Abstract
Arthroscopic rotator cuff repair is being performed by an ever-increasing number of surgeons. With an ageing population and growing patient expectations it is crucial that clinical outcomes are optimised. Anatomical reduction of the tendon back to its footprint with minimal tension contributes to this, but this can only be achieved if key biomechanical factors are taken into consideration. In this review of the technical aspects of a rotator cuff repair, we focus on: (1) patient positioning, (2) biomechanical principles, (3) optimal visualisation, and (4) repair techniques for both anterior and postero-superior tears.
Keywords: Shoulder arthroscopy, Rotator cuff injuries, Rotator cuff repair, Shoulder pain
1. Introduction
Rotator cuff tears affect 30–50% of persons over the age of 50 years and are a common cause of function-limiting pain and weakness of the shoulder.1,2 Many choose to have surgery due to disabling or progressive symptoms and this has been reflected in a 500% increase in the rate of repair since 2001.3 In the United States, an estimated 75,000 rotator cuff surgeries are performed annually, and this number is likely to rise given an ageing population.4
Arthroscopic rotator cuff repair has gained in popularity over the years because it minimises damage to the deltoid, allows comprehensive visualisation of the glenohumeral joint and permits the identification of concomitant pathology. With an advancement in arthroscopic technology and the development of novel techniques, anatomic repair can be achieved despite tendon retraction and poor tissue quality.5
The purpose of this review is to outline important technical concepts governing arthroscopic rotator cuff repair.
2. Patient positioning
Rotator cuff repair can be performed in either the lateral decubitus or beach-chair positions. Each presents unique challenges, advantages, and disadvantages (Table 1). When placed laterally, the patient is supported on a beanbag with the knees flexed and the head in neutral. If desired, 30° of posterior table tilt will make the glenoid parallel to the floor. The surgical arm is placed in a traction device with a rotating hinge to allow the shoulder to move freely in all directions, providing access to different parts of the humeral head for anchor placement. Traction should be limited to 15–20 lbs to minimise the risk of nerve injury (estimated to occur in up to 10% of cases).6
Table 1.
Comparison of lateral decubitus and beach chair positioning.
| Lateral Decubitus | Beach Chair | |
|---|---|---|
| Advantages | 1. Traction increases space in the glenohumeral joint and subacromial space. 2. Operating table/patient’s head not in the way of postero-superior shoulder. 3. Bubbles move laterally away from the field of view. 4. Decreased risk of cerebral hypoperfusion. |
1. Anatomic position. 2. Anterior portal is easily accessible. 3. No need to re-position if converting to open procedure. |
| Disadvantages | 1. Non-anatomic orientation. 2. Must reach around arm for anterior portal. 3. Risk of neurovascular injury with traction. 4. Must re-position when converting to open procedure. |
1. Risk of cerebral hypoperfusion. 2. Potential mechanical block to using the arthroscope due to the supportive device located at the posterior aspect of the medial border of the scapula. 3. Risk of eye injury with facemask. |
In the beach-chair position, the patient is placed supine on a dedicated operating table (associated with additional costs) and then the table is maneuvered into the desired semi-sitting position. Key considerations include placing a cushion beneath the knees prior to elevating the trunk, maintaining neutral alignment of the head, and using a positioning device to control the arm. Cerebral hypoperfusion is recognised as one of the most serious complications during arthroscopy in the beach-chair position and occurs in up to 80% of patients.7
3. Control of bleeding
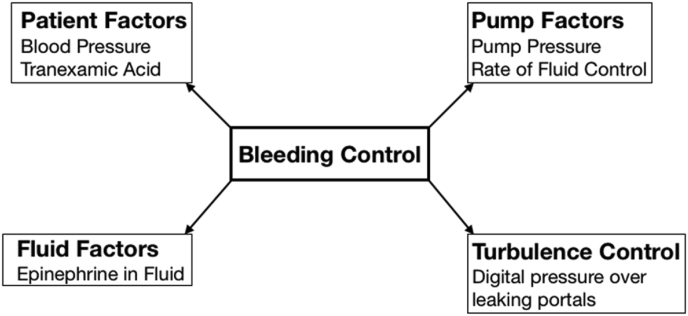
Adequate visualisation is a critical part of any successful arthroscopic operation. One must see the pathology in order to treat it. Control of bleeding is a fundamental component of this. Four factors can be modified to achieve this (Fig. 1): (1) patient factors (blood pressure control and the use of tranexamic acid), (2) pump factors (pump pressure and rate of fluid flow), (3) fluid factors (the use of epinephrine), and (4) turbulence. The most important consideration amongst these is the elimination of turbulence. The Bernoulli effect describes a force at right angles to the arthroscopic fluid that sucks blood from within the capillaries in the subacromial space.8 By applying digital pressure over leaking skin portals, bleeding from turbulence can be effectively controlled. Intra-venous administration of tranexamic acid 10 min before surgery has been shown in a double-blind, prospective randomised controlled trial to significantly improve visual clarity and reduce postoperative analgesic consumption. It should not be used however, in those with pre-existing liver/renal disease, coagulopathy, or concurrent use of anticoagulation medications.9
Fig. 1.
Schematic of factors that can be modified in order to control intra-operative bleeding.
Other measures that may be employed include the addition of epinephrine to the irrigation fluid and increasing pump pressure to create a tamponade effect on bleeding vessels.10 The latter though can paradoxically worsen bleeding in the setting of turbulence due to more blood being drawn out from the capillaries. If these measures collectively fail, then the instruments can be removed and the operation paused for several minutes to allow haemostasis to occur.
4. Reaching the tear
Careful consideration should be given to portal placement which can influence the ease with which the pathology can be approached and treated. Arthroscopic angles encompass both the ‘angle of visualisation’ and the ‘angle of approach.’
The ‘angle of visualisation’ is determined both by the position of the portal used for viewing and the angle of the arthroscope (30° vs 70°). To maximize visualisation, both viewing portals and the angle of the arthroscope can be modified. ‘Angle of approach’ refers to the angle that the instruments approach the tissue and is affected by where a working portal is placed. The internal deltoid fascia can restrict motion in the subacromial space and a small portion may be released to improve mobility. Cannula use can further limit the movement of instruments but is particularly useful when passing sutures and tying knots to prevent the formation of a tissue bridge between sutures.
Accurate portal placement is a foundational skill of arthroscopic shoulder surgery. Planning optimal approach angle and portal placement is assisted with an outside-in technique using an 18-gauge spinal needle. Subsequently “walking” a switching stick down the needle will allow the joint to be entered precisely as planned. Many arthroscopic portals have been described, but Table 2 focusses on those that may be specifically required during a rotator cuff repair.
Table 2.
Common arthroscopic portals used during rotator cuff repair.
| Potential purpose of portal | Portal | Location of portal |
|---|---|---|
| Initial viewing | Posterior | ‘Soft spot’-created by the glenoid medially, humeral head laterally, and rotator cuff superiorly. |
| Approximately 3–4 cm distal and 3–4 cm medial to the posterolateral corner of the acromion. | ||
| Acromioplasty | Anterolateral | In-line with the anterior acromion and 2–3 cm distal to the lateral edge. |
| Debridement of tear and preparation of footprint/viewing the rotator cuff tear | Lateral | In line with the posterior clavicle and 4 cm lateral to the acromion. |
| Subscapularis repair | Anterolateral | In-line with the anterior acromion and 2–3 cm distal to the lateral edge. |
Although the clinical value of subacromial decompression (bursectomy, coracoacromial ligament release, and acromioplasty) may be limited, it increases the working space within the subacromial space thereby enhancing visualisation of the tear, decreasing wear from a type III acromion, and release important growth factors to augment the healing process.11,12 On the other hand, subacromial decompression may contribute antero-superior escape, weakening of the deltoid insertion, and does not result in a better functional outcome following tendon repair.13, 14, 15 With this in mind, we recommend always carrying out a comprehensive bursectomy to aid visualisation whilst reserving a coraco-acromial ligament release ± acromioplasty for those cases where antero-superior escape is not a concern (e.g. partial thickness tears).
5. Biomechanical considerations for repair
The ideal rotator cuff repair construct should optimise suture-to-bone fixation, suture-to-tendon fixation, abrasion resistance of the suture, suture strength, knot security, and loop security.16 Factors influencing this include the repair technique (single row vs double row vs trans-osseous), the suture-tendon interface, type of suture anchor, and arthroscopic knot tying.
5.1. Single row, double row, trans-osseous
Single row repair was the initial technique described for rotator cuff tendon-bone reattachment, but because of the limited ability to restore the anatomic footprint, double row repair configurations were developed. Early double row configurations involved medial mattress sutures not attached to lateral row sutures. More recently, bridge-type constructs link both medial and lateral row anchors. This increases tendon-bone compression, stiffness of the construct, load to failure, and gap formation.17,18 When compared to single row repairs, double row repair typically provides a higher rate of healing.19,20 Therefore, a double row repair is preferred unless it results in a high-tension repair. Over-tensioned double-row repairs as might arise with retracted, immobile tears, and may lead to rotator cuff failure at the musculotendinous junction, medial to the suture fixation.21 To avoid this, a well-executed single row repair may be preferred.21
5.2. Optimizing the suture-tendon interface
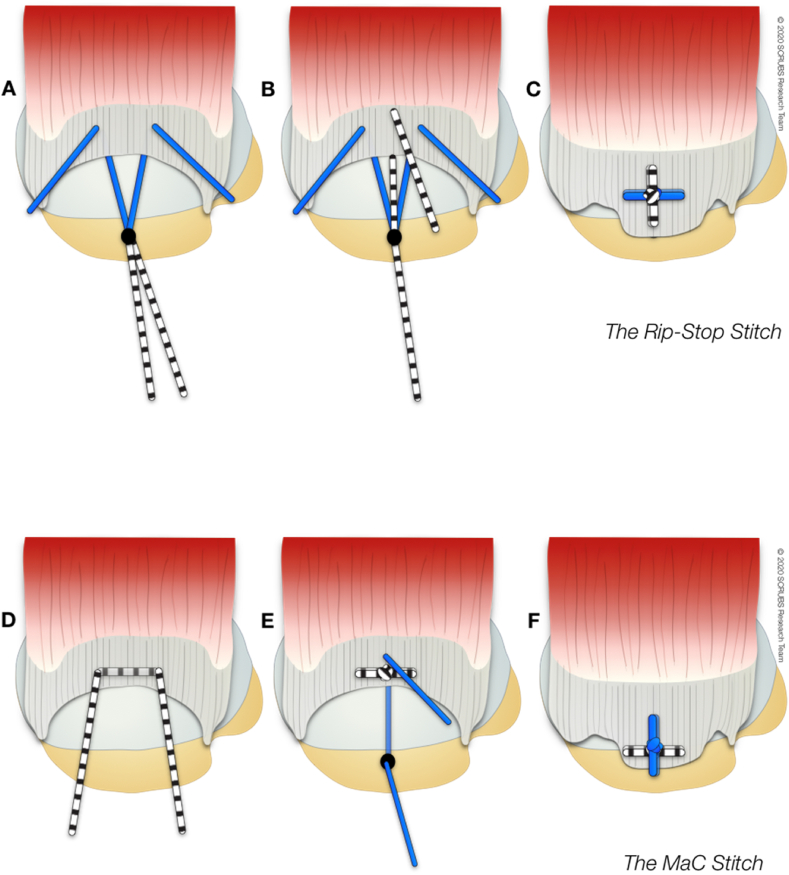
Failure of fixation at the suture-tendon interface has been identified as the most common mechanism of repair failure during revision surgery.22 Use of double or triple loaded anchors is one failure-prevention strategy since doubling the number of suture fixation points reduces the load in each suture by 50%.23 In addition, complex suture configurations have been utilized. While simple and horizontal mattress sutures are a mainstay of securing the tendon to bone, locked suture techniques (i.e. those that involve a vertical limb of suture ‘entrapping’ a horizontal limb of suture) limit gap formation and provide the greatest resistance to repair failure (Fig. 2).24
Fig. 2.
Types of ‘locked’ suture used in arthroscopic rotator cuff repair (A–C) Rip stop25: A load-sharing construct characterised by an inverted horizontal mattress suture (i.e. the ‘rip stop’) secured to a lateral row anchor, combined with a simple suture from a medial row anchor that is placed medial to the horizontal limb. (D–F) Mac stitch/The Massive cuff stitch26: Free horizontal suture loop combined with a simple suture from an anchor. Both sutures are independently tied.
5.3. Anchor insertion
Suture anchor pull-out strength can be optimised by inserting them at a mechanically favourable angle. This ‘deadman angle’ describes both the angle at which the anchor is inserted and the angle that the suture makes with the direction of pull of the rotator cuff. Traditionally, an angle less than 45° has been advocated, but recent evidence suggests that a more vertical entry point corresponding to the angle of applied load more reliably optimises pullout strength.27, 28, 29, 30
5.4. Choice of suture anchor
Suture anchors constitute a strong and stable form of fixation between the torn rotator cuff and its bony footprint. Over the years there has been considerable advancement in their design with the aim of maximising pull-out strength and minimising iatrogenic damage. The long-term arthritic potential of suture anchors is a further concern that is thought to be due to chondral erosion arising from implant migration and breakage.31,32 Recently, vented or coil-type open-architecture suture anchors have been introduced with the potential to induce bony ingrowth into the bone tunnel, release biologically active marrow constituents, and simplify revision. Although clinical outcomes between this novel anchor design and traditional solid screw types are similar, a significantly higher bone mineral density surrounding the coil-type device has been demonstrated.33
5.4.1. Choice of material
The evolution of anchor materials has had the twin purpose of optimizing fixation while addressing problems with existing materials. Early anchors were metallic, and although effective and inexpensive, concerns over migration, interference with diagnostic imaging, and difficulty with revision procedures led to alternative materials being developed. Biodegradable anchors were proposed to overcome these problems, but rapid degradation of early designs caused inflammatory reactions, lytic changes, and the formation of sterile cysts.34 Following degradation, biodegradable anchors are replaced by calcified fibrous tissue rather than bone. These polylactic acid (PLLA) anchors have a degradation time of approximately two years, depending on the polymer used.35 Next, osteoconductive bio-composite materials that encourage bony ingrowth due to the inclusion of β-tricalcium phosphate (β-TCP) and hydroxyapatite (HA) were introduced. These anchors are often reabsorbed 18 months after surgery and have reduced cyst formation over time.36
Polyether ether ketone (PEEK), an alternative to biocomposite materials, is non-degradable plastic which does not interfere with imaging and can be drilled through, aiding revision.31 Studies comparing different types of suture anchor (all-suture vs PEEK vs biocomposite) used for rotator cuff repair have demonstrated variable results with a recent trial finding no significant differences in functional outcome, retear rate, and cyst formation.37
Due to concerns over the arthritic potential of conventional suture anchors, anchors manufactured entirely from suture material were developed. These “soft” anchors consist of a fabric “anchor” sleeve or tape through which an ultrahigh molecular-weight polyethylene-containing suture is woven. When the anchor is inserted into the bone, it is set by pulling the suture limbs, leading to radial expansion and an interference fit in the bone.38 Advantages include a smaller predrilled hole preserving bone, and less disruption to the articular cartilage (a pertinent consideration for a medialised rotator cuff repair at the junction of the articular cartilage and the footprint) due to their smaller size, and softer form.39 A critical factor in the stability of an all-suture anchor is the quality of cortical bone. To optimise this, the ideal angle of insertion should be greater than the conventional 45° (i.e. more vertical) and excessive footprint preparation should be avoided.38 Although there are few clinical studies comparing the differences between different types of suture anchors, all-suture types demonstrate similar biomechanical properties to conventional anchors,38 satisfactory results, and a low rate of retears or loosening needing revision.40
5.5. Type of suture
Failure of the suture-tendon interface is a common mode of cuff repair failure.22,41 Suture factors to consider include material properties, structural design (weaving vs core), and thickness.42 One must also consider suture abrasion effect, ease of tying, and the suture’s effect on knot/loop security. Non-absorbable sutures are usually used for rotator cuff repair, particularly those that are made from ultrahigh molecular weight polyethylene (UHMWP) (e,g, Fiberwire (Arthrex, Naples, USA) and Force Fiber (Wright Medical, USA). However, high strength sutures such as these can potentially cause stress shielding and limit remodelling at the tendon-bone interface.43 Absorbable sutures have shown promise in vitro, resulting in a more mature enthesis.43 In our practice, non-absorbable sutures are used but the lack of comprehensive evidence comparing this to the absorbable equivalent prevents one specific type from being universally advocated.
Sutures may be manufactured as either a standard ‘wire’ or as a wider tape. Tape-type sutures are flat braided materials often used with knotless anchors. A wider contact area at the tendon-bone interface leads to a three-fold increase in footprint contact pressure, load to failure up to 31% higher than a wire, and better force distribution compared to wire sutures.44,45 Collectively, these may prevent suture pull-out but at the expense of creating a larger hole through the tendon that could compromise fixation.42,44,45 Ono et al.42 evaluated the differences between standard suture wire and tape in vitro and demonstrated that whilst tape sutures did initially create a larger hole in the tendon, the hole made by the standard wire suture enlarged during cyclical loading. This implies that tape sutures are protective against suture pull-out and may be useful for larger tears with poor tissue quality. Despite its superior biomechanical properties, rotator cuff repair with a tape does not reduce the retear rate when compared to the standard suture alternatives.45 In our practice, we favour tape sutures in knotless constructs, but use the standard wire sutures for a ‘knotted’ repair because they are pre-loaded within the suture anchors.
5.6. Arthroscopic knots
Following the advent of knotless suture anchors, the need for arthroscopic knots has reduced. It is necessary though to use this form of fixation when faced with certain tear patterns, particularly those that require side to side sutures or marginal convergence. The choice of knot should be based upon knot and loop security. Knot security describes the resistance to slippage when load is applied and depends upon friction, internal interference, and slack between throws. Loop security is the ability to maintain a tight suture loop around tissue as the knot is tied.16 Although many different arthroscopic knots have been described, the surgeons’ static (non-sliding) knot provides an ideal combination of knot and loop security and consists of three half hitches in the same direction followed by three reversing half hitches on alternating posts.46 However, complex sliding knots are popular alternatives and when tied should be ‘locked’ by following it with three reversing half hitches on alternating posts.46
6. Postero-superior tear patterns and repair techniques
Tears of the postero-superior rotator cuff are classified according to the mobility of their free margins and shape. The most frequently encountered types are: (1) crescent-shaped, (2) U-shaped, (3) L-shaped, and (4) massive, contracted, immobile tears.16
6.1. Crescent-shaped tears
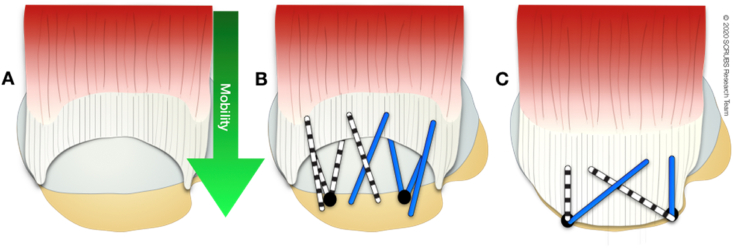
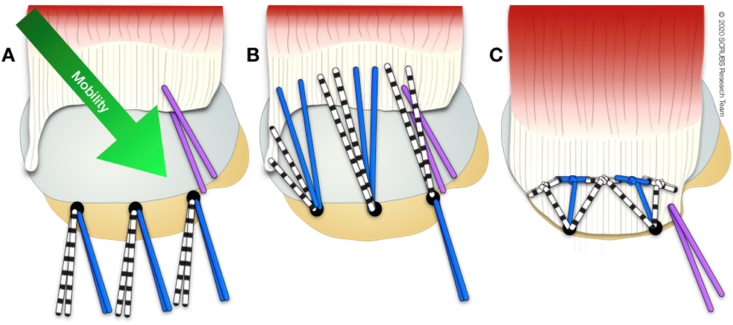
Regardless of size, these tears often exhibit excellent medial-to-lateral mobility and can be repaired directly to bone with minimal tension (Fig. 3).
Fig. 3.
Superior view of a crescent-shaped rotator cuff tear involving supraspinatus and infraspinatus. A: mobility is medial to lateral. B and C: Suture bridge repair is one technique that may be applied to this type of tear. The medial row of pre-loaded anchors is placed and sutures passed. The lateral row is made by crossing sutures and using a knotless anchor.
6.2. U-shaped tears
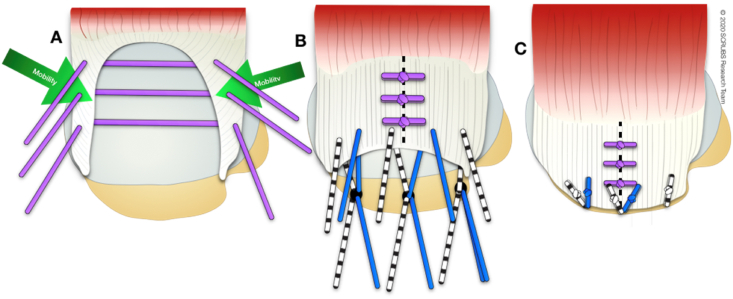
U-shaped tears demonstrate more medial “retraction” and an apex adjacent to the glenoid rim. Recognising this variant is crucial because conventional medial-to-lateral mobilization (as occurs with a crescent shaped tears) results in tensile overload and potential failure. The technique of marginal convergence is useful under these circumstances. Side-to-side (margin convergence) sutures are placed between the anterior and posterior margins of the tear working in a medial-to-lateral direction. This converges the margin of the tear laterally towards the footprint and the free-margin can then be reattached to the bone. A T-shaped repair is the result (Fig. 4).
Fig. 4.
Superior view of a U-shaped rotator cuff tear involving supraspinatus and infraspinatus. A: Mobility is more anterior and posterior to the middle than medial to lateral. This can be closed through margin convergence sutures. B: Side-to-side sutures (margin convergence) create a free margin, which can then be reduced to the bone in a medial to lateral direction. C: In this example, a single row repair technique has been used to finish the repair.
6.3. L-shaped tears
L-shaped and reverse L-shaped tears may look similar to a U-shaped tear, but one of the free margins (i.e. anterior or posterior) is more mobile than the other, allowing it to be reduced more easily to the bone. To achieve this, one must determine which leaf is more mobile and where the apex of the ‘L’ needs to be restored to. A traction suture into the corner of the tear can facilitate this. Side-to-side sutures are then placed along the remaining longitudinal split to achieve marginal convergence. Fixation to bone can then be accomplished (Fig. 5).
Fig. 5.
Superior view of an L-shaped rotator cuff tear involving supraspinatus and infraspinatus. A: The mobility is posteromedial to anterolateral. A traction suture at the anterior corner aids reduction. B and C: In this double-row technique, the medial row of sutures is passed to advance the free edge of the tendon to a reduced position, aided by the traction suture. Once tied, one suture tail from each anchor is placed in a knotless anchor laterally.
6.4. Massive, contracted, immobile tears
Massive, contracted, immobile tears have little to no mobility in both the medial-lateral and anterior-posterior directions, making marginal convergence and direct tendon to bone repair impossible. In these cases, advanced mobilization techniques may be performed to improve mobility of the tissue.
The anterior interval slide involves releasing the tissue (coracohumeral ligament) between supraspinatus and the rotator interval. This is carried out through a laterally-based portal towards the base of the coracoid. Using this method, approximately 1–2 cm of lateral excursion of supraspinatus can be achieved.16 Alternatively, a posterior interval slide releases the interval between the supraspinatus and infraspinatus, and may be performed in isolation or in conjunction with an anterior interval slide. This ‘double interval slide’ is a power tool in the surgeon’s armamentarium and may lead to an extra 4–5 cm of additional mobility of the postero-superior rotator cuff.16 This is particularly relevant for repair of the infraspinatus, which is essential to restoring the action of the posterior cuff.16 The posterior interval slide involves clearing the scapular spine of its fibrofatty tissue, paying particular attention to its junction with the posterior glenoid neck which indicates the position of the suprascapular nerve.
7. Subscapularis repair
Tears of the subscapularis are found in up to 27% of patients undergoing shoulder arthroscopy, with a higher proportion (35%) identified in those with rotator cuff pathology.47 Therefore, identifying and treating associated subscapularis pathology is critical. Tears of subscapularis often begin superiorly and can result in instability of the biceps tendon due to involvement of the medial sling.48 The ‘comma tissue’ signifies the supero-lateral edge of a full-thickness subscapularis tear and represents the confluence between the superior glenohumeral ligament and coracohumeral ligament. It can also help identify the tendon in retracted subscapularis tears. The comma tissue also tethers the anterior leading edge of the supraspinatus tendon to the superior-lateral corner of the subscapularis and represents the anterior rotator cable attachment (important in balancing the force couples of the shoulder). Repairing subscapularis therefore has a number of important contributions: (1) it facilitates repair of the anterior portion of supraspinatus through its tether to the comma tissue, (2) it re-establishes the anterior part of the rotator cable, (3) and the subscapularis may resume its role as the anterior contribution to the force-couple created by the rotator cuff.49
The subscapularis footprint is wider superiorly than it is inferiorly, measuring up to 20 mm in width and 40 mm in length. The tendon inserts on the lesser tuberosity adjacent to the biceps groove along the edge of the articular surface.50 In some patients, contact between the coracoid and lesser tuberosity (sub-coracoid impingement) may be identified due to its potential role in the occurrence of antero-superior rotator cuff tears. Addressing this with a coracoplasty may be considered in a select patient population with narrowing of the coracohumeral interval and corresponding clinical findings (anterior shoulder pain at 120–130° of combined flexion and internal rotation) because it has been demonstrated to reduce pain, increase range of movement, and improve functional outcome.51
7.1. Technical considerations
Visualisation of the subscapularis tear can be challenging and may be improved by placing the arm in traction, forward flexion and internal rotation and by a posteriorly directed force on the upper arm (posterior lever push). A 70° arthroscope may be very useful in this setting as it allows visualisation of the entire footprint and allows one to look “around the corner” of the anterior glenoid.48 Using these techniques, the footprint, medial sling, comma tissue, and position of the biceps can be reliably assessed. In the majority of cases, the medial sling of the biceps will be disrupted and a biceps tenotomy or tenodesis is indicated.
In retracted subscapularis tears, release of the tendon may be required. Placing a traction stitch through the comma sign or upper border of the subscapularis can help pull the medial subscapularis laterally facilitating subsequent releases and repair. A three-sided release of the subscapularis can then be performed. First, an anterior release involves exposing the posterolateral aspect of the coracoid and division of the tissue between the coracoid and the antero-superior subscapularis. A coracoplasty may also be performed as required. Second, a superior release involves resection of the rotator interval and adhesions between the superior border of subscapularis and the inferolateral portion of the coracoid. Finally, a posterior release of the middle glenohumeral ligament, anterior capsule, and posterior border of the subscapularis may be done.
8. Conclusion
Arthroscopic rotator cuff repair is a commonly performed shoulder surgery, and although the technology has evolved over years, this can still be a challenging procedure to execute. It is essential that the surgeon possesses a comprehensive understanding of the biomechanical factors leading to anatomical tendon-bone healing and the techniques that facilitate this. In spite of many potential obstacles during surgery, ensuring adequate visualisation and choosing the most appropriate fixation strategy, can result in a successful outcome and high patient satisfaction.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
None.
Acknowledgements
None.
References
- 1.Zhao S., Zhao J., Dong S. Biological augmentation of rotator cuff repair using bFGF-loaded electrospun poly(lactide-co-glycolide) fibrous membranes. Int J Nanomed. 2014;9:2373–2385. doi: 10.2147/ijn.s59536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isaac C., Gharaibeh B., Witt M., Wright V.J., Huard J. Biologic approaches to enhance rotator cuff healing after injury. J Shoulder Elbow Surg. 2012;21(2):181–190. doi: 10.1016/j.jse.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Hakimi O., Mouthuy P.A., Carr A. Synthetic and degradable patches: an emerging solution for rotator cuff repair. Int J Exp Pathol. 2013;94(4):287–292. doi: 10.1111/iep.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vitale M.A., Vitale M.G., Zivin J.G., Braman J.P., Lu Bigliani, Flatow E.L. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181–187. doi: 10.1016/j.jse.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Baker D.K., Perez J.L., Watson S.L. Arthroscopic versus open rotator cuff repair: which has a better complication and 30-day readmission profile? Arthroscopy. 2017;33(10):1764–1769. doi: 10.1016/j.arthro.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 6.Rains D.D., Rooke G.A., Wahl C.J. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532–541. doi: 10.1016/j.arthro.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Murphy G.S., Szokol J.W., Marymont J.H. Cerebral oxygen desaturation events assessed by near-infrared spectroscopy during shoulder arthroscopy in the beach chair and lateral decubitus positions. Anesth Analg. 2010;111(2):496–505. doi: 10.1213/ANE.0b013e3181e33bd9. [DOI] [PubMed] [Google Scholar]
- 8.Burkhart S.S., Danaceau S.M., Athanasiou K.A. Turbulence control as a factor in improving visualization during subacromial shoulder arthroscopy. Arthroscopy. 2001;17(2):209–212. doi: 10.1053/jars.2001.22298. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y.F., Hong C.K., Hsu K.L. Intravenous administration of tranexamic acid significantly improved clarity of the visual field in arthroscopic shoulder surgery. A prospective, double-blind, and randomized controlled trial. Arthroscopy. 2020;36(3):640–647. doi: 10.1016/j.arthro.2019.10.020. [DOI] [PubMed] [Google Scholar]
- 10.Jensen K.H., Werther K., Stryger V., Schultz K., Falkenberg B. Arthroscopic shoulder surgery with epinephrine saline irrigation. Arthroscopy. 2001;17(6):578–581. doi: 10.1053/jars.2001.23590. [DOI] [PubMed] [Google Scholar]
- 11.Jarvis D.L., Waterman B.R., Verma N.N. Is acromioplasty ever indicated during rotator cuff repair? Arthroscopy. 2019;35(6):1639–1640. doi: 10.1016/j.arthro.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Longo U.G., Petrillo S., Candela V. Arthroscopic rotator cuff repair with and without subacromial decompression is safe and effective: a clinical study. BMC Muscoskel Disord. 2020;21(1):24. doi: 10.1186/s12891-019-3032-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sher J.S., Iannotti J.P., Warner J.J., Groff Y., Williams G.R. Surgical treatment of postoperative deltoid origin disruption. Clin Orthop Relat Res. 1997;343:93–98. [PubMed] [Google Scholar]
- 14.Wiley A.M. Superior humeral dislocation. A complication following decompression and debridement for rotator cuff tears. Clin Orthop Relat Res. 1991;263:135–141. [PubMed] [Google Scholar]
- 15.Milano G., Grasso A., Salvatore M., Zarelli D., Deriu L., Fabbriciani C. Arthroscopic rotator cuff repair with and without subacromial decompression: a prospective randomized study. Arthroscopy. 2007;23(1):81–88. doi: 10.1016/j.arthro.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Burkhart S.S., Lo I.K. Arthroscopic rotator cuff repair. J Am Acad Orthop Surg. 2006;14(6):333–346. doi: 10.5435/00124635-200606000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Lorbach O., Bachelier F., Vees J., Kohn D., Pape D. Cyclic loading of rotator cuff reconstructions: single-row repair with modified suture configurations versus double-row repair. Am J Sports Med. 2008;36(8):1504–1510. doi: 10.1177/0363546508314424. [DOI] [PubMed] [Google Scholar]
- 18.Nelson C.O., Sileo M.J., Grossman M.G., Serra-Hsu F. Single-row modified mason-allen versus double-row arthroscopic rotator cuff repair: a biomechanical and surface area comparison. Arthroscopy. 2008;24(8):941–948. doi: 10.1016/j.arthro.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Chen M., Xu W., Dong Q., Huang Q., Xie Z., Mao Y. Outcomes of single-row versus double-row arthroscopic rotator cuff repair: a systematic review and meta-analysis of current evidence. Arthroscopy. 2013;29(8):1437–1449. doi: 10.1016/j.arthro.2013.03.076. [DOI] [PubMed] [Google Scholar]
- 20.Sugaya H., Maeda K., Matsuki K., Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair. A prospective outcome study. J Bone Joint Surg Am. 2007;89(5):953–960. doi: 10.2106/jbjs.F.00512. [DOI] [PubMed] [Google Scholar]
- 21.Rhee Y.G., Cho N.S., Parke C.S. Arthroscopic rotator cuff repair using modified Mason-Allen medial row stitch: knotless versus knot-tying suture bridge technique. Am J Sports Med. 2012;40(11):2440–2447. doi: 10.1177/0363546512459170. [DOI] [PubMed] [Google Scholar]
- 22.Cummins C.A., Murrell G.A. Mode of failure for rotator cuff repair with suture anchors identified at revision surgery. J Shoulder Elbow Surg. 2003;12(2):128–133. doi: 10.1067/mse.2003.21. [DOI] [PubMed] [Google Scholar]
- 23.Burkhart S.S., Wirth M.A., Simonich M., Salem D., Lanctot D., Athanasiou K. Knot security in simple sliding knots and its relationship to rotator cuff repair: how secure must the knot be? Arthroscopy. 2000;16(2):202–207. doi: 10.1016/s0749-8063(00)90037-9. [DOI] [PubMed] [Google Scholar]
- 24.Koganti A.K., Adamson G.J., Gregersen C.S., Pink M.M., Shankwiler J.A. Biomechanical comparison of traditional and locked suture configurations for arthroscopic repairs of the rotator cuff. Am J Sports Med. 2006;34(11):1832–1838. doi: 10.1177/0363546506289701. [DOI] [PubMed] [Google Scholar]
- 25.Denard P.J., Burkhart S.S. A load-sharing rip-stop fixation construct for arthroscopic rotator cuff repair. Arthrosc Tech. 2012;1(1):e37–e42. doi: 10.1016/j.eats.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma C.B., MacGillivray J.D., Clabeaux J., Lee S., Otis J.C. Biomechanical evaluation of arthroscopic rotator cuff stitches. J Bone Joint Surg Am. 2004;86(6):1211–1216. doi: 10.2106/00004623-200406000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Burkhart S.S. The deadman theory of suture anchors: observations along a south Texas fence line. Arthroscopy. 1995;11(1):119–123. doi: 10.1016/0749-8063(95)90100-0. [DOI] [PubMed] [Google Scholar]
- 28.Green R.N., Donaldson O.W., Dafydd M., Evans S.L., Kulkarni R. Biomechanical study: determining the optimum insertion angle for screw-in suture anchors-is deadman’s angle correct? Arthroscopy. 2014;30(12):1535–1539. doi: 10.1016/j.arthro.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Clevenger T.A., Beebe M.J., Strauss E.J., Kubiak E.N. The effect of insertion angle on the pullout strength of threaded suture anchors: a validation of the deadman theory. Arthroscopy. 2014;30(8):900–905. doi: 10.1016/j.arthro.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 30.Strauss E., Frank D., Kubiak E., Kummer F., Rokito A. The effect of the angle of suture anchor insertion on fixation failure at the tendon-suture interface after rotator cuff repair: deadman’s angle revisited. Arthroscopy. 2009;25(6):597–602. doi: 10.1016/j.arthro.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 31.Visscher L.E., Jeffery C., Gilmour T., Anderson L., Couzens G. The history of suture anchors in orthopaedic surgery. Clin Biomech. 2019;61:70–78. doi: 10.1016/j.clinbiomech.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Godinho G.G., França F.O., Alves Freitas J.M., Aguiar P.N., de Carvalho Leite M. Complications resulting from the use OF metal anchors IN shoulder arthroscopy. Rev Bras Ortop. 2009;44(2):143–147. doi: 10.1016/s2255-4971(15)30061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chahla J., Liu J.N., Manderle B. Bony ingrowth of coil-type open-architecture anchors compared with screw-type PEEK anchors for the medial row in rotator cuff repair: a randomized controlled trial. Arthroscopy. 2020;36(4):952–961. doi: 10.1016/j.arthro.2019.11.119. [DOI] [PubMed] [Google Scholar]
- 34.Barber F.A. Complications of biodegradable materials: anchors and interference screws. Sports Med Arthrosc Rev. 2015;23(3):149–155. doi: 10.1097/jsa.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 35.Barber F.A., Herbert M.A., Richards D.P. Sutures and suture anchors: update 2003. Arthroscopy. 2003;19(9):985–990. doi: 10.1016/j.arthro.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 36.Chung S.W., Lee Y.S., Kim J.Y. Changes in perianchor cyst formation over time after rotator cuff repair: influential factors and outcomes. Am J Sports Med. 2019;47(1):165–172. doi: 10.1177/0363546518810517. [DOI] [PubMed] [Google Scholar]
- 37.Ro K., Pancholi S., Son H.S., Rhee Y.G. Perianchor cyst formation after arthroscopic rotator cuff repair using all-suture-type, bioabsorbable-type, and PEEK-type Anchors. Arthroscopy. 2019;35(8):2284–2292. doi: 10.1016/j.arthro.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 38.Ergün S., Akgün U., Barber F.A., Karahan M. The clinical and biomechanical performance of all-suture anchors: a systematic review. Arthrosc Sports Med Rehabil. 2020;2(3):e263–e275. doi: 10.1016/j.asmr.2020.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazzocca A.D., Chowaniec D., Cote M.P. Biomechanical evaluation of classic solid and novel all-soft suture anchors for glenoid labral repair. Arthroscopy. 2012;28(5):642–648. doi: 10.1016/j.arthro.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 40.Van der Bracht H., Van den Langenbergh T., Pouillon M., Verhasselt S., Verniers P., Stoffelen D. Rotator cuff repair with all-suture anchors: a midterm magnetic resonance imaging evaluation of repair integrity and cyst formation. J Shoulder Elbow Surg. 2018;27(11):2006–2012. doi: 10.1016/j.jse.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 41.Trantalis J.N., Boorman R.S., Pletsch K., Lo I.K. Medial rotator cuff failure after arthroscopic double-row rotator cuff repair. Arthroscopy. 2008;24(6):727–731. doi: 10.1016/j.arthro.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 42.Ono Y., Joly D.A., Thornton G.M., Lo I.K.Y. Mechanical and imaging evaluation of the effect of sutures on tendons: tape sutures are protective to suture pulling through tendon. J Shoulder Elbow Surg. 2018;27(9):1705–1710. doi: 10.1016/j.jse.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 43.Su W., Qi W., Li X., Zhao S., Jiang J., Zhao J. Effect of suture absorbability on rotator cuff healing in a rabbit rotator cuff repair model. Am J Sports Med. 2018;46(11):2743–2754. doi: 10.1177/0363546518787181. [DOI] [PubMed] [Google Scholar]
- 44.De Carli A., Lanzetti R.M., Monaco E., Labianca L., Mossa L., Ferretti A. The failure mode of two reabsorbable fixation systems: swivelock with Fibertape versus Bio-Corkscrew with Fiberwire in bovine rotator cuff. J Orthop Sci. 2012;17(6):789–795. doi: 10.1007/s00776-012-0275-z. [DOI] [PubMed] [Google Scholar]
- 45.Liu R.W., Lam P.H., Shepherd H.M., Murrell G.A.C. Tape versus suture in arthroscopic rotator cuff repair: biomechanical analysis and assessment of failure rates at 6 months. Orthop J Sports Med. 2017;5(4) doi: 10.1177/2325967117701212. 2325967117701212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lo I.K., Burkhart S.S., Chan K.C., Athanasiou K. Arthroscopic knots: determining the optimal balance of loop security and knot security. Arthroscopy. 2004;20(5):489–502. doi: 10.1016/j.arthro.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Bennett W.F. Subscapularis, medial, and lateral head coracohumeral ligament insertion anatomy. Arthroscopic appearance and incidence of "hidden" rotator interval lesions. Arthroscopy. 2001;17(2):173–180. doi: 10.1053/jars.2001.21239. [DOI] [PubMed] [Google Scholar]
- 48.Burkhart S.S., Brady P.C. Arthroscopic subscapularis repair: surgical tips and pearls A to Z. Arthroscopy. 2006;22(9):1014–1027. doi: 10.1016/j.arthro.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 49.Burkhart S.S., Esch J.C., Jolson R.S. The rotator crescent and rotator cable: an anatomic description of the shoulder’s "suspension bridge". Arthroscopy. 1993;9(6):611–616. doi: 10.1016/s0749-8063(05)80496-7. [DOI] [PubMed] [Google Scholar]
- 50.Curtis A.S., Burbank K.M., Tierney J.J., Scheller A.D., Curran A.R. The insertional footprint of the rotator cuff: an anatomic study. Arthroscopy. 2006;22(6):609–e1. doi: 10.1016/j.arthro.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Lo I.K., Parten P.M., Burkhart S.S. Combined subcoracoid and subacromial impingement in association with anterosuperior rotator cuff tears: an arthroscopic approach. Arthroscopy. 2003;19(10):1068–1078. doi: 10.1016/j.arthro.2003.10.016. [DOI] [PubMed] [Google Scholar]