Key Points
Question
Will 10-kHz spinal cord stimulation improve pain relief for patients with painful diabetic neuropathy refractory to medical management?
Findings
In this randomized clinical trial including 216 patients, there was a significant benefit of 10-kHz spinal cord stimulation, with 79% of treatment responders whose underlying neurological deficits did not worsen compared with 5% of controls treated with conventional medical management.
Meaning
Patients with painful diabetic neuropathy with inadequate pain relief despite best available medical treatments should be considered for 10-kHz spinal cord stimulation.
This randomized clinical trial evaluates whether 10-kHz spinal cord stimulation improves outcomes for patients with painful diabetic neuropathy refractory to conventional medical management.
Abstract
Importance
Many patients with diabetic peripheral neuropathy experience chronic pain and inadequate relief despite best available medical treatments.
Objective
To determine whether 10-kHz spinal cord stimulation (SCS) improves outcomes for patients with refractory painful diabetic neuropathy (PDN).
Design, Setting, and Participants
The prospective, multicenter, open-label SENZA-PDN randomized clinical trial compared conventional medical management (CMM) with 10-kHz SCS plus CMM. Participants with PDN for 1 year or more refractory to gabapentinoids and at least 1 other analgesic class, lower limb pain intensity of 5 cm or more on a 10-cm visual analogue scale (VAS), body mass index (calculated as weight in kilograms divided by height in meters squared) of 45 or less, hemoglobin A1c (HbA1c) of 10% or less, daily morphine equivalents of 120 mg or less, and medically appropriate for the procedure were recruited from clinic patient populations and digital advertising. Participants were enrolled from multiple sites across the US, including academic centers and community pain clinics, between August 2017 and August 2019 with 6-month follow-up and optional crossover at 6 months. Screening 430 patients resulted in 214 who were excluded or declined participation and 216 who were randomized. At 6-month follow-up, 187 patients were evaluated.
Interventions
Implanted medical device delivering 10-kHz SCS.
Main Outcomes and Measures
The prespecified primary end point was percentage of participants with 50% pain relief or more on VAS without worsening of baseline neurological deficits at 3 months. Secondary end points were tested hierarchically, as prespecified in the analysis plan. Measures included pain VAS, neurological examination, health-related quality of life (EuroQol Five-Dimension questionnaire), and HbA1c over 6 months.
Results
Of 216 randomized patients, 136 (63.0%) were male, and the mean (SD) age was 60.8 (10.7) years. Additionally, the median (interquartile range) duration of diabetes and peripheral neuropathy were 10.9 (6.3-16.4) years and 5.6 (3.0-10.1) years, respectively. The primary end point assessed in the intention-to-treat population was met by 5 of 94 patients in the CMM group (5%) and 75 of 95 patients in the 10-kHz SCS plus CMM group (79%; difference, 73.6%; 95% CI, 64.2-83.0; P < .001). Infections requiring device explant occurred in 2 patients in the 10-kHz SCS plus CMM group (2%). For the CMM group, the mean pain VAS score was 7.0 cm (95% CI, 6.7-7.3) at baseline and 6.9 cm (95% CI, 6.5-7.3) at 6 months. For the 10-kHz SCS plus CMM group, the mean pain VAS score was 7.6 cm (95% CI, 7.3-7.9) at baseline and 1.7 cm (95% CI, 1.3-2.1) at 6 months. Investigators observed neurological examination improvements for 3 of 92 patients in the CMM group (3%) and 52 of 84 in the 10-kHz SCS plus CMM group (62%) at 6 months (difference, 58.6%; 95% CI, 47.6-69.6; P < .001).
Conclusions and Relevance
Substantial pain relief and improved health-related quality of life sustained over 6 months demonstrates 10-kHz SCS can safely and effectively treat patients with refractory PDN.
Trial Registration
ClincalTrials.gov Identifier: NCT03228420
Introduction
The World Health Organization estimates a total of 422 million adults with diabetes worldwide and a worldwide prevalence (8.5%) that has nearly doubled over 4 decades.1 Diabetes may cause systemic damage with profound impact on health-related quality of life and is potentially life threatening. Diabetic peripheral neuropathy is a common complication presenting as pain and other dysesthesias, including numbness, burning, or tingling. Approximately 20% of patients with diabetes will develop painful diabetic neuropathy (PDN), a progressive, potentially debilitating chronic neuropathic pain condition.2
Current PDN treatments include neuropathic pain medications, such as gabapentinoids, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, opioids, and topical solutions.3,4 High-quality randomized clinical trials (RCTs) demonstrate limited efficacy of these medications with high incidence of adverse effects. Gabapentinoids may increase the risk of respiratory depression, a serious concern for patients taking opioids or with underlying respiratory impairment.5,6,7 Systematic review and meta-analysis of neuropathic pain medication RCTs reported a number needed to treat ranging from 3.6 to 7.7, with a number needed to harm ranging from 11.8 to 25.6.8
Gabapentin and pregabalin are commonly prescribed for PDN, but long-term adherence can be poor, with more than 60% of patients discontinuing by 6 months.9 Duloxetine reveals a similar pattern, with 50% discontinuing by 6 months.9 Most of these patients do not switch to an alternative therapy, leaving their progressive neuropathic pain condition untreated. This represents a large patient population with significant unmet needs.
Nonpharmacological PDN treatment with spinal cord stimulation (SCS) devices was first reported in 1996.10,11 Two prior RCTs demonstrated moderate utility of low-frequency (40- to 60-Hz) SCS with smaller samples (36 to 60 participants) and 6-month to 24-month follow-up.12,13,14 Long-term follow-up of low-frequency SCS shows responder attrition within 12 months.15 Observational data suggest high-frequency (10-kHz) SCS provides substantial pain relief for patients with PDN without generating paresthesias required for other types of SCS.16,17,18 Previous results support 10-kHz SCS as a superior treatment compared with low-frequency SCS for chronic low back and leg pain and effective for nonsurgical low back pain, upper limb and axial neck pain, and neuropathic limb pain while reducing opioid dosages.19,20,21,22,23,24,25,26,27,28
The Comparison of 10 kHz SCS Combined With CMM to CMM Alone in the Treatment of Neuropathic Limb Pain (SENZA-PDN) RCT29 extends observations from low-frequency SCS studies in, to our knowledge, the largest RCT to date to test the hypothesis that 10-kHz SCS combined with conventional medical management (CMM) provides meaningful pain relief compared with CMM alone for patients with refractory PDN.
Methods
Detailed methods have been previously described.29 The Western Institutional Review Board and local institutional review boards approved the protocol, consent form, and study documents prior to study commencement at each site. Participants were enrolled after providing written informed consent. The study was conducted in accordance with the Declaration of Helsinki30 as well as good clinical practices and reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. Participants reported demographic information, including race/ethnicity, and those data were summarized. The study protocol can be found in Supplement 1, and the statistical analysis plan can be found in Supplement 2. The study design has been previously published.29
Design and Outcomes
Patients with PDN were recruited across multiple sites in the US. Key inclusion criteria were PDN diagnosis with symptoms for 12 months or more that was refractory to treatment with gabapentin or pregabalin and at least 1 other class of analgesic, lower limb pain intensity of 5 cm or more on a 10-cm visual analogue scale (VAS), and medically suitable for the proposed procedure. All patients were psychologically evaluated and reviewed by independent medical monitors prior to randomization. Key exclusion criteria were hemoglobin A1c (HbA1c) greater than 10%, body mass index (calculated as weight in kilograms divided by height in meters squared) greater than 45, daily opioid dosage greater than 120 mg morphine equivalents, and upper limb pain intensity of 3 cm or more on a VAS. The primary measure for pain was the VAS.31 Additional pain qualities were assessed by Short-Form McGill Pain Questionnaire (SF-MPQ-2), Douleur Neuropathique (DN4), and modified Neuropathy Symptom Score.32,33,34 Health-related quality of life measures included the Pain and Sleep Questionnaire, Global Assessment of Functioning, EuroQol 5-Dimension Questionnaire (EQ-5D-5L), and patient satisfaction.35,36 Patient safety was assessed via adverse event (AE) monitoring and thorough neurological assessment. A clinical events committee provided oversight of AEs.
Two independent neurologists designed the standardized neurological examination and trained investigators. Lower limb motor function, light touch sensation, and reflexes were assessed in standard fashion developed in collaboration with the US Food and Drug Administration for a prior study.19,20 The neurological assessment also included a 10-point diabetic foot examination with pinprick and Semmes-Weinstein 10-g monofilament testing (eFigure 1 in Supplement 3). Neuropen (Owen Mumford Ltd) was used to deliver consistent, reproducible pressure for testing these modalities, as this has been shown most accurate among commercially available products.37 The neurological assessment was conducted at baseline and follow-up visits and is consistent with American Diabetes Association recommendations for assessing loss of protective sensation as well as American Academy of Neurology’s definition of distal symmetric polyneuropathy.38,39 Investigators used clinical judgment to determine if observed changes were clinically meaningful.
Randomization and Follow-up
Treatment allocations were concealed with computer assignment 1:1 to CMM or 10-kHz SCS plus CMM. Randomization was performed using a random-sized block method by site and stratified by pain severity (VAS) and glycemic control (HbA1c). Data collection will continue for 24 months. Patients could opt to cross over to the other treatment arm at 6 months if they had insufficient pain relief (less than 50% improvement), were dissatisfied with treatment, and were appropriate to proceed as determined by their physician.
SCS Treatment
Patients assigned to the SCS treatment group underwent temporary trial stimulation for 5 to 7 days with percutaneous leads placed epidurally along T8 to T11 (eFigure 2 in Supplement 3). Patients reporting 50% or more pain relief using the VAS were eligible for permanent SCS device implant (Nevro Corp), which included 2 percutaneous leads placed epidurally connected to an implantable pulse generator typically placed in the low back. Stimulation parameters included 10-kHz frequency, 30-μs pulse width delivered via bipole, and amplitude range of 0.5 to 3.5 mA. Optimal bipole location and amplitude were adjusted per patient feedback, as previously described.19
Statistical Analysis
Baseline characteristics were compared for potential imbalance by a standardized difference effect size index (Cohen d). Primary end point outcome was a composite of effectiveness and stable neurological examination requiring 50% or more pain relief by VAS without a meaningful worsening of baseline neurological deficits at 3-month follow-up. Primary analysis involved the intention-to-treat population with known status, with a secondary analysis in the per-protocol population of patients who completed 3-month follow-up as assigned, assessed by Fisher exact test with 2-sided α level of .05. The effects of missing outcome data on the primary end point comparison between groups were examined in sensitivity analyses (eTable 1 in Supplement 3). Beyond primary end point analysis of the intention-to-treat population, outcomes are reported for the per-protocol population as means with 95% CIs. A hierarchical closed testing procedure was performed to control type I error in evaluating significant differences in secondary end points. If the primary end point was met, there were 8 prespecified secondary end points that were tested successively with Fisher exact test or t test, as appropriate, until significance could not be demonstrated at a 2-sided α level of .05.29 Results through 6 months are reported, including the primary end point at 3 months and all secondary end points: 2 assessed at 3 months and 6 assessed at 6 months. We conducted analyses using SPSS Statistics version 25 (IBM).
Results
Patients were enrolled at 18 research sites across the US, including academic centers and independent pain clinics, between August 28, 2017, and August 23, 2019. Screening 430 candidates resulted in 216 patients with PDN who were randomized (Figure 1). Of these 216 randomized patients, 136 (63.0%) were male, and the mean (SD) age was 60.8 (10.7) years. Screening failures included ineligibility per inclusion or exclusion criteria (n = 146) and patient decision (n = 65). Randomized patients included 103 assigned to CMM and 113 assigned to 10-kHz SCS plus CMM.
Figure 1. Disposition of All Patients Screened for Study Participation.
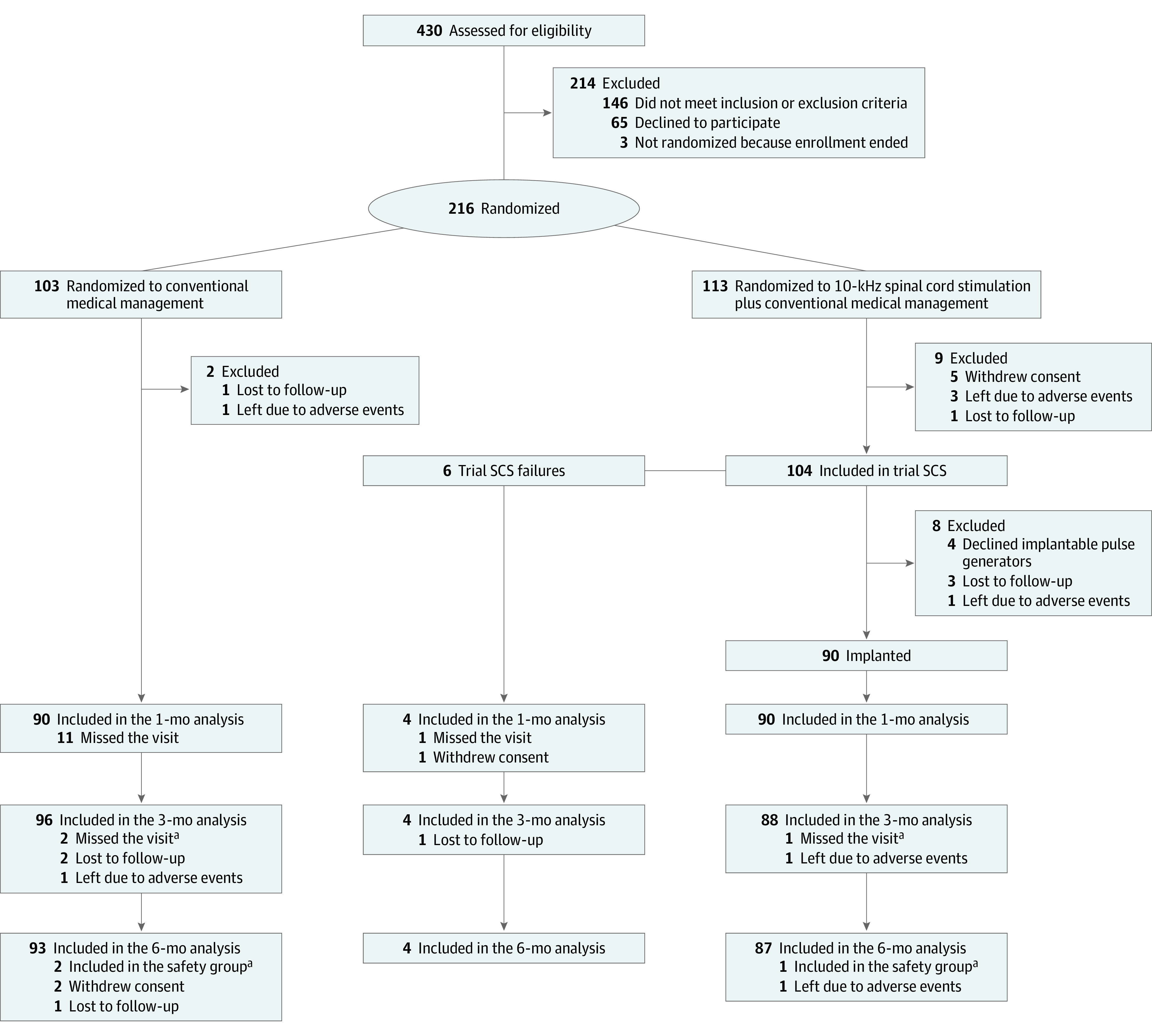
aPatients who missed the 3-month primary end point assessment (2 patients in the conventional medical management group, 1 in the 10-kHz spinal cord stimulation plus conventional medical management group) were considered part of the safety population but excluded from the per-protocol population for other outcome assessments even though they completed the 6-month visit.
Among 216 randomized patients, the mean (SD) HbA1c was 7.4% (1.2) and mean (SD) body mass index was 33.7 (5.3). A total of 130 patients (60.2%) had suboptimally controlled diabetes. The median (interquartile range) duration of diabetes and peripheral neuropathy were 10.9 (6.3-16.4) years and 5.6 (3.0-10.1) years, respectively, before enrollment. There was a similar distribution of sex between groups (Table). Patients presented with moderate to severe neuropathic pain indicated by a mean (SD) baseline VAS score of 7.3 (1.6) cm and a mean (SD) DN4 score of 6.6 (1.8).
Table. Baseline Characteristics for All Randomized Patients.
| Characteristic | No. (%) | Standardized differencea | |
|---|---|---|---|
| CMM (n = 103) | 10-kHz SCS plus CMM (n = 113) | ||
| Age, y | |||
| Mean (SD) | 60.8 (9.9) | 60.7 (11.4) | 0.01 |
| Median (IQR) | 62.0 (55.0-67.5) | 61.0 (55.0-70.0) | |
| Sex | |||
| Male | 66 (64.1) | 70 (61.9) | 0.04 |
| Female | 37 (35.9) | 43 (38.1) | |
| Race | |||
| White | 85 (82.5) | 87 (77.0) | 0.14 |
| Black or African American | 13 (12.6) | 18 (15.9) | |
| Native Hawaiian or other Pacific Islander | 1 (1.0) | 3 (2.7) | |
| American Indian or Alaska Native | 0 | 2 (1.8) | |
| Asian | 1 (1.0) | 1 (0.9) | |
| Other | 3 (2.9) | 2 (1.8) | |
| Diabetes | |||
| Type 1 | 3 (2.9) | 8 (7.1) | 0.19 |
| Type 2 | 100 (97.1) | 105 (92.9) | |
| Duration, y | |||
| Diabetes | |||
| Mean (SD) | 12.2 (8.5) | 12.9 (8.5) | 0.09 |
| Median (IQR) | 10.4 (6.3-15.2) | 12.0 (6.4-18.6) | |
| Peripheral neuropathy | |||
| Mean (SD) | 7.1 (5.1) | 7.4 (5.7) | 0.06 |
| Median (IQR) | 5.4 (2.9-10.0) | 5.7 (3.1-10.1) | |
| Lower limb pain VAS | |||
| Mean (SD), cm | 7.1 (1.6) | 7.5 (1.6) | 0.22 |
| Median (IQR), cm | 7.2 (6.2-8.2) | 7.5 (6.6-8.6) | |
| <7.5 cm | 57 (55.3) | 54 (47.8) | 0.15 |
| ≥7.5 cm | 46 (44.7) | 59 (52.2) | |
| HbA1c | |||
| Mean (SD), % | 7.4 (1.2) | 7.3 (1.1) | 0.11 |
| Median (IQR), % | 7.3 (6.6-8.2) | 7.3 (6.3-8.2) | |
| <7.0% | 40 (38.8) | 46 (40.7) | 0.04 |
| ≥7.0% | 63 (61.2) | 67 (59.3) | |
| BMIb | |||
| Mean (SD) | 33.9 (5.2) | 33.6 (5.4) | 0.06 |
| Median (IQR) | 34.3 (30.9-37.1) | 33.6 (29.8-36.3) | |
| Severity of neuropathic pain | |||
| DN4 | |||
| Mean (SD) | 6.5 (1.9) | 6.6 (1.7) | 0.12 |
| Median (IQR) | 6 (5-8) | 7 (5-8) | |
| <3 | 3 (2.9) | 1 (0.9) | 0.15 |
| ≥3 | 99 (97.1) | 112 (99.1) | |
| mNSS | |||
| Mean (SD) | 6.9 (1.1) | 6.8 (1.3) | 0.05 |
| Median (IQR) | 7 (6-8) | 7 (6-8) | |
| Mild (3-4) | 2 (2.0) | 2 (1.8) | NA |
| Moderate (5-6) | 33 (32.4) | 46 (40.7) | |
| Severe (7-9) | 67 (65.7) | 65 (57.5) | |
| Pain medications | |||
| Anticonvulsants | |||
| Gabapentin | 50 (48.5) | 63 (55.8) | 0.14 |
| Pregabalin | 29 (28.2) | 25 (22.1) | 0.14 |
| Antidepressants | |||
| SNRIs | 29 (28.2) | 25 (22.1) | 0.14 |
| TCAs | 14 (13.6) | 10 (8.8) | 0.15 |
| Opioids | 44 (42.7) | 50 (44.2) | 0.03 |
| Topicals | 9 (8.7) | 11 (9.7) | 0.03 |
| Diabetes medications | |||
| Insulin | 47 (45.6) | 51 (45.1) | 0.01 |
| Oral and noninsulin injectable medications | 84 (81.6) | 88 (77.9) | 0.09 |
Abbreviations: BMI, body mass index; CMM, conventional medical management; DN4, Douleur Neuropathique; HbA1c, hemoglobin A1c; IQR, interquartile range; mNSS, modified Neuropathy Symptom Score; NA, not applicable; SCS, spinal cord stimulation; SNRI, serotonin-norepinephrine reuptake inhibitor; TCA, tricyclic antidepressant; VAS, visual analogue scale.
Possible imbalances in baseline characteristics were evaluated with a standardized difference effect size index (Cohen d). Index scores less than 0.20 suggest the groups are well matched, whereas scores of 0.20 or greater indicate small differences, of 0.50 or greater indicate medium differences, and of 0.80 or greater indicate large differences between the groups.
Calculated as weight in kilograms divided by height in meters squared.
Primary End Point Assessment
In the CMM group, 5 of 94 patients (5%) met the composite primary end point of 50% or more pain relief using the VAS without observed deterioration on neurological examination compared with 75 of 95 in the 10-kHz SCS plus CMM group (79%; difference, 73.6%; 95% CI, 64.2-83.0; P < .001). Sensitivity analyses considered varying assumptions for missing data with no effect on the conclusion that the treatment effect for 10-kHz SCS plus CMM was superior to CMM alone (eTable 1 in Supplement 3). The intention-to-treat population with known status included temporary trial stimulation failures (n = 6) and patients who exited the study due to an AE (n = 2) as nonresponders in the 10-kHz SCS plus CMM group. One patient in the 10-kHz SCS plus CMM group and 2 in the CMM group missed 3-month follow-up but continued in the study. These patients were considered part of the safety population but excluded from the defined per-protocol population, with data reported separately (eTable 2 in Supplement 3).
Safety
There were no study-related AEs reported for the CMM group as the protocol did not require any specific treatments, while there were 18 AEs reported among 14 patients in the 10-kHz SCS plus CMM group (eTable 3 in Supplement 3). There were 3 study-related AEs for infection, 2 for wound dehiscence, and 1 for impaired healing among 5 of 90 patients (6%). Of 90 total implanted patients, 2 (2%) required explant. There were no stimulation-related neurological deficits in the 10-kHz SCS plus CMM group. A clinical events committee reviewed all AEs periodically with no concerns about the conduct of the study.
Secondary End Point Assessments
A summary table of all secondary end point outcomes is shown in order of hierarchical analysis in eTable 4 in Supplement 3.
Pain Relief During Trial SCS
In the 10-kHz SCS plus CMM treatment arm, 104 patients completed a temporary trial with SCS, with 98 achieving 50% or more pain relief using the VAS (94% success) and eligible for implantation of a permanent system (Figure 1). The mean pain VAS score at the end of the trial was 1.3 cm (95% CI, 1.0-1.6), a mean 82.3% (95% CI, 78.5-86.1) reduction from baseline. The 6 patients who failed temporary trial SCS continued with CMM (eTable 5 in Supplement 3). A total of 90 patients in the 10-kHz SCS plus CMM group received permanent device implants.
Pain Relief at 3 and 6 Months
At 3-month follow-up, 5 of 96 in the CMM group (5%) had pain VAS scores of 3 cm or less compared with 69 of 88 in the 10-kHz SCS plus CMM group (78%; difference, 73.2%; 95% CI, 63.5-82.9; P < .001) (Figure 2A). At 6-month follow-up, there was no change in mean pain VAS scores for the CMM group, with a baseline mean of 7.0 cm (95% CI, 6.7-7.3) and a 6-month mean of 6.9 cm (95% CI, 6.5-7.3); however, lower limb pain VAS scores decreased by a mean of 76.3% (95% CI, 70.8-81.8) for the implanted group. Patients in the 10-kHz SCS plus CMM group had a mean baseline pain VAS score of 7.6 cm (95% CI, 7.3-7.9) and a 6-month mean pain VAS score of 1.7 cm (95% CI, 1.3-2.1) (Figure 2B). Individual responses revealed worsening pain for 48 of 93 patients in the CMM group (52%) and 2 of 87 in the 10-kHz SCS plus CMM group (2%) (Figure 2C). The proportion of responders, defined as 50% or more pain relief from baseline VAS, was 5% (5 of 93) in the CMM group compared with 85% (74 of 87) in the 10-kHz SCS plus CMM group at 6 months (P < .001) (Figure 2C). In addition, 53 of 88 patients in the 10-kHz SCS plus CMM group (60%) achieved remission of pain, defined as VAS score of 3 cm or less sustained for 6 months,40 compared with 1 of 95 in the CMM group (1%; P < .001).
Figure 2. Pain Relief Over Time Measured by a 10-cm Visual Analogue Scale (VAS).
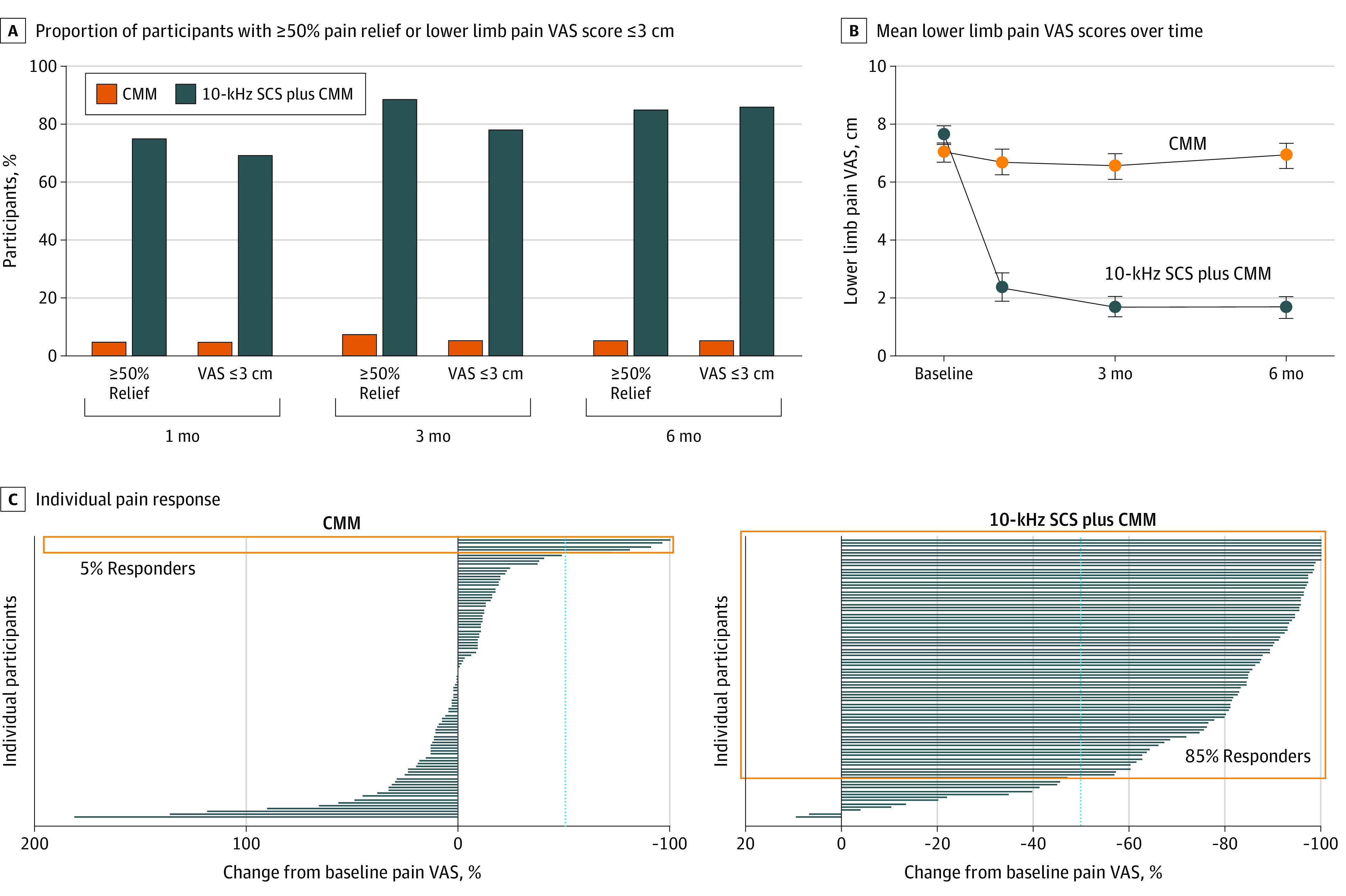
A, Proportion of patients with at least 50% pain relief on a VAS from baseline or lower limb pain of 3 cm or less using the VAS at 1, 3, and 6 months for conventional medical management (CMM) and 10-kHz spinal cord stimulation (SCS) plus CMM. B, Mean lower limb pain scores on the VAS over time for 93 patients in the CMM group and 87 patients in the 10-kHz SCS plus CMM group. Error bars indicate 95% CIs. C, Individual pain response. Each line represents the change in a single patient’s lower limb pain VAS score at 6 months relative to baseline for 93 patients in the CMM group and 87 in the 10-kHz SCS plus CMM group. The dotted blue line represents the threshold for treatment responders of at least 50% pain relief. In the CMM group, 5% of patients were responders compared with 85% of patients in the 10-kHz SCS plus CMM group (orange boxes).
Neurological Assessment at 3 and 6 Months
Investigators assessed meaningful worsening or improvement in motor, sensory, or reflex testing. Improvement from baseline without any worsening on examination was noted in 6 patients in the CMM group (6%) and 63 in the 10-kHz SCS plus CMM group (72%) at 3 months (difference, 66.0%; 95% CI, 55.4-76.6; P < .001) and 3 patients in the CMM group (3%) and 52 in the 10-kHz SCS plus CMM group (62%) at 6 months (difference, 58.6%; 95% CI, 47.6-69.6; P < .001) (Figure 3A). Most of the improvements were in sensory assessment. A total of 17 patients in the CMM group (19%) and 5 in the 10-kHz SCS plus CMM group (6.0%) demonstrated a meaningful deficit at 6 months compared with baseline.
Figure 3. Changes in Neurological Assessment and Quality of Pain.
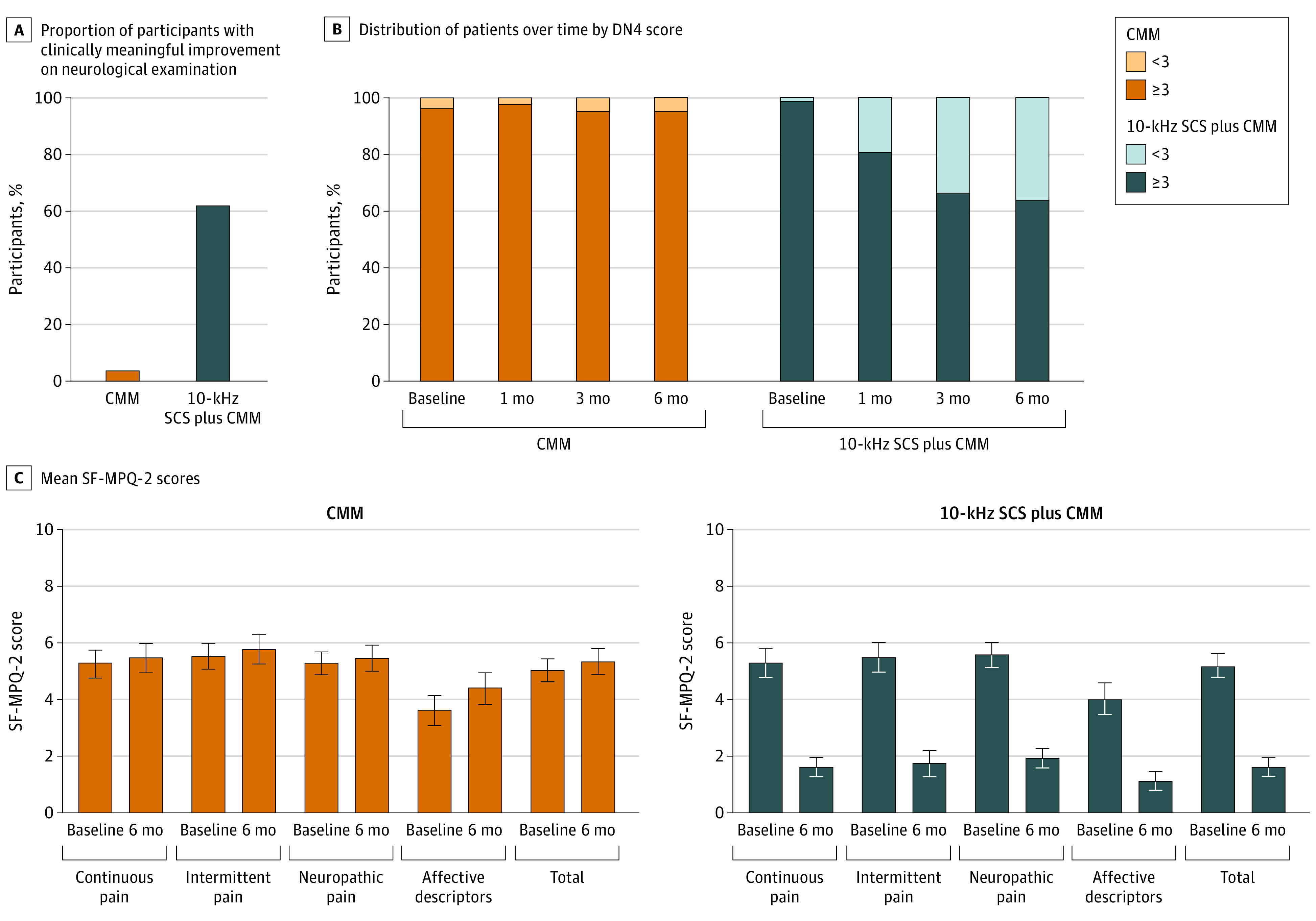
A, Proportion of patients with clinically meaningful improvement in motor, sensory, or reflex neurological examination scores and without a clinically meaningful deficit in any category as determined by the investigator at 6 months compared with baseline for 92 patients in the conventional medical management (CMM) group and 84 in the 10-kHz spinal cord stimulation (SCS) plus CMM group. B, Distribution of patients over time with Douleur Neuropathique (DN4) score of less than 3 and 3 or more for 91 patients in the CMM group and 84 in the 10-kHz SCS plus CMM group. DN4 score measures the severity of neuropathic pain. C, Mean Short-Form McGill Pain Questionnaire (SF-MPQ-2) scores for each subscale at baseline and 6 months for 93 patients in the CMM group and 87 in the 10-kHz SCS plus CMM group. SF-MPQ-2 is a patient-reported measure of the intensity of pain descriptors. Error bars indicate 95% CIs.
A DN4 score of 3 or more is consistent with clinically confirmed PDN.41 The mean DN4 score for patients in the CMM group was 6.4 (95% CI, 6.2-6.6) at baseline and 6.5 (95% CI, 6.3-6.7) at 6 months. A total of 88 of 91 patients in the CMM group had a DN4 baseline score of 3 or more. This decreased by 1.1% to 87 of 91 patients at 6 months (Figure 3B). For those in the 10-kHz SCS plus CMM group, the mean score was 6.5 (95% CI, 6.3-6.7) at baseline and 3.5 (95% CI, 3.2-3.8) at 6 months. A total of 83 of 84 patients in this group had a baseline score of 3 or more. This decreased by 34.5% to 54 of 84 patients at 6 months.
Treatment groups were well matched for intensity in all 4 SF-MPQ-2 subscales at baseline: continuous pain, intermittent pain, neuropathic pain, and affective descriptors (Figure 3C). There was no change for those in the CMM group over 6-month follow-up, whereas all subscales improved for those in the 10-kHz SCS plus CMM group, including a mean 67.0% (95% CI, 58.6-75.4) improvement in the intensity of affective descriptors.
Health-Related Quality of Life at 6 Months
Each group rated overall health equivalently at baseline on EQ-5D-5L VAS (Figure 4A). There was no change for those in the CMM group but a mean 16-point (95% CI, 11.3-20.5) improvement for those in the 10-kHz SCS plus CMM group (P < .001). The mean EQ-5D-5L index score was 0.630 (95% CI, 0.600-0.660) for those in the CMM group at baseline and decreased to 0.599 (95% CI, 0.566-0.632) at 6 months (Figure 4A). For those in the 10-kHz SCS plus CMM group, the mean EQ-5D-5L index score of 0.636 (95% CI, 0.604-0.668) at baseline improved to 0.765 (95% CI, 0.737-0.793; P < .001) at 6 months, a difference of 0.129. The minimally important difference is estimated between 0.03 to 0.05 in the type 2 diabetes population.42
Figure 4. Health-Related Quality of Life Outcomes and Patient Satisfaction.
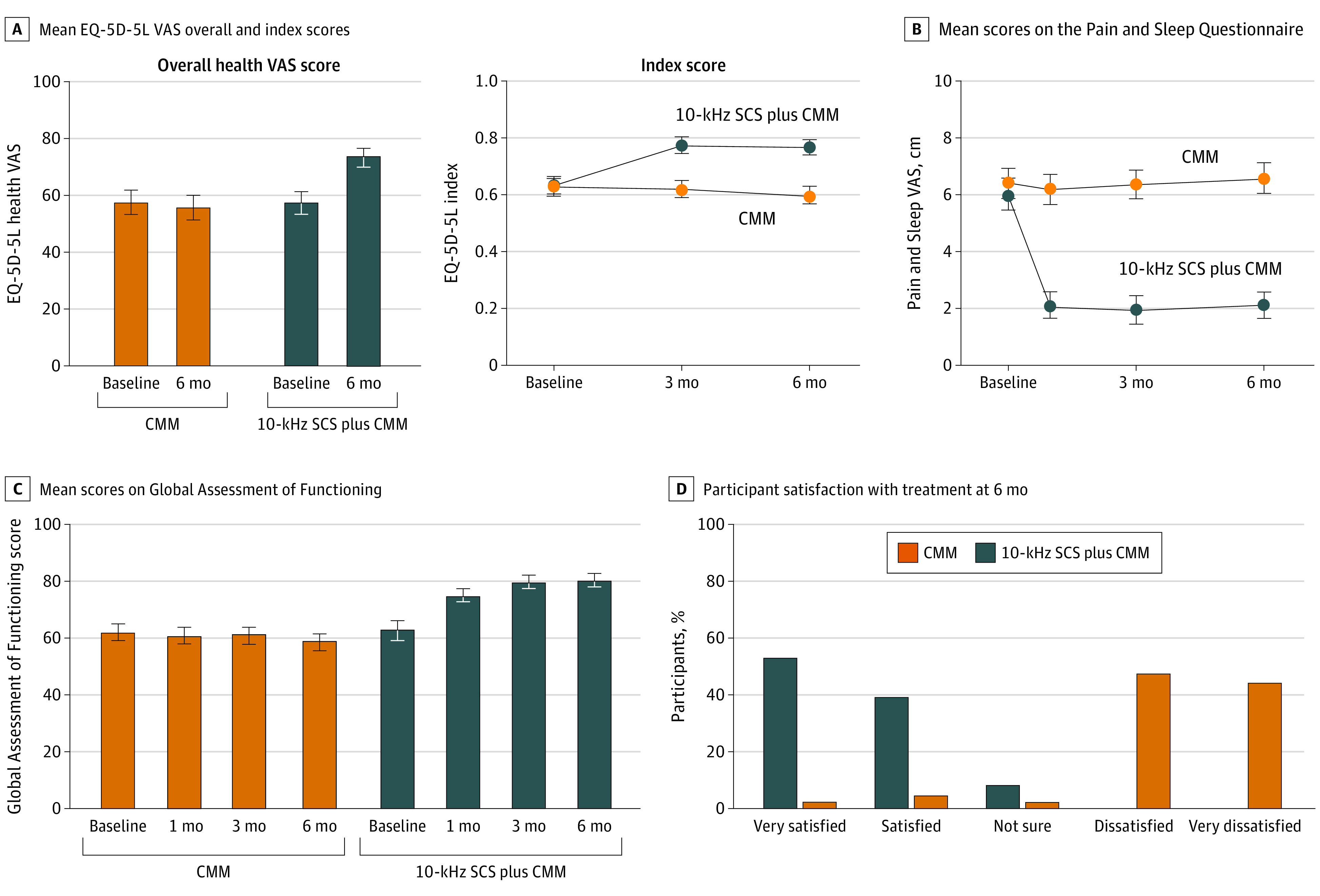
A, Mean EuroQol 5-Dimension Questionnaire (EQ-5D-5L) overall health visual analogue scale (VAS) score (left) and index score (right) for 92 patients in the conventional medical management (CMM) group and 87 in the 10-kHz spinal cord stimulation (SCS) plus CMM group from baseline to 6 months. The minimally important difference in index scores is estimated between 0.03 to 0.05. B, Mean scores for the Pain and Sleep Questionnaire assessing how often pain disturbs sleep. A score of 0 indicates never and a score of 10 indicates always. Scores are shown for 93 patients in the CMM group and 87 in the 10-kHz SCS plus CMM group. C, Mean scores on Global Assessment of Functioning at baseline and at 1, 3, and 6 months for 91 patients in the CMM group and 86 in the 10-kHz SCS plus CMM group. The Global Assessment of Functioning represents the physician’s evaluation of how much a patient’s symptoms affect psychological, social, and occupational functioning. D, Patient satisfaction with treatment at 6 months. Scores are shown for 93 patients in the CMM group and 87 in the 10-kHz SCS plus CMM group. Error bars indicate 95% CIs.
The effect of pain on sleep quality was evident in both arms at baseline (Figure 4B). In the CMM group, sleep disturbance due to pain increased by 5.3% (95% CI, −15.0 to 4.4), while in the 10-kHz SCS plus CMM group, there was a mean 61.9% (95% CI, 54.4-69.4) reduction. Investigators evaluated patients’ well-being with the Global Assessment of Functioning (Figure 4C). No change was observed for those in the CMM group, while patients in the 10-kHz SCS plus CMM group had a mean improvement of 17.7 points (95% CI, 13.8-21.6). At 6-month follow-up, 80 of 87 in the 10-kHz SCS plus CMM group (92%) reported being satisfied or very satisfied with their treatment compared with 6 of 93 in the CMM group (6%) (Figure 4D).
For patients in the CMM group, the mean HbA1c level was 7.4% (95% CI, 7.1-7.6) at baseline, with a mean increase of 2.6% (95% CI, −0.7 to 5.8) over 6 months. In the 10-kHz SCS plus CMM group, the mean HbA1c was 7.4% (95% CI, 7.1-7.6) at baseline, with a mean increase of 1.5% (95% CI, −1.8 to 4.7) over 6 months. There was no difference between the groups in the mean change over 6 months (P = .65).
Patients could opt to cross over to the other treatment arm at 6 months if they met all 3 prespecified criteria: less than 50% pain relief using the VAS from baseline, dissatisfaction with the current treatment, and investigator agreement the patient was medically appropriate to proceed. None of the patients in the 10-kHz SCS plus CMM group met the criteria for crossover, whereas 76 of 93 in the CMM group (82%) both met criteria and elected to cross over (P < .001).
Discussion
This study—the largest RCT for SCS treatment of PDN to date—was designed as a pragmatic study to provide high-level evidence in guiding clinical decision-making. Participants had long-standing diabetes and well-established PDN. Inclusion and exclusion criteria were consistent with the greater population of patients with refractory PDN. Previous treatments, including medications, followed best-practice guidelines.3,4
The proportion of treatment responders to high-frequency (10-kHz) SCS at 6 months (85%) surpasses that of 2 published RCTs comparing low-frequency SCS with CMM.12,13,14 de Vos et al12 reported a 69% responder rate at 6 months among 36 individuals with PDN. Slangen and colleagues14 reported a 56% responder rate at 6 months among 16 individuals with PDN. Long-term follow-up of 22 patients with PDN reveals 36% responders to low-frequency SCS at 5 years with severity of neuropathy predictive of treatment failure.15 Improvements in neurological function were not reported in prior low-frequency SCS studies. Additionally, 10-kHz SCS provides pain relief without exacerbating underlying paresthesias as it is the only paresthesia-independent SCS device.18 This study provides level I evidence supporting the addition of 10-kHz SCS to CMM for patients with PDN with symptoms refractory to medical management.
There are several RCTs evaluating pharmacological PDN treatments demonstrating lower responder rates than SCS. Meta-analysis of pregabalin data with 4-week to 12-week follow-up yielded a responder rate of 47% for 600 mg daily.43 Studies for duloxetine with 8-week to 12-week follow-up reported 42% to 59% responders.44 Tapentadol therapy resulted in 40% responders at 15 weeks.45 Longer-term data are lacking for PDN pharmacotherapy.
Effectiveness of 10-kHz SCS PDN treatment exceeds results reported for other pain etiologies. A large RCT demonstrated responder rates of 76% for back pain and 81% for leg pain at 6 months.19 A prospective, multicenter, observational study reported 78% responders for axial neck pain and 88% for upper limb pain at 6 months.23 Nonsurgical patients with refractory back pain treated with 10-kHz SCS resulted in 75% responders at 6 months and 80% at 36 months.22,46 Two prior studies have described long-term benefit of 10-kHz SCS for peripheral polyneuropathy.16,17 The current study extends these initial observations with level I evidence.
Wound complications are a primary concern when performing surgical procedures for patients with diabetes. Combined incidence of wound dehiscence, impaired healing, or infection was seen in 5 of 90 patients with permanent SCS implant, a 5.6% wound complication rate. This is consistent with the risk for SCS wound complications in general and suggests implantation of an SCS device can be safely accomplished in patients with diabetes.47
Improvements in sensation, as observed by investigators in most patients receiving 10-kHz SCS plus CMM, is unreported with other SCS therapies and supports similar findings in observational studies of this treatment in those with peripheral polyneuropathy of various etiologies.16,17 Existing pharmacological treatments for PDN may mitigate pain symptoms without effect on underlying pathophysiology.3,48 Interestingly, changes in sensation were observed often by the end of trial stimulation and persisted over the course of follow-up. Further studies are needed to elucidate potential mechanisms of action but may involve increased blood flow to the periphery, improvement in peripheral or central sensory processing, and/or changes in intraepidermal nerve fiber density.49,50 Improved sensation has potential advantages for patients with diabetic peripheral neuropathy that could aid in foot ulcer or infection reduction and possibly enhance proprioception that would reduce risk of falling and potential injury. This study applied standard clinical assessments of neurological function; however, assessor variability can be significant, and the findings of neurological improvements should be interpreted in the context of this limitation. Further study including more objective measures will be required to validate these observations.
Chronic pain, regardless of etiology, negatively affects sleep quality. This can be especially true for patients with PDN with symptoms classically worse at night. Prior RCT data with low-frequency SCS reported half the participants achieved significant pain relief at night.14 Sleep deprivation exacerbates underlying disease, affects mental health, and daytime functioning. This study indicates that 10-kHz SCS greatly improves sleep for patients with PDN. In addition, patients improved across a variety of health-related quality of life measures, suggesting a broad effect of this treatment on patients’ lives.
Limitations
This study had limitations. Comparing an implanted medical device with best available medical treatment made blinding impossible. Steps were taken to mitigate bias, including random sequence generation and concealed treatment allocation. The cohorts were well matched at baseline, attrition was acceptable, missing data were unlikely to have a meaningful effect on the outcomes, and all primary and secondary outcomes have been reported as prespecified. Nonetheless, lack of blinding may influence patients and investigators. Potential placebo effects in this study may be significant with an active device treatment. Long-term follow-up may mitigate concerns about a placebo effect.
This study included patients with symptoms refractory to evidence-based CMM at baseline—10-kHz SCS was added in the treatment arm to explore if satisfactory benefit could be achieved. The control arm reflects a sizable patient population for whom currently available treatments provide insufficient pain relief; however, generalizing to all patients with PDN warrants caution. The findings of neurological improvements should be interpreted in the context of these limitations.
Conclusions
Patients with PDN refractory to best available treatments can be safely and effectively treated with high-frequency (10-kHz) SCS. Evidence-based treatment guidelines should contemplate positioning of 10-kHz SCS in the continuum of care. Follow-up of this study population will continue for 24 months and establish potential durability of this treatment beyond 6 months. Patients experienced substantial, sustained pain relief as well as clinically assessed improvements in neurological function and improved health-related quality of life. In practice, patients with PDN with inadequate response to conventional treatments should be considered for 10-kHz SCS.
Study protocol.
Statistical analysis plan.
eTable 1. Primary end point sensitivity analysis results.
eTable 2. Pain visual analogue scale scores for patients excluded from the per-protocol population.
eTable 3. Summary of study-related adverse events.
eTable 4. Summary of secondary end point analyses.
eTable 5. Pain visual analogue scale scores for patients who failed temporary trial spinal cord stimulation.
eFigure 1. Diabetic foot examination.
eFigure 2. Spinal cord stimulation lead placement.
Data sharing statement.
References
- 1.World Health Organization . Global report on diabetes, 2016. Accessed November 9, 2018. https://apps.who.int/iris/rest/bitstreams/909883/retrieve
- 2.Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain. 2002;18(6):350-354. doi: 10.1097/00002508-200211000-00002 [DOI] [PubMed] [Google Scholar]
- 3.Pop-Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154. doi: 10.2337/dc16-2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bril V, England J, Franklin GM, et al. ; American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation . Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76(20):1758-1765. doi: 10.1212/WNL.0b013e3182166ebe [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gabapentin and risk of severe respiratory depression. Drug Ther Bull. 2018;56(1):3-4. doi: 10.1136/dtb.2018.1.0571 [DOI] [PubMed] [Google Scholar]
- 6.Meisenberg B, Ness J, Rao S, Rhule J, Ley C. Implementation of solutions to reduce opioid-induced oversedation and respiratory depression. Am J Health Syst Pharm. 2017;74(3):162-169. doi: 10.2146/ajhp160208 [DOI] [PubMed] [Google Scholar]
- 7.Eipe N, Penning J. Postoperative respiratory depression with pregabalin: a case series and a preoperative decision algorithm. Pain Res Manag. 2011;16(5):353-356. doi: 10.1155/2011/561604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173. doi: 10.1016/S1474-4422(14)70251-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang M, Qian C, Liu Y. Suboptimal treatment of diabetic peripheral neuropathic pain in the United States. Pain Med. 2015;16(11):2075-2083. doi: 10.1111/pme.12845 [DOI] [PubMed] [Google Scholar]
- 10.Tesfaye S, Watt J, Benbow SJ, Pang KA, Miles J, MacFarlane IA. Electrical spinal-cord stimulation for painful diabetic peripheral neuropathy. Lancet. 1996;348(9043):1698-1701. doi: 10.1016/S0140-6736(96)02467-1 [DOI] [PubMed] [Google Scholar]
- 11.Kumar K, Toth C, Nath RK. Spinal cord stimulation for chronic pain in peripheral neuropathy. Surg Neurol. 1996;46(4):363-369. doi: 10.1016/S0090-3019(96)00191-7 [DOI] [PubMed] [Google Scholar]
- 12.de Vos CC, Meier K, Zaalberg PB, et al. Spinal cord stimulation in patients with painful diabetic neuropathy: a multicentre randomized clinical trial. Pain. 2014;155(11):2426-2431. doi: 10.1016/j.pain.2014.08.031 [DOI] [PubMed] [Google Scholar]
- 13.van Beek M, Slangen R, Schaper NC, et al. Sustained treatment effect of spinal cord stimulation in painful diabetic peripheral neuropathy: 24-month follow-up of a prospective two-center randomized controlled trial. Diabetes Care. 2015;38(9):e132-e134. doi: 10.2337/dc15-0740 [DOI] [PubMed] [Google Scholar]
- 14.Slangen R, Schaper NC, Faber CG, et al. Spinal cord stimulation and pain relief in painful diabetic peripheral neuropathy: a prospective two-center randomized controlled trial. Diabetes Care. 2014;37(11):3016-3024. doi: 10.2337/dc14-0684 [DOI] [PubMed] [Google Scholar]
- 15.van Beek M, Geurts JW, Slangen R, et al. Severity of neuropathy is associated with long-term spinal cord stimulation outcome in painful diabetic peripheral neuropathy: five-year follow-up of a prospective two-center clinical trial. Diabetes Care. 2018;41(1):32-38. doi: 10.2337/dc17-0983 [DOI] [PubMed] [Google Scholar]
- 16.Sills S. Treatment of painful polyneuropathies of diabetic and other origins with 10 kHz SCS: a case series. Postgrad Med. 2020;132(4):352-357. doi: 10.1080/00325481.2020.1732065 [DOI] [PubMed] [Google Scholar]
- 17.Galan V, Scowcroft J, Chang P, et al. 10-kHz Spinal cord stimulation treatment for painful diabetic neuropathy: results from post-hoc analysis of the SENZA-PPN study. Pain Manag. 2020;10(5):291-300. doi: 10.2217/pmt-2020-0033 [DOI] [PubMed] [Google Scholar]
- 18.De Carolis G, Paroli M, Tollapi L, et al. Paresthesia-independence: an assessment of technical factors related to 10 kHz paresthesia-free spinal cord stimulation. Pain Physician. 2017;20(4):331-341. [PubMed] [Google Scholar]
- 19.Kapural L, Yu C, Doust MW, et al. Novel 10-kHz high-frequency therapy (HF10 therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA-RCT randomized controlled trial. Anesthesiology. 2015;123(4):851-860. doi: 10.1097/ALN.0000000000000774 [DOI] [PubMed] [Google Scholar]
- 20.Kapural L, Yu C, Doust MW, et al. Comparison of 10-kHz high-frequency and traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: 24-month results from a multicenter, randomized, controlled pivotal trial. Neurosurgery. 2016;79(5):667-677. doi: 10.1227/NEU.0000000000001418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Kaisy A, Van Buyten J-P, Smet I, Palmisani S, Pang D, Smith T. Sustained effectiveness of 10 kHz high-frequency spinal cord stimulation for patients with chronic, low back pain: 24-month results of a prospective multicenter study. Pain Med. 2014;15(3):347-354. doi: 10.1111/pme.12294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Kaisy A, Palmisani S, Smith TE, et al. Long-term improvements in chronic axial low back pain patients without previous spinal surgery: a cohort analysis of 10-kHz high-frequency spinal cord stimulation over 36 months. Pain Med. 2018;19(6):1219-1226. [DOI] [PubMed] [Google Scholar]
- 23.Amirdelfan K, Vallejo R, Benyamin R, et al. High-frequency spinal cord stimulation at 10 kHz for the treatment of combined neck and arm pain: results from a prospective multicenter study. Neurosurgery. 2020;87(2):176-185. doi: 10.1093/neuros/nyz495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Kaisy A, Palmisani S, Smith T, Harris S, Pang D. The use of 10-kilohertz spinal cord stimulation in a cohort of patients with chronic neuropathic limb pain refractory to medical management. Neuromodulation. 2015;18(1):18-23. doi: 10.1111/ner.12237 [DOI] [PubMed] [Google Scholar]
- 25.Al-Kaisy A, Van Buyten JP, Carganillo R, et al. 10 kHz SCS therapy for chronic pain, effects on opioid usage: post hoc analysis of data from two prospective studies. Sci Rep. 2019;9(1):11441. doi: 10.1038/s41598-019-47792-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Kaisy A, Van Buyten JP, Amirdelfan K, et al. Opioid-sparing effects of 10 kHz spinal cord stimulation: a review of clinical evidence. Ann N Y Acad Sci. 2020;1462(1):53-64. doi: 10.1111/nyas.14236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El Majdoub F, Neudorfer C, Richter R, Schieferdecker S, Maarouf M. 10 kHz Cervical SCS for chronic neck and upper limb pain: 12 months’ results. Ann Clin Transl Neurol. 2019;6(11):2223-2229. doi: 10.1002/acn3.50915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DiBenedetto DJ, Wawrzyniak KM, Schatman ME, Kulich RJ, Finkelman M. 10 kHz Spinal cord stimulation: a retrospective analysis of real-world data from a community-based, interdisciplinary pain facility. J Pain Res. 2018;11:2929-2941. doi: 10.2147/JPR.S188795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mekhail NA, Argoff CE, Taylor RS, et al. High-frequency spinal cord stimulation at 10 kHz for the treatment of painful diabetic neuropathy: design of a multicenter, randomized controlled trial (SENZA-PDN). Trials. 2020;21(87):1-12. doi: 10.1186/s13063-019-4007-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 31.McCormack HM, Horne DJ, Sheather S. Clinical applications of visual analogue scales: a critical review. Psychol Med. 1988;18(4):1007-1019. doi: 10.1017/S0033291700009934 [DOI] [PubMed] [Google Scholar]
- 32.Dworkin RH, Turk DC, Revicki DA, et al. Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2). Pain. 2009;144(1-2):35-42. doi: 10.1016/j.pain.2009.02.007 [DOI] [PubMed] [Google Scholar]
- 33.Bouhassira D, Attal N, Alchaar H, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005;114(1-2):29-36. doi: 10.1016/j.pain.2004.12.010 [DOI] [PubMed] [Google Scholar]
- 34.Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993;36(2):150-154. doi: 10.1007/BF00400697 [DOI] [PubMed] [Google Scholar]
- 35.Ayearst L, Harsanyi Z, Michalko KJ. The Pain and Sleep Questionnaire three-item index (PSQ-3): a reliable and valid measure of the impact of pain on sleep in chronic nonmalignant pain of various etiologies. Pain Res Manag. 2012;17(4):281-290. doi: 10.1155/2012/635967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727-1736. doi: 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Booth J, Young MJ. Differences in the performance of commercially available 10-g monofilaments. Diabetes Care. 2000;23(7):984-988. doi: 10.2337/diacare.23.7.984 [DOI] [PubMed] [Google Scholar]
- 38.Boulton AJ, Armstrong DG, Albert SF, et al. ; American Diabetes Association; American Association of Clinical Endocrinologists . Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31(8):1679-1685. doi: 10.2337/dc08-9021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.England JD, Gronseth GS, Franklin G, et al. ; American Academy of Neurology; American Association of Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation . Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2005;64(2):199-207. doi: 10.1212/01.WNL.0000149522.32823.EA [DOI] [PubMed] [Google Scholar]
- 40.Amirdelfan K, Gliner BE, Kapural L, et al. A proposed definition of remission from chronic pain, based on retrospective evaluation of 24-month outcomes with spinal cord stimulation. Postgrad Med. 2019;131(4):278-286. doi: 10.1080/00325481.2019.1592401 [DOI] [PubMed] [Google Scholar]
- 41.Spallone V, Morganti R, D’Amato C, Greco C, Cacciotti L, Marfia GA. Validation of DN4 as a screening tool for neuropathic pain in painful diabetic polyneuropathy. Diabet Med. 2012;29(5):578-585. doi: 10.1111/j.1464-5491.2011.03500.x [DOI] [PubMed] [Google Scholar]
- 42.McClure NS, Sayah FA, Ohinmaa A, Johnson JA. Minimally important difference of the EQ-5D-5L index score in adults with type 2 diabetes. Value Health. 2018;21(9):1090-1097. doi: 10.1016/j.jval.2018.02.007 [DOI] [PubMed] [Google Scholar]
- 43.Freeman R, Durso-Decruz E, Emir B. Efficacy, safety, and tolerability of pregabalin treatment for painful diabetic peripheral neuropathy: findings from seven randomized, controlled trials across a range of doses. Diabetes Care. 2008;31(7):1448-1454. doi: 10.2337/dc07-2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hossain SM, Hussain SM, Ekram AR. Duloxetine in painful diabetic neuropathy: a systematic review. Clin J Pain. 2016;32(11):1005-1010. doi: 10.1097/AJP.0000000000000343 [DOI] [PubMed] [Google Scholar]
- 45.Vinik AI, Shapiro DY, Rauschkolb C, et al. A randomized withdrawal, placebo-controlled study evaluating the efficacy and tolerability of tapentadol extended release in patients with chronic painful diabetic peripheral neuropathy. Diabetes Care. 2014;37(8):2302-2309. doi: 10.2337/dc13-2291 [DOI] [PubMed] [Google Scholar]
- 46.Al-Kaisy A, Palmisani S, Smith TE, et al. 10 kHz High-frequency spinal cord stimulation for chronic axial low back pain in patients with no history of spinal surgery: a preliminary, prospective, open label and proof-of-concept study. Neuromodulation. 2017;20(1):63-70. doi: 10.1111/ner.12563 [DOI] [PubMed] [Google Scholar]
- 47.Eldabe S, Buchser E, Duarte RV. Complications of spinal cord stimulation and peripheral nerve stimulation techniques: a review of the literature. Pain Med. 2016;17(2):325-336. [DOI] [PubMed] [Google Scholar]
- 48.Boulton AJ, Kempler P, Ametov A, Ziegler D. Whither pathogenetic treatments for diabetic polyneuropathy? Diabetes Metab Res Rev. 2013;29(5):327-333. doi: 10.1002/dmrr.2397 [DOI] [PubMed] [Google Scholar]
- 49.van Beek M, Hermes D, Honig WM, et al. Long-term spinal cord stimulation alleviates mechanical hypersensitivity and increases peripheral cutaneous blood perfusion in experimental painful diabetic polyneuropathy. Neuromodulation. 2018;21(5):472-479. doi: 10.1111/ner.12757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castellanos J, Kuo D, Zardouz S, et al. Evaluation of high frequency spinal cord stimulation (SCS) on corneal confocal microscopy and intraepidermal nerve fibers in diabetic peripheral neuropathy: preliminary findings. Paper presented at: The 23rd Annual Meeting of the North American Neuromodulation Society; January 24, 2020; Las Vegas, NV. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study protocol.
Statistical analysis plan.
eTable 1. Primary end point sensitivity analysis results.
eTable 2. Pain visual analogue scale scores for patients excluded from the per-protocol population.
eTable 3. Summary of study-related adverse events.
eTable 4. Summary of secondary end point analyses.
eTable 5. Pain visual analogue scale scores for patients who failed temporary trial spinal cord stimulation.
eFigure 1. Diabetic foot examination.
eFigure 2. Spinal cord stimulation lead placement.
Data sharing statement.


