Abstract
We describe a case of severe neuroexcitatory reaction with hyperthermia after administration of propofol in a 43-year-old patient suspected of a relapse of sarcoidosis who underwent bronchoscopy. This resulted in a lengthy stay in intensive care and long-term neuropsychological impairment. A review of the literature shows that severe neuroexcitatory symptoms (seizure-like phenomena, abnormal hypertonic, and/or jerky movements) occur rarely after propofol administration and may be life-threatening. Due to the paucity of data, the treatment is mostly empirical. The diagnosis can also be delayed owing to underrecognition. We conclude that health practitioners who frequently use propofol should be aware of this specific manifestation of drug toxicity, which albeit rare can be devastating for the patient.
Keywords: propofol, neuroexcitatory reaction, sarcoidosis
Case Presentation
A 43-year-old patient of Eritrean origin, diagnosed in 2014 with sarcoidosis leading to restrictive lung disease and a voluminous mediastinal lymphadenopathy is admitted to hospital in August 2017 after she presents to the emergency room with a 24-hour fever of unknown etiology. Of note, she was untreated for her sarcoidosis due to severe side effects of all previous immunosuppressive agents including glucocorticoids, azathoprine, methotrexate, and mycophenolate. She is currently on Losartan 50 mg, Febuxostat 40 mg, and Calcitriol 25 µg twice weekly.
An infectious process is ruled out with large microbiological and molecular investigations. A chest and abdominal computed tomography (CT) reveals worsening of the lymphadenopathies, suggesting a relapse of sarcoidosis. Therefore, a diagnostic bronchoscopy with lung and lymph node biopsies is planned. The procedure is performed under conventional general anesthesia with propofol (approximate dosage 750 mg over 90 minutes), remifentanil (435 micrograms), and rocuronium.
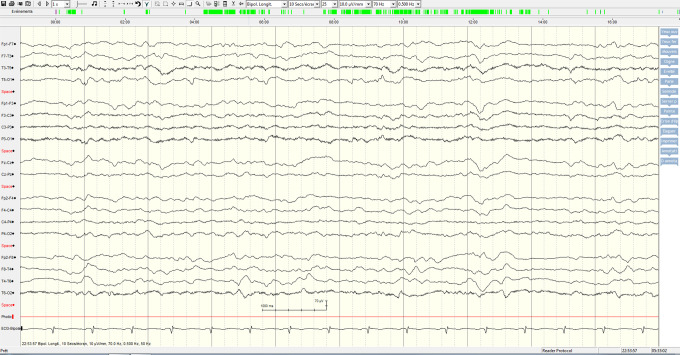
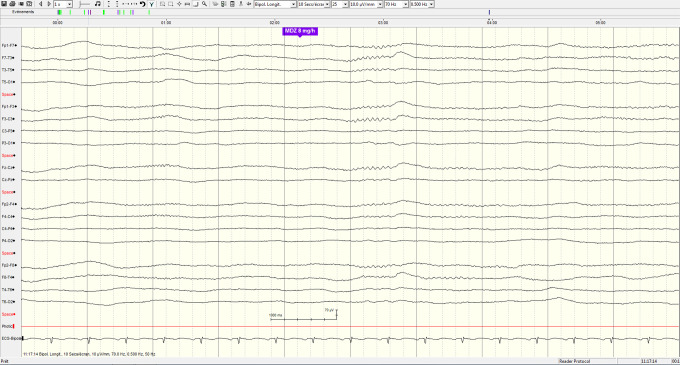
The bronchoalveolar lavage is within normal limits, whereas the transbronchial biopsies and lymph node needle aspirations show noncaseating granulomas without malignant cells, confirming the diagnosis of sarcoidosis. At the end of the procedure, the patient opens her eyes on command and shows a cough reflex on the endotracheal tube. She is extubated but rapidly loses contact and develops an abnormal and rapid increase in muscle tone accompanied by psychomotor agitation and loss of urine. A brain CT and magnetic resonance imaging show only known subcortical white matter lesions in the context of probable neurosarcoidosis (Figures 1 and 2: TSE-T2 axial and T2 TIRM DF coronal images on day 11 showing signal abnormalities involving the retro-atrial white matter). The cerebrospinal fluid shows a markedly increased protein content (1320 mg/L, normal reference <450 mg/L) with a normal cell count. A few hours later, she develops a high fever and decrease in oxygen saturation, prompting her transfer to the intensive care unit (ICU), where she is reintubated. The electroencephalogram (EEG) demonstrates a moderate nonspecific encephalopathy without epileptic activity (Figures 3 and 4: Selected EEG recording snapshots on day 4 without sedation and 11 under midazolam). Values of creatine kinase (CK) and thyroid-stimulating hormone (TSH) are within normal limits and repeated blood cultures fail to show any bacteremia. A trial with biperidene has no effect on the patient’s neurological state.
Figure 1.
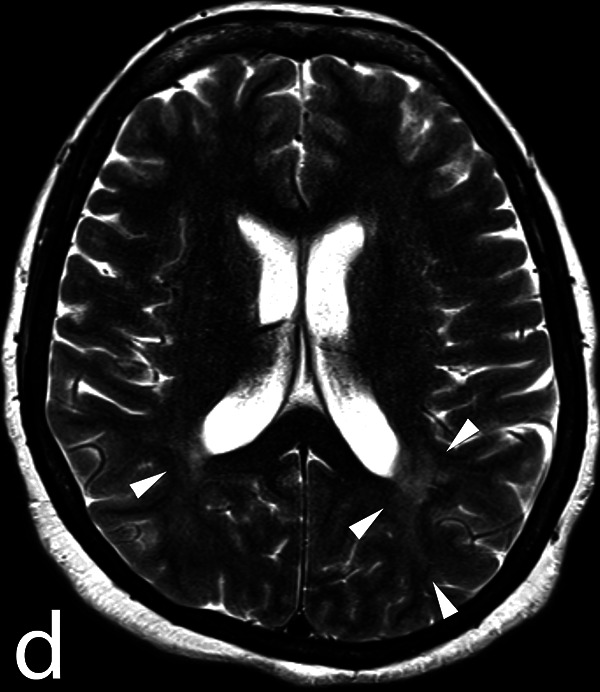
Turbo-spin echo T2 weighted images on day 11 showing signal abnormalities (arrowheads) involving the retro-atrial white matter.
Figure 2.
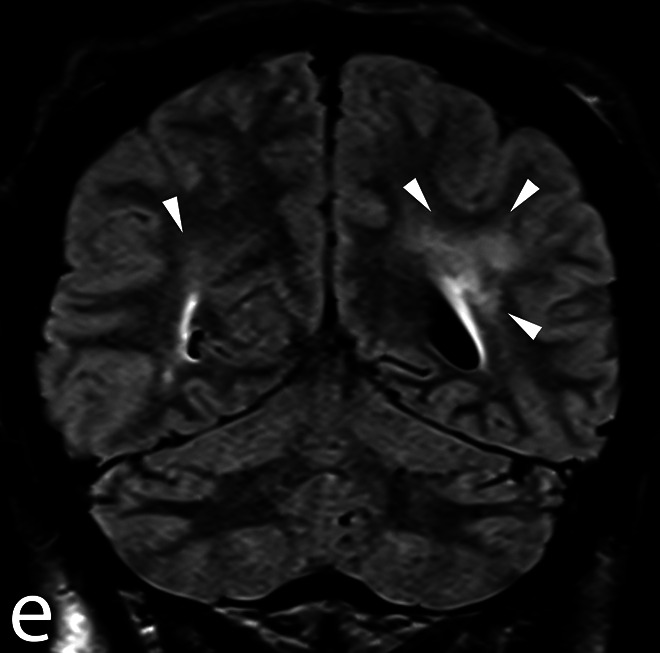
T2 turbo inversion recovery magnitude dark fluid coronal images on day 11 showing the same white matter lesions.
Figure 3.
Electroencephalogram recording snapshot on day 4 without sedation.
Figure 4.
Electroencephalogram recording snapshot on day 11 under midazolam.
Hyperthermia is persistent despite maximal intravascular cooling through a Coolguard device, with temperature values around 39 °C. The neurological status is characterized by a lack of any contact or ocular pursuit, with normal pupillary reflexes and a weak oculocephalic reflex. There is also diffuse muscular rigidity (without frank opisthotonos) and hyperreflexia with provoked clonus in all 4 limbs (Supplemental Digital Content: video 1 showing a severe rigidity, clonus, and polykinetic reflexes). No autonomic lability is observed, with values of blood pressure ranging from 100/60 to 140/80 mm Hg and heart rate from 100 to 120 bpm. Immunological investigation and muscle biopsy are inconclusive and only confirm the known sarcoidosis.
Malignant hyperthermia is ruled out because of the atypical clinical picture, the absence of CK elevation and the absence of drugs known to precipitate this condition. A trial administration of dantrolene at standard doses has no clinically sustainable effect. A possible serotoninergic reaction to remifentanyl is considered, as indeed both the Sternbach and the Hunter Serotonin Toxicity Criteria are met. However, the severity and duration of the reaction in the absence of any predisposing treatments, recreational drugs, or cytochrome inhibitors, the lack of some cardinal clinical manifestations (mydriasis, diaphoresis, diarrhea, skin flushing) and the relatively low dose of remifentanyl administered decrease the likelihood of the diagnosis. A trial of cyproheptadine is also entirely ineffective. A neuroleptic malignant syndrome is incompatible with hyperreflexia and myoclonus. An anticholinergic reaction is unlikely in view of the duration and absence of clinical response to biperidene. Finally, a diagnosis of propofol infusion syndrome is ruled out due to the lack of rhabdomyolysis, acidosis, conduction abnormalities, and cardiac or renal insufficiency. In view of the clinical presentation and the exclusion of all other likely explanations after a comprehensive work-up, a severe neuroexcitatory reaction to propofol is diagnosed.
Sedation is maintained exclusively with midazolam at doses of 5 to 10 mg/h with good effect on muscle tone and daily windows show a rapid increase of rigidity without any return of conscious awareness. The use of opiates (fentanyl) is kept at a minimum.
The patient is successfully weaned from the ventilator 23 days after the initial event. The level of consciousness gradually improves, with reestablished eye contact followed by a recuperation of language functions. At the end of her hospital stay, the patient shows executive dysfunction and impairment of memory and language. She returns home after a 64-day hospital stay including 23 days in the ICU, on an immunosuppressive treatment of mycophenolate which is well tolerated.
Discussion
This well-illustrated case of severe neuroexcitatory reaction after propofol infusion demonstrates a rare, underrecognized, and life-threatening condition secondary to a frequently used drug. The literature about this syndrome is scarce, probably reflecting its relatively low incidence despite the rapid spread of propofol use. Neurological symptoms suggesting hyperexcitatory effects (myoclonic jerks, twitching, and seizures) have been reported however in up to 1% of cases. Severe neuroexcitatory reactions are characterized by hypertonic muscles and/or jerky movements, sometimes combined with unconsciousness, developing in relation to propofol administration.1 They may present during induction, maintenance or emergence from anaesthesia, or even as delayed reactions.2
A large spectrum of abnormal movements has been reported in this setting: myoclonus,3 increase in muscle tone ranging from diffuse rigidity and extensor movements to opisthotonos4 and dystonic reactions that can be either focal—for example masseteric or laryngeal5,6 or generalized.7,8 These abnormal movements are generally accompanied by disorders of consciousness, ranging from intermittent loss of consciousness to marked agitation and even delirium, sometimes referred to as “propofol frenzy.”9 Since there are no uniform diagnostic criteria for this condition, we think that all these cases may have been labeled as severe neuroexcitatory reactions.
The only case series available in the literature recording severe neuroexcitatory symptoms is a drug registry of 44 cases of abnormal propofol reactions.10 There was a female predominance of 2:1 and a median age of 27 years. As in our case, the onset of symptoms can be delayed, from 60 minutes to more than 6 hours after anesthesia and may last up to several weeks.
The pathophysiology of this reaction is not completely understood. The anesthetic properties of propofol are complex. Its hypnotic action is centrally mediated by the potentialization of GABAergic channels, but other mechanisms include direct activation of sodium channels,11 potentialization of the inhibitory effect of glycine receptors,12,13 inhibition of N-methyl-D-aspartate (NMDA) glutamate receptors,14 and possible activity via the endocannabinoid system.15
Among the various mechanisms that have been proposed for these neuroexcitatory symptoms are antagonism of glycine and dopamine receptors,12 hyposensitization of GABAergic pathways and dysregulated inhibition of NMDA glutamate receptors.9 Rapid changes in propofol concentration have been thought to be precipitating factors for seizure-like phenomena1 and the variety of receptor subunits may account for the variability of clinical manifestations.16 In our case, the clinical manifestations of the reaction were probably compounded by the underlying neurosarcoidosis, although the localization of the brain lesions and the fulminant course of the neurological impairment argue in favor of an additional causative process. This is consistent with the longer, more severe, and disabling propofol-induced reactions observed in patients with preexisting brain involvement.10
Health practitioners who frequently use propofol should be aware of this specific manifestation of drug toxicity, which albeit rare can be devastating for the patient. Our case evolved favorably with high doses of continuous intravenous midazolam for 3 weeks, however its optimal management remains unclear. Larger scale registries as well as the use of advanced brain imaging (positron emission tomography [PET], single photon emission computed tomography [SPECT], magnetic resonance spectroscopy [MRS]) are warranted to increase the recognition of severe neuroexcitatory reactions after propofol administration, to gain insight into their pathophysiology and ultimately to improve their treatment.
Footnotes
Authors’ Note: The study was conducted at Hôpital du Valais, Sion, Switzerland. Informed consent was obtained from the patient for the study, including publication of video recorded material. A signed copy can be produced on demand.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Linos Pantelakis, MD  https://orcid.org/0000-0001-5290-9847
https://orcid.org/0000-0001-5290-9847
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Walder B, Tramèr MR, Seeck M. Seizure-like phenomena and propofol: a systematic review. Neurology. 2002;58(9):1327–1332. [DOI] [PubMed] [Google Scholar]
- 2. Sneyd JR. Excitatory events associated with propofol anaesthesia: a review. J R Soc Med. 1992;85(5):288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hughes NJ, Lyons JB. Prolonged myoclonus and meningism following propofol. Can J Anaesth. 1995;42(8):744–746. [DOI] [PubMed] [Google Scholar]
- 4. Ries CR, Scoates PJ, Puil E. Opisthotonos following propofol: a nonepileptic perspective and treatment strategy. Can J Anaesth. 1994;41(5 pt 1):414–419. [DOI] [PubMed] [Google Scholar]
- 5. LeRiger M, Williams J, Duncan-Wiebel G, Shukry M. Acute masseter dystonia in a pediatric patient receiving aripiprazole and methylphenidate following induction of general anesthesia. Paediatr Anaesth. 2017;27(8):863–864. ISSN. 155-5645. [DOI] [PubMed] [Google Scholar]
- 6. Steckelberg R, Tsiang D, Pettijohn K, Mendelsohn A, Hoftman N. Acute vocal fold dystonic reaction to propofol: a case report. Am J Otolaryngol. 2015;36(2):303–305. [DOI] [PubMed] [Google Scholar]
- 7. Schramm B, Orser B. Dystonic reaction to propofol attenuated by benztropine (Cogentin). Anesth Analg. 2002;94(5):1237–1240. [DOI] [PubMed] [Google Scholar]
- 8. Saravanakumar K, Venkatesh P, Bromley P. Case report: delayed onset refractory dystonic movements following propofol anesthesia. Paediatr Anaesth. 2005;15(7):597–601. [DOI] [PubMed] [Google Scholar]
- 9. Carvalho DZ, Townley RA, Christopher M. Propofol frenzy: clinical spectrum in 3 patients. Mayo Clin Proc. 2017;92(11):1682–1687. [DOI] [PubMed] [Google Scholar]
- 10. Islander G, Vinge E. Severe neuroexcitatory symptoms after anaesthesia—with focus on propofol anaesthesia. Acta Anaesthesiol Scand. 2000;44(2):144–149. [DOI] [PubMed] [Google Scholar]
- 11. Ouyang W, Wang G, Hemmings HC. Isoflurane and propofol inhibit voltage-gated sodium channels in isolated rat neurohypophysial nerve terminals. Mol Pharmacol. 2003;64(2):373–381. [DOI] [PubMed] [Google Scholar]
- 12. Dolin SJ, Smith MB, Soar J, Morris PJ. Does glycine antagonism underlie the excitatory effects of methohexitone and propofol? Br J Anaesth. 1992;68(5):523–526. [DOI] [PubMed] [Google Scholar]
- 13. Hales TG, Lambert JJ. The actions of propofol on inhibitory amino acid receptors ofbovine adrenomedullary chromaffin cells and rodent central neurones. Br J Pharmacol. 1991;104(3):619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buggy DJ, Beverly N, Rowbotham DJ, Lambert DG. Effects of intravenous anesthetic agents on glutamate release. Anesthesiology. 2000;92(4):1067–1073. [DOI] [PubMed] [Google Scholar]
- 15. Fowler CG. Possible involvement of the endocannabinoid system in the actions of three clinically used drugs. Trends Pharmacol Sci. 2004;25(2):59–61. [DOI] [PubMed] [Google Scholar]
- 16. Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14(6):383–400. [DOI] [PubMed] [Google Scholar]