Abstract
Background
Photophobia is a potentially debilitating symptom often found in dry eye disease (DE), migraine and traumatic brain injury (TBI).
Methods
We conducted a review of the literature via a PubMed search of English language articles with a focus on how photophobia may relate to a shared pathophysiology across DE, migraine and TBI.
Results
DE, migraine and TBI are common conditions in the general population, are often comorbid, and share photophobia as a symptom. Across the three conditions, neural dysregulation of peripheral and central nervous system components is implicated in photophobia in various animal models and in humans. Enhanced activity of the neuropeptide calcitonin gene-related peptide (CGRP) is closely linked to photophobia. Current therapies for photophobia include glasses which shield the eyes from specific wavelengths, botulinum toxin, and inhibition of CGRP and its receptor. Many individuals have persistent photophobia despite the use of these therapies, and thus, development of new therapies is needed.
Conclusions
The presence of photophobia in DE, migraine and TBI suggests shared trigeminothalamic pathophysiologic mechanisms, as explained by central neuroplasticity and hypersensitivity mediated by neuropeptide CGRP. Treatment strategies which target neural pathways (ie, oral neuromodulators, transcutaneous nerve stimulation) should be considered in patients with persistent photophobia, specifically in individuals with DE whose symptoms are not controlled with traditional therapies.
INTRODUCTION
Clinicians have long considered dry eye disease (DE), migraine and traumatic brain injury (TBI) to be independent conditions with distinct clinical manifestations that are treated symptomatically in isolation. Classically, DE is considered an ocular surface disease, migraine a neurologic disease and TBI a neurologic injury. However, DE symptoms, especially sensitivity to light or photophobia, often coexist with migraine and TBI, implicating a shared underlying pathophysiology. Literature suggests that neuroplasticity within peripheral and central trigeminal neural pathways underlies photophobia and links the three diseases. This concept has both diagnostic and therapeutic implications, mostly in the management of DE. In this review, we outline the connections between DE, migraine and TBI and discuss pathophysiologic mechanisms that underlie the shared symptom of photophobia. We propose that symptoms common to DE, migraine and TBI are manifestations of overlapping pathophysiological processes which may respond to the same treatments.
Dry eye disease
DE represents a spectrum of clinical manifestations including symptoms such as dryness, discomfort, and pain sensations and ocular surface disturbances such as increased tear evaporation or decreased production. Ocular pain sensations may occur spontaneously, or be evoked by a stimulus, such as wind or light. The latter represents the ocular equivalent of hyperalgesia (eg, increased pain from a stimulus that normally provokes pain) and allodynia (eg, pain due to a stimulus that does not normally provoke pain), respectively.1 Several clinical signs are associated with DE, such as unstable osmolarity, ocular surface inflammation, rapid tear film breakup time, corneal staining and low tear production.2 A known reality in DE is that symptoms of disease correlate poorly with ocular surface signs.3 This disconnect suggests that factors beyond tear film and ocular health drive ocular pain symptoms such as trigeminal nerve dysfunction in peripheral and central nerves connecting the cornea to the brain.2
Migraine
Migraine is diagnosed clinically in the setting of recurrent attacks of unilateral or bilateral pulsatile or throbbing headaches that may last hours to days, with about 25% of patients experiencing aura or sensory symptoms experienced before or during the migraine episode. Migraines can present with a variable constellation of motor (ie, speech disturbance) and sensory (ie, auditory or visual changes) symptoms and are frequently associated with nausea or vomiting, phonophobia and photophobia.4
Genetic, environmental, dietary and metabolic factors are thought to incite pathologic activation of areas of the hypothalamus and neurologic circuits between the thalamus and cortex. Excitation may continue to parts of the cortex, brainstem and cervical nerves. Diffuse activation is hypothesised to manifest as a concentric wave of neuronal and glial depolarisation through the majority of one cortical hemisphere (known as cortical spreading depolarisation). This phenomenon is suspected to be the physiologic manifestation of migraine aura.5 Associated excitation of trigeminal sensory nerves, which innervate the dura as well as cerebral and meningeal arteries, and release of neuropeptides may play a role in the onset of headache.6
Traumatic brain injury
TBI is a major cause of disability, morbidity and mortality among civilians and military personnel. TBI may be grouped into closed head TBI (ie, blunt trauma), penetrating TBI (ie, foreign body penetration, such as by gunshot) and explosive blast TBI (ie, wartime explosives causing rapid pressure shock waves in the parenchyma) subtypes. Closed head TBI is the most common and frequently results from motor vehicle accidents, sports injuries and falls. Clinical features are variable and may include headache, nausea, seizures, speech disturbances, amnesia, behavioural changes, visual disturbances including photophobia7 and coma; these generally manifest within seconds or minutes of the inciting incident but may persist for months and years.8 Headache developing within seven days after a head injury or after regaining consciousness from a head injury, not better defined by another headache condition, can be classified as an acute headache attributable to TBI, often referred to as post-traumatic headache (PTH).9
Pathophysiological mechanisms of TBI are twofold—damage from direct mechanical forces during the incident (primary) and the ensuing tissue and cellular reactions (secondary). Primary damage includes focal injuries, such as localised necrosis and inflammation following a gunshot wound, and diffuse axonal injury (DAI), such as ischemia and oedema from shearing forces during rapid acceleration/deceleration that compromise axons and cerebral vasculature. DAI occurs in approximately 70% of moderate-to-severe TBI cases.10 Secondary brain injuries can last from hours to years, representing a spectrum of inflammation and dysfunctional healing that occurs in cerebral tissues and cells. These mechanisms include breakdown of the blood–brain barrier, excessive glutamate release, mitochondrial dysfunction, lipid peroxidation, neuroinflammation, axonal degeneration, neuronal excitotoxicity and apoptosis.8,11 Evidence suggests that TBI-induced hypersensitivity of the trigeminal sensory system (trigeminal nucleus, thalamus and sensory cortex) plays a significant role in chronic TBI pain disability.12
Epidemiology of DE, migraine and TBI
DE, migraine and TBI are highly prevalent. The Tear Film & Ocular Surface Society reported that the global prevalence of DE varied from 5% to 50% depending on the population studied and the definition of disease.13 The frequency of DE increases with age and is more common in women.14 Migraine is also a common condition with an estimated global prevalence of 1.04 billion individuals, as of 2016.15 The US prevalence of migraine was from 11.7% to 16.2%.16 Migraine is also more prevalent in women with a lifetime cumulative incidence of 43% in women compared to 18% in men.17 The prevalence of TBI is lower than that of DE and migraine; yet, an estimated 69 million individuals globally sustain a TBI from any cause each year, with a nearly three times greater prevalence in low-income and middle-income countries compared to high-income nations.18 In the US, 5.3 million Americans live with a TBI-related disability (1.7% of all Americans) and 1.7 million individuals experience a TBI each year.19 Among veterans, the prevalence of TBI is even higher. In a study of 3,265,894 veterans seen over a 5-year period, 4% carried a diagnosis of TBI.20
Photophobia and neural networks
As noted above, DE, migraine and TBI have photophobia as a shared feature. Other studies have reviewed neural pathways implicated in photophobia.21–23 As such, we will concisely summarise several candidate pathways that have been identified (figure 1).
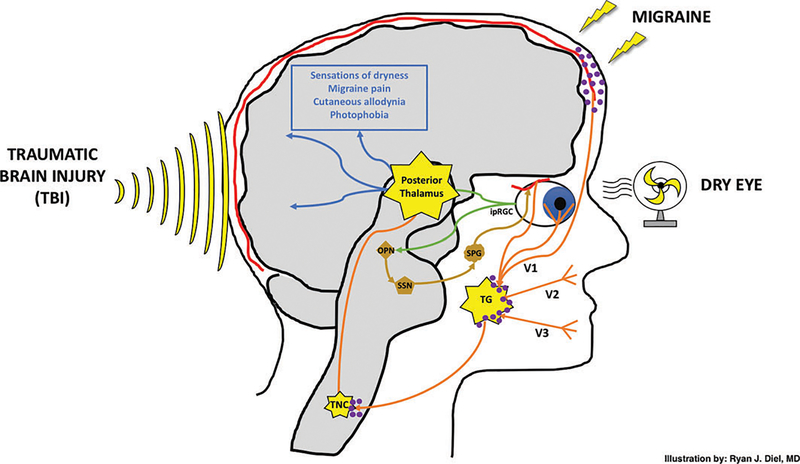
Figure 1.
Photophobia is a manifestation of dry eye, migraine and traumatic brain injury. Melanopsin-containing intrinsically photosensitive retinal ganglion cells (ipRGCs) transmit light-evoked signals (green pathway) to the olivary pretectal nucleus (OPN) and posterior thalamus. A parasympathetic-mediated vasodilatory response (gold pathway) is transmitted via the superior salivatory nucleus (SSN) and sphenopalatine ganglia (SPG) to ocular blood vessels. Nociceptive signals from ocular blood vessels, corneal surface and dural meninges are all transmitted via the ophthalmic branch (V1) of the trigeminal nerve (orange pathway) before converging at the trigeminal ganglia (TG), trigeminal nucleus caudalis (TNC) and posterior thalamus. Dry eye, migraine and traumatic brain injury all represent inputs which may act to sensitise the aforementioned underlying neuronal networks via calcitonin gene-related peptide (purple molecules). The TG, TNC and posterior thalamus are potential areas of neuroplasticity/sensitisation governing the sensations finally culminating as dryness, migraine pain, allodynia and photophobia (blue pathway). Direct activation of trigeminal afferents by an independent melanopsinmediated pathway originating from the iris and/or cornea is not shown. Light-sensing pathways mediating systemic hypothalamic-sympathetic and hypothalamic-parasympathetic pathways are also not shown. Illustration by Ryan J. Diel, MD.
A special population of retinal ganglion cells termed intrinsically sensitive retinal ganglion cells (ipRGCs), also known as melanopsin-containing retinal ganglion cells, lie at the centre of photophobia models. ipRGCs respond best to blue light with a peak wavelength sensitivity at 479 nm in humans and 484 nm in mice.24 In mice, when light strikes the retina, light-evoked potentials, mostly from ipRGCs,25 are transmitted to the olivary pretectal nucleus and then the superior salivatory nucleus, which in turn exerts a parasympathetic-mediated vasodilatory response on ocular blood vessels.26 Vasodilation of blood vessels then sends nociceptive signals through the trigeminothalamic pathway via the trigeminal nerve afferents to the trigeminal nucleus caudalis (TNC), posterior thalamus (the pain relay centre of the body) and higher cortical centres. Evidence for the existence of this circuit comes from a study that found blue light, which activates ipRGCs, but not yellow light, induced inflammation in the trigeminal ganglia.27
Another pathway directly connects ipRGCs to the pulvinar nuclei of the posterior thalamus28,29 and from there to somatosensory and visual cortices.30 In rats, histopathologic studies demonstrated close apposition of dura-sensitive neurons and light-sensitive neurons, specifically ipRGCs, which projected directly to the posterior thalamus.28,29 In mice, anterograde labelling of intravitreal injections demonstrated labelled axons in the ventral and dorsal posterior thalamus. Similarly, retrograde labelling of individual axons of the posterior thalamus was traced to cell bodies in the TNC and ipRGCs. Using the same model, cortical mapping demonstrated termination of axons within the somatosensory cortex.29 Activation of this pathway has been demonstrated in humans, specifically in a patient with long-standing idiopathic photophobia using functional MRI (fMRI).31 Taken together, these data provide good evidence of connections between ipRGC and the thalamus, both in animal models and in humans.
Another postulated pathway connects melanopsin-containing cells in the iris and/or cornea to trigeminal nerve afferents.32–34 In fact, trigeminal neurons in humans have been found to contain melanopsin, and isolated trigeminal ganglion cell neurons have been found to be responsive to light via melanopsin pigment.33 This pathway may function independently, but less data is available on its role in pain transmission. Other players may also be involved in photophobia, including retinal cone cells35 and the hypothalamus, with secondary sympathetic and parasympathetic responses to light.36
METHODS
Given the overlapping symptom of photophobia in the three conditions, the aim of this review was to evaluate the literature and summarise overlapping pathologic mechanisms that may explain the shared feature, with the goal of guiding therapy in appropriate individuals. As such, a PubMed search was conducted using the terms ‘photophobia’ AND either ‘dry eye’, ‘migraine’, ‘traumatic brain injury’, ‘sensitisation’, ‘allodynia’, ‘hyperalgesia’ and ‘CGRP’. All published scientific articles were considered including original research, meta-analyses and systematic reviews. All searches were limited to the English language. Articles were excluded if they studied ocular pain associated with trigeminal neuralgia and postherpetic neuralgia. Eligible articles were reviewed and summarised.
RESULTS
Comorbidity of DE, migraine and TBI
DE, migraine and TBI are comorbid. First, patients with migraine are more likely to have DE symptoms and signs when compared to controls,37–40 which significantly impact visual quality of life.41 In two studies, patients with migraine had significantly higher Ocular Surface Disease Index (OSDI) scores, lower Schirmer scores and lower tear break-up times compared to individuals without migraine.37,38 Likewise, headache and migraine are among the most common sequela of TBI.20 Many patients with TBI progress to having chronic headaches persisting months to years following initial insult.8 In one systematic review of 23 independent studies, the prevalence of headache after TBI was 57.8% [95% CI 55.5% to 60.2%].42 In a prospective study of over 200 TBI subjects, nearly 50% of PTH were classified as migraine or probable migraine, and of those reporting several headaches/week, 62% were classified as having migraine.43 Veterans with TBI were also more likely to have a diagnosis of DE compared with their counterparts without TBI (37% vs 29%, p<0.0005).20 The prevalence of migraine and TBI in patients with DE has not been systematically evaluated, and the literature does not suggest a causative relationship between DE, migraine and TBI. However, the shared symptoms between them suggest a common underlying pathophysiology. Among these symptoms is photophobia.
Photophobia is common to DE, migraine and TBI
Photophobia is a symptom of many ocular, neurological and psychiatric conditions and is also associated with certain medications.22 It is often debilitating and difficult to manage.2 In this review, we focused on photophobia in DE, migraine and TBI because the conditions are common, are often comorbid and may have similar underlying neural pathophysiology.
In a retrospective review of 124 patients with photophobia presenting to a major academic centre, over one-third of cases were attributed to DE and 12.9% attributed to other ocular causes, including blepharitis (4.8%).44 We have also demonstrated that men with persistent DE symptoms (Dry Eye Questionnaire (DEQ)-5 scores ≥6 over a 2-year period) were more likely to report photophobia than those without persistent DE symptoms (OR=5.6; 95% CI 2.0 to 123, p=0.009).45 In fact, the first question on the OSDI, the most commonly used DE symptom questionnaire, asks about light sensitivity,46 further demonstrating the importance of photophobia as a component of DE symptoms.
Photophobia is also a defining characteristic of migraine, both during and between attacks.47,48 In fact, photophobia was found to be the ‘most bothersome symptom’ of migraine in 6,045 respondents from the Migraine in America: Symptoms and Treatment study.49 Our group demonstrated that of 117 patients with chronic migraine, greater than 80% rated their photophobia as severe (score ≥7 out of 10).48 In another study, 99 individuals tested during a migraine-free interval reported higher levels of photophobia and were less tolerant to visual stress with black/white contrast compared to 101 healthy controls.50
TBI is also associated with a myriad of visual complaints including photophobia. A 50%–74% of people with TBI endorse visual complaints, of which approximately 55% report photophobia.51–53 In a case–control comparison study of US military personnel with and without blast-induced mild TBI, photophobia was significantly greater among those with TBI.53 In another study of 40 individuals, the prevalence of photophobia was 4.5 times higher in those with TBI as compared to controls.54 In a retrospective review of 62 patients with TBI and photophobia, 26% were sensitive to fluorescent lights and 21% reported sensitivity to all light sources.55 Taken together, these data show that photophobia is common in DE, migraine and following TBI. Recent advances have found that central neurologic abnormalities that underlie photophobia physiologically link these three conditions together.
Photophobia in humans correlates with central neuroplasticity
Photophobia has been linked to central nervous system (CNS) abnormalities in humans. In one study, 18% of individuals (41 of 224) with DE symptoms were found to have persistent ocular pain after placement of topical anaesthesia.56 This implies an abnormality within central neurons, as peripheral corneal afferents should be completely quieted by sodium channel inhibition. Individuals with persistent pain after topical anaesthesia also reported higher intensities of pain to wind and light compared to their counterparts with DE symptoms but without persistent pain, thus linking photophobia to central neuron changes.56 We also found that individuals with wind hyperalgesia and photophobia had increased pain sensitivity at the forearm,57 suggesting system-wide increased sensitivity to pain. Individuals with photophobia also exhibited other signs suggestive of CNS dysfunction, including aftersensations and increased ‘wind up’ with recurrent pain.57
Furthermore, two case studies described fMRI activation of the trigeminothalamic pathway including central changes in two individuals, one with photophobia in the setting of a corneal injury58 and another with congenital photophobia.31 Specifically, in the individual with a hard contact lens-induced corneal injury, bright light induced pain and activated neurons at the level of the trigeminal ganglia, TNC and ventroposteromedial thalamus,58 areas participating in the photophobia pathways described above. After the injury healed, the same light stimulus no longer produced pain and these areas were not activated. Together, these studies suggest a relationship between corneal nociception, RGCs and ipRGCs. Taken together, these data suggest that pathways for photophobia involve central nerve dysfunction and activation of the trigeminothalamic pathway.
Pathologic neuronal dysregulation likely underlies photophobia in DE, migraine and TBI
In DE, migraine and TBI, the common denominator linking light to pain is the trigeminothalamic pathway.23 When we touch a hot stove, acute nociceptive pain signals generate a withdrawal reflex that effectively removes our hand from the heat, although with accompanying pain. Under normal physiologic conditions, barring no tissue damage, withdrawal from the painful stimuli halts further nociceptive input and no additional pain is elicited. However, synapses are subject to use-dependent plasticity.59,60 A sudden disruption to neurons (as is seen in TBI) or chronic activation of neurons (as is seen in DE and migraine) can lead to permanent morphological and molecular changes in neurons (further discussed below).
Neuroplasticity of primary afferent sensory neurons (eg, corneal nerves) and central neurons in second-order and third-order trigeminal neuron projections can underlie abnormal pain responses.61,62 With peripheral neuroplasticity, the threshold of peripheral nociceptive activation is lowered and hyperalgesia is generally restricted to the site of tissue injury. With central neuroplasticity, there is increased membrane excitability, facilitated synaptic transmission, reduced inhibition and enlargement of receptive fields.59,60 The resulting phenotype is that normally innocuous stimuli may generate pain (allodynia), painful stimuli may be exaggerated (hyperalgesia) and/or pain may extend beyond the site of initial insult (secondary hyperalgesia). In fact, allodynia is a hallmark of central neuroplasticity or sensitisation.59,63 Studies have found that specific neuropeptides are involved in the manifestations described above.
Biological role of calcitonin gene-related peptide (CGRP) in mediating central neuroplasticity and linking DE, migraine and TBI
CGRP is a multifunctional neuropeptide identified in distinct regions of the peripheral nervous system and CNS. CGRP has been implicated in cardiovascular, gastrointestinal, endocrine and neurologic activities and was one of the earliest examples of cellular alternative splicing in the literature. Recognised members of the CGRP neuropeptide include calcitonin, α-CGRP and β-CGRP, amylin, adrenomedullin and intermedin (or adrenomedullin 2). The CGRP receptor is a G protein-coupled receptor with three subunits: calcitonin-like receptor, receptor component protein and receptor activity-modifying protein 1 (RAMP1). This receptor primarily activates a cyclic AMP-signalling pathway to regulate gene expression and cellular activity. Human α-CGRP is encoded by the CALCA gene and is the predominant form expressed in trigeminal ganglia. Trigeminal CGRP is a mediating factor in vasodilation and neurogenic inflammation.64,65 There has been a significant exploration into CGRP as a common culprit in photophobia in the setting of DE, migraine and TBI.66–68
While the development of central neuroplasticity is complex, involving a variety of different receptors affecting downstream cellular mechanisms which alter gene expression and receptor activation, CGRP is highly implicated in this process.64 Specifically, within the trigeminal system at the level of the trigeminal ganglia, CGRP acts via autocrine69 and paracrine70,71 mechanisms to activate mitogen-activated protein kinases which induce transcription factors involved in the inflammatory cascade (cytokines and interleukins).71 This in turn stimulates surrounding glial cells to release nitric oxide, further potentiating an inflammatory state.70 Over-stimulation of peripheral nociceptors and neuroplasticity in recipient central neurons, whether it be from chronic ocular surface disruption, chronic migraine or TBI, can sensitise neurons which can cause spontaneous72 unpleasant sensations from normally innocuous inputs, including photophobia.63,73
CGRP is a key mediator of photophobia
In mice, CGRP administration alone leads to photophobia. First, genetically modified mice with upregulated RAMP1, a required subunit of the CGRP receptor, were more photophobic than controls, as measured by light aversion behaviour. Specifically, light aversion behaviour was measured as reduced time spent in a light chamber, number of transitions between dark and light chambers and time latencies from entry to second entry into a light compartment.74 Second, wild-type mice were more photophobic than controls when CGRP was administered centrally via intracerebroventricular injection.72 Third, transgenic and wild-type mice were more photophobic than controls when CGRP was administered peripherally via intraperitoneal injection.68 In both mouse models, photophobic behaviour following administration of CGRP, whether centrally or peripherally, was attenuated with coadministration of triptans (well-known abortive migraine medications).68,72 In addition, peripheral administration of an anti-CGRP antibody attenuated the peripheral CGRP-induced photophobic response.68
Humans who receive CGRP infusions also develop photophobia. In human volunteers, significant photophobia developed within 30 min of CGRP infusion, well before the median onset of headache which occurred at 3 hours. While this study did not have a control group, these results suggest that not only does CGRP trigger migraine in humans, but it may also trigger photophobia before the onset of migraine.75 The multifunctional nature of CGRP has also implicated it in several pain processes, including corneal sensation.
Neuroplasticity and CGRP are involved in corneal nociception and DE
Lacrimal gland excision is one method used to induce aqueous tear deficiency (ATD) in animal models.76 Studies have found that corneal nerve structure and function are altered after lacrimal gland excision.77 These changes were accompanied by increased corneal sensitivity, demonstrated by increased eye-wiping behaviour following administration of hypertonic saline as compared to control mice.78 Central changes were also noted in the form of periorbital receptor field expansion, as measured by neuronal electrophysiologic recordings at the trigeminal subnucleus interpolaris/subnucleus caudalis transition (Vi/Vc) and trigeminal caudalis/cervical transition (Vc/C1) transition regions of the trigeminal nucleus. This effect was abolished after administration of a synaptic blocking agent (CoCl2) administered into the Vi/Vc transition region.79 Taken together, these findings demonstrate long-term changes in nerve structure and function after induction of ATD in both peripheral and central nerves.
Photophobia and corneal nociception are connected via the Vi/Vc and Vc/C1 transition regions of the trigeminal nucleus. Bright light activation of ipRGCs stimulates neurons in the Vi/Vc and the Vc/C1,26 areas that also receive information from corneal nociception.80,81 In mouse models, benzalkonium chloride-induced corneal surface damage increased corneal mechanosensitivity and induced light aversive behaviour when compared to control mice, effects that were not seen in mice lacking ipRGCs.82 These data suggest that via ipRGCs, mechanical injury to the cornea induced photophobia and initiated activation of the trigeminothalamic pathway. As such, persistent and inappropriate activation of these networks may produce a clinical picture of chronic DE symptoms and photophobia, even without overt clinical signs.
CGRP is likely involved in the above processes. The cornea is innervated by three broad classes of neurons: polymodal nociceptors, cold thermoreceptors and mechano-nociceptors. Polymodal nociceptors are characterised by the transient receptor potential vanilloid type 1 (TRPV1) channel, which becomes sensitised when stimulated by repeated noxious stimuli.83 CGRP (and other inflammatory molecules) result in upregulation of TRPV1 in the trigeminal ganglia,84 and CGRP has been shown to colocalise with TRPV1.85 Additionally, activated corneal nerves release CGRP.86 This neuropeptide also enhances activity of N-methyl-D-aspartate receptors,87 a key player in the central neuroplasticity of trigeminal subnuclei.79,88 Though studies have shown decreased levels of CGRP within the tear film of individuals with DE (defined as a Dry Eye Workshop DE severity grading score ≥189),90 there may be CGRP-mediated increases in neural responsiveness at the level of the trigeminal ganglion and more centrally. CGRP-induced changes also play a role in other neurologic disease states, like migraine.
Neuroplasticity and CGRP are involved in migraine
Activation and sensitisation of the trigeminovascular system, including the TNC and thalamus, are involved in the pathophysiology of migraine (figure 2).73,91,92 First, in mouse models, chemical stimulation of the dural meninges increased neuronal firing rates in trigeminal neurons in response to innocuous mechanical stimulation of the face (brush and pressure).93 Second, in rats, an inflammatory ‘soup’ (histamine, serotonin, bradykinin and prostaglandin E2) was placed on the dural meninges, resulting in hypersensitivities to light touch, pinch and heat noted outside the trigeminal distribution (contralateral paw). The thalamus also exhibited increased firing rates and magnitude.63 Together, these studies demonstrate that neuroplasticity in migraine occurs at multiple sites.63,91
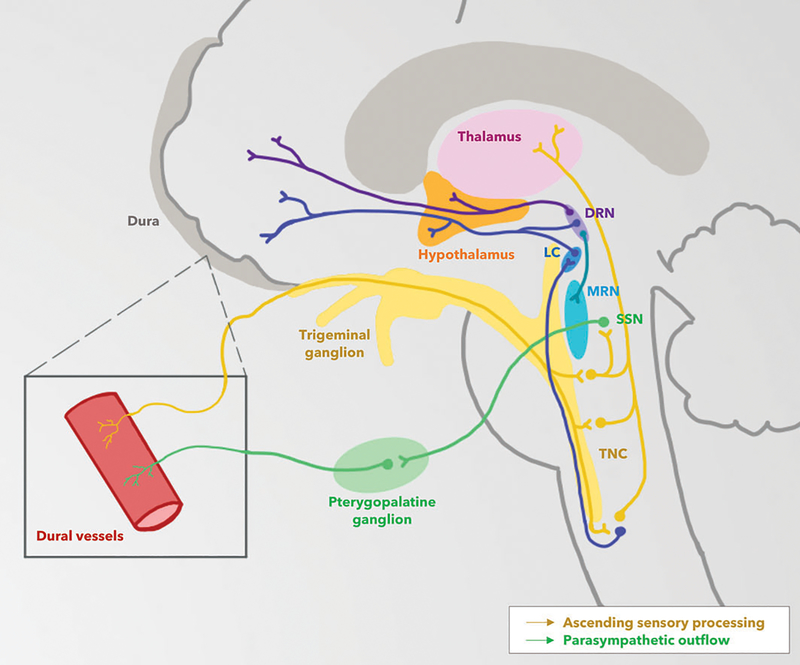
Figure 2.
The trigeminovascular system in migraine. The dysfunctions of several brainstem pathways have been implicated in the pathogenesis of migraine. Trigeminovascular input from meningeal vessels passes through the ophthalmic branch of the trigeminal nerve and trigeminal ganglion before synapsing at the trigeminal nucleus caudalis (TNC). These neurons decussate in the brainstem and synapse at the thalamus. Brain imaging studies have revealed nociceptive modulatory activity involving the magnus raphe nucleus (MRN), locus ceruleus (LC) and dorsal raphe nucleus (DRN). A reflex neural connection from the pons, originating at the superior salivatory nucleus (SSN), is responsible for cranial parasympathetic output to dural vessels, as mediated by the pterygopalatine, or sphenopalatine, ganglion; this trigemino-autonomic pathway may play a role in migraine. Genetic, environmental, dietary and metabolic factors in migraine are thought to ultimately incite pathologic activation of areas of the hypothalamus, limbic system and cortex. Illustration by Divy Mehra.
Not surprisingly, human migraineurs also experience cutaneous allodynia of the scalp, and self-reported photophobia is a prominent accompanying symptom.94,95 In a cross-sectional questionnaire-based study of 456 consecutive patients with migraine, 248 (54%) reported allodynia of the scalp (with touching/brushing hair, wearing glasses or lying with head resting on the affected side) and 385 (84%) reported photophobia. Among patients with chronic migraine (n=126), allodynia prevalence was greater in photophobic compared to non-photophobic individuals (67% vs 40%, p=0.029).95 These anatomical relationships are also supported clinically in humans, as even blind individuals (visual acuity <20/200) continue to experience migraine-associated photophobia, in the setting of extensive rod/cone dysfunction but intact inner retinal ipRGCs.29 The association between allodynia and photophobia in chronic migraine and the presence of persistent photophobia in blind patients suggest that the above neuronal networks are chronically altered such that normally innocuous light may be perceived as painful.
CGRP is highly implicated in migraine.66,96 In cats, stimulation of the trigeminal nucleus resulted in increased cerebral blood flow and CGRP in the ipsilateral external jugular vein.97 In humans, individuals with migraine have significantly higher levels of CGRP within the external jugular vein when compared to controls,98 and human volunteers develop migraine after receiving intravenous infusions of CGRP.99 In summary, these above studies demonstrate that central neural changes and CGRP are involved in migraine. Similar data has been found in TBI.
Neuroplasticity and CGRP are involved in TBI
The symptoms associated with TBI are complex and various mechanisms have been proposed. An array of inflammatory processes and neural dysfunctions ultimately results in an increase in neuronal activation, which may be mediated, in part, by CGRP.
Mice develop cutaneous allodynia and photophobia after head injury. First, in a mouse model of controlled-cortical impact (CCI), test mice were subjected to a stereotaxic impactor applied directly to the somatosensory cortex unilaterally, while control mice experienced an incision only. Mechanical allodynia was measured in the periorbital region (V1) using predefined allodynia thresholds defined as the smallest force needed to elicit a limb withdrawal reaction. Following CCI insult, control mice demonstrated no significant differences in mechanical sensitivity when compared to baseline. Mice exposed to CCI developed significant allodynic responses—mechanical thresholds were reduced nearly 10-fold when compared to control mice, an effect that persisted for up to 28 days at locations ipsilateral and contralateral to the impact site.67 Second, other mouse models involving blunt closed head injury (weight drop) demonstrated similar findings of increased periorbital tactile sensitivity when compared to controls.9 Third, in addition to periorbital allodynia, mice subjected to direct somatosensory cortical impact also developed significant light aversive behaviours to bright light (4000 lux) when compared to controls.100 Contralateral involvement and the persistence of symptoms beyond initial insult support the hypothesis of underlying central neuroplasticity after TBI.
Humans also develop cutaneous hyperalgesia and photophobia following TBI. In humans with PTH, the three most frequently reported areas of headache pain are within the trigeminal distribution: the temple (82%), the forehead (76.5%) and the periorbital region (49%). Individuals with TBI demonstrated higher thermal pain thresholds but lower pressure pain thresholds on the head and dorsal hand compared to controls, suggesting neural dysregulation of different pain pathways following TBI. Furthermore, bright light exacerbated headache symptoms in 65% of subjects with PTH.101 While photophobia severity scores in patients with TBI declined over time, 42% of individuals experienced persistent photophobia extending beyond a year after inciting injury.55 As above, this persistence suggests that photophobia is a manifestation of permanent, rather than transient, changes in the CNS.
CGRP likely plays a key role in mediating peripheral and central neuroplasticity and thus photophobia in TBI. In mouse models, CCI resulted in increased levels of CGRP and substance P within the TNC and significantly reduced periorbital mechanical thresholds (a marker of allodynia). Moreover, reduced mechanical thresholds of the periorbital region correlated with elevated CGRP (r = −0.65, p < 0.0001).67 In similar animal models, allodynia was abolished in 57% and 75% of mice following administration of triptans and CGRP monoclonal antibodies (mAb), respectively. Photophobic behaviour, as measured by percentage of time spent in a bright light chamber (4000 lux) compared to an ambient light chamber (400 lux), was also reduced by administration of a monoclonal CGRP antibody.100 Finally, long-term CGRP mAb treatment given at the time of injury was effective in preventing allodynia.9
Taken together, these studies implicate increased inflammatory mediators, notably CGRP, acting at various levels in the afferent trigeminal system to produce allodynia and photosensitivity following TBI. This pathophysiological mechanism has prompted CGRP to become one of several targets in the treatment of photophobia associated with DE, migraine and TBI. Assessing for the presence and severity of photophobia is an important first step in guiding treatment in these conditions.
Clinical assessment of photophobia
It is important to evaluate photophobia in a standardised way. This can easily be done in the clinical setting using any of a number of questionnaires. Specifically, the presence and intensity of photophobia can be assessed by looking at answers to specific questions within DE (Question 1 of the OSDI46) and migraine (Question 2 of ID Migraine102) questionnaires. Alternatively, questionnaires have been validated specifically for photophobia such as the Visual Light Sensitivity Questionnaire-8 (VLSQ-8),103 the Utah Photophobia Symptom Impact Scale 17104 and the Korean Photophobia Questionnaire.105 These questionnaires use a series of questions using a Likert scale to assess the severity of a wide variety of photophobia complaints. A Likert scale (5-point or 7-point scale) grades how much an individual agrees or disagrees with a statement, assuming that the intensity of a sensation such as light sensitivity is linear and assumes that sensitivity can be subjectively and accurately assessed by the subject.
In the research arena, groups are studying other approaches to quantifying photophobia. One group developed and validated an ocular photosensitivity system which uses precisely controlled light stimuli ranging from 0.1 to 32 000 lux to determine at which light intensity the subject feels pain. The light intensity starts at a low level and is increased gradually. Individuals are instructed to press a button when the light stimulus becomes painful. This machine has demonstrated that individuals with light sensitivity from achromatopsia or TBI are more photophobic than healthy controls.106
Another objective approach for assessing light sensitivity is recording pupil and facial electromyogram (EMG) responses to increasing light stimuli using a Ganzfeld dome. In one study, subjects with a history of migraine not currently experiencing a headache were found to have a normal pupil response to red and blue light, but an exaggerated EMG response from the procerus and orbicularis regions compared to normal age-matched subjects.107 Concomitant measurement of heart rate variability demonstrated increased parasympathetic and reduced sympathetic nerve activity at baseline in individuals with photophobia. These findings may be explained by the postulated role of the hypothalamus in photophobia pathways, as described above. Identifying the presence of photophobia in DE, migraine and TBI can help improve treatments of these conditions.
Treatment of photophobia in DE, migraine and TBI
First, therapies have been studied specifically for photophobia. For example, tinted lenses reduce the severity of photophobia and can be used as an adjuvant treatment in DE, migraine and TBI. Specifically, the tinted lens FL-41 minimises transmittance of blue wavelengths around 480 nm, which corresponds to the spectral sensitivity of melanopsin, the photopigment contained within ipRGCs that activates trigeminothalamic pathways. FL-41 lenses reduced the frequency of migraine attacks in schoolchildren following 4 months of daily wear (frequency was reduced from 6.2/month to 1.6/month), but did not improve attack intensity or duration.108 FL-41 tinted lenses have also shown therapeutic benefit for photophobia in patients with benign essential blepharospasm109 and idiopathic photophobia.31 While FL-41 lenses have not been systematically evaluated for photophobia related to DE or TBI, a variety of coloured lens tints have been found to reduce photophobia symptoms in patients with TBI.110
Given CGRP’s relevance in migraine pathogenesis, mAbs that block CGRP were approved by the US Food and Drug Administration including eptinezumab (intravenous infusion once every 3 months), fremanezumab (monthly or quarterly subcutaneous injection) and galcanezumab (monthly subcutaneous injection). Erenumab (monthly subcutaneous injection) is an antibody that blocks the CGRP receptor.111 Prophylactic CGRP antagonists have similar individual efficacy to other preventative agents (ie, topiramate, propranolol) and may be used in combination in episodic and chronic migraine.111 Another category of oral medications (eg, rimegepant and ubrogepant) was recently approved as abortive agents that also block the CGRP receptor. In the ACHIEVE I clinical trial, 1672 patients with migraine were randomised to ubrogepant 100 mg (n=557), to ubrogepant 50 mg (n=556) and to placebo (n=559) for acute treatment. At 2 hours postdose, higher proportions of ubrogepant-treated individuals reported normal functioning as compared to placebo (ubrogepant 100 mg, 43% vs 30%, p<0.0001; ubrogepant 50 mg, 41% vs 30%, p=0.0012).112 These data support the adjunctive use of anti-CGRP agents in select patients. To date, these treatments have not been investigated in DE or TBI.
However, lessons learnt from treating migraine and TBI can be applied to individuals with DE symptoms that persist despite traditional therapies, specifically to consider treatments that modulate peripheral and central nerve function. For individuals with photophobia in the setting of ocular surface pathology, autologous blood products including serum tears (ASTs) and platelet-rich plasma are good options. The effects of blood products are thought to be partially mediated by growth factors (nerve growth factor and platelet-derived growth factor) that have been found to improve corneal nerve density and function after injury.113 In fact, ASTs are often used to treat persistent corneal epithelial defects in the setting of neurotrophic keratitis.114 One small retrospective series of 16 individuals with photophobia from diverse aetiologies, including DE, found that ASTs reduced light sensitivity and improved corneal nerve anatomy in a majority of individuals.115
However, given that central nerve abnormalities accompany photophobia, agents that modulate central nerve function should be considered. Gabapentin (600–900 mg three times daily) and pregabalin (150 mg two times per day) are first-line options for suspected neuropathic DE symptoms, acting as α2γ ligands that modulate central voltage-dependent calcium channels. In a case series of seven individuals with severe chronic ocular pain presumed to be neuropathic in origin (ie, symptoms of photophobia and burning, ineffective treatment with therapies targeting the ocular surface and tears, persistent pain with topical anaesthetic), oral gabapentin and pregabalin regimens were associated with pain improvements in the majority of patients after being titrated to relatively high doses (600–900 mg three times per day for gabapentin, 150 mg two times per day for pregabalin). Two patients noted complete ocular pain resolution (Numerical Rating Scale (NRS) score of 0 out of 10), two noted significant improvements (NRS≤2), one noted slight but noticeable improvement (NRS of 10 at baseline, 7 at follow-up) and two noted no improvement.116 This data supports the efficacy of α2γ ligands in targeting photophobia and other neuropathic ocular pain (NOP) symptoms. Tricyclic antidepressant, such as nortriptyline (10–100 mg daily), is another therapeutic option in individuals with chronic NOP, alone or in conjunction with α2γ ligands.117,118 Additional support for the hypothesis that similar mechanisms underlie symptoms of migraine and DE comes from data that migraine-approved medications may have a beneficial effect on DE symptoms. For example, we found that botulinum toxin not only reduced the severity of migraine pain, but also had a positive effect on photophobia and a marginal effect on symptoms of dryness.48 These positive effects were found to be independent of tear volume.119
In addition to quantifying pain and photophobia, physicians should evaluate for the symptoms of depression and anxiety in individuals with DE, migraine and TBI. Animal and human studies have discovered links between photophobia and the limbic system.120 In a study of neonatal rats (which have undeveloped rod and cone photoreceptors but intact ipRGCs), LED lights evoked aversive behaviour (vigorous reorientation away from the light source) in all wild-type rats (n=22). Light exposure was also found to increase phosphorylation of ERK, a marker of neuronal activation, in the amygdala compared to controls.121 These data tie photophobia to activation of the amygdala, a component of the limbic system pivotal to the pathogenesis of several anxiety disorders.122,123 In humans, individuals with migraine and interictal photophobia (n=16) had higher depression and anxiety scores than individuals with migraine without photophobia (n=16) (depression 13±10 vs 6±4.7, p=0.021; anxiety 16±9.8 vs 4±3.4, p<0.001), as well as healthy controls (n=16) (depression 13±10 vs 6±8.8, p=0.0245; anxiety 16 ±9.8 vs 5±6.9, p<0.001).120
Similar findings have been found in individuals with DE symptoms and photophobia.124,125 In a study of 181 patients with DE, individuals were classified into ‘low NOP’ (n=130) and ‘high NOP’ (n=51) groups based on the presence and severity (NRS scale 0–10) of spontaneous burning ocular pain and pain evoked by wind, light and temperature (air conditioning air or warm weather). The high NOP group demonstrated significantly greater light sensitivity (NRS; 7.6±2.2 vs 2.0±2.2, p<0.0001) and greater depression scores via patient health questionnaire 9 compared to the low NOP group (13.5±8.1 vs 7.6±7.2, p<0.0005).124 These findings suggest a relationship between pain severity, photophobia and depression. Individuals with DE symptoms and ocular pain also have dysfunctional psychological coping mechanisms, specifically catastrophising, as seen by correlations between the Pain Catastrophising Scale and DEQ-5 scores (r=0.41, p<0.0005), and Neuropathic Pain Symptom Inventory-Eye scores (r=0.48, p<0.0005).126 The link between emotion and light sensitivity highlights the importance of screening individuals for depression and anxiety and referring appropriate individuals to mental health providers.
CONCLUSION
To conclude, our review highlights that DE, migraine and TBI are often comorbid with photophobia being a shared feature. As such, the presence of migraine, TBI and other pain complaints should be identified in individuals with DE. Validated questionnaires (ie, OSDI, VLSQ-8) can easily be incorporated into the assessment of patients in the eye clinic to quantify the severity of photophobia. In individuals with DE symptoms, the presence of photophobia, migraine or TBI should alert the eye care provider to the possibility that central nerve abnormalities in trigeminothalamic pathways are involved in the disease process. This can then help guide therapy, especially in individuals who fail traditional therapies such as artificial tears and topical anti-inflammatory therapies. In these individuals, therapies that modulate peripheral and central nerves can be considered, such as ASTs or gabapentin. In addition, treatments specific to photophobia (such as tinted lenses) can help decrease disease morbidity in all three conditions. Given the role of CGRP in modulating photophobia and pain, medications that target CGRP, such as botulinum toxin or the newer CGRP antagonists, may be considered, although there is a paucity of evidence on their effects in DE and TBI. As such, future studies are needed to investigate which approaches that address pain and photophobia in migraine can improve disease morbidity in DE and TBI. In addition, investigations into new treatment modalities that target photophobia are needed. Overall, as with any chronic pain condition, an interdisciplinary approach is often needed that includes a patient’s primary care physician, neurologist, mental health provider and ophthalmologist.127
Acknowledgments
Funding Supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research I01 CX002015 (Dr Galor), Biomedical Laboratory R&D (BLRD) Service I01 BX004893 (Dr Galor), Department of Defense GW190010 (Dr Galor), R01EY026174 (Dr Galor), NIH Center Core Grant P30EY014801 and Research to Prevent Blindness Unrestricted Grant, and discretionary funds from the Department of Anesthesiology, Boston Children’s Hospital (Dr Moulton). RR&D Iowa City Center for the Prevention and Treatment of Visual Loss C9251-C (RX003002) (Dr. Kardon), C2978-R (Dr. Kardon), 2 I01 RX000889-05A2 (Dr. Kardon), Department of Defense/VA/Chronic Effects of Neurotrauma Consortium W81XWH-13-2-0095, I01 CX001135 (Dr. Kardon), NIH (NEI) 1R01EY031544-01 FAIN: R01EY031544 (Dr. Kardon).
Footnotes
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement All data collected are being presented in this review.
REFERENCES
- 1.IASP terminology. Available http://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698 (accessed Jan 2019).
- 2.Galor A, Moein H-R, Lee C, et al. Neuropathic pain and dry eye. Ocul Surf 2018;16:31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galor A, Feuer W, Lee DJ, et al. Ocular surface parameters in older male veterans. Invest Ophthalmol Vis Sci 2013;54:1426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fraser CL, Hepschke JL, Jenkins B, et al. Migraine aura: pathophysiology, mimics, and treatment options. Semin Neurol 2019;39:739–48. [DOI] [PubMed] [Google Scholar]
- 5.Charles A The pathophysiology of migraine: implications for clinical management. Lancet Neurol 2018;17:174–82. [DOI] [PubMed] [Google Scholar]
- 6.Iyengar S, Johnson KW, Ossipov MH, et al. CGRP and the trigeminal system in migraine. Headache 2019;59:659–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong RA. Visual problems associated with traumatic brain injury. Clin Exp Optometry 2018;101:716–26. [DOI] [PubMed] [Google Scholar]
- 8.Ng SY, Lee AYW. Traumatic brain injuries: pathophysiology and potential therapeutic targets. Front Cell Neurosci 2019;13:528–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bree D, Levy D. Development of CGRP-dependent pain and headache related behaviours in a rat model of concussion: implications for mechanisms of post-traumatic headache. Cephalalgia 2018;38:246–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skandsen T, Kvistad KA, Solheim O, et al. Prevalence and impact of diffuse axonal injury in patients with moderate and severe head injury: a cohort study of early magnetic resonance imaging findings and 1-year outcome. J Neurosurg 2010;113:556–63. [DOI] [PubMed] [Google Scholar]
- 11.Mares C, Dagher JH, Harissi-Dagher M. Narrative review of the pathophysiology of headaches and photosensitivity in mild traumatic brain injury and concussion. Can J Neurol Sci 2019;46:14–22. [DOI] [PubMed] [Google Scholar]
- 12.Mustafa G, Hou J, Tsuda S, et al. Trigeminal neuroplasticity underlies allodynia in a preclinical model of mild closed head traumatic brain injury (cTBI). Neuropharmacology 2016;107:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf 2017;15:334–65. [DOI] [PubMed] [Google Scholar]
- 14.Farrand KF, Fridman M, Stillman IO, et al. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 years and older. Am J Ophthalmol 2017;182:90–8. [DOI] [PubMed] [Google Scholar]
- 15.Stovner LJ, Nichols E, Steiner TJ, et al. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol 2018;17:954–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smitherman TA, Burch R, Sheikh H, et al. The prevalence, impact, and treatment of migraine and severe headaches in the United States: a review of statistics from national surveillance studies. Headache 2013;53:427–36. [DOI] [PubMed] [Google Scholar]
- 17.Stewart WF, Wood C, Reed ML, et al. Cumulative lifetime migraine incidence in women and men. Cephalalgia 2008;28:1170–8. [DOI] [PubMed] [Google Scholar]
- 18.Dewan MC, Rattani A, Gupta S, et al. Estimating the global incidence of traumatic brain injury. 2018;130:1080. [DOI] [PubMed] [Google Scholar]
- 19.Roozenbeek B, Maas AI, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol 2013;9:231–6. [DOI] [PubMed] [Google Scholar]
- 20.Lee CJ, Felix ER, Levitt RC, et al. Traumatic brain injury, dry eye and comorbid pain diagnoses in US veterans. Br J Ophthalmol 2018;102:667–73. [DOI] [PubMed] [Google Scholar]
- 21.Digre KB. More than meets the eye: the eye and migraine-what you need to know. J Neuroophthalmol 2018;38:237–43. [DOI] [PubMed] [Google Scholar]
- 22.Digre KB, Brennan KC. Shedding light on photophobia. J Neuro-ophthalmol 2012;32:68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz BJ, Digre KB. Diagnosis, pathophysiology, and treatment of photophobia. Surv Ophthalmol 2016;61:466–77. [DOI] [PubMed] [Google Scholar]
- 24.Bailes HJ, Lucas RJ. Human melanopsin forms a pigment maximally sensitive to blue light (lambdamax approximately 479 nm) supporting activation of G(q/11) and G(i/o) signalling cascades. Proc Biol Sci 2013;280:20122987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hattar S, Kumar M, Park A, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol 2006;497:326–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okamoto K, Thompson R, Tashiro A, et al. Bright light produces Fos-positive neurons in caudal trigeminal brainstem. Neuroscience 2009;160:858–64. [DOI] [PubMed] [Google Scholar]
- 27.Marek V, Reboussin E, Degardin-Chicaud J, et al. Implication of melanopsin and trigeminal neural pathways in blue light photosensitivity in vivo. Front Neurosci 2019;13:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noseda R, Burstein R. Advances in understanding the mechanisms of migraine-type photophobia. Curr Opin Neurol 2011;24:197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noseda R, Kainz V, Jakubowski M, et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci 2010;13:239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noseda R, Copenhagen D, Burstein R. Current understanding of photophobia, visual networks and headaches. Cephalalgia 2019;39:1623–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panorgias A, Lee D, Silva KE, et al. Blue light activates pulvinar nuclei in long-standing idiopathic photophobia: a case report. Neuroimage Clin 2019;24:102096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xue T, Do MT, Riccio A, et al. Melanopsin signalling in mammalian iris and retina. Nature 2011;479:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matynia A, Nguyen E, Sun X, et al. Peripheral sensory neurons expressing melanopsin respond to light. Front Neural Circuits 2016;10:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Semo M, Gias C, Ahmado A, et al. A role for the ciliary marginal zone in the melanopsin-dependent intrinsic pupillary light reflex. Exp Eye Res 2014;119:8–18. [DOI] [PubMed] [Google Scholar]
- 35.Noseda R, Bernstein CA, Nir RR, et al. Migraine photophobia originating in cone-driven retinal pathways. Brain 2016;139:1971–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burstein R, Noseda R, Fulton AB. Neurobiology of photophobia. J Neuroophthalmol 2019;39:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarac O, Kosekahya P, Yildiz Tasci Y, et al. The prevalence of dry eye and sjogren syndrome in patients with migraine. Ocul Immunol Inflamm 2016;1–6. [DOI] [PubMed] [Google Scholar]
- 38.Koktekir BE, Celik G, Karalezli A, et al. Dry eyes and migraines: Is there really a correlation? Cornea 2012;31:1414–16. [DOI] [PubMed] [Google Scholar]
- 39.Kinard KI, Smith AG, Singleton JR, et al. Chronic migraine is associated with reduced corneal nerve fiber density and symptoms of dry eye. Headache 2015;55:543–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farhangi M, Diel RJ, Buse DC, et al. Individuals with migraine have a different dry eye symptom profile than individuals without migraine. Br J Ophthalmol 2019. [DOI] [PubMed] [Google Scholar]
- 41.Ozudogru S, Neufeld A, Katz BJ, et al. Reduced visual quality of life associated with migraine is most closely correlated with symptoms of dry eye. Headache 2019;59:1714–21. [DOI] [PubMed] [Google Scholar]
- 42.Nampiaparampil DE. Prevalence of chronic pain after traumatic brain injury: a systematic review. JAMA 2008;300:711–9. [DOI] [PubMed] [Google Scholar]
- 43.Lucas S, Hoffman JM, Bell KR, et al. A prospective study of prevalence and characterization of headache following mild traumatic brain injury. Cephalalgia 2014;34:93–102. [DOI] [PubMed] [Google Scholar]
- 44.TDk B, Warner JE, Katz BJ. A review of the symptoms of photophobia in an eye clinic. Neurology 2009;72:184. [Google Scholar]
- 45.Galor A, Zlotcavitch L, Walter SD, et al. Dry eye symptom severity and persistence are associated with symptoms of neuropathic pain. Br J Ophthalmol 2015;99:665–8. [DOI] [PubMed] [Google Scholar]
- 46.Schiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the ocular surface disease index. Arch Ophthalmol 2000;118:615–21. [DOI] [PubMed] [Google Scholar]
- 47.International Headache Society. Headache classification committee of the international headache society (IHS) the international classification of headache disorders, 3rd edition. Cephalalgia 2018;38:1–211. [DOI] [PubMed] [Google Scholar]
- 48.Diel RJ, Kroeger ZA, Levitt RC, et al. Botulinum toxin a for the treatment of photophobia and dry eye. Ophthalmology 2018;125:139–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Munjal S, Singh P, Reed ML, et al. Most bothersome symptom in persons with migraine: results from the migraine in America Symptoms and Treatment (MAST) Study. Headache 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mulleners WM, Aurora SK, Chronicle EP, et al. Self-reported photophobic symptoms in migraineurs and controls are reliable and predict diagnostic category accurately. Headache 2001;41:31–9. [DOI] [PubMed] [Google Scholar]
- 51.Goodrich GL, Kirby J, Cockerham G, et al. Visual function in patients of a polytrauma rehabilitation center: a descriptive study. J Rehabil Res Dev 2007;44:929–36. [DOI] [PubMed] [Google Scholar]
- 52.Bulson R, Jun W, Hayes J. Visual symptomatology and referral patterns for operation Iraqi freedom and operation enduring freedom veterans with traumatic brain injury. J Rehabil Res Dev 2012;49:1075–82. [DOI] [PubMed] [Google Scholar]
- 53.Capo-Aponte JE, Urosevich TG, Temme LA, et al. Visual dysfunctions and symptoms during the subacute stage of blast-induced mild traumatic brain injury. Mil Med 2012;177:804–13. [DOI] [PubMed] [Google Scholar]
- 54.Truong JQ, Ciuffreda KJ. Objective pupillary correlates of photosensitivity in the normal and mild traumatic brain injury populations. Mil Med 2016;181:1382–90. [DOI] [PubMed] [Google Scholar]
- 55.Truong JQ, Ciuffreda KJ, Han MH, et al. Photosensitivity in mild traumatic brain injury (mTBI): a retrospective analysis. Brain Injury 2014;28:1283–7. [DOI] [PubMed] [Google Scholar]
- 56.Crane AM, Feuer W, Felix ER, et al. Evidence of central sensitisation in those with dry eye symptoms and neuropathic-like ocular pain complaints: incomplete response to topical anaesthesia and generalised heightened sensitivity to evoked pain. Br J Ophthalmol 2017;101:1238–43. [DOI] [PubMed] [Google Scholar]
- 57.Galor A, Levitt RC, McManus KT, et al. Assessment of somatosensory function in patients with idiopathic dry eye symptoms. JAMA Ophthalmol 2016;134:1290–8. [DOI] [PubMed] [Google Scholar]
- 58.Moulton EA, Becerra L, Borsook D. An fMRI case report of photophobia: activation of the trigeminal nociceptive pathway. Pain 2009;145:358–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011;152:S2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science 2000;288:1765–9. [DOI] [PubMed] [Google Scholar]
- 61.Rosenthal P, Borsook D, Moulton EA. Oculofacial pain: corneal nerve damage leading to pain beyond the eye. Invest Ophthalmol Vis Sci 2016;57:5285–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosenthal P, Borsook D. The corneal pain system. Part I: the missing piece of the dry eye puzzle. Ocul Surf 2012;10:2–14. [DOI] [PubMed] [Google Scholar]
- 63.Burstein R, Jakubowski M, Garcia-Nicas E, et al. Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol 2010;68:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Benarroch EE. CGRP: sensory neuropeptide with multiple neurologic implications. Neurology 2011;77:281–7. [DOI] [PubMed] [Google Scholar]
- 65.Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol 2015;55:533–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Durham PL. Diverse physiological roles of calcitonin gene-related peptide in migraine pathology: modulation of neuronal-glial-immune cells to promote peripheral and central sensitization. Curr Pain Headache Rep 2016;20:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elliott MB, Oshinsky ML, Amenta PS, et al. Nociceptive neuropeptide increases and periorbital allodynia in a model of traumatic brain injury. Headache 2012;52:966–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mason BN, Kaiser EA, Kuburas A, et al. Induction of migraine-like photophobic behavior in mice by both peripheral and central CGRP mechanisms. J Neurosci 2017;37:204–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Z, Winborn CS, Marquez de Prado B, et al. Sensitization of calcitonin gene-related peptide receptors by receptor activity-modifying protein-1 in the trigeminal ganglion. J Neurosci 2007;27:2693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li J, Vause CV, Durham PL. Calcitonin gene-related peptide stimulation of nitric oxide synthesis and release from trigeminal ganglion glial cells. Brain Res 2008;1196:22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vause CV, Durham PL. CGRP stimulation of iNOS and NO release from trigeminal ganglion glial cells involves mitogen-activated protein kinase pathways. J Neurochem 2009;110:811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaiser EA, Kuburas A, Recober A, et al. Modulation of CGRP-induced light aversion in wild-type mice by a 5-HT(1B/D) agonist. J Neurosci 2012;32:15439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.PJ G, PR H, Martins-Oliveira M, et al. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev 2017;97:553–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Recober A, Kaiser EA, Kuburas A, et al. Induction of multiple photophobic behaviors in a transgenic mouse sensitized to CGRP. Neuropharmacology 2010;58:156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guo S, Vollesen AL, Olesen J, et al. Premonitory and nonheadache symptoms induced by CGRP and PACAP38 in patients with migraine. Pain 2016;157:2773–81. [DOI] [PubMed] [Google Scholar]
- 76.Stevenson W, Chen Y, Lee SM, et al. Extraorbital lacrimal gland excision: a reproducible model of severe aqueous tear-deficient dry eye disease. Cornea 2014;33:1336–41. [DOI] [PubMed] [Google Scholar]
- 77.Kovacs I, Luna C, Quirce S, et al. Abnormal activity of corneal cold thermoreceptors underlies the unpleasant sensations in dry eye disease. Pain 2016;157:399–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meng ID, Barton ST, Mecum NE, et al. Corneal sensitivity following lacrimal gland excision in the rat. Invest Ophthalmol Vis Sci 2015;56:3347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rahman M, Okamoto K, Thompson R, et al. Sensitization of trigeminal brainstem pathways in a model for tear deficient dry eye. Pain 2015;156:942–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bereiter DA, Hathaway CB, Benetti AP. Caudal portions of the spinal trigeminal complex are necessary for autonomic responses and display Fos-like immunoreactivity after corneal stimulation in the cat. Brain Res 1994;657:73–82. [DOI] [PubMed] [Google Scholar]
- 81.Okamoto K, Bereiter DF, Tashiro A, et al. Ocular surface-evoked Fos-like immunoreactivity is enhanced in trigeminal subnucleus caudalis by prior exposure to endotoxin. Neuroscience 2009;159:787–94. [DOI] [PubMed] [Google Scholar]
- 82.Matynia A, Parikh S, Deot N, et al. Light aversion and corneal mechanical sensitivity are altered by intrinsically photosensitive retinal ganglion cells in a mouse model of corneal surface damage. Exp Eye Res 2015;137:57–62. [DOI] [PubMed] [Google Scholar]
- 83.Belmonte C, Nichols JJ, Cox SM, et al. TFOS DEWS II pain and sensation report. Ocul Surf 2017;15:404–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goto T, Oh SB, Takeda M, et al. Recent advances in basic research on the trigeminal ganglion. J Physiol Sci 2016;66:381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Murata Y, Masuko S. Peripheral and central distribution of TRPV1, substance P and CGRP of rat corneal neurons. Brain Res 2006;1085:87–94. [DOI] [PubMed] [Google Scholar]
- 86.Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res 2004;78:513–25. [DOI] [PubMed] [Google Scholar]
- 87.Iyengar S, Ossipov MH, Johnson KW. The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. Pain 2017;158:543–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kurose M, Meng ID. Corneal dry-responsive neurons in the spinal trigeminal nucleus respond to innocuous cooling in the rat. J Neurophysiol 2013;109:2517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Diagnostic Methodology Subcommittee of the International Dry Eye Workshop. Methodologies to diagnose and monitor dry eye disease: Report of the diagnostic methodology subcommittee of the International Dry Eye Workshop (2007). Ocul Surf 2007;5:108–52. [DOI] [PubMed] [Google Scholar]
- 90.Lambiase A, Micera A, Sacchetti M, et al. Alterations of tear neuromediators in dry eye disease. Arch Ophthalmol 2011;129:981–6. [DOI] [PubMed] [Google Scholar]
- 91.Bernstein C, Burstein R. Sensitization of the trigeminovascular pathway: perspective and implications to migraine pathophysiology. J Clin Neurol 2012;8:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Goadsby PJ, Lipton RB, Ferrari MD. Migraine: current understanding and treatment. N Engl J Med 2002;346:257–70. [DOI] [PubMed] [Google Scholar]
- 93.Burstein R, Yamamura H, Malick A, et al. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol 1998;79:964–82. [DOI] [PubMed] [Google Scholar]
- 94.Lovati C, D’Amico D, Bertora P, et al. Acute and interictal allodynia in patients with different headache forms: an Italian pilot study. Headache 2008;48:272–7. [DOI] [PubMed] [Google Scholar]
- 95.Lovati C, Mariotti C, Giani L, et al. Central sensitization in photophobic and non-photophobic migraineurs: possible role of retino nuclear way in the central sensitization process. Neurol Sci 2013;34:S133–5. [DOI] [PubMed] [Google Scholar]
- 96.Edvinsson L The CGRP pathway in migraine as a viable target for therapies. Headache 2018;58:33–47. [DOI] [PubMed] [Google Scholar]
- 97.Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol 1993;33:48–56. [DOI] [PubMed] [Google Scholar]
- 98.Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol 1990;28:183–7. [DOI] [PubMed] [Google Scholar]
- 99.Lassen LH, Haderslev PA, Jacobsen VB, et al. CGRP may play a causative role in migraine. Cephalalgia 2002;22:54–61. [DOI] [PubMed] [Google Scholar]
- 100.Daiutolo BV, Tyburski A, Clark SW, et al. Trigeminal pain molecules, allodynia, and photosensitivity are pharmacologically and genetically modulated in a model of traumatic brain injury. J Neurotrauma 2016;33:748–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Defrin R, Gruener H, Schreiber S, et al. Quantitative somatosensory testing of subjects with chronic post-traumatic headache: implications on its mechanisms. Eur J Pain 2010;14:924–31. [DOI] [PubMed] [Google Scholar]
- 102.Lipton RB, Dodick D, Sadovsky R, et al. A self-administered screener for migraine in primary care: the ID migraine validation study. Neurology 2003;61:375–82. [DOI] [PubMed] [Google Scholar]
- 103.Verriotto JD, Gonzalez A, Aguilar MC, et al. New methods for quantification of visual photosensitivity threshold and symptoms. Transl Vis Sci Technol 2017;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cortez MM, Digre K, Uddin D, et al. Validation of a photophobia symptom impact scale. Cephalalgia 2019;39:1445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Choi JY, Oh K, Kim BJ, et al. Usefulness of a photophobia questionnaire in patients with migraine. Cephalalgia 2009;29:953–9. [DOI] [PubMed] [Google Scholar]
- 106.Aguilar MC, Gonzalez A, Rowaan C, et al. Automated instrument designed to determine visual photosensitivity thresholds. Biomed Opt Express 2018;9:5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Anderson SC, Poolman P, Full JA, et al. The light induced electromyogram shows exaggerated response in migraine. Assoc Res Vis Ophthalmol (Abstract) 2012. [Google Scholar]
- 108.Good PA, Taylor RH, Mortimer MJ. The use of tinted glasses in childhood migraine. Headache 1991;31:533–6. [DOI] [PubMed] [Google Scholar]
- 109.Blackburn MK, Lamb RD, Digre KB, et al. FL-41 tint improves blink frequency, light sensitivity, and functional limitations in patients with benign essential blepharospasm. Ophthalmology 2009;116:997–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Clark J, Hasselfeld K, Bigsby K, et al. Colored glasses to mitigate photophobia symptoms posttraumatic brain injury. J Athl Train 2017;52:725–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Scuteri D, Adornetto A, Rombolà L, et al. New trends in migraine pharmacology: targeting calcitonin gene-related peptide (CGRP) with monoclonal antibodies. Front Pharmacol 2019;10:363–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dodick DW, Lipton RB, Ailani J, et al. Ubrogepant, an acute treatment for migraine, improved patient-reported functional disability and satisfaction in 2 single-attack phase 3 randomized trials, ACHIEVE I and II. Headache 2020;60:686–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ma K, Yan N, Huang Y, et al. Effects of nerve growth factor on nerve regeneration after corneal nerve damage. Int J Clin Exp Med 2014;7:4584–9. [PMC free article] [PubMed] [Google Scholar]
- 114.Mixon W, Angelle PP, Chang RI. Autologous eye drops for the treatment of dry eye and neurotrophic keratitis. Int J Pharm Compd 2009;11:506–15. [PubMed] [Google Scholar]
- 115.Aggarwal S, Kheirkhah A, Cavalcanti BM, et al. Autologous serum tears for treatment of photoallodynia in patients with corneal neuropathy: efficacy and evaluation with in vivo confocal microscopy. Ocul Surf 2015;13:250–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.LR S, Galor A, ER F, et al. Oral gabapentinoids and nerve blocks for the treatment of chronic ocular pain. Eye Contact Lens 2019. [DOI] [PubMed] [Google Scholar]
- 117.Galor A, Levitt RC, Felix ER, et al. What can photophobia tell us about dry eye? Expert Rev Ophthalmol 2016;11:321–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ozmen MC, Dieckmann G, Rashad R, et al. Nortriptyline is effective in ameliorating symptoms of neuropathic corneal pain. Invest Ophthalmol Vis Sci 2019;60:4732–32. [Google Scholar]
- 119.Diel RJ, Hwang J, Kroeger ZA, et al. Photophobia and sensations of dryness in patients with migraine occur independent of baseline tear volume and improve following botulinum toxin A injections. Br J Ophthalmol 2019;103:1024–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Llop SM, Frandsen JE, Digre KB, et al. Increased prevalence of depression and anxiety in patients with migraine and interictal photophobia. J Headache Pain 2016;17:34–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Delwig A, Logan AM, Copenhagen DR, et al. Light evokes melanopsin-dependent vocalization and neural activation associated with aversive experience in neonatal mice. PLoS One 2012;7:e43787–e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Ann N Y Acad Sci 2003;985:389–410. [DOI] [PubMed] [Google Scholar]
- 123.Kim JE, Dager SR, Lyoo IK. The role of the amygdala in the pathophysiology of panic disorder: evidence from neuroimaging studies. Biol Mood Anxiety Disord 2012;2:20–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Crane AM, Levitt RC, Felix ER, et al. Patients with more severe symptoms of neuropathic ocular pain report more frequent and severe chronic overlapping pain conditions and psychiatric disease. Br J Ophthalmol 2017;101:227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Galor A, Feuer W, Lee DJ, et al. Depression, post-traumatic stress disorder, and dry eye syndrome: a study utilizing the national United States veterans affairs administrative database. Am J Ophthalmol 2012;154:340–46.e2. [DOI] [PubMed] [Google Scholar]
- 126.Patel S, Felix ER, Levitt RC, et al. Dysfunctional coping mechanisms contribute to dry eye symptoms. J Clin Med 2019;8:901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Agostoni EC, Barbanti P, Calabresi P, et al. Current and emerging evidence-based treatment options in chronic migraine: a narrative review. J Headache Pain 2019;20:92. [DOI] [PMC free article] [PubMed] [Google Scholar]