Abstract
OBJECTIVE
Sudden unexpected death in epilepsy (SUDEP) is the leading cause of death for patients with refractory epilepsy, and there is increasing evidence for a centrally mediated respiratory depression as a pathophysiological mechanism. The brain regions responsible for a seizure’s inducing respiratory depression are unclear—the respiratory nuclei in the brainstem are thought to be involved, but involvement of forebrain structures is not yet understood. The aim of this study was to analyze intracranial EEGs in combination with the results of respiratory monitoring to investigate the relationship between seizure spread to specific mesial temporal brain regions and the onset of respiratory dysfunction and apnea.
METHODS
The authors reviewed all invasive electroencephalographic studies performed at Northwestern Memorial Hospital (Chicago) since 2010 to identify those cases in which 1) multiple mesial temporal electrodes (amygdala and hippocampal) were placed, 2) seizures were captured, and 3) patients’ respiration was monitored. They identified 8 investigations meeting these criteria in patients with temporal lobe epilepsy, and these investigations yielded data on a total of 22 seizures for analysis.
RESULTS
The onset of ictal apnea associated with each seizure was highly correlated with seizure spread to the amygdala. Onset of apnea occurred 2.7 ± 0.4 (mean ± SEM) seconds after the spread of the seizure to the amygdala, which was significantly earlier than after spread to the hippocampus (10.2 ± 0.7 seconds; p < 0.01).
CONCLUSIONS
The findings suggest that activation of amygdalar networks is correlated with central apnea during seizures. This study builds on the authors’ prior work that demonstrates a role for the amygdala in voluntary respiratory control and suggests a further role in dysfunctional breathing states seen during seizures, with implications for SUDEP pathophysiology.
Keywords: apnea, amygdala, intracranial EEG, stereo EEG, SUDEP, epilepsy
Sudden un expected death in epilepsy (SUDEP) is the most common cause of death in patients with refractory epilepsy.22Growing evidence suggests that centrally mediated apnea could be responsible for some SUDEP cases.29 It has been known that ictal respiratory dysfunction can occur in some patients,5,30 but only recently has the prevalence of this phenomenon been truly appreciated; in a recently published study,ictal central apnea was found to occur in nearly half of a multicenter epilepsy monitoring unit (EMU) population of focal epilepsy patients.19 Severe and prolonged peri-ictal central apnea could lead to death, as in SUDEP and near-SUDEP cases observed in EMUs.29 Thus, the underpinnings of the circuit that is responsible for central apnea during seizures may have importance in elucidating the pathophysiology of SUDEP.
Central control of respiration is thought to be represented through multiple forebrain structures and ultimately extending at several levels of the neuraxis to the final pathways for respiratory and autonomic control represented in brainstem areas in the pons and medulla.28 Temporal lobe and limbic areas are also involved in breathing,35and patients with temporal lobe epilepsy (TLE) are more likely to have both substantial desaturation and ictal central apnea.4,19 However, the precise temporal regions that mediate central control of respiration remain largely unknown in humans. Some studies have provided evidence that the amygdala in particular may be involved—electrical stimulation of this structure consistently elicits central apnea events and in one patient produced apnea upon seizure spread.10,20,23 Amygdaloid structures are intimately connected to the brainstem respiratory areas, and preclinical studies show a clear role for these structures in mediating respiratory control.3,36 There is thus a clear role for amygdaloid nuclei in voluntary respiratory control and the potential of the amygdala as a critical mediator of seizure-related central apnea. In the context of a clinical seizure, associated with reduced arousal and brainstem suppression, the patient may not be able to overcome the loss of respiratory drive, which may lead to respiratory failure a nd SUDEP.22
Intracranial seizure recordings in combination with respiratory monitoring provide a unique opportunity to evaluate the timing of apnea onset and how it relates to seizure spread to various temporal structures. This approach can be used to determine which brain regions may be important for seizure-induced central apnea. In this study, we used intracranial electroencephalography (EEG) techniques combined with simultaneous respiratory monitoring to support the hypothesis that the onset of seizure-related central apnea tightly correlates to the spread of seizure activity to the amygdala, rather than other temporal structures.
Methods
All methods were approved by the Northwestern Feinberg School of Medicine’s institutional review board.
Patient Selection
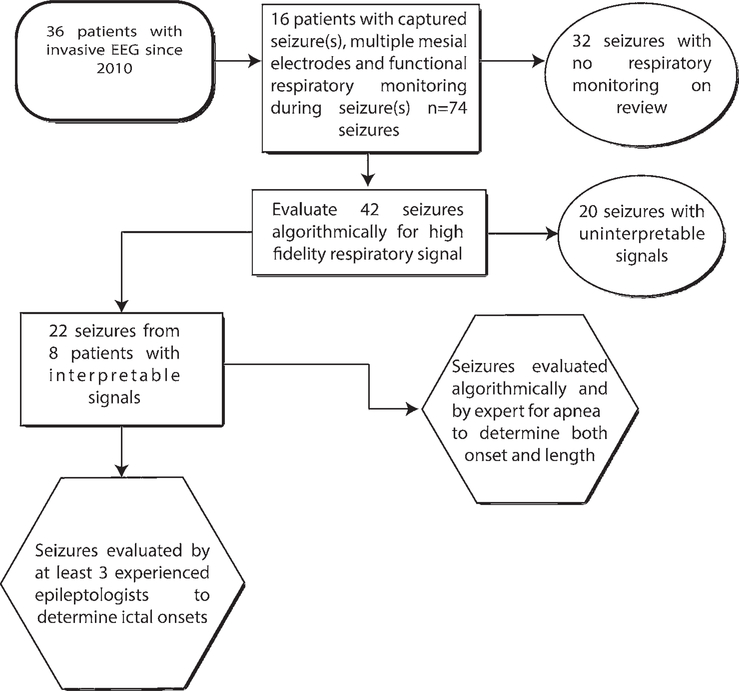
Patients were identified for potential inclusion in the data analysis through review of all cases in which patients were admitted for invasive EEG evaluation at Northwestern Memorial Hospital (NMH) from 2010 until June 2018 (Fig. 1). The initial inclusion criteria were presence of multiple mesial electrodes, presence of at least 1 recorded electroclinical seizure, and functional respiratory monitoring (inductance plethysmography with abdominal and/ or thoracic belts and/or nasal flow sensor). A total of 16 patients met these criteria, and these patients had a total of 74 seizures recorded. Each seizure from this initial group was reviewed for functional respiratory monitoring. Of the 74 seizures group, 42 occurred during respiratory monitoring. These 42 seizure events were then reviewed algorithmically for the presence of a high-fidelity signal and also confirmed to have unobscured video (important for precisely distinguishing artifact from movement/ speech from true apnea). A total of 22 seizures in 8 patients met the criteria and were used for apnea determination and seizure evaluation. All 8 patients had TLE. Chart review was performed for these 8 patients to determine demographic and clinical characteristics of each (Table 1), including seizure types, medications, and comorbidities. SUDEP risk was determined using the SUDEP-7 Risk Inventory score.25
FIG. 1.
Flowchart of study design and patient selection.
TABLE 1.
Patient characteristics
| Pt No. | Age (yrs), Sex | Flandedness | Age of Onset | Epilepsy Type | Ictal Onset Zone | MRI Findings | AEDs | Comorbidities; SSRI Tx | SUDEP Risk | No. of Clinical Sz | No. of Sz w/Interpretable Resp Signal | No. of Sz w/Apnea |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 36, M | Rt | 2 | Rt TLE | Mesial HC | MTS, perinatal ischemia | Oxcarbazepine, topiramate, pregabalin, clonazepam | Anxiety & ADHD; N | High | 5 | 1 | 1 |
| 2 | 48, M | Rt | 37 | Rt TLE | Pst/mesial HC | None | Levetiracetam, lamotrigine, oxcarbazepine | OSA & depression; Y | Low | 12 | 4 | 3 |
| 3 | 35, F | Rt | 32 | Rt TLE | Mesial temp | FCD* | Levetiracetam, clonazepam | None; N | Low | 4 | 1 | 1 |
| 4 | 28, F | Rt | 1 | Lt TLE | Ant basal temp | MTS & FCD* | Lamotrigine, topiramate, levetiracetam | None; N | High | 4 | 2 | 2 |
| 5 | 59, F | Rt | 12 | Lt TLE | Mesial temp | None | Valproic acid, lacosamide, primidone | None; N | High | 9 | 9 | 9 |
| 6 | 34, M | Rt | 24 | Lt TLE | Pst basal temp | Likely FCD | Levetiracetam, carbamazepine | None; N | Low | 5 | 2 | 0 |
| 7 | 26, F | Rt | 9 | Lt TLE | Ant HC | MTS | Levetiracetam, lamotrigine, carbamazepine, zonisamide | Depression; Y | High | 12 | 1 | 0 |
| 8 | 25, F | Rt | 22 | Lt TLE | Ant basal temp | FCD* | Levetiracetam, lamotrigine | Anxiety; Y | Low | 4 | 2 | 1 |
ADHD = attention deficit hyperactivity disorder; AED = anti-epileptic drug; Ant = anterior; FCD = focal cortical dysplasia; HC = hippocampus; MTS = mesial temporal sclerosis; N = no; OSA = obstructive sleep apnea; pst = posterior; pt = patient; resp = respiratory; SSRI = selective serotonin reuptake inhibitor; Sz = seizure(s); temp = temporal; Tx = therapy; Y = yes.
Confirmed on pathological examination.
Electrodes
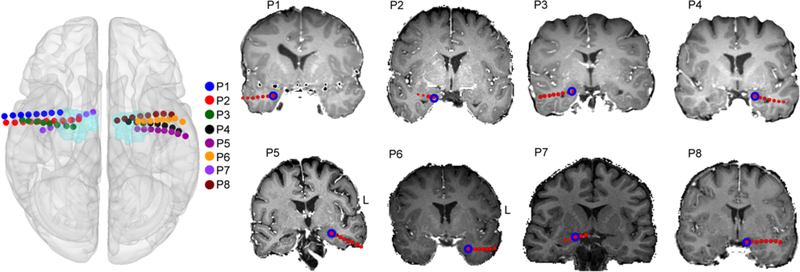
Surgically implanted electrodes were used to record local field potential (LFP) data with the intent of recording seizures and interictal abnormalities. Electrodes for this study included 8-contact depth electrodes (Integra Epilepsy) utilizing the Leksell frame (Elekta) and Brainlab planning system (iPlan Stereotaxy 3.0; Brainlab). Electrodes had 10-mm center-to-center spacing between adjacent contacts. Extratemporal and temporal subdural electrodes were also utilized in some patients. Preoperative structural MR images were acquired in all cases with either a 1.5-T or 3-T MRI scanner. CT scans were acquired postoperatively with depth electrodes in place, clearly showing electrode positions with respect to the patient’s skull geometry. Electrodes were localized using preoperative structural MRI scans and postoperative CT scans with the FSL (FMRIB Software Library) registration tool FLIRT. The individual CT image was registered to the MRI image as previously described.23 Finally, the electrodes were localized by thresholding the raw CT image, and the unweighted mass center of each electrode was calculated and converted into standard MNI (Montreal Neurological Institute) space using MATLAB (MathWorks) and FSL (Fig. 2).
FIG. 2.
Electrode placement. Electrode coordinates for each patient were converted into MNI space. The 3D brain surface plot (left) shows the position of the amygdala depth wire in each patient (P1, P2, etc.). The light blue areas indicate the amygdala mask from the Harvard-Oxford atlas (HarvardOxford-submaxprob-thr0–1 mm) implemented in FSL. The coronal brain slices from T1-weighted MR images (right) show the location of amygdala contacts (blue circles) in each patient in native anatomical space.
Physiological Recordings
As standard clinical protocol at NMH, respiratory monitoring is routinely conducted for all surgical epilepsy patients. To monitor breathing during the hospital stay, chest and abdominal excursions are recorded using respiratory inductance plethysmography belts (Ambu). One belt is placed around the chest and the other around the abdomen. Respiratory belts provide a measure of tidal volume during breathing. To measure airflow through the nose, patients are also fitted with a nasal cannula attached to a piezoelectric sensor (Salter Labs). The majority of patients (6 of 8) were not compliant with instructions regarding use of the nasal cannula. In analyzing respiratory data for the purposes of our study, nasal cannula data and respiratory belts were used in tandem with preference given to nasal cannula measurements when available because they have been shown to be more sensitive and accurate than belt data.14
Determination of Channels Involved at Seizure Onset and Spread
As we aimed to determine whether onset of apnea was influenced by seizure onset and spread to potential respiration-related brain structures we used expert EEG review to assess the electrode contacts involved at seizure onset and spread to the contacts in the hippocampal and amygdalar electrodes and insular electrodes (if present). At least 3 electroencephalographers who were experienced in invasive EEG evaluations (J.T., K.G., E.G., S.V., and W.N.) reviewed each recording. The reviewers were blinded to patients’ names and were given a short history, including type of epilepsy, patient age, and electrode locations. Each reviewer was given an unmarked electroencephalogram in a bipolar montage showing all electrodes for each patient’s seizure. They were not shown the respiratory signals or allowed to change montages. Channels showing the first unequivocal electroencephalographic change from the background activity that led to a seizure discharge were determined as channels involved at seizure onset, with spread of high-frequency fast activity generally used as a marker of onset and involvement of the seizure in other brain regions, as in prior studies of invasive EEG.13,16 These criteria were also used to determine seizure spread to other brain regions. We required agreement of at least 2 of the 3 reviewers to sufficiently determine onset and spread. Agreement was defined as seizure onset and spread times marked within 1 second of each other. There was good interrater agreement, with unanimous agreement among the 3 reviewers regarding 10 seizures and 2 of 3 reviewers agreeing on another 8 seizures. There was clear discrepancy in 5 instances, and in those cases a fourth reviewer was enlisted to evaluate and in each case sided with one of the prior reviewers. For onset times we present the mean and standard error of the mean (SEM) of the reviewer-marked times (Tables 2 and 3).
TABLE 2.
Apneic seizure characteristics
| Pt & Sz No. | Ictal Onset Zone | Sz Type | Delay From Sz Onset to Apnea (seo) | Apnea Duration (seo) | % O2 Saturation Nadir During Apnea (baseline) | Delay From Amygdala Spread to Apnea (seo) | Delay From HC Spread to Apnea (seo)* | Delay From Insula Spread to Apnea (seo) | Sz Out of Sleep |
|---|---|---|---|---|---|---|---|---|---|
| Pt 1 | |||||||||
| Sz 1 | Mid HC | FOIA w1 motor onset (automatisms) | 3.3 ± 0.3 | 11.39 | — | 2.8 ± 0.5 | 3.3 ± 0.3 | 10.2 ± 0.5 | Y |
| Pt 2 | |||||||||
| Sz 1 | Pst HC | FOA (aura) | 15.2 ± 0.4 | 9.8 | — | −14.5 ± 0.6 | 15.2 ± 0.4 | NA | Y |
| Sz 2 | Pst HC | FOA (aura) | 18.7 ± 0.4 | 18.4 | — | −3.5 ± 0.1 | 18.7 ± 0.4 | NA | Y |
| Pt 3 | |||||||||
| Sz 1 | Mesial temp | FOA (aura) → FOIA w/ motor onset (automatisms) | 6.5 ± 0.1 | 15 | 98 (99) | 6.4 ± 0.1 | 6.4 ± 0.1 | 11.6 ± 0.2 | Y |
| Pt 4 | |||||||||
| Sz 1 | Ant basal temp | FOA (aura) → FOIA nonmotor (dialepsis) | 5.6 ± 0.2 | 50.9 | 89 (100) | 4.3 ± 0.2 | 5.2 | NA | N |
| Sz 2 | Ant basal temp | FOIA w/ motor onset (automatisms) | 5.8 ± 0.1 | 34.2 | 89 (100) | 4.8 ± 0.1 | 5.6 | NA | N |
| Pt 5 | |||||||||
| Sz 1 | Mesial temp | FOIA nonmotor (dialepsis) | 12.1 ± 0.4 | 18.9 | — | 1.1 ± 0.2 | 10.5 ± 0.5 | NA | Y |
| Sz 2 | Basal temp/mesial temp | FOIA w1 motor onset (clonic) | 10.4 ± 0.5 | 19.5 | — | 2.9 ± 0.6 | 10.1 ± 0.3 | NA | Y |
| Sz 3 | Mesial temp | FOIA w/ motor onset (clonic) | 18.4 ± 0.5 | 9.9 | — | 4.9 ± 0.5 | 17.3 ± 0.1 | NA | Y |
| Sz 4 | Mesial temp | FOIA w1 motor onset (clonic) | 11.8 ± 0.7 | 14.6 | — | 0.6 ± 0.4 | 10.9 ± 0.2 | NA | Y |
| Sz 5 | Basal temp/mesial temp | FOIA w/ motor onset (clonic) | 9.3 ± 0.1 | 17.1 | — | 0.1 ± 0.1 | 12.5 ± 0.1 | NA | Y |
| Sz 6 | Mesial temp/ant HC | FOIA w1 motor onset (clonic) | 13.1 ± 0.1 | 14.2 | — | 0.6 | 13.1 ± 0.1 | NA | Y |
| Sz 7 | Mesial temp/ant HC | FOIA nonmotor (dialepsis) | 8.4 ± 0.1 | 11.4 | — | 1.3 ± 0.1 | 8.4 ± 0.1 | NA | Y |
| Sz 8 | Mesial temp/ant HC | FOIA nonmotor (dialepsis) | 8.2 | 11.3 | — | 3.5 ± 0.5 | 8.2 | NA | Y |
| Sz 9 | Mesial temp | FOIA nonmotor (dialepsis) | 10.1 ± 0.7 | 16.3 | — | −0.2 ± 0.4 | 10.4 ± 0.3 | NA | Y |
| Pt 8 | |||||||||
| Sz 1 | Ant basal temp | FOIA nonmotor (dialepsis) | 38.8 ± 0.5 | 15.7 | 97 (100) | 5.7 ± 0.5 | 13.3 ± 0.5 | NA | Y |
FOA = focal onset aware; FOIA = focal onset impaired awareness; NA = not applicable.
Data are presented as mean and standard error of the mean of the reviewer-marked times. When no SEM value is given, the agreement was 100%.
TABLE 3.
Non-apneic seizure characteristics
| Pt & Sz No. | Ictal Onset Zone | Seizure Type | Delay From Onset to Amygdala Spread (sec) | Delay From Onset to HC Spread (sec) | Seizure Out of Sleep |
|---|---|---|---|---|---|
| Pt 2 | |||||
| Sz 3 | Mid/pst HC | FOIA w/ motor onset (automatismo) → GTC | 1.5 | 0 | Y |
| Sz 4 | Mid HC | FOA (aura) | NA (amygdala not active) | 0 | Y |
| Pt 6 | |||||
| Sz 1 | Pst basal temp | FOIA nonmotor (dialepsis) | 21 | 8 | N |
| Sz 2 | Pst basal temp | FOIA nonmotor (dialepsis) | 14 | 2 | N |
| Pt 7 | |||||
| Sz 1 | Ant HC | FOA (aura)→ FOIA w/ motor onset (automatisms) | NA (amygdala not active) | 0 | N |
| Pt 8 | |||||
| Sz 2 | Ant basal temp | Aphasic | NA (amygdala not active) | NA (HC not active) | Y |
GTC = generalized tonic-clonic.
Determination of Apnea Events
Respiratory channels for each seizure were exported and analyzed by a custom algorithm in MATLAB, BreathMetrics (MathWorks),24 to aid in apnea determination. Following initial algorithmic determination, experts (C.Z. and G.L.) manually reviewed the respiratory signal from each patient to confirm the beginning and end of each apnea event (when possible and applicable). We required a clear lack of breathing for at least 9 seconds to classify pauses as apnea events.6
Statistical Analysis
Mann-Whitney nonparametric U-tests were used to determine statistical significance. Mean values are provided with standard errors of the mean (SEMs) in the text. Mean, median, interquartile range, and 5th through 95th percentiles are presented in box-and-whisker plots.
Results
Association of Seizures With Central Apnea
In line with the literature, the majority of the seizures evaluated (16 of 22) were associated with a central apnea event. As expected for TLE patients, all seizures were focal in onset and the majority were associated with impaired awareness at onset (Table 2). Apnea lasted between 9.8 and 50.9 seconds (mean 18 ± 2.6 seconds). All apnea events occurred after an ictal onset was seen on the intracranial EEG, with the average time from ictal onset to apnea being 12.2 seconds. Unfortunately, only 4 seizures were recorded when the patient was wearing a pulse oximeter. There were only very subtle drops in oxygen saturation with the brief apnea events measured and only moderate drops even with longer apnea events (an 89% nadir was recorded with apnea of 34.2 and 50.9 seconds). Heart rate increased during seizure onset in all cases, as expected.9 Because we required high-quality respiratory signals devoid of artifacts, most of the seizures that qualified for our study occurred during sleep, when motion artifact was minimized. During sleep, respiratory signals tend to be cleaner because patients are stationary, there is no interaction with any visitors during seizure onset, and support staff and technicians are slower to enter the room. Only two of the included seizures occurred while patients were awake.
Correlation of Apnea Onset With Seizure Spread to the Amygdala
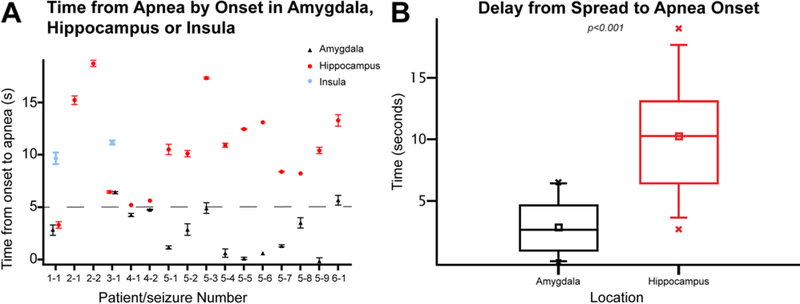
Among the seizures with central apnea events, there was a strong correlation between seizure spread to the amygdala depth electrodes and the onset of apnea (Table 2). There was only 1 patient (patient 2) whose apnea preceded amygdala involvement, and this patient had 2 seizures recorded. Curiously, these were the only seizures that consisted of an aura without any impairment of awareness during the clinical event. These were also the only seizures with an onset in the posterior hippocampus. Overall, onset of apnea occurred 2.7 ± 0.4 seconds after the spread of the seizure to the amygdala, which was significantly different compared to spread to the hippocampus where time from spread to onset was 10.2 ± 0.7 seconds (p < 0.01) (Figs. 3 and 4; see also Supplementary Fig. 1). In all but 2 seizures, the time from amygdala onset to apnea was less than 5 seconds. The ictal onset zone for the majority of the seizures was in the mesial temporal structures, with a significant minority of seizures also arising from the anterior basal temporal region (Fig. 4).
FIG. 3.
A: Scatterplot of delay in apnea onset for each seizure. Delay in onset from the amygdala is not included for the seizures from patient 2 because the apnea preceded the spread to the amygdala, as noted in Table 2. B: Box plot of delay in apnea onset after spread to either the amygdala or hippocampus (squares indicate mean values, X’s signify extremes, boxes indicate the interquartile range [25%–75%], and whiskers indicate 5%–95%).
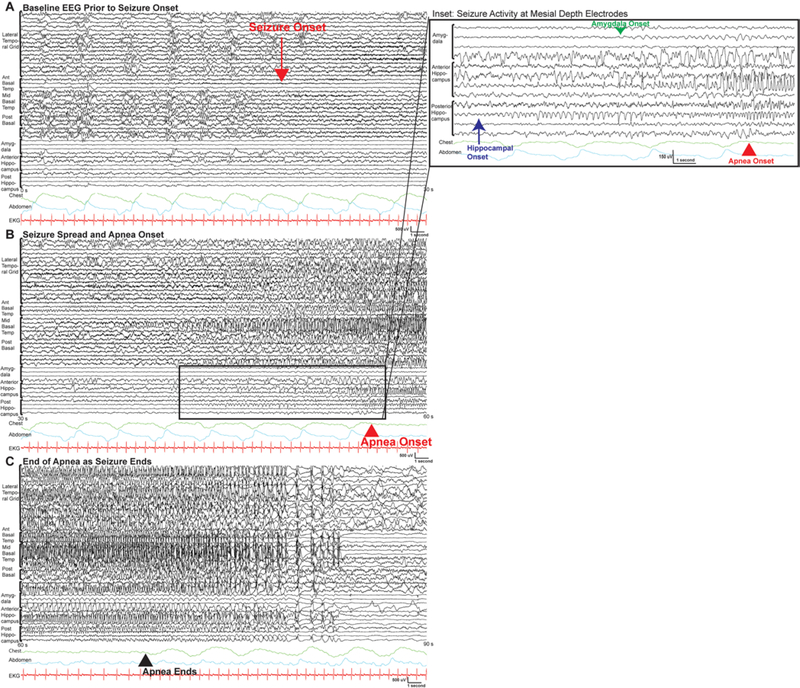
FIG. 4.
Apnea onset and seizure spread: example of invasive EEG of a left temporal seizure with an apnea event (patient 8, seizure 1) shown in 3 consecutive 30-second pages. A bipolar montage is shown, with all electrodes visible. In panel A, prior to the onset of the seizure the patient is sleeping with normal respirations as noted by the chest (green) and abdomen (teal) respiratory belts. The onset of the seizure is noted by the red arrow. Focal onset can be seen, with building rhythmic low-voltage fast activity in the basal temporal grid. Panel B shows the seizure spread and the onset of the apnea—visible in traces from the respiratory leads—about 39 seconds after seizure onset. Panel C shows the diffuse seizure spread to the other electrodes and the apnea ending 16 seconds after it started. The inset for panel B shows a detailed look at traces from the hippocampal and amygdala depth electrodes. The presence of rhythmic activity emerging from the background is seen first in the posterior hippocampus, as noted by the blue arrow. Similar activity is seen emerging in the amygdala, as noted by the green arrow. This precedes the apnea onset, as noted by the respiratory traces. Electrode locations: lateral temporal grid, middle basal temporal grid, anterior basal temporal strip, posterior basal temporal strip, amygdala depth, anterior hippocampus depth, and posterior hippocampus depth. All channels are shown. Ant = anterior; EKG = electrocardiogram; post = posterior; s = seconds.
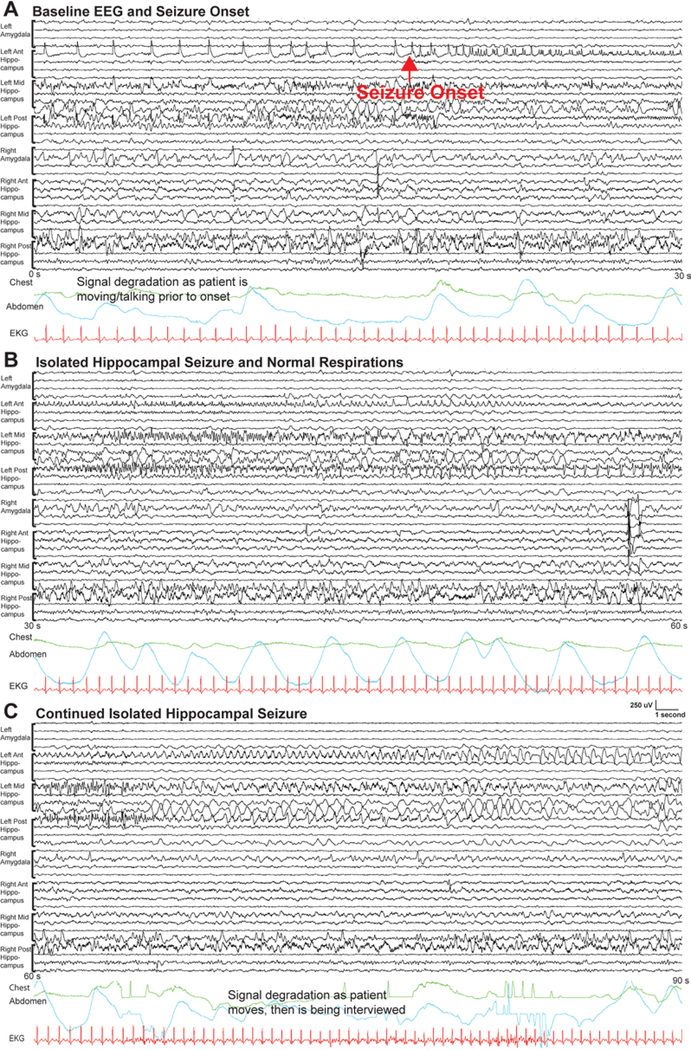
Only 6 of the 22 seizures recorded had no clear apnea (Table 3). Two of these seizures had no amygdala involvement (Fig. 5; see also Supplementary Fig. 2), and one had no amygdala or hippocampal involvement and remained isolated in the anterior basal temporal electrodes. Two of the 3 seizures without apnea events that had amygdala involvement only involved the amygdala after 14 and 20 seconds from the ictal onset. The final seizure without apnea had very quick generalization (within 5 seconds from onset in the hippocampus and only 3.5 seconds from onset in the amygdala); after generalization it was difficult to determine from the signal whether there was any late apnea.
FIG. 5.
Example of invasive EEG of a left mesial temporal seizure without an apnea event (patient 7, seizure 1) shown in 3 consecutive 30-second pages. A bipolar montage is shown, with all electrodes visible. In panel A, prior to the onset of the seizure the patient is awake and interacting with family in the room, with some irregular respirations as noted by the chest (green) and abdomen (teal) respiratory belts. The onset of the seizure is noted by the red arrow. Focal onset can be seen with building spikes in the left anterior hippocampus. Panel B shows the seizure continuing in the left hippocampal electrodes and normal respirations, which are visible in the respiratory leads. Panel C shows the seizure continuing and then some degradation in the signal as the patient is being interviewed and moves, prior to again returning to normal respirations. Electrode locations: left amygdala depth, left anterior hippocampus depth, left middle hippocampus depth, left posterior hippocampus depth, right amygdala depth, right anterior hippocampus depth, right middle hippocampus depth, and right posterior hippocampus depth. All channels are shown.
Discussion
There is now strong clinical and preclinical evidence for seizure-induced central respiratory dysfunction as a pathophysiological mechanism for SUDEP.11,18,29,33 This study suggests that in a series of TLE patients seizure propagation to the amygdala, rather than the hippocampus or other mesial temporal structures, is intimately associated with the onset of central apnea.
We note that in our population central apnea events were even more prevalent than in the literature,19 perhaps owing to the fact that our patients all had refractory epilepsy, as compared to the general EMU population previously investigated. Refractory epilepsy may produce structural and functional circuit changes that place patients at increased risk for apnea. Characteristics of patients with ictal central apnea have not been investigated, but our study suggests that this entity may be more prevalent in patients with refractory epilepsy.
The majority of seizures that were not associated with central apnea (6 seizures) had either no amygdala involvement (3 seizures) or late amygdala involvement (2 seizures). Those seizures not associated with apnea but with amygdala involvement occurred in patients with low SUDEP risk, again potentially implicating central apnea with more refractory epilepsy. Our prior report, which replicates more preclinical data, suggests that particular amygdala regions may be critical for mediating apnea23—through this mechanism there may be seizure spread to the amygdala and an absence of apnea, as long as critical regions are not involved.
Overall, our study solidifies the previously reported findings implicating the amygdala as a critical component in the circuit mediating this effect as all seizures with ictal central apnea events showed amygdala involvement.10,20,23 This study expands on a prior case report implicating seizure spread to the amygdala with apnea10 and agrees with prior case series showing a clear effect of amygdala stimulation on breathing.10,20,23 It does, however, seemingly conflict with an earlier case series showing seizure-induced apnea tightly correlated with contralateral ictal spread.31 These findings were in a population without specific amygdalar depth electrode coverage and the study was not designed to investigate seizure spread to specific regions; furthermore, the authors were evaluating only bilateral invasive EEG investigations, so the study population was enriched for bitemporal epilepsy.
Our study, like these previous reports, also suffers from inherent selection and observational biases. While there was a clear effect in our population, these observations could be made only due to a combination of clinical factors—all of our patients had refractory epilepsy requiring invasive EEG evaluation with multiple electrodes. While our patients had extensive electrode coverage for seizure evaluation, the sites were still limited to what was clinically relevant based on the patient’s prior presurgical evaluation. Thus seizure spread to regions beyond the coverage area may correlate with apnea as well—this seems like it may be the case in patient 2, whose 2 seizures were associated with apnea that substantially preceded the spread of the seizure to either the amygdala or hippocampus. Perhaps a region such as the insula, anterior cingulate, or smaller extended amygdala regions (not investigated in this patient) may be important in this case and may play a role in others. Another natural inquiry is whether patients with an isolated epileptic onset zone in the amygdala may be at higher risk for ictal central apnea—unfortunately our patient population did not include any such patients. These issues underlie the complex nature of both epilepsy and respiratory control, and animal models may be required to help tease them out. Finally, our study is limited in that we report only monitoring of TLE patients, this being the most common adult epilepsy and thus the most likely to receive invasive presurgical evaluation at our institution. In the end, it is difficult to know whether these findings can be extrapolated to broader populations of epilepsy patients.
The amygdala is a natural candidate region for mediating central apnea during ictal events. There are multiple possible effector sites that may result in respiratory dysfunction and apnea downstream of the amygdala. The extended amygdalar nuclei have dense projections to brainstem regions that generate respiratory rhythms such as the ventral medullary group (including the pre-Bötziner complex) and the pontine respiratory group, which can provide overriding feedback to this circuit.3,7,36 Preclinical studies in rodent models have shown that these afferents from the extended amygdala have functional control over respiratory efforts,17 but more precise investigation is necessary to fully determine the critical pathways. The extended amygdala also provides major input to the arousal network of the brain including the serotonergic raphe nuclei27,32 and the parabrachial nucleus in the pons, both of which can modulate the respiratory cycle and directly sense hypercapnia and influence arousal.15 It is possible that the amygdala is involved in both the onset of apnea with seizures and the impairment of arousal systems. Plasticity induced by repeated seizures in this circuit, as has been described in other brainstem regions in epilepsy,21 could put patients at increasing risk for apnea and SUDEP.
Ever-emerging data implicate brainstem circuitry as a risk for seizure-induced apnea and SUDEP.1 In our study, these central apnea events were largely brief and self-limited, resolving prior to completion of the seizure. The invasive EEG reflects the spread of the seizure into the amygdala with an intensity sufficient for an initial respiratory disruption. As seizures propagate, the intensity of a specific clinical activation may resolve or continue to build into a bilateral tonic-clonic convulsion. This spread and more intense activation may be needed to result in the widespread brainstem depression seen in animal models and correlating to a sustained apnea.29 It appears that the amygdala is central to the initial loss of respiratory drive and likely crucial to our understanding of why some patients’ respiration does not recover.
From a broader standpoint, evidence exists that activity of the amygdala may be involved in the respiratory rhythm thought to be mediated in the brainstem—amygdala activity is tied to the respiratory cycle, suggesting that the unconscious and conscious control of respiratory function may be related to this limbic circuit.26,35 One intriguing possibility is that the amygdala’s role in respiratory control relates to its primary cortical position within the olfactory system. In primates and likely humans,2,8 the olfactory bulb projects directly to the central amygdala. The inextricable link between nasal inhalation and olfactory sampling suggests the need for rapid, threat-related access to brainstem respiratory control centers within this system.
Identifying brain regions where seizure spread can produce respiratory dysfunction may help with the development of noninvasive biomarkers for SUDEP. Brainstem volume loss has been observed in MRI studies of SUDEP patients and increased amygdala volume has been reported in association with increased risk of SUDEP.34 Perhaps structural or functional connectivity in the amygdala-to-brainstem circuit can imply greater risk? More research remains to be done on this subject. The presence of apneic seizures themselves may be a risk factor—it is reasonable to think that seizure-induced apnea and subsequent impaired oxygenation combined with poor recovery may be the critical cascade leading to SUDEP. Thus the presence of apneic seizures may serve as another marker to complement ongoing imaging studies; it also argues for more widespread respiratory monitoring in EMUs.12 Overall, we suspect that increased knowledge of ictal apnea and the brain regions responsible will lead toward identifying patients at risk for SUDEP and therapeutic strategies for prevention.
Conclusions
Our study expands on the increasing literature regarding the amygdala and respirations, clearly suggesting a role of the amygdala in not just physiological breathing but also dysfunctional breathing states seen during seizures. Seizure spread to the amygdala may be part of a critical cascade leading to apnea events and increasing risk for SUDEP. Further work is necessary—both clinical and preclinical—to test these hypotheses and determine how ictal apneas may relate to SUDEP risk. Identifying the underlying circuit leading to apnea is a critical initial step to developing effective therapies to minimize risk of SUDEP.
Supplementary Material
Acknowledgments
We thank Jeremy Eagles, Navid Shadlou, Jacob Stolz, Timothy Hanson, and George Culler for technical assistance and support.
This work was supported by grants to C.Z. from the National Institute on Deafness and Other Communication Disorders (R00DC012803 and R01DC016364) and by a grant to W.N. from Citizens United for Research in Epilepsy (CURE) (Taking Flight Award) and a pilot grant from the National Institute of Neurological Disorders and Stroke’s Center for SUDEP Research (CSR).
ABBREVIATIONS
- EEG
electroencephalography
- EMU
epilepsy monitoring unit
- FSL
FMRIB Software Library
- MNI
Montreal Neurological Institute
- NMH
Northwestern Memorial Hospital
- SUDEP
sudden unexpected death in epilepsy
- TLE
temporal lobe epilepsy
Footnotes
Disclosures
Dr. Gerard reports being a speaker for UCB-China and receiving study-related clinical or research support from SAGE and Sunovion.
Supplemental Information
Online-Only Content Supplemental material is available with the online version of the article.
Supplementary Figs. 1 and 2. https://thejns.org/doi/suppl/10.3171/2019.1.JNS183157.
References
- 1.Aiba I, Noebels JL: Spreading depolarization in the brainstem mediates sudden cardiorespiratory arrest in mouse SUDEP models. Sci Transl Med 7:282ra46, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison AC: The secondary olfactory areas in the human brain. J Anat 88:481–488, 1954 [PMC free article] [PubMed] [Google Scholar]
- 3.Applegate CD, Kapp BS, Underwood MD, McNall CL: Autonomic and somatomotor effects of amygdala central N. stimulation in awake rabbits. Physiol Behav 31:353–360, 1983 [DOI] [PubMed] [Google Scholar]
- 4.Bateman LM, Li CS, Seyal M: Ictal hypoxemia in localization-related epilepsy: analysis of incidence, severity and risk factors. Brain 131:3239–3245, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bateman LM, Spitz M, Seyal M: Ictal hypoventilation contributes to cardiac arrhythmia and SUDEP: report on two deaths in video-EEG-monitored patients. Epilepsia 51:916–920, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Berry RB: Monitoring respiration—event definitions and examples, in Berry RB (ed): Fundamentals of Sleep Medicine. St Louis: WB Saunders, 2012, pp 119–140 [Google Scholar]
- 7.Bowman BR, Kumar NN, Hassan SF, McMullan S, Goodchild AK: Brain sources of inhibitory input to the rat rostral ventrolateral medulla. J Comp Neurol 521:213–232, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Carmichael ST, Clugnet MC, Price JL: Central olfactory connections in the macaque monkey. J Comp Neurol 346:403–434, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Devinsky O: Effects of seizures on autonomic and cardiovascular function. Epilepsy Curr 4:43–46, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dlouhy BJ, Gehlbach BK, Kreple CJ, Kawasaki H, Oya H, Buzza C, et al. : Breathing inhibited when seizures spread to the amygdala and upon amygdala stimulation. J Neurosci 35:10281–10289, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dlouhy BJ, Gehlbach BK, Richerson GB: Sudden unexpected death in epilepsy: basic mechanisms and clinical implications for prevention. J Neurol Neurosurg Psychiatry 87:402–413, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Gehlbach BK, Sainju RK, Tadlock DK, Dragon DN, Granner MA, Richerson GB: Tolerability of a comprehensive cardiorespiratory monitoring protocol in an epilepsy monitoring unit. Epilepsy Behav 85:173–176, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grinenko O, Li J, Mosher JC, Wang IZ, Bulacio JC, Gonzalez-Martinez J, et al. : A fingerprint of the epileptogenic zone in human epilepsies. Brain 141:117–131, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson BN, Russell C, Khan RM, Sobel N: A comparison of methods for sniff measurement concurrent with olfactory tasks in humans. Chem Senses 31:795–806, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Kaur S, Wang JL, Ferrari L, Thankachan S, Kroeger D, Venner A, et al. : A genetically defined circuit for arousal from sleep during hypercapnia. Neuron 96:1153–1167, 1167. e1–1167.e5, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khoo HM, von Ellenrieder N, Zazubovits N, Dubeau F, Gotman J: Epileptic networks in action: synchrony between distant hemodynamic responses. Ann Neurol 82:57–66, 2017 [DOI] [PubMed] [Google Scholar]
- 17.Kim SY, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, et al. : Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature 496:219–223, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim Y, Bravo E, Thirnbeck CK, Smith-Mellecker LA, Kim SH, Gehlbach BK, et al. : Severe peri-ictal respiratory dysfunction is common in Dravet syndrome. J Clin Invest 128:1141–1153, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lacuey N, Zonjy B, Hampson JP, Rani MRS, Zaremba A, Sainju RK, et al. : The incidence and significance of periictal apnea in epileptic seizures. Epilepsia 59:573–582, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lacuey N, Zonjy B, Londono L, Lhatoo SD: Amygdala and hippocampus are symptomatogenic zones for central apneic seizures. Neurology 88:701–705, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lui S, Torontali Z, Tadjalli A, Peever J: Brainstem nuclei associated with mediating apnea-induced respiratory motor plasticity. Sci Rep 8:12709, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massey CA, Sowers LP, Dlouhy BJ, Richerson GB: Mechanisms of sudden unexpected death in epilepsy: the pathway to prevention. Nat Rev Neurol 10:271–282, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nobis WP, Schuele S, Templer JW, Zhou G, Lane G, Rosenow JM, et al. : Amygdala-stimulation-induced apnea is attention and nasal-breathing dependent. Ann Neurol 83:460–471, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noto T, Zhou G, Schuele S, Templer J, Zelano C: Automated analysis of breathing waveforms using BreathMetrics: a respiratory signal processing toolbox. Chem Senses 43:583–597, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novak JL, Miller PR, Markovic D, Meymandi SK, DeGiorgio CM: Risk assessment for sudden death in epilepsy: the SUDEP-7 Inventory. Front Neurol 6:252, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramirez JM, Baertsch N: Defining the rhythmogenic elements of mammalian breathing. Physiology (Bethesda) 33:302–316, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren J, Friedmann D, Xiong J, Liu CD, Ferguson BR, Weerakkody T, et al. : Anatomically defined and functionally distinct dorsal raphe serotonin sub-systems. Cell 175:472–487, 487. e1–487.e20, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richter DW, Smith JC: Respiratory rhythm generation in vivo. Physiology (Bethesda) 29:58–71, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryvlin P, Nashef L, Lhatoo SD, Bateman LM, Bird J, Bleasel A, et al. : Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol 12:966–977, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Schuele SU, Afshari M, Afshari ZS, Macken MP, Asconape J, Wolfe L, et al. : Ictal central apnea as a predictor for sudden unexpected death in epilepsy. Epilepsy Behav 22:401–403, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Seyal M, Bateman LM: Ictal apnea linked to contralateral spread of temporal lobe seizures: Intracranial EEG recordings in refractory temporal lobe epilepsy. Epilepsia 50:2557–2562, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Smith HR, Leibold NK, Rappoport DA, Ginapp CM, Purnell BS, Bode NM, et al. : Dorsal raphe serotonin neurons mediate CO2-induced arousal from sleep. J Neurosci 38:1915–1925, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sowers LP, Massey CA, Gehlbach BK, Granner MA, Richerson GB: Sudden unexpected death in epilepsy: fatal post-ictal respiratory and arousal mechanisms. Respir Physiol Neurobiol 189:315–323, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wandschneider B, Koepp M, Scott C, Micallef C, Balestrini S, Sisodiya SM, et al. : Structural imaging biomarkers of sudden unexpected death in epilepsy. Brain 138:2907–2919, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zelano C, Jiang H, Zhou G, Arora N, Schuele S, Rosenow J, et al. : Nasal respiration entrains human limbic oscillations and modulates cognitive function. J Neurosci 36:12448–12467, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang JX, Harper RM, Ni HF: Cryogenic blockade of the central nucleus of the amygdala attenuates aversively conditioned blood pressure and respiratory responses. Brain Res 386:136–145, 1986 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.