Abstract
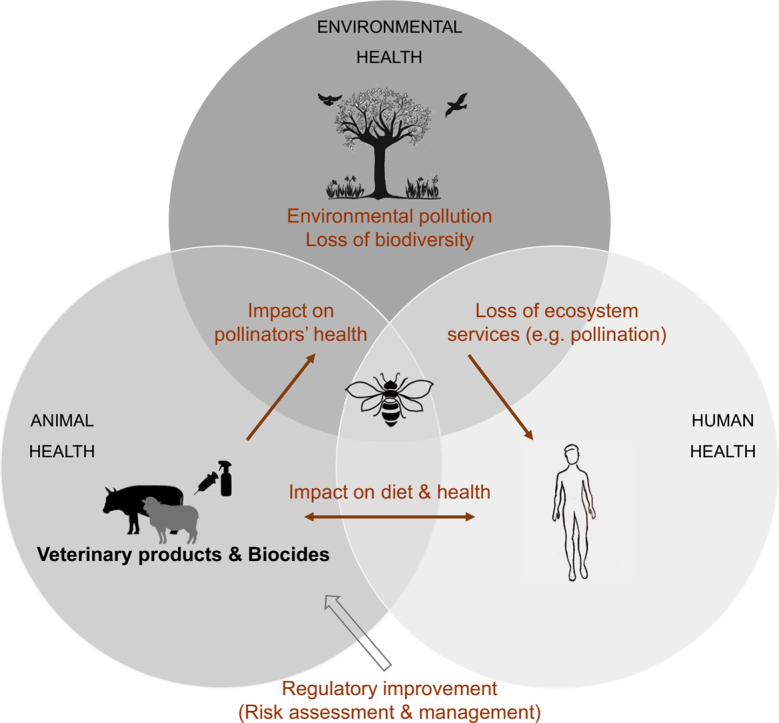
The One Health approach acknowledges that human health is firmly linked to animal and environmental health. It involves using animals such as bees and other pollinators as sentinels for environmental contamination or biological indicators. Beekeepers noticed intoxications of apiaries located in the vicinity of sheep and cattle farms, which led to the suspicion of bees' intoxication by the products used for livestock: veterinary medicinal products (VMPs) and Biocides, confirmed by laboratory analysis. We review the legal context of VMPs and Biocidal products considering Europe as a case study, and identify shortcomings at the environmental level. We describe the possible ways these products could intoxicate bees in the vicinity of livestock farms. We also illustrate the way they may impact non-target species. The cases of ivermectin and abamectin as VMPs, deltamethrin and permethrin as Biocides are considered as case studies. We show bees can be exposed to new and unrecognized routes of exposure to these chemicals, and demonstrate that their application in livestock farming can affect the survival of pollinators, such as bees. We conclude that: (1) figures on the marketing/use of these chemicals should be harmonized, centralized and publicly available, (2) research should be devoted to clarifying how pollinators are exposed to VMPs and Biocides, (3) toxicity studies on bees should be carried out, and (4) pollinators should be considered as non-targeted species concerning the environmental risk assessment before their marketing authorization. We propose the term “Multi-use substances” for active ingredients with versatile use.
Keywords: Pesticide, Livestock, Bees, Ecotoxicology, Environmental health, Risk assessment, Multi-use substances
Abbreviations: BTV, Bluetongue virus; ECHA, European Chemical Agency; EIA, environmental impact assessment; EMA, Environmental Medicine Agency; ERA, environmental risk assessment; MA, market authorisation; PEC, predicted environmental concentration; PNEC, predicted no effect concentration; VMPs, veterinary medicinal products; SPs, synthetic pyrethroids; RQ, risk quotient; VICH, International Cooperation on Harmonization of Technical Requirements for Registration of Veterinary Medicinal Products.
Graphical abstract
Highlights
-
•
We evaluate the impact that VMPs and Biocides have on bees using the One Health concept.
-
•
We show that VMPs and Biocides involve health risks to pollinators, which remain unconsidered.
-
•
Lack of data and knowledge gaps should be filled regarding exposure and toxicity to pollinators of VPMs and Biocides
-
•
Bees ought to be included in environmental risk assessment of VMPs and Biocides
-
•
Term “Multi-use substances”, for referring to a product with versatile usage: VMPs, Biocides, and pesticides.
1. Introduction
Approaches integrating interdisciplinary perspectives are required to address the current complex health and environmental challenges. Environmental issues such as global warming, pollution, emerging pathogens, and biodiversity loss are presently at the heart of today's preoccupations. The One Health approach acknowledges that human health is firmly linked to the health of animals and the environment [1,2]. Its basis is grounded on the interdependence of humans with their natural systems. One Health fosters an interdisciplinary collaboration locally, nationally and globally to attain optimal health. The scope of One Health's discipline includes using animals as sentinels for detecting environmental agents and contaminants [3]. Some animal species could be described as efficient “indicators” to assess infectious environmental hazards [4]. Bees and other pollinators are some examples of such indicators [[5], [6], [7]].
Bees and other insect pollinators can serve as bio-monitoring tools. They ensure the function of pollination that is essential for the reproduction of a large proportion of plants, and is crucial for sustaining ecosystems [8]. They provide agricultural services through crop pollination, estimated at approximately 351 billion dollars per year [8]. Additionally, bees and insect pollinators are sources of income, inspiration, and of cultural value for many [8,9]. Unfortunately, they are subject to several anthropogenic pressures such as agricultural intensification (land use), the introduction of invasive species, climate change, pollution, emerging pathogens, and pesticides [10,11]. Without close regulation, the decline of pollinators might, consequently, introduce severe global health burdens [12], for example, leading to dietary change and micronutrient deficiencies, resulting from a decrease in pollinator-dependent crop yields. As a result, this can increase the risks of humans contracting diseases [13]. As Deem et al., (2018) state: “the loss of pollinators, with their ecosystem services, may result in a cost to human health and the environment” [14].
Anthropogenic pressures are affecting ecosystems worldwide. Human activities generate the release of chemical substances into the environment, affecting animals in a complicated way [15]. We can explore the link between human activities, animal health, and environmental health from a One Health perspective. However, there is a lack of a systematic understanding of how VMPs and Biocides contribute to the decline of non-target species such as bees, particularly the European honeybee (Apis mellifera). In France, this problem has appeared in the Department of Ariège (winter 2008–2009), where over 4000 hives located in remote mountains, far from crops, suffered a sudden loss of honeybee colonies.
Independent veterinary experts confirmed observing the intoxication of bees (Colin, 2020, pers. comm.). The affected hives were in the vicinity of sheep and cattle farms which led to a strong suspicion of the bees' intoxication from the products used for livestock [16]. Analyses performed in bee matrices (honey and bees) by a specialized laboratory of the Centre National de la Recherche Scientifique (CNRS) in France confirmed this suspicion. CNRS found 0.25 ng/bee of bifenthrin and 0.1 to 0.5 ng/bee of permethrin in dead bees, and 17 to 57 ng/g of deltamethrin in honey (CNRS – analysis report N° 09/00088, N°09/00023, N°09/00703).
VMPs and Biocides can affect terrestrial and aquatic environments due to their broad applicability [17]. Products such as permethrin are also harmful to human health. They are classified as carcinogens, endocrine disruptors, or neurotoxicants [18]. Therefore, VMPs and Biocides have shown their ability to affect biodiversity, wildlife and human health [19,20]. Numerous papers have been published on how wildlife and other ecosystems could be exposed, either directly or indirectly, to pharmaceutical products [19,21,22]. Furthermore, VMPs' residues found in sheep and cattle dung have a sublethal effect on the community of dung arthropods [23], which provides numerous ecosystem services, including the decomposition of fecal matter. Additionally, the decline of the dung beetle species also affects the upper trophic level [24]. Dung beetles are resources for various bird species, mammals such as bats, skunks, badgers and hedgehogs, some amphibians and reptile species [24]. However, only a few studies have investigated the ecotoxicological effect of VMPs on wildlife. To date, this issue has received scant attention from the scientific community.
To our knowledge, no study has evaluated the impact of chemicals used in livestock farming practices such as VMPs and Biocides, especially their effect on bees and other insect pollinators under the scope of the One Health concept. We explore the available knowledge regarding the potential links between the use of VMPs and Biocides, and their impacts on pollinators. In the following, we will describe a new route where bees come to be exposed to such chemical products. Additionally, we list the main knowledge gaps regarding this matter.
The first section of this paper reviews the legal context of VMPs and Biocides in the EU and the identified shortcomings at the environmental level in the current regulatory framework. Considering Europe as our case study, the following section elucidates the possible way bees can be exposed and contaminated by VMPs and Biocidal products in the vicinity of sheep and cattle farms. The third part illustrates how these products can also impact non-targeted species (such as dung beetles) and insect pollinators (such as bees);. scientific literature exists on the ecotoxicological impact of pharmaceutical products on dung beetles [25,26]. We describe notable examples of VMPs such as ivermectin and abamectin and Biocidal products such as deltamethrin and permethrin. We then consider the legal context of VMPs and Biocides identifying shortcomings at the environmental level in the current regulatory framework. Finally, we briefly highlight the knowledge and data gaps that exist regarding risks to pollinators from using these products.
2. Legal framework of VMPs and Biocides; Europe as a case study
The International Cooperation on Harmonization of Technical Requirements for Registration of Veterinary Medicinal Products (VICH) defines the methodology for performing risk assessments of VMPs in Europe, Japan, and the United States [27,28]. VICH is a program made for the regulatory authorities and aims to harmonize the technical requirements for veterinary drug registration [29]. For VICH signatories, among the requirements before the marketing authorization of VMPs, is the mandatory performance of an environmental risk assessment (ERA) consisting of 2 phases [29].
In Europe, the regulation of VMPs is managed by the European member states and the European Commission, assisted by the risk assessment of the European Medicine Agency (EMA). Before the marketing authorization (MA), active substances of VMPs must be subject to a benefit/risk analysis in conformity with the European Regulation 2019/6 that has repealed the Directive 2001/82/EC [26]. Among the requirements for authorization, it is mandatory to perform an ERA following the VICH guidelines.
According to the VICH GL6 guidelines, Phase-I consists of an environmental impact assessment (EIA) that studies the exposure scenario of non-targeted species to VMPs [28]. The exposure scenario depends on the application and properties of the VMPs. An estimation of the Predicted Environmental Concentration (PEC) is calculated from the dose and application frequency [28]. The PEC represents the estimated parent compound and its metabolite concentrations in each environmental compartment (water, soil and sediment). Phase-II, Tier A is an EIA set of guidelines for aquatic, terrestrial and pasture animals, and assesses the impact that VMPs have on non-targeted organisms. As few species are selected as indicators or models, they represent the essential taxa in the studied ecosystem, or those most notably sensitive to ecotoxicity risks when exposed to VMPs. The Predicted No Effect Concentration (PNEC) is estimated using acute and subacute toxicity studies for each chosen species [30]. The PNEC represents the active substance concentration below which the VMPs have no observable effect on the indicator species.
For each environmental compartment, a comparison of the PEC and PNEC of the non-targeted organisms is made. The ratio PEC/PNEC is calculated as a Risk Quotient (RQ). If the value of the RQ is less than 1 for a species, the concentration of the active substances used is not considered harmful for the studied organism. Otherwise, the VMP under consideration presents a risk for this species in this environment and needs further assessment. Phase-II, Tier B is then performed, including a chronic exposure assessment [30]. Should a product exceed its trigger value in Tier B, further testing may be required, or risk mitigation measures need to be implemented to obtain an MA. For Phase-II, Tiers A and B, dung beetles are among the non-target model organisms tested in the ERA of VMPs used for pasture animals [27,30]. However, there is no assessment planned for pollinators (including managed honeybees).
The approval of the Biocidal active substances and their management, is coordinated by the European Commission, which is assisted by the risk assessment of the European Chemical Agency (ECHA). Its ERA follows the ECHA guidelines [31]. As is the case for the ERA of VMPs, a tiered approach is applied, starting with the PEC's estimation and is followed by an ecotoxicity assessment (PNEC). Then, an RQ for each non-targeted organism has to be evaluated [31]. Similar to the assessment of VMPs, neither bees nor other pollinators are currently covered in the Biocides' risk assessment [31]. Nonetheless, the European Commission has mandated the ECHA to start developing guidance measures to assess the risk to bees and other arthropod pollinators from the use of Biocides [32].
3. Environmental fate of VMPs and Biocides
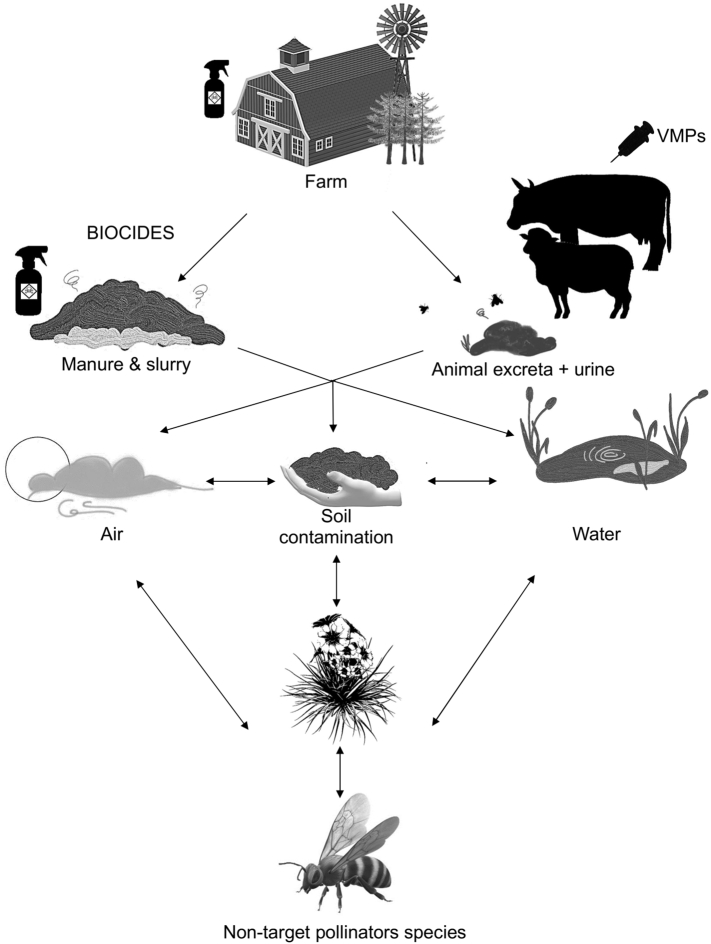
Animals treated with antiparasitic compounds can excrete non-metabolized products in their urine and feces. Up to 98% of macrocyclic lactone released through feces remains unmetabolized or is excreted as active metabolites [17]. Due to the broad applicability of VMPs and Biocides, they can affect terrestrial and aquatic environments [17]. Environmental contamination of non-target species from VMPs probably occurs through slurry and manure or dung and urine from grazing animals on pasture. This pathway could end in the contamination of soil and surface water by leaching, run-off or drainage [22]. However, few studies are addressing the concentration of anthelmintic in the environment [17]. Recent contaminations were evidenced in ribwort plantains growing on artificial media contaminated with ivermectin [33].
Concentrations of 199,2 ppb were detected in plants growing close to ivermectin-contaminated feces 60 days after deposition [34]. Therefore, it may be deduced that pollinators get exposed to environmental pollutants through diverse pathways. While they are out of the hive looking for resources like water, nectar, pollen or resin, honeybees can come into contact with pollutants dissolved in the air [35], or conveyed by wind through dust particles [36,37]. Both metabolized and unmetabolized VMPs excreted by cattle in urine and feces can be transported outside the pasture boundaries [38]. Peterson et al., (2020) studied the aerial transport of VMPs via particulate matter (PM) from beef cattle feed yards in America. The resulting PM (669,000 kg) contained sufficient insecticides enough to kill more than a billion honeybees (Apis mellifera) per day. Permethrin was the most commonly identified molecule in PM (67.6%), with the highest concentration of 28,929 ng/m3 having been aerially propagated from feed yards. Also, ivermectin (49%) and neonicotinoid (<27%) were found in all PM samples taken from feed yards [38].
Residue found in environmental matrices like water and soil, and vegetal matrices like nectar, pollen or resin may either affect the forager bees directly or be brought to the colony, thereby exposing all the in-hive bees and larvae as well. Also, honeybees prefer to collect water from different sources, such as rainwater gutters containing decaying organic matter, and puddles that form on the top of cow dung and sewage effluent [39]. Additionally, water-foraging honeybees are often observed collecting brackish water, and they could be directly affected by the contaminated excreta because of their attraction to water enriched with mineral salts [40] (Fig. 1). Butler, (1940) studied the preferences of honeybees among forty various solutions containing different substances and concentrations, including cow dung and urine distillate [39]. They showed a higher degree of preference for cow dung water distillate, cow dung water, and urine distillate compared to distilled water. Therefore, bees foraging water in cattle excreta may get contaminated by residual VMPs. Another route of exposure is possible since the plants growing close to these excreta are contaminated more than two months after the deposition of feces, which raises questions on the safety of the foraging activity of pollinators (vide infra) [39].
Fig. 1.
Environmental fate of VMPs (veterinary medicine products) and Biocides by which pollinators can be exposed.
A wide range of knowledge exists on the exposure pathways to pesticides of social bees, such as bumblebees, honeybees and stingless bees [41,42]. Here, caste, developmental stage, climatic conditions, and seasonality determine the level of exposure [43].
From over 20,000 species of bees worldwide, most of them solitary, 65% of them excavate their nests underground [42]. The exposure of wild bees to agricultural pesticides has been explored in several studies, revealing that soil is a relevant route of exposure for bee species nesting underground [42,44]. Among the non-social bee species, we find some either living underground, or using soil for building their nests (e.g. Osmia spp., Megachile rotundata, Nomia malanderi). As a result, they are more exposed to the terrestrial compartment [42,45]. For example, Osmia cornuta can collect 2.2–4.4 g (dry weight) of mud for the constructing of their nests [46]. In this way, they can be exposed to residue of VMPs and Biocides in contaminated soil (Fig. 1). Other insect orders play an important role in pollination, such as Diptera. Among them, hoverflies (Syrphidae) are one of the largest families and can be exposed to contaminated animal excreta and manure. Adult Rhingia campestris feed on pollen and nectar or honeydew, but their larvae are coprophagous. As a result, adults lay their eggs on the grass around cow manure [47,48]. For other pollinators such as Lepidoptera, besides air and water contamination, the soil compartment can be a means of exposure. Indeed, during the spring, the larvae of moth species dwell in the upper layer of the soil surface [49].
Furthermore, residue in soil has proved to be absorbed by plants, making them available to pollinators as they appear in the pollen or nectar of flowers [50]. The persistence or solubility in water of VMPs can lead to the contamination of flowers in the meadow where animals graze, or contaminate wild flowers in the surroundings. For example, Gassner et al., (1997) assessed the persistence of ear-tags permethrin (40:60 cis-trans-permethrin) in the environment after treatment. In the grass where the animal rested, 1 μg/g of permethrin was measured six weeks after treatment, and 0.5 μg/g in the bark of a pine two weeks after the animal had left the pasture [51]. In this way, other organisms can come to be exposed for an extended period to low doses of insecticide still present in the grass. In another example, 19 residual pesticides along with fipronil sulfone compound (1.7–3.6 μg/kg) and imidacloprid (1.1–5.7 μg/kg) were found in pollen loads collected by honeybees in France [52].
Finally, pharmaceutical-laden dust loaded with antiparasitic substances can arise from feces contaminated with products [53,54], which may drift, deposit, and accumulate on flowers foraged by bees. By way of illustration, Peterson et al. (2017) show how VMPs can contaminate wildflowers near cattle feeding yards through dust [55] (Fig. 1).
4. Ways in which pollinators are exposed to chemical products
Bees are exposed to multiple stressors in fluctuating environmental conditions. As for pesticides, the exposure pathways of pollinators to chemical products, VMPs, and Biocides depend on their biology and social behavior [56]. Residues of VMPs and Biocides can generate a range of effects which depends on the characteristics of the active substance, its concentration, pharmaceutical preparation, mode of administration, and the species tested. These chemical products can be distributed in different environmental compartments depending on their physicochemical properties, application quantities and pattern, and environmental characteristics. Biocidal risk assessment includes aquatic, terrestrial, and atmospheric compartments, while VMPs' risk assessment includes soil, water and sediment compartments [30,31]. As previously stated, pollinators are exposed to three environmental compartments (terrestrial, aquatic, air). The sediment compartment remains a reservoir of residues that could eventually release chemical compounds in the aforementioned three [57]. It is fair to consider that pollinators are exposed to all environmental compartments considered in the risk assessment of VMPs and Biocides considering the large distribution, biology, and biotopes of different pollinator species. The relevance of the exposure will depend on the considered species (Table 1).
Table 1.
Potential exposure pathways of pollinators to VMPs and Biocides considering all environmental compartments (+: assumed low route of exposure, ++: assumed moderate route of exposure, +++: assumed relevant route of exposure, ++++: assumed high route of exposure).
| Potential compartments of exposure | ||||||
|---|---|---|---|---|---|---|
| Pollinator groups | Water (including animal excreta) | Soil | Plants | Air | References | |
| Hymenoptera | Social bees (honeybees, bumblebees, stingless bees) | +++ | + | +++ | +++ | [42,101] |
| Ground-nesting bees (mining bees, sweat bees) | ++ | ++++ | +++ | +++ | [[42], [43], [44], [45]] | |
| Other Hymenoptera (wasp) | ++ | + | +++ | +++ | ||
| Diptera (hoverflies, flies, dagger flies) | ++++ | ++++ | +++ | +++ | [47,48,102] | |
| Lepidoptera (butterflies, moths) | ++ | +++ | +++ | +++ | [49] | |
| Coleoptera (beetles) | ++ | ++++ | +++ | +++ | [25,66,70] | |
VMPs and Biocides broadly used in livestock and affecting non-targeted species such as dung beetles and aquatic organisms can also affect bees. The VMPs considered in this study, being antiparasitic animal drugs used in livestock farming, pose a risk to bees. However, that does not exclude the potential impacts of other VMPs on pollinators.
The term Biocides has many definitions. The first use of the term Biocides dates back to 1947 and describes substances that destroy or inhibit the growth or activity of living organisms [58]. However, the Regulation (EU) No. 528/2012 restricts the definition of Biocidal products to “active substances and preparation composed of one or further active substance assembled in the form in which it is supplied to the user, designed to render harmless, destroy, prevent an action, or else apply a controlling effect on any harmful organism by chemical or biological means” [59]. For our study, the term “Biocides” designates all product types with repellent and insecticidal qualities used on livestock premises and the equipment used to control flies and other insects [31]. This type of Biocides is classified by the European Chemical Agency (ECHA) as “product type 3 and 18” [31].
VMPs, such as ivermectin and abamectin, are among the most extensively used endectocidal drugs. In Europe, they are also authorized as Biocides and pesticides to treat plants against pests [60,61]. They belong to the group of avermectins used in agriculture [62]. Hence, ivermectin is a VMP, but due to its broad spectrum of biological uses, we consider it as a Biocide, even though it is not authorized as such in Europe because it does not conform to the legal definition (Table 2). Permethrin and deltamethrin are farm premise disinfectants and insecticides used to fight against biting midges (Culicoides spp.) [63], and, therefore these products are authorized as Biocides. Besides, permethrin and deltamethrin asVMPs are directly administrable on cattle and small animals as a pour-on insecticide as flea and tick control agents [63,64].
Table 2.
Chemical products and their main use.
| VMPs | Biocides | Phytosanitary and Plant protection product (PPP) | References | |
|---|---|---|---|---|
| Ivermectin | Endectocides* | Biocide in the antifouling system, pre-registered substances as Biocides at the ECHA | – | [69,82,103] |
| Abamectin | Endectocides | insecticides, acaricides, arthropod control (Product type 18**) | PPP | [69,104,105] |
| Permethrin | Flea and tick controls | insecticides, acaricides, arthropod controls (Product type 18) | – | [63,106,107] |
| Deltamethrin | Ectoparasites control in ruminants | insecticides, acaricides, arthropod control (Product type 18) | PPP | [63,81,108,109] |
* Against internal and external parasites; ** Product type – 18: pest control product approved by the ECHA.
Some active substances such as those in Table 2 can be used as VMPs and Biocides, even as Plant Protection Products, and can have therapeutic, prophylactic or phytosanitary uses and are toxic to the targeted and non-targeted species. From a One Health perspective, we propose to use the term “Multi-use substances” for active ingredients with this versatility of use, whose residues end up in the environment endangering non-target species. Table 3 shows some examples of insecticidal substances used in animal husbandry and some toxicological effects on bees as described in the literature.
Table 3.
Examples of active substances authorized as VMP, Biocide and pesticide, and their sublethal effects and acute toxicity values on bees (LC50 and LD50: lethal concentration and lethal dosage leading to the death of 50% of the studied population).
| Active substances | Families | Authorization framework | Sublethal effects | Acute toxicity | Species | References |
|---|---|---|---|---|---|---|
| Ivermectin | Macrocyclic lactone | VMP | Inhibition of long-term olfactory memory | LC50 = 570 ng/mL (24 h, oral) LD50 = 1.316 μg a.i/bee (topical) |
Apis mellifera | [76,77] |
| Abamectin | Macrocyclic lactone | VMP/Biocide/ Pesticide | Digestive disorder affecting colonies' health and vitality | LD50 = 0.011 μg a.i/bee (oral) LD50 = 7.8 μg a.i/bee (topical) |
Apis mellifera | [75,110,111] |
| Deltamethrin | Pyrethroid | VMP/Biocide/ Pesticide | Neurotoxic symptoms, decreased foraging activity, alteration of learning performance, disorientation, decreased fertility | LD50 = 0.850 μg a.i/bee (oral) LD50 = 0.0016 ng/bee (topical) |
Apis mellifera Megachile rotundata |
[94,[111], [112], [113], [114]] |
| Permethrin | Pyrethroid | VMP/Biocide | Lower learning response, Disruption of odor perception |
LD50 = 0.057 μg/bee (topical) | Megachile rotundata | [94,115,116] |
| Imidacloprid | Neonicotinoid | VMP/Biocide/ Pesticide | Disruption of olfactory learning, decreased foraging activity, reduced climbing ability, neurotoxic symptoms | LD50 = 5.4 ng/bee (24 h, oral) LD50 = 6.7 ng/bee (24 h, topical) |
Apis mellifera | [113,117,118] |
5. “Multi-use substances”: case studies on their environmental impact and effect on bees
5.1. Case study 1: ivermectin & abamectin
Ivermectin and abamectin are active ingredients within the family of avermectin. They belong to the class of macrocyclic lactones, and are used in nematicidal, acaricidal and insecticidal activities [65]. They are the most broadly used compounds within the avermectin family [66], which can be one reason why there exists a large body of literature showing investigations of their ecotoxicological profile and impact on terrestrial and aquatic environments [26,66,67]. Abamectin (avermectin B1) is a natural compound extracted from the fermentation of Streptomyces avermitilis [68]. Ivermectin is produced from a catalytic reduction of a 22,23-double bond of abamectin [67]. It is composed of 80% of 22,23-dihydro avermectin B1a and 20% of 22,23-dihydro avermectin B1b [66]. Ivermectin and abamectin are classified as neurotoxins. They alter the nervous system function of invertebrates by blocking the ion tropic α-aminobutyric acid (GABA), which may lead to paralysis [26,66,68]. As previously mentioned, they are used as endectocides to control internal and external parasites of livestock [26]. They have injectable and topical formulations, the latter being poured along the grazing animal's dorsal midline [67,69].
Ivermectin's effect on non-targeted species such as dung beetles is well-documented [25,66,70]. As was shown in the Lumaret et al., (1993) study, the exposure of dung beetles (Euoniticellus fulvus) to dung from cattle treated with ivermectin (0.20 mg/kg b.w) caused a delay in the development of the beetles 30 days post cattle injection [70]. Pour-on cattle application of ivermectin affected the survival of adult dung beetles (Liatongus minutus) during the first two weeks post-treatment and possibly delayed their development [71].
Avermectins are highly toxic to Apis mellifera, with acute toxicity resulting in an average LD50 of 0.04 μg/bee [72]. Oral toxicity of bees to abamectin showed an LD50 of 0.009 μg/bee [73]. An LD50 of 0.002 and 0.017 μg/bee at 24 and 48 h respectively resulted from contact toxicity to bees from foliage treated with abamectin. Acute oral toxicity of abamectin on bumblebees resulted in an LD50 of 0.014 μg/bee calculated at 72 h after ingestion. In comparison, the acute topical toxicity showed an LD50 of 0.07 μg/bee (within 72 h) [74]. Toxicity studies with abamectin (at 0.1 ppm) and deltamethrin (at 2.50 ppm) on the forager species Apis mellifera jementica showed lethal times (LT50) of 21.02 h and 72.01 h, respectively [74]. These chemicals also alter the midgut cells, possibly leading to digestive disorders and malnutrition, thus impacting honeybee colonies and their vitality [75].
The study carried out by Hassani et al., (2008) shows that a low dose of ivermectin (0.01 ng/bee) impaired the glutamate-mediated long term olfactory memory of honeybees, while a higher dose of 0.05 ng/bee had no effect [76]. The potential interaction of several compounds (fumagillin, pristine, quercetin) with specific enzymes (multidrug resistance transporters) can raise Ivermectin's toxicity to honeybees, thus increasing their mortality [77]. Nonetheless, ivermectin alone is falsely considered by Guseman et al., (2016) as a non-significant threat to honeybees because it is not considered by them as a crop pesticide, even though it is a widely distributed VMP.
5.2. Case study 2: deltamethrin and permethrin.
Deltamethrin and permethrin belong to the family of synthetic pyrethroids (SPs), which are synthetic derivatives of natural pyrethrins from the plant Chrysanthemum cinerariaefolium [78]. They have neurotoxic insecticidal properties and can disrupt nerve function [79]. However, these SPs have been widely classified as two types based on the symptomatology of insects and mammals exposed to the products [80]. Permethrin belongs to type I pyrethroids which causes tremor type symptoms in mammals (T). In contrast, deltamethrin belongs to type II pyrethroids, which contain a moiety of α-cyano-3-phenoxybenzyl alcohol and generally induce choreoathetosis/salivation (CS) [78,80]. Deltamethrin is the active substance found in different Biocidal commercial products in Europe [81]. ECHA classifies it as Biocides Product-Type 18, i.e. all chemical products used as acaricides, insecticides and products to control other arthropods [82]. Permethrin is also a Biocide used in insecticides, acaricides, and for arthropods control [83].
Deltamethrin and permethrin are approved insecticidal substances used on livestock premises as disinfectants (Biocides) [63], animal treatments (VMPs) [84,85], or plant treatments (pesticides) [86]. As Biocides, they have been used on ruminants to control the “Bluetongue disease”, which is a vector-borne disease caused by the bluetongue virus (BTV), and transmitted to ruminants through the bite of midges (Culicoides spp.) [87].
Deltamethrin and permethrin are used topically as VMPs (spray, dip, pour-on or ear-tags) to control sheep and cattle ectoparasites. Between 96 and 98% of deltamethrin residues used as pour-on treatment in cattle are excreted in the feces. With 0.1 g deltamethrin poured, a concentration of 0.44 μg/ml feces was found three days after treatment [88]. A deltamethrin residue of 0.02–0.1 ppm (mg per liter of dung) can significantly decrease the number of beetles (families: Hydrophilidae, Scarabidae and Staphilinidae) that feed off the dung [89]. A single treatment of cattle (10 ml/100 kg live weight) may cause up to a 75% reduction of dung beetle activity by the end of a season [90]. Similarly, permethrin has shown to be extremely toxic for dung beetles [91].
The acute oral toxicity of deltamethrin in honeybees is an LD50 of 0.08 μg/bee, and its acute contact toxicity is an LD50 of 0.001 μg/bee [92]. Sublethal doses of deltamethrin on honeybee foragers proved to have a detrimental behavioral effect on their learning, memory, and homing behavior by inducing an exacerbated phototropism and thermoregulation of the exposed workers [93]. Piccolomini et al., (2018) studied the effect of synthetic pyrethroid on alfalfa leaf-cutting bees (Megachile rotundata) by measuring their respiration rate to indicate stress response [94]. Deltamethrin showed to be more toxic than permethrin, with an LD50 of 0.0016 μg/bee compared to 0.057 μg/bee, respectively. Likewise, the acute oral toxicity of permethrin to honeybees resulted in an LD50 of 0.3 μg/bee, and its acute contact toxicity led to an LD50 of 0.1 μg/bee [92]. Considering sublethal toxicity, Cox and Wilson (1984) studied the sublethal effects of permethrin on free-flying honeybee foragers inside an insect-proof tunnel. After being exposed to 9 ng/bee, most of the foraging bees could not return to the hive. At the dose of 1 ng/bee, their social activities were reduced, and signs of poisoning were noticed on some individuals [95].
6. Uses of VMPs and Biocides in vector control – environmental impacts
It is required to treat animals and disinfect livestock premises, their surroundings and means of transport with insecticides to prevent “Bluetongue disease” outbreaks (Council Directive 200/75/EC, Article 4, d) iii). Council Directive 200/75/EC is implemented by Regulation n°1266/2007, which states that “the treatment with authorized insecticides of animals, premises and their surroundings in infected holdings should only be carried out following a defined protocol based on the positive outcome of a case-by-case risk assessment which takes into account geographical, epidemiological, ecological, environmental, entomological data and a cost/benefit assessment”. Thanks to the progress made with vaccines, disease control is today less dependent on chemical VMPs and Biocides. BTV vaccines have been developed since 2005, first with a modified live virus, and later, inactivated vaccines for livestock were made available [96]. Vaccination strategies were set up depending on the epidemiological situation of the affected area and the purpose of its possible use, such as decreasing BTV circulation to prevent clinical disease and allowing for safe movement of animals between BTV-affected zones to BTV-free zones [96].
Consequently, Commission Regulation n°1266/2007 was amended by Regulation n°456/2012, which states that the use of authorized insecticides or repellents should not be required as it provides limited additional safety. Still, treatment is required for the animal in transit.
As previously stated, insecticides such as deltamethrin and permethrin are extremely toxic to pollinators [97], which may induce adverse consequences to the environment and non-targeted species. However, no specific evidence has been found regarding the efficacy of these insecticides on Culicoides midges control [63]. Therefore, decreasing the use of these insecticides can drastically limit environmental contamination, provided that Bluetongue spread is controlled through other methods.
7. Data/Knowledge gap
The present study describes the uses, potential exposure and impacts of chemicals used in livestock farming, such as VMPs and Biocides, on bees and other insect pollinators. Based on the One Health approach, since several active ingredients could be considered in this paper as “Multi-use substances”, we highlight critical knowledge gaps:
-
•
To the best of the authors' knowledge, harmonized and centralized statistics of consumption or use of VMPs or Biocides at the national and EU levels are not publicly available. Member States and pharmaceutical industries may have the data, but due to commercial secrets, the data are not accessible to the public. Kools et al., (2008) have reviewed some of the available sources and did an estimation of some families of VMP consumption in Europe [98]. Still, the data per active ingredient is not available.
-
•
Considering the multivalent purpose of many active substances, the overall exposure level and impact of the chemicals released on the environment is unknown. Indeed, the logic surrounding the current regulatory framework involves an in-silo approach to the risks of molecules authorized as VMPs, Biocides or pesticides.
-
•
We know little about the fate of the residues of these active ingredients in the environment and the subsequent levels of exposure of non-target organisms, such as bees.
-
•
Interaction of the multivalent active substances considered in this study with other stressors such as diseases, other pesticides and their potential synergistic effects on bees are rarely studied.
-
•
The research on long term cumulative toxicity, not only for honeybees but also for wild bees and other insect pollinators, is lacking.
-
•
The sublethal effects of active substances approved as VMPs and Biocides on bees and other non-targeted insects are poorly studied.
8. Conclusion
This study highlights that the application of VMPs and Biocides in livestock farming may involve health risks to pollinators, such as bees, which remain unconsidered to date. As a result of their broad application and versatile use, we propose the term “Multi-use substances” for active ingredients authorized as VMPs and Biocides, even Plant Protection Products, that can contaminate the environment and threaten human, animal, and environmental health. Thus, this study is intrinsically linked to the One Health approach.
The EFSA has already carried out a thorough work on risk assessment of pesticides on bees [99]. A new methodology for risk assessment was proposed, including improvements to exposure, toxicity and risk assessment, such as integration of exposure through dust, guttation, water, succeeding crops, and the evaluation of acute, chronic, lethal and sublethal toxicity on adult bees and larvae [100]. We have shown that the regulatory framework of VMPs and Biocides have their shortcomings and how these products affect bees with even a low dose (e.g. an LD50 0.0016 ng/bee of deltamethrin is inducing neurotoxicity symptoms in Apis mellifera [90,[107], [108], [109], [110]]). Therefore, our study has significant implications for understanding how bees face new and unrecognized exposure routes to these chemical products and their potential effects.
Finally, we give an account of the knowledge gaps regarding the matter. Based on our analyses, we conclude that: (1) figures on the marketing/use of these chemicals should be harmonized, centralized, and publicly available, (2) research should be devoted to clarifying the ways of exposure of pollinators to these chemicals, (3) toxicity studies on bees should be carried out, and (4) pollinators should be considered as non-targeted species for the environmental risk assessment before the marketing authorization of VMPs and Biocidal products. We propose the term “Multi-use substances” for active ingredients with versatile use, i.e. authorized as VMPs, Biocides or even Plant Protection Products.
Funding
UNAF (VZ master scholarship), UNAF, BeeLife, Pollinis & TFSP (publication fees); the funders had no role in the conception or drafting and decision to publish this manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Acknowledgements
The authors would like to thank the Union Nationale de l'Apiculture Française (UNAF, France), Fédération Française d'Apiculteur Professionnels (FFAP), Ms. Nicole Russier, The Task Force on Systemic Pesticides (TFSP), The Centre Apicole de Recherche et d'information (CARI, Belgium), BeeLife European Beekeeping Coordination, The Ecole Normale Supérieure (ENS, France) for their support in this project, Mrs. Sara Tan for proofreading this paper, and the reviewers for their feedback. We also thank N. Artiges-Maunoury for our fruitful discussions.
References
- 1.Mackenzie J.S., Jeggo M. The One Health approach—why is it so important? Trop. Med. Infect. Dis. 2019;4 doi: 10.3390/tropicalmed4020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.One Health EJP Project Promoting One Health in Europe through Joint Actions on Foodborne Zoonoses, Antimicrobial Resistance and Emerging Microbiological Hazards. 2020. https://cordis.europa.eu/project/id/773830 (accessed May 22, 2020)
- 3.National Research Council . The National Academies Press; Washington, DC: 1991. Animals as Sentinels of Environmental Health Hazards. [DOI] [PubMed] [Google Scholar]
- 4.Reif J.S. Animal sentinels for environmental and Public Health. Public Health Rep. 2011;126:50–57. doi: 10.1177/00333549111260S108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kevan P.G. Pollinators as bioindicators of the state of the environment: species, activity and diversity. Agric. Ecosyst. Environ. 1999;74:373–393. doi: 10.1016/S0167-8809(99)00044-4. [DOI] [Google Scholar]
- 6.Porrini C., Sabatini A.G., Girotti S., Fini F., Monaco L., Bortolotti L., Ghini S. Vol. 56. 2003. The Death of Honeybees and Environmental Pollution By pesticides: the Honeybees as Biological Indicators; pp. 147–152. [Google Scholar]
- 7.Schindler M., Diestelhorst O., Haertel S., Saure C., Scharnowski A., Schwenninger H.R. Monitoring agricultural ecosystems by using wild bees as environmental indicators. BioRisk. 2013;8:53–71. doi: 10.3897/biorisk.8.3600. [DOI] [Google Scholar]
- 8.Lautenbach S., Seppelt R., Liebscher J., Dormann C.F. Spatial and temporal trends of global pollination benefit. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallai N., Salles J.-M., Settele J., Vaissière B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009;68:810–821. doi: 10.1016/j.ecolecon.2008.06.014. [DOI] [Google Scholar]
- 10.Matias D.M.S., Leventon J., Rau A.-L., Borgemeister C., von Wehrden H. A review of ecosystem service benefits from wild bees across social contexts. Ambio. 2017;46:456–467. doi: 10.1007/s13280-016-0844-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanbergen A.J., the I.P. Initiative Threats to an ecosystem service: pressures on pollinators. Front. Ecol. Environ. 2013;11:251–259. doi: 10.1890/120126. [DOI] [Google Scholar]
- 12.Smith C. 2016. What do Pollinator Declines Mean for Human Health? p. 2. [Google Scholar]
- 13.Smith M.R., Singh G.M., Mozaffarian D., Myers S.S. Effects of decreases of animal pollinators on human nutrition and global health: a modelling analysis. Lancet. 2015;386:1964–1972. doi: 10.1016/S0140-6736(15)61085-6. [DOI] [PubMed] [Google Scholar]
- 14.Deem S.L., Lane-deGraaf K.E., Rayhel E.A. Wiley-Blackwell, John Wiley & Sons; 2018. Introduction to One Health: An Interdisciplinary Approach to Planetary Health. [Google Scholar]
- 15.Halfwerk W., Slabbekoorn H. Pollution going multimodal: the complex impact of the human-altered sensory environment on animal perception and performance. Biol. Lett. 2015;11:20141051. doi: 10.1098/rsbl.2014.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Union Nationale de l'’Apiculture Française UNAF . 2018. How Pesticides Used in Livestock Farming Threaten Bees. [Google Scholar]
- 17.Horvat A.J.M., Babić S., Pavlović D.M., Ašperger D., Pelko S., Kaštelan-Macan M., Petrović M., Mance A.D. Analysis, occurrence and fate of anthelmintics and their transformation products in the environment. TrAC Trends Anal. Chem. 2012;31:61–84. doi: 10.1016/j.trac.2011.06.023. [DOI] [Google Scholar]
- 18.I.U. of P. and A.C. IUPAC permethrin, Int. Union Pure Appliend Chem. 2019. https://sitem.herts.ac.uk/aeru/iupac/Reports/515.htm#3 (accessed July 9, 2020)
- 19.Arnold K.E., Brown A.R., Ankley G.T., Sumpter J.P. Medicating the environment: assessing risks of pharmaceuticals to wildlife and ecosystems. Philos. Trans. R. Soc. B Biol. Sci. 2014;369 doi: 10.1098/rstb.2013.0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morand S., Lajaunie C. 5 - anthropogenic stress. In: Morand S., Lajaunie C., editors. Biodivers. Health. Elsevier; 2018. pp. 63–81. [DOI] [Google Scholar]
- 21.Boxall A.B.A., Fogg L.A., Blackwell P.A., Kay P., Pemberton E.J., Croxford A. Veterinary medicines in the environment. Rev. Environ. Contam. Toxicol. 2004;180:1–91. doi: 10.1007/0-387-21729-0_1. [DOI] [PubMed] [Google Scholar]
- 22.Kools S.A.E., Boxall A., Moltmann J.F., Bryning G., Koschorreck J., Knacker T. A ranking of European veterinary medicines based on environmental risks. Integr. Environ. Assess. Manag. 2008;4:399–408. doi: 10.1897/IEAM_2008-002.1. [DOI] [PubMed] [Google Scholar]
- 23.Davis A.L.V., Scholtz C.H., Kryger U., Deschodt C.M., Strümpher W.P. Dung beetle assemblage structure in Tswalu Kalahari reserve: responses to a mosaic of landscape types, vegetation communities, and dung types. Environ. Entomol. 2010;39:811–820. doi: 10.1603/EN09256. [DOI] [PubMed] [Google Scholar]
- 24.Young O.P. Predation on dung beetles (Coleoptera: Scarabaeidae): a literature review. Trans. Am. Entomol. Soc. 2015;141:111–155. doi: 10.3157/061.141.0110. [DOI] [Google Scholar]
- 25.Jacobs C.T., Scholtz C.H. A review on the effect of macrocyclic lactones on dung-dwelling insects: Toxicity of macrocyclic lactones to dung beetles. Onderstepoort J. Vet. Res. 2015;82 doi: 10.4102/ojvr.v82i1.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lumaret J.-P., Errouissi F., Floate K., Wardhaugh K. A review on the toxicity and non-target effects of macrocyclic lactones in terrestrial and aquatic environments. Curr. Pharm. Biotechnol. 2012;13:1004–1060. doi: 10.2174/138920112800399257. http://www.eurekaselect.com/96888/article (accessed June 4, 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.European Commission . 2018. Regulation (EU) 2019/6 of the European Parliament and of the Council of 11 December 2018 on veterinary medicinal products and repealing Directive 2001/82/EC (Text with EEA relevance) p. 125.http://data.europa.eu/eli/reg/2019/6/oj ELI. [Google Scholar]
- 28.VICH . 2000. Environmental impact assessment (EIAs) for veterinary medicinal products (VMPs) – Phase I. VICH GL 6 (Ecotoxicity Phase I) p. 10. [Google Scholar]
- 29.Robinson J.A. Assessing environmental impacts of veterinary products: lessons from VICH. Drug Inf. J. DIJ Drug Inf. Assoc. 2007;41:169–185. doi: 10.1177/009286150704100208. [DOI] [Google Scholar]
- 30.VICH . VICH GL 38 Ecotoxicity Phase II. 2004. Environmental Impact Assessment for Veterinary Medicinal Products Phase II Guidance; p. 39. [Google Scholar]
- 31.ECHA Guidance on the Biocidal Products Regulation. 2017. https://echa.europa.eu/documents/10162/23036412/biocides_guidance_human_health_ra_iii_part_bc_en.pdf/30d53d7d-9723-7db4-357a-ca68739f5094 (accessed June 3, 2020)
- 32.ECHA News - ECHA Weekly - 11 December 2019. 2019. https://echa.europa.eu/view-article/-/journal_content/title/echa-weekly-11-december-2019 (accessed June 30, 2020)
- 33.Navrátilová M., Raisová Stuchlíková L., Skálová L., Szotáková B., Langhansová L., Podlipná R. Pharmaceuticals in Environment: The Effect of Ivermectin on Ribwort Plantain (Plantago Lanceolata L.) Environ. Sci. Pollut. Res. Int. 2020;27(25):31202–31210. doi: 10.1007/s11356-020-09442-4. https://europepmc.org/article/med/32483720 [DOI] [PubMed] [Google Scholar]
- 34.Iglesias L.E., Saumell C., Sagüés F., Sallovitz J.M., Lifschitz A.L. Ivermectin dissipation and movement from feces to soil under field conditions. J. Environ. Sci. Health Part B. 2018;53:42–48. doi: 10.1080/03601234.2017.1371554. [DOI] [PubMed] [Google Scholar]
- 35.Geoghegan T., Kimberly J., Scheringer M. 2013. Predicting Honeybee Exposure to Pesticides from Vapour Drift using a Combined Pesticide Emission and Atmospheric Transport Model; p. 174.https://scholar.google.com/scholar_lookup?journal=Melbourne&title=Predicting+honeybee+exposure+to+pesticides+from+vapour+drift+using+a+combined+pesticide+emission+and+atmospheric+transport+model.+SETAC+Australasia%E2%80%94Multidisciplinary+approaches+to+managing+environmental+pollution&author=T+Geoghegan&author=J+Kimberly&author=M+Scheringer&publication_year=2013&pages=174& Melbourne. (accessed October 16, 2020) [Google Scholar]
- 36.Girolami V., Mazzon L., Squartini A., Mori N., Marzaro M., Di Bernardo A., Greatti M., Giorio C., Tapparo A. Translocation of neonicotinoid insecticides from coated seeds to seedling guttation drops: a novel way of intoxication for bees. J. Econ. Entomol. 2009;102:1808–1815. doi: 10.1603/029.102.0511. [DOI] [PubMed] [Google Scholar]
- 37.Greatti M., Sabatini A.G., Barbattini R., Rossi S., Stravisi A. Risk of environmental contamination by the active ingredient imidacloprid used for corn seed dressing. Preliminary results. Bull. Insectology. 2003:69–72. [Google Scholar]
- 38.Peterson E.M., Green F.B., Smith P.N. Pesticides used on beef cattle feed yards are aerially transported into the environment via particulate matter. Environ. Sci. Technol. 2020;54:13008–13015. doi: 10.1021/acs.est.0c03603. [DOI] [PubMed] [Google Scholar]
- 39.Butler C.G. The choice of drinking water by the honeybee. J. Exp. Biol. 1940;17:253–261. [Google Scholar]
- 40.Lau P.W., Nieh J.C. Salt preferences of honeybee water foragers. J. Exp. Biol. 2016;219:790–796. doi: 10.1242/jeb.132019. [DOI] [PubMed] [Google Scholar]
- 41.Mommaerts V., Smagghe G. Side-effects of pesticides on the pollinator bombus: An overview. In: Stoytcheva M., editor. Pestic. Mod. World - Pests Control Pestic. Expo. Toxic. Assess. InTech; 2011. [DOI] [Google Scholar]
- 42.Sgolastra F., Hinarejos S., Pitts-Singer T.L., Boyle N.K., Joseph T., Lūckmann J., Raine N.E., Singh R., Williams N.M., Bosch J. Pesticide exposure assessment paradigm for solitary bees. Environ. Entomol. 2019;48:22–35. doi: 10.1093/ee/nvy105. [DOI] [PubMed] [Google Scholar]
- 43.Boyle N.K., Pitts-Singer T.L., Abbott J., Alix A., Cox-Foster D.L., Hinarejos S., Lehmann D.M., Morandin L., O’Neill B., Raine N.E., Singh R., Thompson H.M., Williams N.M., Steeger T. Workshop on pesticide exposure assessment paradigm for non-Apis bees: foundation and summaries. Environ. Entomol. 2019;48:4–11. doi: 10.1093/ee/nvy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson N.L., Harmon-Threatt A.N. Chronic contact with realistic soil concentrations of imidacloprid affects the mass, immature development speed, and adult longevity of solitary bees. Sci. Rep. 2019;9:3724. doi: 10.1038/s41598-019-40031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kopit A.M., Pitts-Singer T.L. Routes of pesticide exposure in solitary, cavity-nesting bees. Environ. Entomol. 2018;47:499–510. doi: 10.1093/ee/nvy034. [DOI] [Google Scholar]
- 46.Bosch J., Vicens N. Sex allocation in the solitary bee Osmia cornuta: do females behave in agreement with Fisher’s theory? Behav. Ecol. Sociobiol. 2005;59:124. doi: 10.1007/s00265-005-0017-8. [DOI] [Google Scholar]
- 47.Klecka J., Hadrava J., Biella P., Akter A. Flower visitation by hoverflies (Diptera: Syrphidae) in a temperate plant-pollinator network. PeerJ. 2018;6 doi: 10.7717/peerj.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sommaggio D. Syrphidae: can they be used as environmental bioindicators? Agric. Ecosyst. Environ. 1999;74:343–356. doi: 10.1016/S0167-8809(99)00042-0. [DOI] [Google Scholar]
- 49.Pisa L.W., Amaral-Rogers V., Belzunces L.P., Bonmatin J.M., Downs C.A., Goulson D., Kreutzweiser D.P., Krupke C., Liess M., McField M., Morrissey C.A., Noome D.A., Settele J., Simon-Delso N., Stark J.D., Van der Sluijs J.P., Van Dyck H., Wiemers M. Effects of neonicotinoids and fipronil on non-target invertebrates. Environ. Sci. Pollut. Res. 2015;22:68–102. doi: 10.1007/s11356-014-3471-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bonmatin J.M., Moineau I., Charvet R., Fleche C., Colin M.E., Bengsch E.R. A LC/APCI-MS/MS method for analysis of imidacloprid in soils, in plants, and in pollens. Anal. Chem. 2003;75:2027–2033. doi: 10.1021/ac020600b. [DOI] [PubMed] [Google Scholar]
- 51.Gassner B., Wüthrich A., Lis J., Scholtysik G., Solioz M. Topical application of synthetic pyrethroids to cattle as a source of persistent environmental contamination. J. Environ. Sci. Health Part B. 1997;32:729–739. doi: 10.1080/03601239709373111. [DOI] [PubMed] [Google Scholar]
- 52.Chauzat M.-P., Faucon J.-P., Martel A.-C., Lachaize J., Cougoule N., Aubert M. A survey of pesticide residues in pollen loads collected by honey bees in France. J. Econ. Entomol. 2006;99:253–262. doi: 10.1093/jee/99.2.253. [DOI] [PubMed] [Google Scholar]
- 53.Blackwell B.R., Wooten K.J., Buser M.D., Johnson B.J., Cobb G.P., Smith P.N. Occurrence and characterization of steroid growth promoters associated with particulate matter originating from beef cattle Feedyards. Environ. Sci. Technol. 2015;49:8796–8803. doi: 10.1021/acs.est.5b01881. [DOI] [PubMed] [Google Scholar]
- 54.Sandoz M.A., Wooten K.J., Clendening S.L., Hensley L.L., Smith L.R., Smith P.N. Transport mechanisms for veterinary pharmaceuticals from beef cattle feedyards to wetlands: is aerial deposition a contributing source? Agric. Ecosyst. Environ. 2018;252:14–21. doi: 10.1016/j.agee.2017.09.016. [DOI] [Google Scholar]
- 55.Peterson E.M., Wooten K.J., Subbiah S., Anderson T.A., Longing S., Smith P.N. Agrochemical mixtures detected on wildflowers near cattle feed yards. Environ. Sci. Technol. Lett. 2017;4:216–220. doi: 10.1021/acs.estlett.7b00123. [DOI] [Google Scholar]
- 56.Sponsler D.B., Grozinger C.M., Hitaj C., Rundlöf M., Botías C., Code A., Lonsdorf E.V., Melathopoulos A.P., Smith D.J., Suryanarayanan S., Thogmartin W.E., Williams N.M., Zhang M., Douglas M.R. Pesticides and pollinators: a socioecological synthesis. Sci. Total Environ. 2019;662:1012–1027. doi: 10.1016/j.scitotenv.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 57.Bonmatin J.-M., Noome D.A., Moreno H., Mitchell E.A.D., Glauser G., Soumana O.S., Bijleveld van Lexmond M., Sánchez-Bayo F. A survey and risk assessment of neonicotinoids in water, soil and sediments of Belize. Environ. Pollut. 2019;249:949–958. doi: 10.1016/j.envpol.2019.03.099. [DOI] [PubMed] [Google Scholar]
- 58.Webster M. Definition of BIOCIDE. 2020. https://www.merriam-webster.com/dictionary/biocide (accessed March 11, 2021)
- 59.Regulation (EU) Commission Implementing Regulation (EU) No 456/2012 of 30 May 2012 amending Regulation (EC) No 1266/2007 on implementing rules for Council Directive 2000/75/EC as regards the control, monitoring, surveillance and restrictions on movements of certain animals of susceptible species in relation to bluetongue Text with EEA relevance. 2012. http://data.europa.eu/eli/reg_impl/2012/456/oj/eng (accessed July 2, 2020)
- 60.Liebig M., Fernandez Á.A., Blübaum-Gronau E., Boxall A., Brinke M., Carbonell G., Egeler P., Fenner K., Fernandez C., Fink G., Garric J., Halling-Sørensen B., Knacker T., Krogh K.A., Küster A., Löffler D., Cots M.Á.P., Pope L., Prasse C., Römbke J., Rönnefahrt I., Schneider M.K., Schweitzer N., Tarazona J.V., Ternes T.A., Traunspurger W., Wehrhan A., Duis K. Environmental risk assessment of ivermectin: a case study. Integr. Environ. Assess. Manag. 2010;6:567–587. doi: 10.1002/ieam.96. [DOI] [PubMed] [Google Scholar]
- 61.Silva H.C., Prette N., Lopes W.D.Z., Sakamoto C.A.M., Buzzulini C., dos Santos T.R., Cruz B.C., Teixeira W.F.P., Felippelli G., Carvalho R.S., Maciel W.G., Soares V.E., da Costa A.J. Endectocide activity of a pour-on formulation containing 1.5 per cent ivermectin +0.5 per cent abamectin in cattle. Vet. Rec. Open. 2015;2 doi: 10.1136/vetreco-2014-000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wislocki P.G., Grosso L., Dybas R.A. Springer-Verlag; 1989. Environmental Aspects of Abamectin in Crop Protection. [Google Scholar]
- 63.EFSA Bluetongue: control, surveillance and safe movement of animals. EFSA J. 2017;15:126. doi: 10.2903/j.efsa.2017.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lecru L.-A., Combarros D., Castilla-Castaño E., Navarro C., Cadiergues M.C. Treatment of Harvest mite infestation in dogs using a permethrin 54.5% and fipronil 6.1% (Effitix®) topical spot-on formulation. Vet. Sci. 2019;6 doi: 10.3390/vetsci6040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lasota J.A., Dybas R.A. Avermectins, a novel class of compounds: implications for use in arthropod pest control. Annu. Rev. Entomol. 1991;36:91–117. doi: 10.1146/annurev.en.36.010191.000515. [DOI] [PubMed] [Google Scholar]
- 66.Bai S.H., Ogbourne S. Eco-toxicological effects of the avermectin family with a focus on abamectin and ivermectin. Chemosphere. 2016;154:204–214. doi: 10.1016/j.chemosphere.2016.03.113. [DOI] [PubMed] [Google Scholar]
- 67.Halley B.A., VandenHeuvel W.J.A., Wislocki P.G. Environmental effects of the usage of avermectins in livestock. Vet. Parasitol. 1993;48:109–125. doi: 10.1016/0304-4017(93)90149-H. [DOI] [PubMed] [Google Scholar]
- 68.Mossa A.-T.H., Mohafrash S.M.M., Chandrasekaran N. Safety of natural insecticides: toxic effects on experimental animals. Biomed. Res. Int. 2018;2018 doi: 10.1155/2018/4308054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Floate K.D. Endectocide use in cattle and fecal residues: environmental effects in Canada. Can. J. Vet. Res. 2006;70:1–10. [PMC free article] [PubMed] [Google Scholar]
- 70.Lumaret J.P., Galante E., Lumbreras C., Mena J., Bertrand M., Bernal J.L., Cooper J.F., Kadiri N., Crowe D. Field effects of ivermectin residues on dung beetles. J. Appl. Ecol. 1993;30:428–436. doi: 10.2307/2404183. [DOI] [Google Scholar]
- 71.Iwasa M., Nakamura T., Fukaki K., Yamashita N. Nontarget effects of ivermectin on coprophagous insects in Japan. Environ. Entomol. 2005;34:1485–1492. doi: 10.1603/0046-225X-34.6.1485. [DOI] [Google Scholar]
- 72.Sánchez-Bayo F. Insecticides mode of action in relation to their toxicity to non-target organisms. J. Environ. Anal. Toxicol. S4. 2012 doi: 10.4172/2161-0525.S4-002. [DOI] [Google Scholar]
- 73.Rugg D., Buckingham S.D., Sattelle D.B., Jansson R.K. 5.2 - the insecticidal macrocyclic lactones. In: Gilbert L.I., editor. Compr. Mol. Insect Sci. Elsevier; Amsterdam: 2005. pp. 25–52. [DOI] [Google Scholar]
- 74.Marletto F., Patetta A., Manino A. Laboratory assessment of pesticide toxicity to bumblebees. Bull. Insectology. 2003:155–158. [Google Scholar]
- 75.Aljedani D.M. Effects of abamectin and deltamethrin to the foragers honeybee workers of Apis mellifera jemenatica (Hymenoptera: Apidae) under laboratory conditions. Saudi J. Biol. Sci. 2017;24:1007–1015. doi: 10.1016/j.sjbs.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.El Hassani A.K., Giurfa M., Gauthier M., Armengaud C. Inhibitory neurotransmission and olfactory memory in honeybees. Neurobiol. Learn. Mem. 2008;90:589–595. doi: 10.1016/j.nlm.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 77.Guseman A.J., Miller K., Kunkle G., Dively G.P., Pettis J.S., Evans J.D., Van Engelsdorp D., Hawthorne D.J. Multi-drug resistance transporters and a mechanism-based strategy for assessing risks of pesticide combinations to honey bees. PLoS One. 2016;11 doi: 10.1371/journal.pone.0148242. e0148242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chrustek A., Hołyńska-Iwan I., Dziembowska I., Bogusiewicz J., Wróblewski M., Cwynar A., Olszewska-Słonina D. Current research on the safety of pyrethroids used as insecticides. Medicina (Mex.). 2018;54 doi: 10.3390/medicina54040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Casida J.E., Durkin K.A. Neuroactive insecticides: targets, selectivity, resistance, and secondary effects. Annu. Rev. Entomol. 2013;58:99–117. doi: 10.1146/annurev-ento-120811-153645. [DOI] [PubMed] [Google Scholar]
- 80.Soderlund D.M., Bloomquist J.R. Neurotoxic actions of pyrethroid insecticides. Annu. Rev. Entomol. 1989;34:77–96. doi: 10.1146/annurev.en.34.010189.000453. [DOI] [PubMed] [Google Scholar]
- 81.ECHA Deltamethrin, Active Substances Factsheet. 2020. https://echa.europa.eu/nl/information-on-chemicals/biocidal-active-substances/-/disas/factsheet/24/PT18
- 82.Product-types - ECHA 2020. https://echa.europa.eu/regulations/biocidal-products-regulation/product-types (accessed May 27, 2020)
- 83.Michalak I., Chojnacka K. Biocides. In: Wexler P., editor. Encycl. Toxicol. Third ed. Academic Press; Oxford: 2014. pp. 461–463. [DOI] [Google Scholar]
- 84.(European Medicine Agency) EMA Deltamethrin, Summary Report, Committee for Medicinal Products for Veterinary Use (Extrapolation to all ruminants) 2004. https://www.ema.europa.eu/en/documents/mrl-report/deltamethrin-extrapolation-all-ruminants-summary-report-4-committee-veterinary-medicinal-products_en.pdf (accessed July 2, 2020)
- 85.(European Medicine Agency) EMA Permethrin, summary report , Committee for veterinary medicinal products. 2002. https://www.ema.europa.eu/en/documents/mrl-report/permethrin-summary-report-3-committee-veterinary-medicinal-products_en.pdf (accessed July 2, 2020)
- 86.European Commission Commission Implementing Regulation (EU) 2019/1589 of 26 September 2019 amending Implementing Regulation (EU) No 540/2011 as regards the extension of the approval periods of the active substances amidosulfuron, beta-cyfluthrin, bifenox, chlorotoluron, clofentezine, clomazone, cypermethrin, daminozide, deltamethrin, dicamba, difenoconazole, diflubenzuron, diflufenican, fenoxaprop-P, fenpropidin, fludioxonil, flufenacet, fosthiazate, indoxacarb, lenacil, MCPA, MCPB, nicosulfuron, picloram, prosulfocarb, pyriproxyfen, thiophanate-methyl, triflusulfuron and tritosulfuron (Text with EEA relevance.) 2019. http://data.europa.eu/eli/reg_impl/2019/1589/oj/eng (accessed September 25, 2020)
- 87.Wilson A.J., Mellor P.S. Bluetongue in Europe: past, present and future. Philos. Trans. R. Soc. B Biol. Sci. 2009;364:2669–2681. doi: 10.1098/rstb.2009.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Venant A., Belli P., Borrel S., Mallet J. Excretion of deltamethrin in lactating dairy cows. Food Addit. Contam. 1990;7:535–543. doi: 10.1080/02652039009373916. [DOI] [PubMed] [Google Scholar]
- 89.Chihiya J., Gadzirayi C.T., Mutandwa E. Effect of three different treatment levels of deltamethrin on the numbers of dung beetles in dung pats. Afr. J. Agric. Res. 2006;1:074–077. [Google Scholar]
- 90.Wardhaugh K., Longstaff B., Lacey M. Effects of residues of deltamethrin in cattle feces on the development and survival of three species of dung breeding insect. Aust. Vet. J. 1998;76:273–280. doi: 10.1111/j.1751-0813.1998.tb10159.x. [DOI] [PubMed] [Google Scholar]
- 91.Lumaret J.-P., Errouissi F. Use of anthelmintics in herbivores and evaluation of risks for the non target fauna of pastures. Vet. Res. 2002;33:547–562. doi: 10.1051/vetres:2002038. [DOI] [PubMed] [Google Scholar]
- 92.International Commission for Bee Botany . International Commission for Bee Botany, S.l; 1982. Symposium on the harmonization of methods for testing the toxicity of pesticides to bees, Second symposium on the harmonization of methods for testing the toxicity of pesticides to bees. [Google Scholar]
- 93.Belzunces L., Tchamitchian S., Brunet J.-L. Neural effects of insecticides in the honeybee. Apidologie. 2012;43:348–370. doi: 10.1007/s13592-012-0134-0. [DOI] [Google Scholar]
- 94.Piccolomini A.M., Whiten S.R., Flenniken M.L., O’Neill K.M., Peterson R.K.D. Acute toxicity of permethrin, Deltamethrin, and Etofenprox to the alfalfa Leafcutting bee. J. Econ. Entomol. 2018;111:1001–1005. doi: 10.1093/jee/toy014. [DOI] [PubMed] [Google Scholar]
- 95.Cox R.L., Wilson W.T. Effects of Permethrin on the Behavior of Individually Tagged Honey Bees, Apis mellifera L. (Hymenoptera: Apidae) Environ. Entomol. 1984;13:375–378. doi: 10.1093/ee/13.2.375. [DOI] [Google Scholar]
- 96.Zientara S., Sánchez-Vizcaíno J.M. Control of bluetongue in Europe. Vet. Microbiol. 2013;165:33–37. doi: 10.1016/j.vetmic.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 97.Maund S.J., Campbell P.J., Giddings J.M., Hamer M.J., Henry K., Pilling E.D., Warinton J.S., Wheeler J.R. Ecotoxicology of synthetic pyrethroids. Top. Curr. Chem. 2012;314:137–165. doi: 10.1007/128_2011_260. [DOI] [PubMed] [Google Scholar]
- 98.Kools S.A.E., Moltmann J.F., Knacker T. Estimating the use of veterinary medicines in the European union. Regul. Toxicol. Pharmacol. 2008;50:59–65. doi: 10.1016/j.yrtph.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 99.EFSA Scientific Opinion on the science behind the development of a risk assessment of Plant Protection Products on bees (Apis mellifera, Bombus spp. and solitary bees) EFSA J. 2012;10:2668. doi: 10.2903/j.efsa.2012.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.EFSA Guidance on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees) EFSA J. 2013;11:3295. doi: 10.2903/j.efsa.2013.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Simon-Delso N., San Martin G., Bruneau E., Delcourt C., Hautier L. The challenges of predicting pesticide exposure of honeybees at landscape level. Sci. Rep. 2017;7:3801. doi: 10.1038/s41598-017-03467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Basley K., Davenport B., Vogiatzis K., Goulson D. Effects of chronic exposure to thiamethoxam on larvae of the hoverfly Eristalis tenax (Diptera, Syrphidae) PeerJ. 2018;6 doi: 10.7717/peerj.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu C., Yan B., Duan J., Hou B. Biofilm inhibition effect of an ivermectin/silyl acrylate copolymer coating and the colonization dynamics. Sci. Total Environ. 2020;736:139599. doi: 10.1016/j.scitotenv.2020.139599. [DOI] [PubMed] [Google Scholar]
- 104.Anastassiadou M., Bernasconi G., Brancato A., Cabrera L.C., Greco L., Jarrah S., Kazocina A., Leuschner R., Magrans J.O., Miron I., Nave S., Pedersen R., Reich H., Rojas A., Sacchi A., Santos M., Stanek A., Theobald A., Vagenende B., Verani A. Setting of import tolerances for abamectin in various crops. EFSA J. 2020;18 doi: 10.2903/j.efsa.2020.6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.ECHA Guidance on the Biocidal Products Regulation. 2020. https://echa.europa.eu/documents/10162/23036412/bpr_guidance_vol_i_parts_abc_en.pdf/31b245e5-52c2-f0c7-04db-8988683cbc4b (accessed June 3, 2020)
- 106.ECHA Assessment Report of Prmethrin, Product-Type 18 (Insecticides, Acaricides and Products to Control Other Arthropods) 2014. http://dissemination.echa.europa.eu/Biocides/ActiveSubstances/1342-18/1342-18_Assessment_Report.pdf
- 107.Navarro C., Reymond N., Crastes N., Bonneau S. Efficacy and safety of a permethrin-fipronil spot-on solution (Effitix®) in dogs naturally infested by ticks in Europe. Biomed. Res. Int. 2016;2016 doi: 10.1155/2016/9498604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brancato A., Brocca D., Lentdecker C.D., Erdos Z., Ferreira L., Greco L., Jarrah S., Kardassi D., Leuschner R., Lythgo C., Medina P., Miron I., Molnar T., Nougadere A., Pedersen R., Reich H., Sacchi A., Santos M., Stanek A., Sturma J., Jose T., Anne T., Vagenende B., Verani A., Villamar-Bouza L. Modification of the existing maximum residue level for deltamethrin in kale. EFSA J. 2018;16 doi: 10.2903/j.efsa.2018.5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marrone R., Ramkumar A., Smaldone G., Rufrano D., Chirollo C., Veneziano V., Danaher M., Anastasio A. Deltamethrin residues in milk and cheese of lactating goats (Capra hircus) Molecules. 2019;24 doi: 10.3390/molecules24030517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Domatskaya T.F., Domatskiy A.N., Levchenko M.A., Silivanova E.A. Acute contact toxicity of insecticidal baits on honeybees Apis Mellifera: a laboratory study. Ukr. J. Ecol. 2018;8:887–891. [Google Scholar]
- 111.Sarto M., Oliveira E., Guedes R., Campos L. Differential insecticide susceptibility of the Neotropical stingless bee Melipona quadrifasciata and the honeybee Apis mellifera. Apidologie. 2014;45:626–636. doi: 10.1007/s13592-014-0281-6. [DOI] [Google Scholar]
- 112.Dai P.-L., Wang Q., Sun J.-H., Liu F., Wang X., Wu Y.-Y., Zhou T. Effects of sublethal concentrations of bifenthrin and deltamethrin on fecundity, growth, and development of the honeybee Apis mellifera ligustica. Environ. Toxicol. Chem. 2010;29:644–649. doi: 10.1002/etc.67. [DOI] [PubMed] [Google Scholar]
- 113.Decourtye A., Devillers J., Cluzeau S., Charreton M., Pham-Delègue M.-H. Effects of imidacloprid and deltamethrin on associative learning in honeybees under semi-field and laboratory conditions. Ecotoxicol. Environ. Saf. 2004;57:410–419. doi: 10.1016/j.ecoenv.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 114.Vandame R., Meled M., Colin M.-E., Belzunces L.P. Alteration of the homing-flight in the honeybee Apis mellifera L. Exposed to sublethal dose of deltamethrin. Environ. Toxicol. Chem. 1995;14:855–860. doi: 10.1002/etc.5620140517. [DOI] [Google Scholar]
- 115.Haynes K.F. Sublethal effects of neurotoxic insecticides on insect behavior. Annu. Rev. Entomol. 1988;33:149–168. doi: 10.1146/annurev.en.33.010188.001053. [DOI] [PubMed] [Google Scholar]
- 116.Mamood A.N., Waller G.D. Recovery of learning responses by honeybees following a sublethal exposure to permethrin. Physiol. Entomol. 1990;15:55–60. doi: 10.1111/j.1365-3032.1990.tb00492.x. [DOI] [Google Scholar]
- 117.Suchail S., Guez D., Belzunces L.P. Characteristics of imidacloprid toxicity in two Apis mellifera subspecies. Environ. Toxicol. Chem. 2000;19:1901–1905. doi: 10.1002/etc.5620190726. [DOI] [Google Scholar]
- 118.Wu Y.-Y., Luo Q.-H., Hou C.-S., Wang Q., Dai P.-L., Gao J., Liu Y.-J., Diao Q.-Y. Sublethal effects of imidacloprid on targeting muscle and ribosomal protein related genes in the honeybee Apis mellifera L. Sci. Rep. 2017;7:15943. doi: 10.1038/s41598-017-16245-0. [DOI] [PMC free article] [PubMed] [Google Scholar]