Abstract
Background
The decision of whether frozen embryo transfer (FET) should be performed in the cycle immediately after OPU or at least one cycle later is controversial. FET could improve pregnancy rates in IVF; however, how much time is needed for the endometrium to return to optimal receptivity after ovarian stimulation is not known.
Methods
Electronic search in MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials to identify studies providing data on the influence of the interval between embryo freezing (or OPU) and FET in FET cycles published between January 1, 2007, and February 1, 2020.
Main findings
Data analyzed indicated that in the immediate FET cycles, there was a trend to an increased biochemical pregnancy rate (RR = 1.08; CI = 1.00‐1.18), whereas the clinical pregnancy rate was somewhat higher, but without reaching statistical significance (RR = 1.07; CI = 0.99‐1.15). The live birth rate was similar in the two groups (RR = 1.05; CI = 0.95‐1.15), as was the implantation rate (RR = 0.98; CI = 0.83‐1.16). Stratifying by embryo stage or FET type (freeze‐all or FET after failed fresh transfer) showed no differences.
Conclusion
Systematically delaying FET does not offer benefits to IVF outcomes. In addition, immediate transfer is associated with a nonsignificant trend to better clinical pregnancy rate and it also avoids the psychological effects of prolonging the stress on prospective parents.
Keywords: delayed transfer, frozen embryo transfer, immediate transfer, IVF, pregnancy rates
Short abstract
These meta‐analysis showed that systematically delaying FET does not offer benefits to IVF outcomes. In addition, immediate transfer is associated with a non‐significant trend to better clinical PR and it also avoids the psychological effects of prolonging the stress on prospective parents.
1. INTRODUCTION
Since embryo freezing (EF) is performed by vitrification, 1 frozen embryo transfer (FET) is increasingly common. 2 Its efficacy and safety with surplus embryo are well known. 2 Some authors have recommended the “freeze‐all” strategy for all patients, 3 , 4 while others have suggested using this strategy in selected groups of patients: women with preimplantation genetic diagnosis cycles 5 ; or women with high ovarian response, 6 polycystic ovarian syndrome, 7 , 8 high progesterone levels, 9 and conditions appearing during stimulation such as polyps, 10 thin endometrium, 11 or hydrosalpinges. 12
Ovarian stimulation has been associated with a number of detrimental changes in the endometrium, impairing implantation. Some of these adverse effects are related to a hyperestrogenic state, 13 the production of reactive oxygen species, 14 immunological changes in endometrial natural killer cells and cytokines, 15 and advanced endometrial maturation. 16 The aforementioned adverse effects are one of the main reasons for “freeze‐all” programs. Nonetheless, it has not been studied whether these detrimental changes persist in subsequent cycles. In particular, there are no prospective randomized trials assessing the influence of EF‐embryo transfer (ET) interval on pregnancy rates (PRs). In this context, the ideal interval between embryo freezing and ET has not been established. In a Web‐based study, reporting data from 179 ART centers in 58 countries, the majority of responders (61%) preferred a washout period between fresh and FET cycles. 17
In retrospective studies, some authors have found higher PRs when ET is performed in the immediately following menstrual cycle. 18 , 19 , 20 , 21 On the other hand, there have also been reports of better results when ET is delayed ≥ 1 menstrual cycle 22 or better clinical results in delayed FET, though the difference did not reach significance, 23 while others have reported no differences. 9 , 12 , 24 , 25 , 26 , 27 Further, the differences in some of the reports that were reported in favor of immediate FET disappeared when controlling for associated variables. 20
The aim of our study was to perform a systematic review of the literature and meta‐analysis of results found concerning the influence of EF‐FET interval on IVF pregnancy outcomes.
2. MATERIALS AND METHODS
The literature search was performed in MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials, with the following key words: "Time"+"interval"+"cycle"+"ivf" or "immediate"+"embryo"+ "transfer" or "immediate"+"vs"+"delayed"+"ivf" or "optimal"+"time"+"between"+"ivf" or "delay"+"ivf"+"cycle" or “interval” +” transfer”+ “IVF.” The search was restricted to articles published between January 1, 2007, and February 1, 2020, written in English or Spanish. In addition, reference lists of the publications included were screened. Our search was submitted to the PROSPERO database (CRD42020204072). The meta‐analysis was performed following the PRISMA methodology. 28
The inclusion criteria were as follows: (1) cycles of ovarian stimulation for an IVF/ICSI in which at least one embryo was cryopreserved; (2) performance of FET in a cycle other than the cycle in which oocyte pick‐up (OPU) was performed; and (3) collection of data on IVF outcomes allowing comparison between at least two EF‐FET interval groups. Thus, two types of patients were included: (a) patients receiving fresh ET in the OPU cycle who did not become pregnant and received FET in a subsequent cycle, and (b) patients undergoing “freeze‐all” protocols.
The exclusion criteria were as follows: (1) fresh transfers in the second ET; (2) non‐human studies; (3) failure to report data on PRs; and (4) lack of data to allow comparisons by EF‐ET interval.
2.1. Data extraction
The following data were extracted from every article: author, publication year, country where the study took place, number of cycles included in the study, mean age of the women, number of oocytes retrieved, IVF management, endometrial preparation and luteal phase support, exclusion criteria, EF‐FET interval(s), number of cryopreserved embryos transferred, frozen embryo outcomes (implantation rate, biochemical pregnancy, clinical pregnancy, live birth rate), and the study conclusions. In one case, the authors were contacted to obtain additional information. 24 The search was conducted by the same investigator (SB).
2.2. Study quality assessment
Two reviewers (RM and IP) used the Newcastle‐Ottawa Scale (NOS) for observational studies to assess the quality of the manuscripts included. 29 With this scale, each category is rated by awarding a maximum of one star, except “comparability by design or analysis” for which two stars may be given. A maximum of nine points is assigned indicating the least risk of bias (Table S1).
2.3. Statistical analysis
For the statistical analysis, the following were calculated: relative risk (RR) and the corresponding 95% confidence interval (CI), and the p‐value for each parameter under study. For each parameter, a "forest plot" was used to present the RR and CI for each of the studies. The threshold for statistical significance was set at P < .05. The heterogeneity of the results was assessed with the χ 2 and I 2 tests.
Statistical analysis was performed with Stata 15.1
3. RESULTS
The flow of studies through the review process is shown in a PRISMA diagram 28 presented in Figure 1. The search strategy yielded 520 records (516 after removal of duplicates), of which 499 were excluded after reading of the title and/or abstract, indicating that they did not fulfill the selection criteria. Finally, 17 studies were screened and 12 were included in the meta‐analysis. The characteristics of studies excluded 30 , 31 , 32 , 33 , 34 are listed in Table S2. All the studies included scored well on the NOS, achieving a score of between 7 and 9 (Table S1).
FIGURE 1.
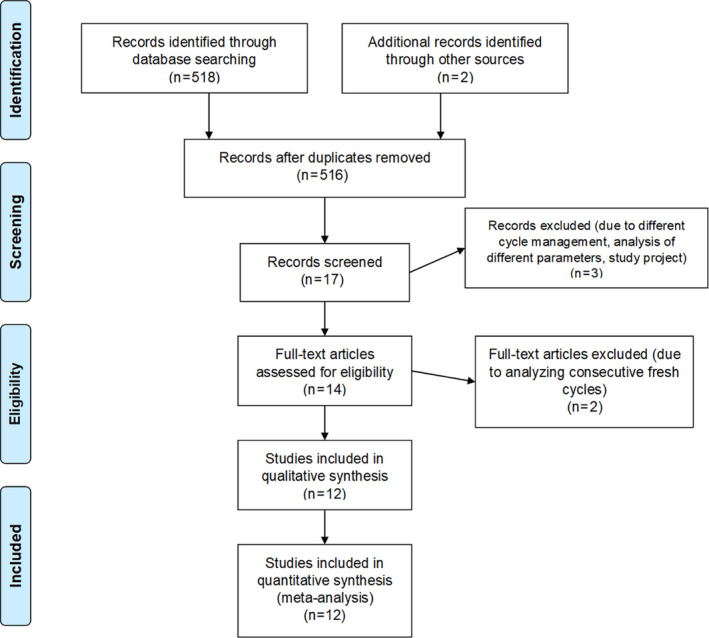
PRISMA flowchart
3.1. Study characteristics
3.1.1. Origin
Our search identified 12 studies, 11 of them corresponding to full‐length articles and 1 to an abstract. 21 As we can see in Table 1, the 12 studies included a total of 18 127 FET cycles (5457 immediate and 12 570 delayed) carried out in women from centers in 6 different countries (Belgium, Spain, Australia, China, Israel, USA, and Turkey). Ten of the publications reported single‐center studies, 9 , 27 while one was based on a multisite IVF clinic 18 and one 12 included two centers.
TABLE 1.
Characteristics of the studies included
| Author | Year, country (region) | Study | Number of cycles (immediate + delayed transfers) | Age (y) | OPU cycle management | Endometrial preparation/ luteal phase support | Day of embryo development | Inclusion criteria | Exclusion criteria |
|---|---|---|---|---|---|---|---|---|---|
| He et al | 2020 (2014‐2017), China (Guangzhou) | RC | 3110 GnRH agonist protocol (1585 + 1525) | IT:31.74 ± 4.33 DT: 31.98 ± 4.23 |
Long GnRH agonist protocol (triptorelin [Diphereline]) Recombinant FSH (Gonal‐F or Puregon) Urinary HCG (uHCG) or recombinant HCG (Ovitrelle) |
(a) Natural cycle (b) Daily oral estradiol valerate tablets and 40 mg/d of intramuscular progesterone |
D3 or D5 |
Age: 20‐40 y Normal menstrual cycle. Basal FSH < 12 mIU/mL First FET cycle after whole embryo freezing using vitrification method Analog protocol with GnRh agonists or antagonists |
Donated oocytes PGD cycles Polycystic ovarian syndrome or ovulatory disorders Uterine anomalies Hydrosalpinx Uncontrolled endocrine or immune disorders or other systemic diseases |
|
1294 GnRH antagonist protocol (778 + 516) |
IT:31.37 ± 4.03 DT: 31.53 ± 4.23 |
GnRH antagonist protocol (cetrorelix [Cetrotide]) Recombinant FSH (Gonal‐F or Puregon) Urinary HCG (uHCG) or recombinant HCG (Ovitrelle) |
|||||||
| Higgings et al | 2018 (2000‐2014), Australia (Victoria) | RC |
4994 (635 + 4359) |
IT: 36.0 (26.5‐45.9) DT: 35.5(26.2‐43.9) (P = .001) |
Three GnRH analog protocols were used: (a) Short GnRH agonist (Synarel) protocol (b) GnRH agonist starting in the midluteal phase of the previous cycle (c) GnRH antagonist (Cetrotide, Orgalutran) on day 5 or when the leading follicle was ≥ 14 mm In all cases, ovarian stimulation was performed with rec FSH (400‐600 UI/d) (Gonal, Puregon). In all cases, the beginning of the cycle could be spontaneous or after the intake of contraceptive pills (30 μg ethinyl estradiol and 150 μg levonorgestrel) Triggering with HCG (Ovidrel or Pregnyl) |
(a) Natural cycle (b) Estradiol valerate 6 mg/ day and vaginal progesterone pessaries (400‐800 mg/d) or 8% progesterone gel |
NR | FET cycles with a prior fresh cycle (“fresh cycle‐ FET” interval = 25‐35 d or 50‐70 d) |
Cases from before 2000 or after 2014 FET preceded by another FET Cycles with other gaps |
| Horowitz et al |
2019 (January 2009‐December 2016), Israel (Tel Aviv) |
RC | 198 (118 + 80) | IT: 32.8 ± 5.0 DT: 34.1 ± 5.6 |
GnRH antagonist or agonist protocols. Triggering with rec HCG |
Modified natural cycle Ovulation triggering with hCG Vaginal progesterone: 400 mg Utrogestan/24 h or 200 mg Endometrin (Ferring) or a single daily application of Crinone (Merck) |
Embryos/blastocysts |
Age: 18‐45 y Regular ovulatory cycles First FET cycles after failed fresh embryo transfer |
Cycles involving donors PGD cycles Freeze‐all protocols. |
| Huang et al |
2019 (01/2013‐12/2016), China (Shanghai) |
RC | 2998 (280 + 2718) |
IT: 30.6 ± 4.3 DT: 30.9 ± 4.2 |
(a) GnRH agonist short protocol: 0.1 triptorelin (Decapeptyl; Ferring) Ovarian stimulation with 150‐225 hMG ( hMG; Anhui Fengyuan) Triggering with 5000‐10 000 IU of urinary hCG ( hCG; Lizhu Pharmaceutical Trading) (b) Progestin‐primed ovarian stimulation (10 mg medroxyprogesterone acetate (Shanghai Xinyi Pharmaceutical) Ovarian stimulation with hMG (150‐225 hMG . Triggering with (a) single use of 5000‐1000 IU of urinary hCG, (b) single dose of triptorelin (0.1‐0.2 mg) or (c) dual trigger with 1,000 IU of urinary hCG and 0.1 mg of triptorelin. |
(a) Modified natural cycles were recommended ( in case of regular menstrual cycles) Ovulation was triggered with 5,000 IU of urinary hCG. Luteal phase was supplemented with 40 mg/d of dydrogesterone (Duphaston; Abbott) (b) In irregular cycles 8 mg/d of oral micronized estradiol (Fematon, Abbott) Luteal phase was supplemented with 40 mg/d of oral dydrogesterone and 400 mg/d of vaginal progesterone (Utrogestan) |
Day 3‐4 cleavage stage embryos or day 5‐6 blastocysts |
Infertile women who underwent their first FET after the first IVF/ICSI cycle using the freeze‐all policy Day 3 or day 5 transfer |
Abnormal parental karyotyping Unilateral oophorectomy Recurrent miscarriage Uterine anomalies (congenital or acquired) Donor sperm or testicular/epididymal sperm. Moderate or severe ovarian OHSS during ovarian stimulation cycle Embryo cryopreservation > 120 d Core information missing in medical records Donated oocytes In vitro maturation oocytes PGD |
| Kaye et al | 2018 (2013‐2016), USA | RC | 344 (80 + 264) |
IT: 33.6 ± 3.8 DT: 33.52 ± 3.7 |
GnRH antagonist or agonist (leuprolide) + rFSH and/or, hMG (Gonal‐F, Follistim, Menopur) + hCG (Pregnyl or Novarel) or GnRH agonist or both |
Natural cycle and vaginal progesterone (Crinone; Merck; or Endometrin; Ferring) or GnRH agonist downregulation + oral and/or transdermal estradiol + intramuscular progesterone |
Blastocyst |
Age: 18‐40 y (a) FET after a previous ovarian stimulation cycle with one failed fresh ET (b) Freeze‐all protocol (PGD, ovarian hyperresponse/ OHSS risk, progesterone rise, planned surgery, pregnancy contraindication, lack of suitable D5 with blastocyst cryopreservation on day 6, patient preference) |
Patients without a stimulation cycle before FET (donated oocytes). Patients in whom embryo freezing was performed 120 or more days before the FET Endometrial biopsy or endometrial scratching in the cycle prior to ET |
| Lattes et al |
2017 (1/2012‐12/2014), Spain (Barcelona) |
RC |
512 (263 + 249) |
IT: 34.7 ± 4.13 DT: 35.3 ± 3.98 (P = .067) | GnRH antagonist or GnR agonist protocol, exogenous gonadotropins and GnRH agonist (triptorelin) or rhCG (Ovitrelle) |
Estradiol valerate (6 mg) or transdermal estradiol hemihydrate (150 mg/d) Vaginal micronized progesterone (600 mg/d) |
Day 3/4 embryos | Freeze‐all cycles |
BMI > 30 kg/m2 Endocrine pathologies Uterine abnormalities Chronic, autoimmune, or metabolic diseases Testicular sperm extraction Meiotic chromosomal abnormalities in testicular biopsy or altered sperm FISH Participation within the previous 6 mo, in a clinical trial with medication |
| Mass et al |
2008 (2003‐2007), USA (San Francisco) |
RC |
271 (105 + 166) |
IT: 36.3 ± 5.5 DT: 36.6 ± 5.6 |
NR | NR | Day 5‐6 blastocysts | First FET cycles after unsuccessful fresh ET | |
| Ozgur et al |
2017 (February 2015‐January 2016), Turkey (Antalya) |
RC |
1121 (756 + 365) |
IT:31.5 DT: 31.6 |
GnRH Antagonist (Cetrotide) + rFSH (Gonal‐F) + hMG (Menopur) + hCG/GnRH agonist (Gonapeptyl) or both | GnRH agonist (Lucrin Depot) or Contraceptive pill (Ginera) + oral estradiol in step‐up regime (2‐4‐8 mg) + vaginal progesterone gel (Crinone) | Blastocysts | “Freeze‐all" program |
>42 y Hysteroscopy between follicular aspiration and FET PGD |
| Santos ‐Ribeiro et al (1) |
2016 (January 2010‐November 2014), Belgium (Brussels) |
RC |
1183 (197 + 986) |
IT: 32.4 ± 4.4 DT: 32.5 ± 4.3 (P = .69) | GnRH antagonist (Cetrotide or Ganirelix) + rFSH (Gonal‐F, Puregon, Elonva) + HMG (Menopur) + HCG |
Estradiol valerate (2 mg) Vaginal micronized progesterone |
Day 4 embryos and day 5/6 blastocysts |
GnRH antagonist for downregulation hCG alone for triggering At least one FET after a previous ovarian stimulation cycle with 1 failed fresh ET |
Donated oocytes In vitro maturation PGD Triggering with GnRh agonists (alone or in combination with hCG) hCG administration for reasons other than ovulation triggering FET cycles performed with downregulation with GnRH agonist or with exogenous concomitant ovarian stimulation |
| Santos ‐Ribeiro et al (2) |
2016 (October 2010‐October 2015), Belgium (Brussels), Vietnam (Ho Chi Minh) |
RC |
333 (208 + 125) |
IT: 30.9 ± 4.1 DT: 31.8 ± 4.2 (P = .045, OR 0.97) | GnRH Antagonist (Cetrorelix o Ganirelix). rFSH (Gonal‐F, Puregon, Elonva) + HMG (Menopur )+ GnRH agonist (Triptorelin) |
Estradiol valerate (2 mg) Vaginal micronized progesterone |
D3 and day 5/6 blastocysts | First FET after a freeze‐all protocol. |
Donated oocytes In vitro maturation Blastocyst biopsy for preimplantation genetic diagnosis Previous cycle with fresh ET canceled due to thin endometrium |
| Song et al |
2019 (January 2016‐September 2018), China (Jinan) |
RC | 1540 (385 + 1155) | IT: 31.38 ± 5.19 DT: 30.99 ± 4.45 (P = .19) |
Ultra‐short, short, long and modified ultra‐long GnRH agonist protocol, GnRH antagonist protocol, mini‐stimulation protocol Recombinant FSH (Gonal, Merck or Puregon; MSD (150‐450 IU/d) and urinary hMG (hMG, Livzon) Triggering with GnRH agonist (0.2 mg triptorelin; Decapeptyl) or 250 µg rhCG (Ovitrelle; Merck) |
(a) Artificial cycle (61.7%) Estradiol valerate (2 mg/ twice daily for at least 14‐16 d, adjusting the dose afterward Injectable progesterone (20 mg/d) (b) Natural cycle (29.5% (c) Stimulation cycle (8.8%) |
D3 |
Age < 45 y Stimulation cycle completed with a freeze‐all protocol. D3 cleavage stage embryo transferred |
Patients not undergoing a stimulation cycle prior to FET Donated oocytes Embryos derived from vitrified oocytes Preceding cycles with missing data |
| Volodarsky‐ Perel et al | 2017 (1/2010‐6/2015), Israel (Jerusalem) | RC | 129 (67 + 62) | IT : 29.9 ± 4.4 DT: 29.6 ± 4.2 | GnRH Agonist (triptorelin: 3.75mg im or 0.1 mg/d sc ) + rFSH (Gonal‐F) and HMG (Menogon) and hCG |
Estradiol valerate (4‐6mg/d) Vaginal micronized progesterone (200‐300 mg/d) or intramuscular progesterone |
D 3 and D5 |
First FET cycles after fresh ET with negative β‐hCG Age 20‐38 y FET of 1‐2 vitrified embryos ≤ 3 previous ET Artificial cycle for FET |
Severe OHSS PGD |
Abbreviations: D3, day 3 cleavage embryo; D5, day 5 blastocysts, DT, delayed transfer; ET, embryo transfer; IT, immediate transfer; FET, frozen embryo transfer, FISH, fluorescence in situ hybridization; hCG, human chorionic gonadotropin; hMG, human menopausal gonadotropin; OHSS, ovarian hyperstimulation syndrome; OPU, oocyte pick‐up; PGD, preimplantation genetic diagnosis; RC, retrospective cohort; rFSH, recombinant follicle stimulating hormone.
Two studies were performed by the same group, in different populations. 12 , 26 Regarding the size of the populations included, in two studies, >4000 cases were analyzed, 18 , 24 and in five, <500. 9 , 12 , 21 , 22 , 23 In one study, the interval comparison was made considering two different populations separately, with data provided for: women receiving GnRH agonists and those receiving GnRH antagonists. 24 Since no combined data were given, for the purpose of the meta‐analysis, this research was considered as two different studies: He et al, GnRH agonist protocol; and He et al, GnRH antagonist protocol. Most studies had data collection periods of 3‐5 years, and all can be considered relatively recent (having been published between 2016 and 2020), except that of Maas et al. 21
3.1.2. Inclusion and exclusion criteria
There were six studies including only freeze‐all patients, 19 , 20 , 24 , 25 , 26 , 27 four including only patients with FET after failure of a previous fresh ET 9 , 12 , 21 , 22 and two including both freeze‐all and previously failed fresh transfers 18 , 23 (Table S3).
Six studies reported an upper limit of age in the inclusion criteria: >38 years, 22 >40 years, 23 , 24 >42 years, 25 and >45 years. 9 , 27 Four reported a lower limit: <18 years 9 , 23 and <20 years. 22 , 24
Eight studies excluded preimplantation genetic diagnosis/preimplantation genetic screening cycles, 9 , 12 , 19 , 22 , 24 , 25 , 26 , 27 and five excluded donor oocyte cycles. 9 , 12 , 19 , 24 , 26 The other inclusion/exclusion criteria are listed in Table 1.
3.1.3. Intervals considered
In six studies, FET was considered immediate when performed within the first menstrual cycle after OPU, and delayed when performed after at least 2 menses. 12 , 19 , 20 , 22 , 23 , 27 Another author applied the same criteria but with the limit that in delayed ET, the transferred embryos were cryopreserved for less than 6 months 24 (Table S3).
Other authors established a cutoff of days for immediate and delayed transfer: start of the FET cycle of ≤22 or >22 days after OPU, 9 , 26 fresh ET‐FET interval of ≤50 and >50 days, 21 and EF‐FET interval of 25‐35 days (1 cycle) or 50‐70 days (2 cycles). 18 In another study, the patients were grouped by time interval between OPU and FET [32‐46, 47‐61, 62‐76, 77‐91, and ≥92 days). 25
3.1.4. OPU cycle management
For the study published as an abstract, no data at all were provided concerning cycle management (Table 1). 21 In other cases, regarding the management of the OPU cycle, for downregulation, one study used GnRH agonists in a long protocol, 22 three studies used daily GnRH antagonists, 12 , 25 , 26 one study used either GnRH agonists in a short protocol or priming with progestins, 19 four studies used either GnRH agonists in a long protocol or daily GnRH antagonists, 9 , 20 , 23 , 24 and one study GnRH agonists or antagonists, reporting the data separately for these two groups. 24 Further, in one study, three different protocols were used: boost GnRH agonists, GnRH agonists in a long protocol, or GnRH antagonists, 18 while in another, six different protocols were used: ultra‐short, short, long, and modified ultra‐long GnRH agonist protocols, a GnRH antagonist protocol, or a mini‐stimulation protocol. 27
Ovarian stimulation was performed with recombinant FSH and HMG in six studies. 9 , 12 , 22 , 23 , 25 , 26 , 27 In two studies, moreover long‐acting FSH was also employed. 12 , 26 One study only reported the use of “exogenous gonadotropins”, 20 while only recombinant FSH was used in two studies 18 , 24 and only HMG was employed in another. 19
Triggering was performed with recombinant hCG in three studies, 9 , 22 , 26 with either recombinant hCG or urinary hCG in two, 18 , 24 with GnRH antagonist in one, 12 and with hCG and/or GnRH antagonist in five. 19 , 20 , 23 , 25 , 27
3.1.5. FET cycle management
For the study published as an abstract, no data were given at all concerning the FET cycle management (Table 1). 21 Concerning the FET cycle preparation, GnRH agonist or contraceptive pill pretreatment was used in one study 25 and GnRH agonist downregulation was given to some women in another. 23 In the other publications, no mention was made of GnRH agonists or contraceptive pills for FET cycle preparation.
In four studies, some women underwent FET in a natural cycle 18 , 23 , 24 , 27 or in a modified natural cycle, 19 whereas in one study, all underwent transfer in a modified natural cycle. 9
In five studies, some patients received exogenous estrogens. The dose used was specified in three studies employing oral estradiol, 2 mg/d, 27 6 mg/d, 18 or 8 mg/d, 19 but not reported in two: one employing oral estradiol 24 and the other oral and/or transdermal estradiol. 23 In four other studies, all the women received exogenous estrogens. The doses used were as follows: 2 mg/d, 12 , 26 4‐6 mg, 22 6 mg/d, or 150 mg/d transdermal estradiol hemihydrate. 20
In another, all women received oral estradiol in a step‐up protocol (dose of 2‐4‐8 mg). 25 Lastly, in one report, a small proportion of patients underwent FET in a stimulated cycle.
Concerning luteal phase support, in three studies in which natural cycles were used, no mention was made of progesterone supplementation, 18 , 24 , 27 whereas in one study, vaginal progesterone was used (progesterone gel or micronized progesterone). 23 Regarding the two series of women undergoing modified natural cycle IVF, in one, ovulation was triggered with hCG and the luteal phase supported by micronized vaginal progesterone (200‐400 mg/24 h) or a single daily application of progesterone gel, and in the other, ovulation was triggered with 5000 IU of urinary hCG and the luteal phase supported by 40 mg/d of dydrogesterone. 19 In studies in which exogenous estrogens were used, progesterone supplementation was only given vaginally in five cases: with 600 mg/d of micronized vaginal progesterone in one case, 20 with vaginal micronized progesterone without stating the dose in two cases, 12 , 26 with vaginal progesterone gel in one case, 25 and with vaginal progesterone pessaries 400‐800 mg/d or 8% progesterone gel in one case. 18 In three cases, progesterone was administered intramuscularly: at the dose of 40 mg/d in one study 24 and 20 mg/d in another, 27 while the dose was not reported in the third. 23 In one case, either vaginal micronized progesterone (200‐300 mg/d) or intramuscular progesterone was used, 22 and in another, the oral and vaginal routes were combined, women receiving both 40 mg/d of oral dydrogesterone and 400 mg/d of micronized vaginal progesterone. 19 Regarding the few cases of FET in stimulated cycles, no specific mention was made to luteal phase support. 27
In the study published as an abstract, no mention was made of progesterone supplementation. 21
3.1.6. Embryo characteristics
FET involved only cleavage stage embryos in two studies, 20 , 27 only blastocysts in four, 9 , 21 , 23 , 25 and both cleavage stage embryos and blastocysts in five. 12 , 19 , 22 , 24 , 26 In one study, embryo development stage was not reported 18 (Table S3).
3.2. Homogeneity of study groups
3.2.1. Age
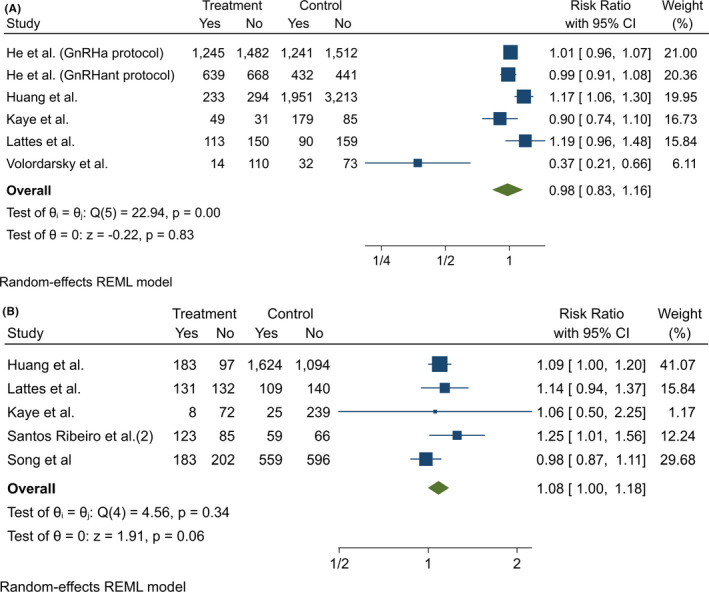
In one study, the age was significantly higher in the immediate transfer group, 18 while in another, the age was significantly higher in the delayed transfer group. 24 The resulting mean age was very similar in both groups (Figure 2A).
FIGURE 2.
Comparison of (A) maternal age, (B) oocytes retrieved, and (C) embryos transferred in the immediate and delayed FET. No significant differences were observed. REML, restricted maximum likelihood
3.2.2. Oocytes retrieved in the OPU cycle
The mean number of oocytes retrieved ranged from nearly 11 19 , 26 to nearly 22. 12 In all the studies, the mean number of oocytes retrieved was similar in the immediate and delayed transfer groups. The meta‐analysis showed no statistical differences (Figure 2B).
3.2.3. Transferred embryos
In three studies, the number of embryos transferred in the FET cycle was significantly higher in delayed transfers, 9 , 12 , 18 whereas in eight, there were no significant differences. 19 , 24 , 26 , 27 In one study, the mean number of embryos transferred was not reported, although it was stated that the frequency of single blastocyst transfer was similar in both groups. 25 The meta‐analysis showed no statistical differences (Figure 2C).
3.3. IVF outcomes
3.3.1. Implantation rate (IR)
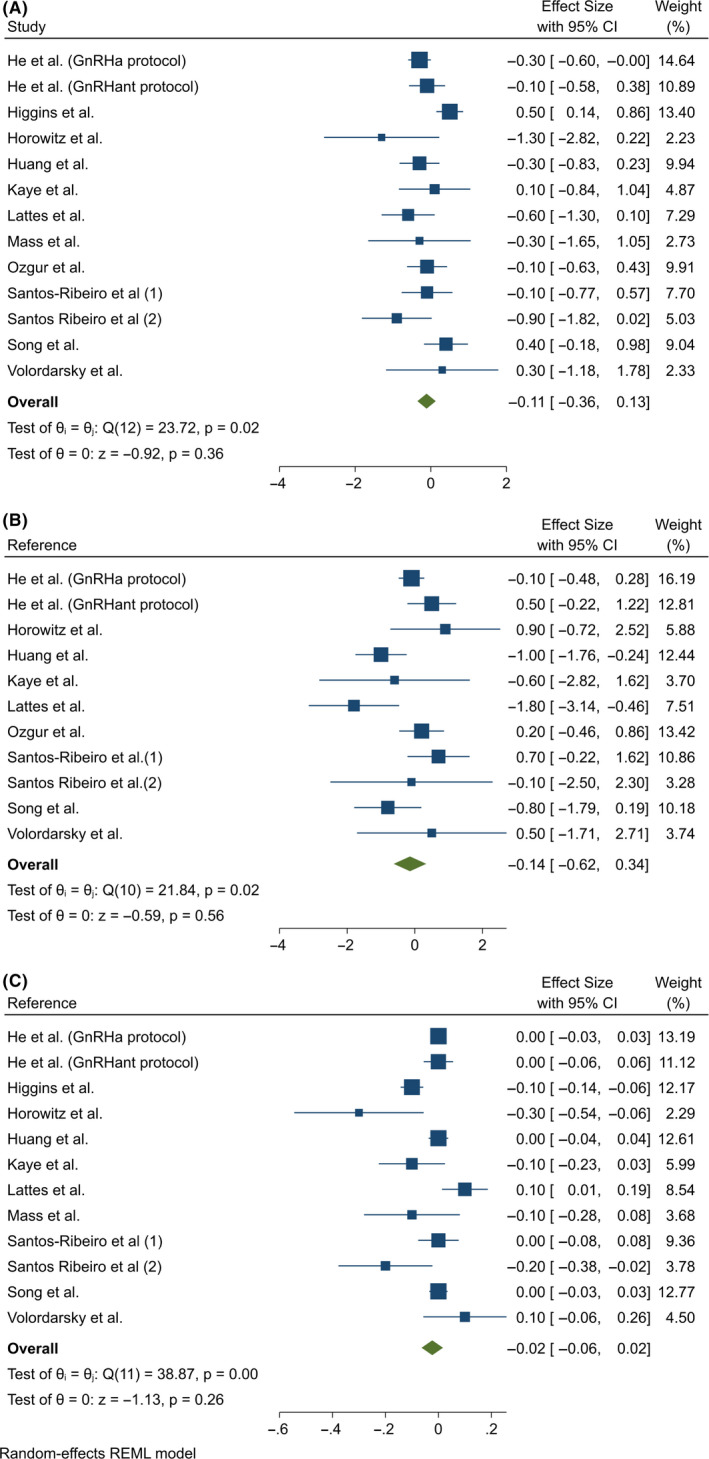
The IR was reported in five of the studies, 19 , 20 , 22 , 23 , 24 one of them 24 being the study that reported results in two populations separately: GnRH agonist and GnRH antagonist groups. In one case, the authors 24 were contacted to correct an inaccuracy in the data (Figure 3A).
FIGURE 3.
(A) Meta‐analysis of studies reporting implantation rates by FET interval. Meta‐analysis of the data from 6 included series (5 publications) that reported implantation rate as an outcome showed that there were no significant differences between immediate and delayed FETs. (B) Meta‐analysis of studies reporting biochemical pregnancy rates by FET interval. Meta‐analysis of the data from 5 publications that reported biochemical pregnancy rate as an outcome showed a RR of 1.08 (CI 1.00‐1.18) in favor of immediate FET. REML, restricted maximum likelihood
The IR was defined differently in three studies as follows: the number of gestational sacs visualized on transvaginal ultrasound divided by the number of embryos transferred, 19 the percentage of gestational sacs per total number of embryos transferred, 22 or the number of embryos transferred that develop to the stage of ultrasound documented gestational sac, divided by the number of transferred embryos, calculated for each woman. 20 In the other two studies, IR was not defined 23 , 24
In one study, the IR was significantly higher in the immediate group, 19 and in another, it was significantly higher in the delayed group. 22 There was statistically significant heterogeneity between the studies included (Cochran's q = 22.94, P < .001), I 2 = 90.7%. Notably, the study by Volordarsky‐Perel et al reported results quite divergent from those of the others. The meta‐analysis obtained a similar RR in the immediate and delayed groups (RR = 0.98 [0.83‐1.16], P = .826).
For subanalysis by embryo development stage (cleavage embryos; blastocysts; cleavage embryo or blastocysts), there was only more than one publication for the subgroup of studies including both cleavage embryos and blastocysts. In this meta‐analysis, no differences reached statistical significance (Figure S1).
Similarly, for subanalysis by FET indication (freeze‐all, FET after failed fresh transfer, both), there was only more than one publication in the case of freeze‐all cycles. Again, no differences reached significance (Figure S2).
3.3.2. Biochemical pregnancy rate
Of the 12 studies analyzed, 5 provided data on biochemical PR 12 , 19 , 20 , 23 , 27 (Figure 3B). In one study, biochemical pregnancy was defined as a serum β‐hCG level ≥ 5 IU/L at 14 days after FET, 19 while in the others, no definition was given. 20 , 23 , 26 , 27
In four studies, a somewhat higher biochemical PR was observed in the immediate group, 12 , 19 , 20 , 23 although the difference was only statistically significant in one. 12 On the other hand, the biochemical PR was somewhat higher in the delayed group in one study. 27
There was no significant heterogeneity in the data analyzed (Cochran's q = 4.56, P = .336), I 2 = 24.5%. The meta‐analysis showed a trend to higher biochemical PRs in the immediate group. The RR was 1.08 (CI = 1.00‐1.18), P = .056.
For subanalysis by embryo development stage, there were two studies concerning cleavage embryos and two concerning studies including both cleavage embryos and blastocysts. No significant differences were detected (Figure S3).
For subanalysis by FET indication, there was only more than one publication concerning freeze‐all cycles. This meta‐analysis showed no significant differences (Figure S4).
3.3.3. Clinical pregnancy rate
In 11 studies, data were provided on clinical PR, and one of them was the study in which data were presented separately for GnRH agonist and antagonist groups. 24 Thus, 12 populations were considered (Figure 4A).
FIGURE 4.
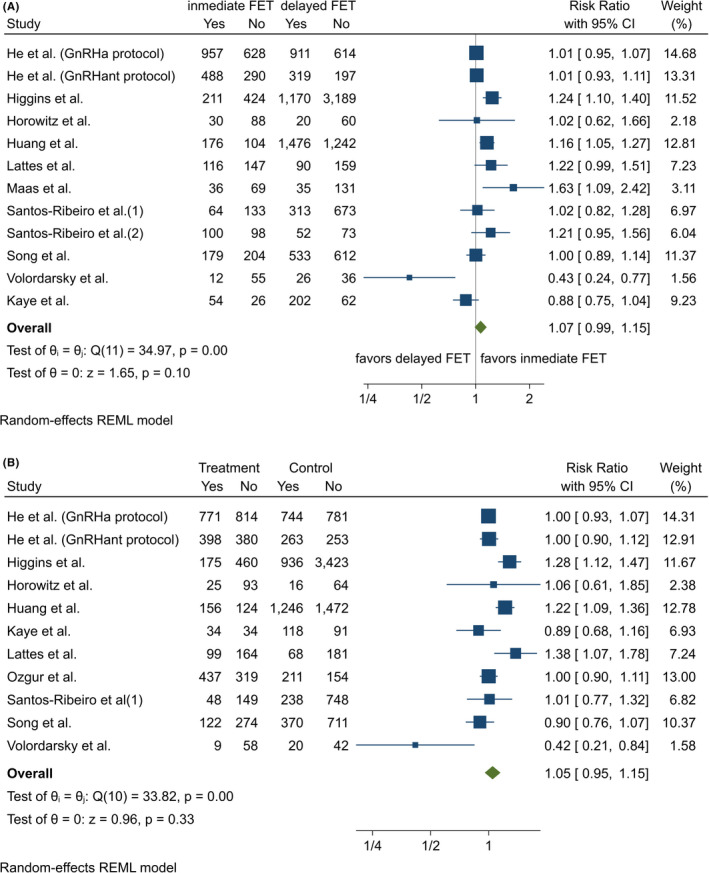
(A) Meta‐analysis of studies reporting clinical pregnancy rates by FET interval. Meta‐analysis of the data from 11 series (10 publications) that reported clinical pregnancy rate as an outcome showed that the clinical pregnancy rate was significantly increased in the immediate FET. (B) Meta‐analysis of studies reporting live birth rates by FET interval. Meta‐analysis of the data from 11 series (10 publications) that reported live birth rate as an outcome showed that there were no significant differences between immediate and delayed FETs. REML, restricted maximum likelihood
The definition of clinical pregnancy differed between the studies. Some authors defined it as the presence of gestational sac on ultrasound at 6‐8 weeks 24 or at 7 weeks of gestation 12 , 18 , 26 or as at least one gestational sac visualized within the uterus on transvaginal ultrasound at 8 weeks of gestation. 23 Similarly, in one other study, clinical pregnancy was defined as at least one gestational sac with or without fetal heart activity at 7 weeks of gestation. 19 On the other hand, other authors defined clinical pregnancy as an intrauterine gestational sac with embryonic cardiac activity at 7 weeks 20 or as an intrauterine gestational sac with fetal heart activity on ultrasound without specifying the gestational age. 9 , 22 Finally, in two reports, no definition was given 21 , 27
In three studies, significantly higher clinical PRs were reported in the immediate group 18 , 19 , 21 whereas in one, that of Volordasky‐Perel et al, a higher clinical PR was reported in the delayed group. In the other eight series, no significant differences were observed.
From a statistical point of view, there was very significant heterogeneity (Cochran's q = 34.97, P < .001), I 2 = 68.03%. Again, the aforementioned study, 22 although small in sample size, obtained results apparently inconsistent with the other studies.
The meta‐analysis showed a trend to somewhat higher clinical PRs in the immediate group, but without statistical significance. The RR was 1.07 (CI = 0.99‐1.15), P = .098.
In the subanalysis by embryo development stage (cleavage embryos; blastocysts; cleavage embryo or blastocysts), the meta‐analysis obtained similar RRs in the immediate and delayed FETs in all three categories considered, none of the differences reaching significance (Figure S5).
When subanalysis was performed by FET indication (freeze‐all, FET after failed fresh transfer, both), the meta‐analysis showed no significant differences between immediate and delayed rates in the freeze‐all or FET‐after‐failed‐fresh‐transfer subgroups (Figure S6). Further, as only one study considered both freeze all and FET after failed fresh transfer, no meta‐analysis could be performed.
3.3.4. Live birth rate
The live birth rate was reported in 10 studies, one of them presenting the data for GnRH agonist and antagonist groups separately (Figure 4B). 24 As for the other variables of interest, definitions of live birth varied between the studies. It was defined as the birth of a live infant at >20 weeks of gestation in one study, 18 at ≥20 weeks of gestation in two, 25 , 27 and at >24 weeks in two. 9 , 22 In contrast, in one other study, it was defined as the delivery of a viable infant at ≥24 weeks. 19 Other authors also set the cutoff at 24 weeks, but additionally considered unknown outcomes (patients lost to follow‐up) as negative. 26 Further, in one study, live birth was defined as the delivery of any viable neonate ≥28 weeks, and twins delivered by one mother were counted as one live birth, 24 while in another, live birth rate was established as the number of live birth deliveries per FET. 20 Finally, live birth rate was not defined in two series. 21 , 23
There were no statistically significant differences in the live birth rate between the immediate and delayed groups. The RR was 1.05 (CI = 0.95‐1.15), P = .335. There was statistically significant heterogeneity (Cochran's q = 33.8, P < .001), I 2 = 72.6%.
In the subanalysis by embryo development stage (cleavage embryos; blastocysts; cleavage embryo or blastocysts), the meta‐analysis obtained similar RRs in the immediate and delayed FETs in all three categories considered, none of the differences reaching significance (Figure S7).
When the subanalysis was performed by FET indication (freeze‐all, FET after failed fresh transfer, both), the RR of the live birth rate in the immediate vs delayed FETs did not differ between the subgroups (Figure S8).
4. DISCUSSION
Embryo freezing/thawing is a widely performed technique, either in selected patients such as those with hyperstimulation risk, 35 endometrial problems, 10 or elevated progesterone levels, 3 patients undergoing preimplantation genetic diagnosis cycles, 20 , 36 , 37 or in all patients as proposed by a number of authors. 38 , 39 , 40
One of the arguments in favor of frozen embryo cycles is that the endometrium would be more favorable to implantation, 41 , 42 , 43 , 44 , 45 since it has not been under the influence of high estrogen levels, especially in hyper‐responders. 20 , 45 Most of the differences in outcomes between fresh ET and FET seem to be due to the effect of ovarian stimulation on endometrial receptivity and embryo‐endometrium synchrony. 46 Nonetheless, it is unknown how much time is required for the expression of endometrial genes and the immunological environment to return to their prestimulation functionality. 20 On the other hand, excessively postponing ET could increase the stress and the anxiety that usually accompany IVF. 47 , 48 , 49 Moreover, although only to a small extent, postponing ET contributes to increase the time to pregnancy, a parameter that is increasingly receiving attention as an important marker of the quality of IVF. 50 , 51
There are a number of recommendations regarding the management of cycles with frozen/thawed embryos, regarding endometrial preparation, progesterone supplementation, cycle monitoring, and implantation window assessment. 52 , 53 , 54 , 55 , 56 Nonetheless, there is no agreement concerning the usefulness of delaying ET.
In our search, from the 520 publications initially retrieved, we found 12 fulfilling the inclusion criteria, these including a total of 17 948 FETs (5443 immediate and 12 505 delayed). Among them, there was a remarkable methodological heterogeneity: Six included only freeze‐all patients, 19 , 20 , 24 , 25 , 26 , 27 four only patients with FET after failure of a previous fresh ET, 9 , 12 , 21 , 22 and two both freeze‐all and previously failed fresh transfers. 18 , 23
It should be highlighted that we did not find any prospective randomized trials or prospective trials. Regarding their quality as assessed by the Newcastle‐Ottawa Scale, eight studies obtained the maximum score ‐9 and four scored ‐7. All of them were retrospective, and all of them were single‐center studies, except one that included two centers. 12 On the other hand, there were marked differences in cycle management, both in the OPU cycle and in the cryotransfer cycle. In cryotransfer cycles, the most commonly used treatment was estradiol valerianate and micronized progesterone, although the doses used varied. FET was considered immediate when performed during the first menstrual cycle following the cycle in which OPU or EF had been performed. On the other hand, transfer was considered delayed when the ET was performed during the second menstrual cycle after OPU/EF or later.
In seven of the studies reviewed, no differences were reported in any of the IFV outcome parameters. 9 , 12 , 23 , 24 , 25 , 26 , 27 At least one IVF outcome parameter was better in the delayed transfer group in just one study, 22 while at least one such parameter was better in the immediate transfer group in four studies. 18 , 19 , 20 , 21 In one of these four, however, the differences disappeared in multivariate analysis, 20 and in another, no multivariate analysis was performed. 21
Since the studies reviewed were not randomized, we performed a separate analysis of three of the most important prognostic factors in IVF, namely maternal age, 57 , 58 the number of oocytes retrieved, 59 and the number of embryos transferred. 60 , 61 All of them were very similar in the immediate and delayed transfer groups, and hence, these were ruled out as confounding factors.
Five studies reported the IR. Better results were obtained in immediate FET in one study 19 and in delayed FET in another, 22 while no differences were seen in the other three. 20 , 23 , 24 The meta‐analysis showed similar IRs in the immediate and delayed FET groups (RR = 0.98; CI = 0.83‐1.16).
Concerning biochemical PR, data were given also in five reports. 12 , 19 , 20 , 23 , 27 The meta‐analysis revealed that there was a trend to higher biochemical PRs in the immediate group. The RR was 1.08 (CI = 1.00‐1.18).
Regarding clinical PR, data were provided on 12 series from 10 publications. 9 , 26 , 27 Two studies found significantly higher clinical PRs in the immediate group, 19 , 21 whereas one observed a higher clinical PR in the delayed group. 22 In the other eight series, no significant differences were observed. Our meta‐analysis revealed that in the immediate FET the clinical pregnancy rate was somewhat higher, with the lower CI close to the unit (RR = 1.07: CI = 0.99‐1.15).
Concerning live birth rate, the gold standard outcome parameter, data were provided in 11 series from 10 publications. 9 , 18 , 20 , 22 , 23 , 24 , 25 , 26 , 27 In three series, the live birth rate was significantly higher in immediate FET, 18 , 19 , 20 and in one, it was significantly higher in delayed FET. 22 Our meta‐analysis reported similar live birth rates in the immediate and delayed FET groups. The RR was 1.05 (CI = 0.95‐1.15).
In brief, none of the IVF outcome parameters studied was better in delayed FET than in immediate FET. On the contrary, clinical PR and biochemical PR were somewhat higher in the immediate FET, but p values were not significant (P = .098). Therefore, our analysis fails to confirm the theoretical beneficial effects (more receptive endometrium, resting ovaries) of waiting ≥ one cycle. Indeed, it could be speculated that there could be a rebound effect similar to that described in the ovary 62 , 63 or with the growth of myomas after stopping medical GnRH analog treatment. 64
When we performed meta‐analyses concerning embryo development stage and FET indication (freeze‐all, FET after failed fresh transfer, or both), results were similar in immediate and delayed FETs.
From our study, it is clear that systematically delaying FET does not offer benefits. First at all, immediate transfer is associated with a nonsignificant trend to better clinical PR. Moreover, it also avoids the psychological effects of prolonging the stress on prospective parents and, in any case, avoids delaying either the pregnancy or a new attempt if the current attempt has not been successful. Finally, it should be noted that the studies included have limitations, particularly due to their retrospective and non‐randomized nature.
DISCLOSURES
Conflict of interest: Roberto Matorras, José Ignacio Pijoan, Irantzu Perez‐Ruiz, Lucía Lainz, Iker Malaina, and Sonia Borjaba declare that they have no conflict of interest. Human/animal rights: This article does not contain any studies with human and animal subjects performed by the any of the authors.
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Fig S6
Fig S7
Fig S8
Table S1
Table S2
Table S3
Matorras R, Pijoan JI, Perez‐Ruiz I, Lainz L, Malaina I, Borjaba S. Meta‐analysis of the embryo freezing transfer interval. Reprod Med Biol. 2021;20:144–158. 10.1002/rmb2.12363
REFERENCES
- 1. Xie X, Zou L, Shen Y, Xiong F, Chen J. Vitrification technology in whole embryo freezing. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2010;35:673‐678. [DOI] [PubMed] [Google Scholar]
- 2. De Geyter C, Calhaz‐Jorge C, Kupka MS, et al. European IVF‐monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). ART in Europe, 2015: results generated from European registries by ESHRE. Hum Reprod Open. 2020;2020:hoz038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roque M, Valle M, Guimarães F, Sampaio M, Geber S. Freeze‐all policy: fresh vs. frozen‐thawed embryo transfer. Fertil Steril. 2015;103:1190‐1193. [DOI] [PubMed] [Google Scholar]
- 4. Zhu Q, Chen Q, Wang L, et al. Live birth rates in the first complete IVF cycle among 20 687 women using a freeze‐all strategy. Hum Reprod. 2018;33:924‐929. [DOI] [PubMed] [Google Scholar]
- 5. McArthur SJ, Leigh D, Marshall JT, de Boer KA, Jansen RP. Pregnancies and live births after trophectoderm biopsy and preimplantation genetic testing of human blastocysts. Fertil Steril. 2005;84:1628‐1636. [DOI] [PubMed] [Google Scholar]
- 6. Griesinger G, Schultz L, Bauer T, Broessner A, Frambach T, Kissler S. Ovarian hyperstimulation syndrome prevention by gonadotropin‐releasing hormone agonist triggering of final oocyte maturation in a gonadotropin‐releasing hormone antagonist protocol in combination with a “freeze‐all” strategy: a prospective multicentric study. Fertil Steril. 2011;95:2029‐2033. [DOI] [PubMed] [Google Scholar]
- 7. Ortega‐Hrepich C, Stoop D, Guzmán L, et al. A "freeze‐all" embryo strategy after in vitro maturation: a novel approach in women with polycystic ovary syndrome? Fertil Steril. 2013;100:1002‐1007. [DOI] [PubMed] [Google Scholar]
- 8. Chen ZJ, Shi Y, Sun Y, et al. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. New Engl J Med. 2016;375:523‐533. [DOI] [PubMed] [Google Scholar]
- 9. Horowitz E, Mizrachi Y, Farhi J, Shalev A, Raziel A, Weissman A. Modified natural‐cycle cryopreserved embryo transfer: is a washout period needed after a failed fresh cycle? Reprod Biomed Online. 2019;39:439‐445. [DOI] [PubMed] [Google Scholar]
- 10. Lass A, Williams G, Abusheikha N, Brinsden P. The effect of endometrial polyps on outcomes of in vitro fertilization (IVF) cycles. J Assist Reprod Genet. 1999;16:410‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu KE, Hartman M, Hartman A. Management of thin endometrium in assisted reproduction: a clinical practice guideline from the Canadian Fertility and Andrology Society. Reprod Biomed Online. 2019;39:49‐62. [DOI] [PubMed] [Google Scholar]
- 12. Santos‐Ribeiro S, Siffain J, Polyzos NP, et al. To delay or not to delay a frozen embryo transfer after a failed fresh embryo transfer attempt? Fertil Steril. 2016;105(5):1202‐1207. [DOI] [PubMed] [Google Scholar]
- 13. Simón C, Cano F, Valbuena D, Remohí J, Pellicer A. Clinical evidence for a detrimental effect on uterine receptivity of high serum oestradiol concentrations in high and normal responder patients. Hum Reprod. 1995;10:2432‐2437. [DOI] [PubMed] [Google Scholar]
- 14. Aurrekoetxea I, Ruiz‐Sanz JI, del Agua AR, et al. Serum oxidizability and antioxidant status in patients undergoing in vitro fertilization. Fertil Steril. 2010;94:1279‐1286. [DOI] [PubMed] [Google Scholar]
- 15. Junovich G, Mayer Y, Azpiroz A, et al. Ovarian stimulation affects the levels of regulatory endometrial NK cells and angiogenic cytokine VEGF. Am J Reprod Immunol. 2011;65:146‐153. [DOI] [PubMed] [Google Scholar]
- 16. Haouzi D, Dechaud H, Assou S, De Vos J, Hamamah S. Insights into human endometrial receptivity from transcriptomic and proteomic data. Reprod Biomed Online. 2012;24:23‐34. [DOI] [PubMed] [Google Scholar]
- 17. Weissman A.IVF worldwide survey results: frozen‐thawed embryo transfer; 2017. https://ivf‐worldwide.com/survey/frozen‐thawed‐embryo‐transfer/results‐frozen‐thawed‐embryo‐transfer.html . Accessed on 24 April 2020.
- 18. Higgins C, Healey M, Jatkar S, et al. Interval between IVF stimulation cycle and frozen embryo transfer: Is there a benefit to a delay between cycles? Aust N Z J Obstet Gynaecol. 2018;58:217‐221. [DOI] [PubMed] [Google Scholar]
- 19. Huang J, Lu X, Xie Q, Lin J, Cai R, Kuang Y. Timing of frozen‐thawed embryo transfer after controlled ovarian stimulation in a non‐elective freeze‐all policy. Ann Transl Med. 2019;7:752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lattes K, Checa MA, Vassena R, Brassesco M, Vernaeve V. There is no evidence that the time from egg retrieval to embryo transfer affects live birth rates in a freeze‐all strategy. Hum Reprod. 2017;32:368‐374. [DOI] [PubMed] [Google Scholar]
- 21. Maas KH, Baker VL, Westphal LM, Lathi RB. Optimal timing of frozen embryo transfer after failed IVF attempt. Fertil Steril. 2008;90:S285. [Google Scholar]
- 22. Volodarsky‐Perel A, Eldar‐Geva T, Holzer HEG, Schonberger O, Reichman O, Gal M. Cryopreserved embryo transfer: adjacent or non‐adjacent to failed fresh long GnRH‐agonist protocol IVF cycle. Reprod BioMed Online. 2017;34:267‐273. [DOI] [PubMed] [Google Scholar]
- 23. Kaye L, Marsidi A, Rai P, et al. Frozen blastocyst transfer outcomes in immediate versus delayed subsequent cycles following GnRH agonist or hCG triggers. J Assist Reprod Genet. 2018;35:669‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. He Y, Zheng H, Du H, et al. Delayed frozen embryo transfer failed to improve live birth rate and neonatal outcomes in patients requiring whole embryo freezing. Reprod Biol Endocrinol. 2020;18(1):PMC6953147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ozgur K, Bulut H, Berkkanoglu M, Humaidan P, Coetzee K. Frozen embryo transfer can be performed in the cycle immediately following the freeze‐all cycle. J Assist Reprod Genet. 2018;35:135‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Santos‐Ribeiro S, Polyzos NP, Lan VTN, et al. The effect of an immediate frozen embryo transfer following a freeze‐all protocol: a retrospective analysis from two centres. Hum Reprod. 2016;31:2541‐2548. [DOI] [PubMed] [Google Scholar]
- 27. Song J, Xiang S, Sun Z. Frozen embryo transfer at the cleavage stage can be performed within the first menstrual cycle following the freeze‐all strategy without adversely affecting the live birth rate: A STROBE‐compliant retrospective study. Medicine. 2019;98:e17329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wells G, Shea B, O’Connell D, et al.The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analysis; 2011. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed on 24 April 2020.
- 30. Barad D, Gleicher N. Effect of dehydroepiandrosterone on oocyte and embryo yields, embryo grade and cell number in IVF. Hum Reprod. 2006;21:2845‐2849. [DOI] [PubMed] [Google Scholar]
- 31. Reichman DE, Chung P, Meyer L, Greenwood E, Davis O, Rosenwaks Z. Consecutive gonadotropin‐releasing hormone‐antagonist in vitro fertilization cycles: does the elapsed time interval between successive treatments affect outcomes? Fertil Steril. 2013;99:1277‐1282. [DOI] [PubMed] [Google Scholar]
- 32. Bayoglu Tekin Y, Ceyhan ST, Kilic S, Korkmaz C. The impact of the time interval on in‐vitro fertilisation success after failure of the first attempt. J Obstet Gynaecol. 2015;35:403‐406. [DOI] [PubMed] [Google Scholar]
- 33. Nouri K, Tempfer CB, Walch K, Promberger R, Dag S, Ott J. Predictive value of the time interval between embryo loading and transfer for IVF/ICSI success: a prospective cohort study. Reprod Biol Endocrinol. 2015;29(13):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li H, Li L, Lu X, Sun X, Ng EHY. Comparison of the effect of immediate versus delayed transfer following a stimulated IVF cycle on the ongoing pregnancy rate of frozen‐thawed embryo transfer cycles: a study protocol for a randomised controlled trial. BMJ Open. 2018;17(8):e020507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wong KM, van Wely M, Mol F, Repping S, Mastenbroek S. Fresh versus frozen embryotransfers in assisted reproduction. Cochrane Database Syst Rev. 2017;3:CD011184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coates A, Kung A, Mounts E, et al. Optimal euploid embryo transfer strategy, fresh versus frozen, after preimplantation genetic screening with next generation sequencing: a randomized controlled trial. Fertil Steril. 2017;107:723‐730. [DOI] [PubMed] [Google Scholar]
- 37. Somigliana E, Busnelli A, Paffoni A, et al. Cost‐effectiveness of preimplantation genetic testing for aneuploidies. Fertil Steril. 2019;111:1169‐1176. [DOI] [PubMed] [Google Scholar]
- 38. Devroey P, Polyzos NP, Blockeel C. An OHSS‐Free Clinic by segmentation of IVF treatment. Hum Reprod. 2011;26:2593‐2597. [DOI] [PubMed] [Google Scholar]
- 39. Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C. Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen thawed embryo transfer in normal responders. Fertil Steril. 2011;96:344‐348. [DOI] [PubMed] [Google Scholar]
- 40. Roque M, Haahr T, Geber S, Esteves SC, Humaidan P. Fresh versus elective frozen embryo transfer in IVF/ICSI cycles: a systematic review and meta‐analysis of reproductive outcomes. Hum Reprod Update. 2019;25:2‐14. [DOI] [PubMed] [Google Scholar]
- 41. Simón C, Velasco JJG, Valbuena D, et al. Increasing uterine receptivity by decreasing estradiol levels during the preimplantation period in high responders with the use of a follicle‐stimulating hormone step‐down regimen. Fertil Steril. 1998;70:234‐239. [DOI] [PubMed] [Google Scholar]
- 42. Nikas G, Develioglu OH, Toner JP, Jones HW Jr. Endometrial pinopodes indicate a shift in the window of receptivity in IVF cycles. Hum Reprod. 1999;14:787‐792. [DOI] [PubMed] [Google Scholar]
- 43. Richter KS, Shipley SK, McVearry I, Tucker MJ, Widra EA. Cryopreserved embryo transfers suggest that endometrial receptivity may contribute to reduced success rates of later developing embryos. Fertil Steril. 2006;86:862‐866. [DOI] [PubMed] [Google Scholar]
- 44. Papanikolaou E, Chartomatsidou T, Timotheou E, et al. In Freeze‐All Strategy, Cumulative Live Birth Rate (CLBR) Is Increasing According to the Number of Blastocysts Formed in Women <40 Undergoing Intracytoplasmic Sperm Injection (ICSI). Front Endocrinol (Lausanne). 2019;10:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Montoya‐Botero P, Polyzos NP. The endometrium during and after ovarian hyperstimulation and the role of segmentation of infertility treatment. Best Pract Res Clin Endocrinol Metab. 2019;33:61‐75. [DOI] [PubMed] [Google Scholar]
- 46. Weinerman R, Mainigi M. Why we should transfer frozen instead of fresh embryos: the translational rationale. Fertil Steril. 2014;102:10‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bringhenti F, Martinelli F, Ardenti R, La Sala G. Psychological adjustment of infertile women entering IVF treatment: differentiating aspect and influencing factors. Acta Obstet Gynecol Scand. 1997;76:431‐443. [DOI] [PubMed] [Google Scholar]
- 48. Matthiesen SM, Frederiksen Y, Ingerslev HJ, Zachariae R. Stress, distress and outcome of assisted reproductive technology (ART): a meta‐analysis. Hum Reprod. 2011;26:2763‐2776. [DOI] [PubMed] [Google Scholar]
- 49. Miller N, Herzberger EH, Pasternak Y, et al. Does stress affect IVF outcomes? A prospective study of physiological and psychological stress in women undergoing IVF. Reprod Biomed Online. 2019;39:93‐101. [DOI] [PubMed] [Google Scholar]
- 50. Bui BN, Torrance HL, Janssen C, et al. Does endometrial scratching increase the rate of spontaneous conception in couples with unexplained infertility and a good prognosis (Hunault > 30%)? Study protocol of the SCRaTCH‐OFO trial: a randomized controlled trial. BMC Pregnancy Childbirth. 2018;18:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ferrick L, Lee YSL, Gardner DK. Reducing time to pregnancy and facilitating the birth of healthy children through functional analysis of embryo physiology. Biol Reprod. 2019;101:1124‐1139. [DOI] [PubMed] [Google Scholar]
- 52. Ghobara T, Gelbaya TA, Ayeleke RO. Cycle regimens for frozen‐thawed embryo transfer. Cochrane Database Syst Rev. 2017;7:CD003414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lawrenz B, Coughlan C, Melado L, Fatemi HM. The ART of frozen embryo transfer: back to nature!. Gynecol Endocrinol. 2020;18:1‐5. [DOI] [PubMed] [Google Scholar]
- 54. Xi Q, Tao Y, Qiu M, Wang Y, Kuang Y. Comparison Between PPOS and GnRHa‐Long Protocol in Clinical Outcome with the First IVF/ICSI Cycle: A Randomized Clinical Trial. Clin Epidemiol. 2020;12:261‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Montoya‐Botero P, Martinez F, Rodríguez‐Purata J, Rodríguez I, Coroleu B, Polyzos NP. The effect of type of oral contraceptive pill and duration of use on fresh and cumulative live birth rates in IVF/ICSI cycles. Hum Reprod. 2020;35(4):826‐836. [DOI] [PubMed] [Google Scholar]
- 56. Mizrachi Y, Horowitz E, Farhi J, Raziel A, Weissman A. Ovarian stimulation for freeze‐all IVF cycles: a systematic review. Hum Reprod Update. 2020;26:118‐135. [DOI] [PubMed] [Google Scholar]
- 57. Harrison KL, Breen TM, Hennessey JF, et al. Patient age and success in a human IVF programme. Aust N Z J Obstet Gynaecol. 1989;29:326‐328. [DOI] [PubMed] [Google Scholar]
- 58. Templeton A, Morris JK, Parslow W. Factors that affect outcome of in‐vitro fertilisation treatment. Lancet. 1996;348:1402‐1406. [DOI] [PubMed] [Google Scholar]
- 59. Connell MT, Richter KS, Devine K, et al. Larger oocyte cohorts maximize fresh IVF cycle birth rates and availability of surplus high‐quality blastocysts for cryopreservation. Reprod Biomed Online. 2019;38:711‐723. [DOI] [PubMed] [Google Scholar]
- 60. Matorras R, Matorras F, Mendoza R, et al. The implantation of every embryo facilitates the chances of the remaining embryos to implant in an IVF programme: a mathematical model to predict pregnancy and multiple pregnancy rates. Hum Reprod. 2005;20:2923‐2931. [DOI] [PubMed] [Google Scholar]
- 61. Matorras R, Otero B, Mendoza R, et al. Quality of additional embryos transferred on pregnancy outcomes in IVF: predictions using a mathematical approach. Reprod Biomed Online. 2014;29:200‐208. [DOI] [PubMed] [Google Scholar]
- 62. Igarashi M. Rebound phenomenon of the human ovarian function and its therapeutic application in female sterility; preliminary report. Fertil Steril. 1957;8:362‐372. [DOI] [PubMed] [Google Scholar]
- 63. Buckler HM, Evans CA, Mamtora H, Burger HG, Anderson DC. Gonadotropin, steroid, and inhibin levels in women with incipient ovarian failure during anovulatory and ovulatory rebound cycles. J Clin Endocrinol Metab. 1991;72:116‐124. [DOI] [PubMed] [Google Scholar]
- 64. Talaulikar VS, Belli A, Manyonda I. GnRH agonists: do they have a place in the modern management of fibroid disease? J Obstet Gynecol India. 2012;62:506‐510. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Fig S6
Fig S7
Fig S8
Table S1
Table S2
Table S3


