgery | 0.01 > | 77.4 | 10 > | 0 | 1403 | CE | 487 | — | 0 | — | — | − | — | — |
| 3 | 14 | 36 | Unknown | 0.02 > | 60.8 | NA | 0 | 297 | CE | 352 | — | 0 | — | — | − | — | — |
| 4 | 11 | 35 | Unknown | 0.1 > | 106.0 | 10 > | 0 | 170 | CE | 903 | — | 0 | — | — | − | — | — |
| 5 | 13 | 33 | Unknown | 0.02 > | 108.0 | 5 > | 0 | 1427 | Estradiol | 119 | — | 0 | — | — | − | — | — |
| 6 | 13 | 27 | Unknown | 0.1 > | 44.6 | 10 > | 0 | 853 | CE | 559 | — | 0 | — | — | − | — | — |
| 7 | 12 | 26 | Unknown | NA | 74.4 | 10 > | 0 | 2951 | CE | 1636 | — | 0 | — | — | − | — | — |
| 8 | 12 | 25 | Radiotherapy | 0.1 > | 19.2 | 36.9 | 1 | 164 | CE | 804 | — | 0 | — | — | − | — | — |
| 9 | 11 | 37 | Ovarian surgery | 0.1 > | 25.0 | 5 > | 0 | 1209 | CE | 1754 | — | 0 | — | — | − | — | — |
| 10 | 11 | 38 | Chemotherapy | NA | 88.0 | 10 > | 0 | 3593 | Estradiol | 1662 | 425 | 1 | — | 1 | − | NC | 8.7 |
| 11 | 14 | 35 | Unknown | 0.01 > | 57.5 | 86.6 | 1 | 134 | Estradiol | 645 | 175 | 1 | ART | — | − | pHMG | 66.0 |
| 12 | 15 | 31 | Unknown | 1.10 | 81.3 | 5 > | 0 | 1296 | CE | 767 | 224 | 1 | ART | — | − | NC | 26.8 |
| 13 | 12 | 37 | Unknown | NA | 104.5 | 10 > | 0 | 622 | Estradiol | 643 | 97 | 1 | TI | — | − | fα | 1.5 |
| 14 | 12 | 38 | Endometriosis | 0.27 | 53.1 | 5 > | 0 | 351 | CE | 323 | 36 | 2 | AIH | — | − | pHMG/fα | 10.9 11.1 |
| 15 | 11 | 33 | Unknown | 0.1 > | 43.0 | 10 > | 0 | 361 | CE | 188 | 41 | 3 | ART | — | − | fα/hMG/hMG | 6.5/0.6/0.1 |
| 16 | 11 | 31 | Unknown | 0.1 > | 11.7 | 10 > | 1 | 268 | CE | 224 | 49 | 4 | TI/AIH | — | − | NC/NC/NC/pHMG | 23.7/7.2/20.8/NA |
| 17 | 10 | 32 | Unknown | 0.1 > | 71.4 | 5 > | 0 | 470 | Estradiol | 335 | 58 | 4 | ART | 3 | − | fα/fα/fα/fα | 3.2/3.4/4.3/10.1 |
| 18 | 15 | 30 | Unknown | 0.02 > | 93.0 | 10 > | 0 | 779 | CE | 671 | 19 | 3 | AIH/ART | — | + | pHMG/pHMG/pHMG | 8.3/10.2/11.8 |
| 19 | 14 | 38 | Unknown | 0.1 > | 102.0 | 10 > | 0 | 344 | CE | 296 | 5 | 3 | TI/ART | — | + | NC/NC/pHMG | 102/11.8/4.8 |
| 20 | 13 | 30 | Unknown | 0.13 | 23.7 | 10 > | 0 | 162 | CE | 839 | 58 | 10 | TI/AIH/ART | 1 | + | NG/pHMG/pHMG/pHMG/pHMG/pHMG/pHMG/pHMG/pHMG/pHMG | NA/24.1/NA/12.6/6.0/16.8/6.5/2.2/NA/12.1 |
Abbreviations: AIH, artificial insemination of husband semen; AMH, anti‐Mullerian hormone; ART, assisted reproductive technology; CE, conjugated estrogen; FSH, follicle‐stimulating hormone; fα, follitropin alfa; HRT, hormone replacement therapy; LSM, the last spontaneous menstruation; NA, not available; NC, natural cycle; pHMG, purified human menopausal gonadotropin; TI, timed intercourse.
The duration of follow‐up period for the two groups, that is, those with and without follicular development, was 644 [318, 855] days. The reasons for the completion of HRT were the termination of fertility treatment, pregnancy, and dropout.
The background characteristics of patients in the two groups are shown in Table 2. The mean age of the study participants was 32.8 ± 4.0 years, the FSH level was 72.9 [44.2, 102.6] mIU/mL, and the AMH level was 0.10 [0.02, 0.10] ng/mL.
TABLE 2.
Comparison of background characteristics
| Background characteristics |
Total (n = 20) |
Non‐follicular development (n = 9) |
Follicular development (n = 11) |
Pregnant (n = 3) |
|---|---|---|---|---|
| Age a (y) | 32.8 ± 4.0 | 31.3 ± 4.4 | 33.9 ± 3.4 | 32.7 ± 4.6 |
| AMH b (ng/mL) | 0.10 [0.02, 0.10] | 0.06 [0.02, 0.10] | 0.10 [0.10, 0.13] | 0.10 [0.06, 0.12] |
| FSH b (mIU/mL) at first visit | 72.9 [44.2, 102.6] | 74.4 [44.6, 108] | 71.4 [48.1, 90.5] | 93.0 [58.4, 97.5] |
| Period from LSM to HRT b (d) | 546 [290, 1323] | 1209 [297, 1427] | 361 [306, 701] | 344 [253, 562] |
| Follow‐up period during HRT (d) | 644 [318, 855] | 804 [487, 1143] | 643 [300, 719] | 671 [484, 755] |
Abbreviations: AMH, anti‐Mullerian hormone; FSH, follicle‐stimulating hormone; HRT, hormone replacement therapy; LSM, the last spontaneous menstruation.
Mean ± SD.
Median [interquartile interval].
There were 9 (45%) women in the non‐follicular development group and 11 (55%) in the follicular development group. There was no significant difference in the background characteristics between the two groups (Table 2). Although there was no significant difference, the period from the LSM to the initiation of HRT tended to be longer in the non‐follicle developing group (1209 [297, 1427] days) than in the follicular development group (361 [306, 700] days; P = .261). The maximum duration of amenorrhea in patients with follicular development was 3593 days. After 9 years of amenorrhea following chemotherapy, follicular development was observed on day 425 after starting observation during HRT.
The follow‐up period during HRT tended to be longer than 1 year in both groups, with 804 [487, 1143] days in the non‐follicular development group and 643 [300, 719] days in the follicular development group (P = .261).
3.2. Non‐follicle development period and subsequent follicular development
Cumulative follicular development rate was shown by totaling the non‐follicle development period during follow‐up (Figure 2). The longer the period during which no follicular development was observed during the follow‐up, the lower the probability that follicular development could be detected thereafter. At 1‐year follow‐up, the cumulative follicular development rate was 70%, and follicular development was observed with a probability of 49.1% at 3 months, 33.4% at 6 months, and 8.3% at 12 months in the follow‐up period. The longest period in which follicular development was observed was 425 days.
FIGURE 2.
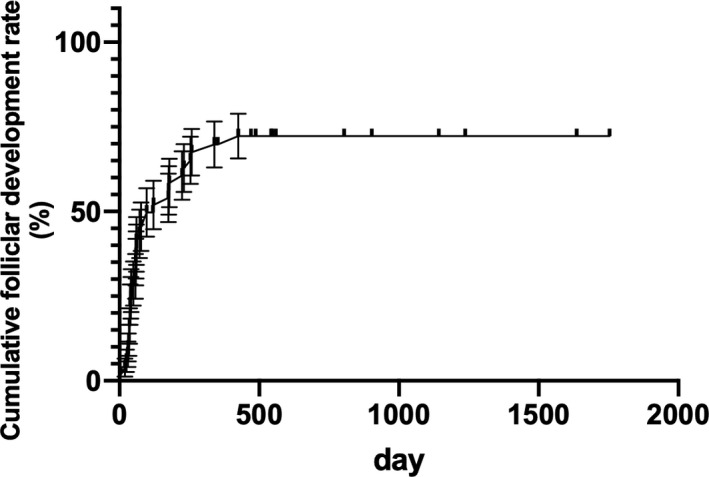
Cumulative follicular development rate during follow‐up during HRT. At 1‐y follow‐up, the cumulative follicular development rate was 70%; follicular development was observed with a probability of 49.1% at 3 mo, 33.4% at 6 mo, and 8.3% at 12 mo in the follow‐up period. The longest period during which follicular development was observed was 425 d
The results of all the 31 follicular development cycles included 5 follicular atresias (16%), 8 TIs (26%), 5 AIHs (16%), and 13 IVF cycles (42%). Three patients became pregnant who did IVF cycles and the pregnancy rates per cycle were 42.9% (the pregnancy rate was calculated per egg acquisition).
3.3. Comparison of FSH levels during menstruation of each cycle in the follicle development group
In the follicle development group, FSH levels during menstruation of each cycle were compared between a cycle in which follicle development was observed and a cycle in which follicle development was not observed. The FSH levels during menstruation of the follicle development cycle (27 cycles) and the non‐follicle development cycle (110 cycles) were 12.7 ± 12.5 mIU/mL and 13.5 ± 11.4 mIU/mL, respectively (P = .739), and no significant difference was observed. Six cycles were not available.
3.4. Pregnant cases
Table 2 shows the background characteristics of the pregnant women. The mean age was 32.7 ± 4.6 years, the FSH level was 93 [58.4, 97.5] mIU/mL, the AMH level was 0.10 [0.06, 0.12] ng/mL, the period from the LSM to the initiation of HRT was 344 [253, 562] days, and the duration of follow‐up period was 671 [484, 755] days. The number of follicular developments was 3 [3, 6.5] in the successful pregnancy and 1.5 [1, 3.3] in the non‐pregnant case, and although there was no significant difference, there was a tendency that the number of developments was high in the pregnant cases.
4. DISCUSSION
A previous observational study had reported about the aggregated ovarian function resumption rate in patients with POI undergoing HRT. 18 However, that study did not investigate when ovarian function recovered. Furthermore, in previous reports, clinicians were not aware of how long the monitoring should be continued during HRT. This study provided information on the follow‐up period and follicular development in patients with POI during HRT.
The present study revealed the following results:
At 1‐year follow‐up, the cumulative follicular development rate was 70%.
The longer the period during which no follicular development was observed during the follow‐up; the lower the probability that follicular development could be detected.
Follicular development was observed with a probability of 49.1% at 3 months, 33.4% at 6 months, and 8.3% at 12 months in the follow‐up period.
The results of this study can be used to counsel the patients, and it is important to decide whether to continue infertility treatment by referring to the probability of follicle development at that time.
In the group with follicular development, there was no significant difference, but the period from the LSM to the start of HRT tended to be short. In long‐term follow‐up of 358 patients with POI, Bidet et al 19 reported that 88% cases with follicular development occurred within the first year and their pregnancy rate was 4.4%. The present study showed a similar trend in terms of the time to follicle development. However, because some patients did not undergo HRT, a direct comparison was not possible. In this study, regular follow‐up of follicle development may have increased the detection rate of follicle development and the rate of pregnancy.
From the viewpoint that POI is disturbed by the number of eggs, not the quality of the eggs, 16 when follicular development can be detected by frequent visits, 14 pregnancy rate may be increased. This study shows the same pregnancy rate as previous reports. 15
Because there is a continuous course from diminished ovarian reserve to the complete depletion of primordial follicles, in patients in whom follicular development does not occur despite the long‐term follow‐up, the remaining follicles might have already disappeared when HRT was initiated.
As shown in this study, POI patients undergoing HRT are less likely to have subsequent follicular development the longer they have the absence of follicular development. However, the follow‐up period tends to be long (Table 2) and the burden on patients increases. At some point, it is necessary to provide information such as recommending the completion of fertility treatment.
Bidet et al 19 reported that AMH was not a predictor of follicle development. Although the detection rate increased when AMH was high, follicle development was observed in six cases (67%) with AMH below the detection sensitivity, and it was considered reasonable to provide regular follow‐up under HRT.
Tartagni et al 17 speculated that a menstrual FSH level of <15 mIU/mL could be a predictor of follicle development in patients with POI during HRT. In the present study, 9 (82%) cases with follicle development had an FSH level of <15 mIU/mL during HRT. However, during examination for each menstrual cycle, follicle development was not observed in 74 (78%) cycles with an FSH level of <15 mIU/mL, and there was no significant difference between the menstrual FSH level in cycles with and without follicle development. One of the reasons why there was no significant difference in both groups was the continuous use of estrogen agents during the period of withdrawal bleeding. Further studies are required to determine whether FSH can be considered as a predictor of follicle development.
In Japan, as in other developed countries, the number of patients who become infertile due to late marriage and delayed childbearing is increasing, 20 and the average age of patients undergoing IVF is 39 years (Japan Society of Obstetrics and Gynecology ART online registration in 2017). With the aging of infertile patients, the opportunity to treat patients with DOR is increasing. Because many Japanese people place importance on blood relations and facilities that can provide eggs are limited, many patients desire to conceive with their gametes even when they are aged >40 years. Oocyte donation is believed to be the only effective treatment for patients with POI, 1 but in countries where such choices are difficult, long‐term follow‐up with HRT is the only option to be pregnant. We expect to accumulate patients and elucidate the characteristics of those with follicular development and pregnancy.
4.1. Limitations
The results cannot be generalized because the study targets only patients who desire to raise their children. In addition, there is a selection bias in terms of pregnancy rate because the study targeted only patients who desired to raise children. In our hospital, chromosome testing and screening for autoimmune diseases are not performed universally for patients with POI. Only two patients underwent chromosome testing, both of whom showed a normal karyotype. Because this study was a small population research targeting Japanese people, it is necessary to examine whether other races show similar results. In observational studies, patients who continued treatment beyond the study period were also included. If the research period was further extended, several follicular development cycles may be recognized. Future follow‐up studies are required to confirm the reported findings.
DISCLOSURES
Conflict of interest: Takuma Sato, Atsuko Kusuhara, Yuta Kasahara, Takayuki Haino, and Hiroshi Kishi declare that they have no conflict of interest. Aikou Okamoto has received a speaker Honorarium from Astra Zeneca K.K. Human rights statements and informed consent: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments. This study was approved by IRB in The Jikei University School of Medicine and the need for informed consent was waived. We provided the patients in this research with the opportunity to opt out. Approval by Ethics Committee: This study was approved by the institutional review board of Jikei University School of Medicine (IRB number 30‐160‐9181).
ACKNOWLEDGMENT
We would like to thank Enago (www.enago.jp) for providing language help.
Sato T, Kusuhara A, Kasahara Y, Haino T, Kishi H, Okamoto A. Follicular development during hormone replacement therapy in patients with premature ovarian insufficiency. Reprod Med Biol. 2021;20:234–240. 10.1002/rmb2.12375
REFERENCES
- 1. European Society for Human R, Embryology Guideline Group on POI , Webber L, Davies M, et al. ESHRE guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31:926‐937. [DOI] [PubMed] [Google Scholar]
- 2. Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67:604‐606. [PubMed] [Google Scholar]
- 3. Atsma F, Bartelink ML, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta‐analysis. Menopause. 2006;13:265‐279. [DOI] [PubMed] [Google Scholar]
- 4. Jacobsen BK, Heuch I, Kvale G. Age at natural menopause and stroke mortality: cohort study with 3561 stroke deaths during 37‐year follow‐up. Stroke. 2004;35:1548‐1551. [DOI] [PubMed] [Google Scholar]
- 5. van der Schouw YT, van der Graaf Y, Steyerberg EW, Eijkemans JC, Banga JD. Age at menopause as a risk factor for cardiovascular mortality. Lancet. 1996;347:714‐718. [DOI] [PubMed] [Google Scholar]
- 6. van Der Voort DJ, van Der Weijer PH, Barentsen R. Early menopause: increased fracture risk at older age. Osteoporos Int. 2003;14:525‐530. [DOI] [PubMed] [Google Scholar]
- 7. McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279‐307. [DOI] [PubMed] [Google Scholar]
- 8. Sujarwoto S, Tampubolon G. Premature natural menopause and cognitive function among older women in Indonesia. J Women Aging. 2019;32:1‐15. [DOI] [PubMed] [Google Scholar]
- 9. Pitkin J, Rees MC, Gray S, et al. Management of premature menopause. Menopause Int. 2007;13:44‐45. [DOI] [PubMed] [Google Scholar]
- 10. Vujovic S, Brincat M, Erel T, et al. EMAS position statement: managing women with premature ovarian failure. Maturitas. 2010;67:91‐93. [DOI] [PubMed] [Google Scholar]
- 11. Faddy MJ, Gosden RG. A model conforming the decline in follicle numbers to the age of menopause in women. Hum Reprod. 1996;11:1484‐1486. [DOI] [PubMed] [Google Scholar]
- 12. Faddy MJ. Follicle dynamics during ovarian ageing. Mol Cell Endocrinol. 2000;163:43‐48. [DOI] [PubMed] [Google Scholar]
- 13. Sowers MR, Eyvazzadeh AD, McConnell D, et al. Anti‐mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab. 2008;93:3478‐3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nelson LM, Covington SN, Rebar RW. An update: spontaneous premature ovarian failure is not an early menopause. Fertil Steril. 2005;83:1327‐1332. [DOI] [PubMed] [Google Scholar]
- 15. Fraison E, Crawford G, Casper G, Harris V, Ledger W. Pregnancy following diagnosis of premature ovarian insufficiency: a systematic review. Reprod Biomed Online. 2019;39:467‐476. [DOI] [PubMed] [Google Scholar]
- 16. Crawford NM, Steiner AZ. Age‐related infertility. Obstet Gynecol Clin North Am. 2015;42:15‐25. [DOI] [PubMed] [Google Scholar]
- 17. Tartagni M, Cicinelli E, De Pergola G, Antonietta M, Lavopa C, Loverro G. Effects of pretreatment with estrogens on ovarian stimulation with gonadotropins in women with premature ovarian failure: a randomized, placebo‐controlled trial. Fertil Steril. 2007;87(4):858‐861. [DOI] [PubMed] [Google Scholar]
- 18. Bachelot A, Nicolas C, Bidet M, et al. Long‐term outcome of ovarian function in women with intermittent premature ovarian insufficiency. Clin Endocrinol (Oxf). 2017;86:223‐228. [DOI] [PubMed] [Google Scholar]
- 19. Bidet M, Bachelot A, Bissauge E, et al. Resumption of ovarian function and pregnancies in 358 patients with premature ovarian failure. J Clin Endocrinol Metab. 2011;96(12):3864‐3872. [DOI] [PubMed] [Google Scholar]
- 20. Evers JL. Female subfertility. Lancet. 2002;360:151‐159. [DOI] [PubMed] [Google Scholar]


