Abstract
In the first year of life, the ability to engage in sustained synchronous interactions develops as infants learn to match social partner behaviors and sequentially regulate their behaviors in response to others. Difficulties developing competence in these early social building blocks can impact later language skills, joint attention, and emotion regulation. For children at elevated risk for autism spectrum disorder (ASD), early dyadic synchrony and responsiveness difficulties may be indicative of emerging ASD and/or developmental concerns.
As part of a prospective developmental monitoring study, infant siblings of children with ASD (high-risk) and typical development (low-risk), 175 infants and their mothers (high-risk group n = 104, low-risk group n = 71) completed a standardized play task when infants were 6, 9, and 12 months of age. These interactions were coded for the frequency and duration of infant and mother gaze, positive affect, and vocalizations, respectively. Using these codes, theory-driven composites were created to index dyadic synchrony and infant/maternal responsiveness.
Multilevel models revealed significant risk group differences in dyadic synchrony and infant responsiveness by 12 months of age. In addition, higher levels of synchrony and responsiveness at 12 months were positively associated with language skills at 36 months, regardless of group. The findings of the present study highlight that promoting dyadic synchrony and responsiveness may aid in advancing optimal development in children at elevated risk for autism.
Keywords: mother-infant interaction, dyadic synchrony, responsiveness, infant sibling, autism spectrum disorder
Lay Summary:
In families raising children with an autism spectrum disorder (ASD), younger siblings are at elevated risks for social communication difficulties. The present study explored whether social-communication differences were evident during a parent-child play task at 6, 9, and 12 months of age. For infant siblings of children with ASD, social differences during play were observed by 12 months of age and may inform ongoing monitoring and intervention efforts.
Introduction
At its core, autism spectrum disorder (ASD) is a social disorder. Difficulty with reciprocal social exchanges is one of the few features that is universal across individuals with ASD. The premise of the present study is that these nuanced social-communication difficulties may be present before a formal diagnosis is established. We aimed to closely assess early dyadic synchrony and responsiveness, within the first year, to examine how and when early social difficulties may emerge, across elevated risk and developmental outcomes (in infant siblings of children with ASD).
Familial-risk designs prospectively track infant siblings of children with ASD and have documented elevated ASD risk, when compared to the general population (Ozonoff et al., 2011). Additionally, these high-risk infants are at elevated risk for a range of developmental concerns beyond ASD (Charman et al., 2017; Miller et al., 2015; Ozonoff et al., 2014; Messinger et al., 2013; Georgiades et al., 2013), which seem to persist once school-aged (Miller et al., 2016; Shephard et al.,2016). To inform early developmental monitoring efforts, previous studies have examined a wide array of prosocial behaviors during play-based interactions using familial-risk (infant sibling) designs. For infants, these prosocial behaviors frequently include gaze patterns (Gangi et al., 2018; Ozonoff et al., 2010), social smiles (Nichols, Ibañez, Foss-Feig, & Stone, 2014; Lambert-Brown et al., 2015), affect (Hutman, Chela, Gillespie-Lynch, & Sigman, 2012; Wan et al., 2013), and vocalizations (Swanson et al., 2019; Talbott, Nelson, & Tager-Flusberg, 2016; Paul, Fuerst, Ramsay, Chawarska, & Klin, 2011). For mothers, they often include vocalizations and gesture use (Jakubowski & Iverson, 2018; Talbott et al., 2015, 2016). Recently dyadic synchrony (Steiner, Gengoux, Smith, & Chawarska, 2018), responsiveness (Harker, Ibañez, Nguyen, Messinger, & Stone, 2016; Leezenbaum, Campbell, Butler, & Iverson, 2014; Schwichtenberg, Kellerman, Young, Miller, & Ozonoff, 2019), and indices of social engagement (Campbell, Leezenbaum, Mahoney, Day, & Schmidt, 2016; Harker et al., 2016; Kellerman, Schwichtenberg, Tonnsen, Posada, & Lane, 2019) have also been indexed. These diverse indices of prosocial behaviors make direct comparisons across studies difficult but general patterns of prosocial trajectories have emerged (described below), demonstrating distinct differences by familial ASD risk and outcome classifications within the first two years.
Familial ASD Risk Group Comparisons
For families raising children with ASD findings regarding dyadic differences are mixed. Three studies document lower maternal sensitive responsiveness and/or higher directiveness in high-risk dyads, when compared to low-risk dyads (Wan et al., 2012; 2013; Harker et al., 2016). However, it is unclear whether mothers were independently less responsive and more directive, or if this is potentially a function of their infants not providing clear social cues. Conversely, Yirmiya et al. (2006) reported no difference in instances of infant-led, mother-led, mutual synchrony, or response time between dyads in high- vs. low-risk groups. Similarly, several studies in the first year have reported no significant high- or low-risk group differences in infant or mother vocalizations, positive affect, use of multimodal bids (Schwichtenberg et al., 2019), parent initiating/directing, praise, scaffolding, warmth, sensitivity (Campbell, Leezenbaum, Mahoney, Moore, & Brownell, 2016), responsiveness (Leezenbaum et al., 2014), or time engaged during play (Steiner et al., 2018). Alternatively, some studies document mothers in the high-risk group as more synchronous (Steiner et al., 2018) and including more gestures (Talbott et al., 2016), when compared to a low-risk group. These diverse findings likely reflect differences in prosocial behavior characterization, differences in the age of assessment, and the relatively small samples used across many of these studies. The current study addresses many of these by using a hybrid approach to prosocial behavior characterization (detailed below) within a relatively large prospective sample across three time points within the first year.
Developmental Progress/Outcome Comparisons
When examining dyadic constructs across outcome classifications, the most consistent or robust group differences are evident by 12–15 months (Gangi et al., 2018; Hutman et al., 2012; Kellerman et al., 2019; Ozonoff et al., 2010; Wan et al., 2013). For example, Gangi et al. (2018) reported infants who developed ASD engaged in less gaze to face behaviors during play at 12 months of age. Similarly, Kellerman and colleagues (2019) documented subclinical dyadic features of ASD evident during play by 15 months of age (i.e., lower infant and maternal responsiveness; lower joint engagement). However, several studies have demonstrated that parents of children with ASD are comparable interactive partners, when compared to parents of children with typical development and those with other developmental concerns (Kasari, Sigman, Mundy, & Yirmiya, 1988; Schwichtenberg et al., 2019; Siller & Sigman, 2002). Notably, many studies go beyond group-based outcome assessments (e.g., ASD vs. Not ASD) to consider continuous measures of developmental competence. The most consistent finding in these studies is a robust association between early prosocial behaviors and later language skills in children with ASD or other developmental concerns and in typical development (e.g., Charman et al., 2009; Northup & Iverson, 2015; Poon, Watson, Baranek, & Poe, 2012; Tamis-LeMonda, Bornstein, & Baumwell, 2001; Young, Merin, Rogers, & Ozonoff, 2009). For example, dyads indexed as more synchronous and responsive, were associated with greater infant expressive and receptive language competence later in development (Siller & Sigman, 2002; 2008). There is a long history of studies documenting the positive developmental influence of dyadic synchrony and responsiveness in other samples/populations as well.
Dyadic Synchrony and Responsiveness
Social interaction promotes opportunities for dyads to create temporal relationships to benefit infants’ development. Within the first six months, infants begin matching their play partners’ behaviors in various modalities (e.g., gaze synchrony), sequentially regulating their behaviors in response to their play partner, and engaging in interaction repairs for mismatched behaviors (Feldman, 2007; Fogel, 1993; Tronick, 1989; Tronick & Cohn, 1989). In typical development, dyadic synchrony relates to infant play skills (Feldman & Greenbaum, 1997), later intelligence (Feldman, Greenbaum, Yirmiya, & Mayes, 1996), and attachment security (Jaffe et al., 2001). Turn taking, a key feature of dyadic synchrony, does not only allow the “intricate dance” (Feldman, 2007) between a mother and her infant, but creates a reciprocal communication ritual that can act as a practice for social conversation (as discussed in Bruner, 1985). Turn-taking and the ability to communicate in a reciprocal fashion constitute the hallmark of successful language interactions; hence, the link between dyadic synchrony and communicative competence is not surprising (Lindsey, Cremeens, Colwell, & Caldera, 2009). Similarly, maternal responsiveness informs the development of infant attention and symbolic play skills (Bornstein & Tamis-LeMonda, 1997), language milestones (Nicely, Tamis-LeMonda, & Bornstein, 1999; Paavola, Kunnari, & Moilanen, 2005; Tamis-LeMonda, et al., 2001; Tamis-LeMonda, Bornstein, Baumwell, & Damast, 1996) social-emotional competence (Denham, 1993), and cognitive development (McFadden & Tamis-LeMonda, 2013). To date, few studies have investigated the influence of infants’ developmental psychopathology on dyadic synchrony (as discussed in Feldman, 2015).
Current Study
Documenting the social communication aspects of dyadic interactions in families raising children with elevated developmental risks is especially salient in identifying developmental risk markers and informing current intervention practices. The present study builds upon seminal work examining dyadic synchrony and responsiveness in infants at elevated risks, by first measuring dyadic synchrony at three time points within the first year of life and by exploring associations with later developmental outcomes. For children at elevated risk for ASD, dyadic synchrony and responsiveness could distinguish which infants later receive an autism diagnosis. In the current study, play interactions were evaluated to (1) assess dyadic synchrony and responsiveness at 6, 9, and 12 months, as a function of risk-status, and (2) to assess if early difficulties with social responsiveness or synchrony precede an ASD diagnosis and/or delayed language skills.
Methods
Procedure
This study was conducted under the approval of the University of California-Davis’ Institutional Review Board. The study was explained to parents orally and in writing, all their questions were answered, and consent was obtained before conducting assessments. Families enrolled in this longitudinal study when their infants were 6 (n = 136) or 9 (n = 39) months of age. Given the nature of the prospective design, visit attendance (though strongly encouraged) is not required for all eight laboratory visits (6, 9, 12, 15, 18, 24, 30, and 36 months of age). To receive an outcome classification, children must have completed at least three visits, including a final diagnostic evaluation between 24–36 months of age. The present study includes parent-child interaction data collected at infants’ 6, 9, and/or 12-month laboratory visits. Child developmental progress and outcome classifications were established at children’s final laboratory visit.
Participants
One hundred and seventy-five families participated in this study. Infant siblings were recruited from families with at least one older child (proband) diagnosed with ASD (high-risk infants: n = 104) or no history of ASD in 1st, 2nd, and 3rd degree relatives (low-risk infants: n = 71). The proband siblings were diagnosed with DSM-IV Autistic Disorder, Asperger Disorder, or Pervasive Developmental Disorder – Not Otherwise Specified (PDD-NOS). Proband diagnostic status was confirmed with the Autism Diagnostic Observation Schedule (ADOS-2; Lord et al., 2012).
Outcomes for infant siblings were categorized at their 36-month visit by the criteria developed by the Baby Siblings Research Consortium (BSRC; Ozonoff et al., 2014). Infants were classified into 1 of 3 groups: Typical Development (TD; n = 116), Non-Typical Development (Non-TD; n = 18), and ASD (n = 17). Infants diagnosed with ASD met DSM-IV criteria for Autistic Disorder or PDD-NOS and received an ADOS-2 calibrated severity score at or above the threshold for an ASD. Calibrated ADOS severity scores were included to account for administered ADOS Module 1–2 variability based on infant language level/use at 36 months (Gotham, Pickles, & Lord, 2009). Algorithmically, children in the Non-TD group did not meet criteria for ASD (i.e., ADOS-2 scores less than 3 points below the ASD cutoff) and either demonstrated two or more MSEL domain scores 1.5 SD below the mean or had one MSEL domain score 2 SD below the mean. Qualitatively, the Non-TD outcome group included children with language delays, behavioral challenges, and/or subthreshold phenotypic characteristics of ASD. Twenty-four infants (13.7% of the sample) did not attend the final laboratory visit, or did not complete all of the necessary assessments at the evaluation. Thus for the purposes of this study, these 24 infants were only included in risk-group analyses for the dyadic constructs. Family demographics stratified by risk status are provided in Table 1.
Table 1.
Demographic information stratified by risk status
| High-Risk | Low-Risk | |
|---|---|---|
| N | 104 | 71 |
| Infant Sex, n(%) | ||
| Male | 62(60%) | 40(56%) |
| Infant Race, n(%) | ||
| African American | 3(3%) | 3(4%) |
| Caucasian | 59(57%) | 47(66%) |
| Multiracial | 18(17%) | 11(15%) |
| Other | 19(18%) | 8(11%) |
| Unreported | 5(5%) | 2(3%) |
| Infant Outcome, n(%) | ||
| ASD | 16(15%) | 1(1%) |
| Non-TD | 16(15%) | 2(3%) |
| TD | 58(56%) | 58(82%) |
| No Outcome | 14(13%) | 10(14%) |
| ADOS Calibrated Severity1, M(SD) | ||
| ASD | 6.40(2.6) | 6.00(0.0) |
| Non-TD | 3.20(1.9) | 1.00(0.0) |
| TD | 1.58(1.0) | 1.28(0.6) |
| Maternal Characteristics | ||
| Maternal age, M(SD) | 34.1(4.9) | 31.7(5.4) |
| Maternal Education, n(%) | ||
| Some high school | 0(0%) | 3(4%) |
| High school or GED | 7(7%) | 7(10%) |
| Trade or vocational | 2(2%) | 2(3%) |
| Some college | 27(26%) | 6(9%) |
| College degree | 48(46%) | 29(41%) |
| Some graduate school | 2(2%) | 3(4%) |
| Graduate degree | 18(17%) | 21(29%) |
Note:
Calibrated Autism Diagnostic Observation Schedule (ADOS) severity scores were derived according to Gotham et al., 2009.
Mother-Child Play Task.
Mother-child dyads participated in a semi-structured free play task when infants were 6, 9, and 12 months of age. A standardized bin of age-appropriate toys was provided and included a doll, bottle, small blanket, car, shape sorter, ball, rattle, and a pair of toy phones. Interactions were videotaped and coded independently in Noldus Observer for the frequency and duration of infant and mother gaze, positive affect, and vocalizations, for 3 consecutive minutes. Once the examiner left the room, the video segment included the first few minutes of active play between mother and child. This did not include any initial toy setup or seating adjustments.
Research assistants were trained to identify when select behaviors (i.e., look face, look object, positive affect, and vocalizations) began (on) and ended (off). For each on and off code, a frequency count of 1 was recorded. Coders received a series of training videos and obtained an initial reliability (intra-class correlation coefficient; ICC) above .70 with a master coder on each code (i.e., gaze, positive affect, and vocalizations). Coders were unaware of risk or outcome group status. Periodic checks of reliability consistently revealed ICCs above .70 (range .70 to .99; M = .88). Detailed coding rules were established in a separate cohort (Ozonoff et al., 2010) to capture behaviors lasting 0.5 seconds or more for total frequencies and duration. For brief shifts in behavior, (e.g., positive affect to look face) the shift must have occurred for at least 0.5 seconds to be counted as a separate behavior.
Using the sequential order of these characterized base codes, theory-driven composites were created in post-processing to assess dyadic synchrony and responsiveness (previously established in Kellerman et al., 2019). Our measurement techniques for creating the composites relied heavily on (1) previous work from Feldman (2007) evaluating temporal relations in early dyadic synchrony by assessing which play partner leads sequences within the interaction; and (2) on multimodal combinations of infant and mother core competency behaviors indicative of prosocial exchanges within the first year (e.g., gaze shifts; social smiling; joint attention). Specifically, dyadic synchrony was indexed by calculating a frequency total of shared eye gazes, shared positive affect expressions, and mother- or child-led gaze to face, positive affect, or vocalizations. To distinguish individual contributions of mother and infant play behaviors within our dyadic synchrony composite, indices of maternal and infant responsiveness were also created to exclusively focus on led exchanges. To be considered a ‘led’ exchange one dyadic partner engaged in the target behavior (e.g., look to face) and the other partner responded with a gaze shift to face, a positive affect expression, or a vocalization, within three seconds. For example, when a mother vocalized and the child shifted his/her gaze to her face, this was summarized as one mother-led vocalization. It is important to note that receiving credit for responsiveness was contingent on the presence of social bid opportunities from the other play partner. A description of the included composite core behaviors to encompass dyadic synchrony, infant responsiveness, and maternal responsiveness is provided in Table 2.
Table 2.
Synchrony and responsiveness composite behaviors
| Frequency | |||
|---|---|---|---|
| Coded Behaviors | Dyadic Synchrony | Infant Responsiveness | Maternal Responsiveness |
| Shared Look Face Gaze | x | ||
| Shared Positive Affect | x | ||
| Mother-Led Look Face Gaze | x | x | |
| Mother-Led Look Object Gaze | x | x | |
| Mother-Led Positive Affect | x | x | |
| Mother-Led Vocalizations | x | x | |
| Infant-Led Look Face Gaze | x | x | |
| Infant-Led Look Object Gaze | x | x | |
| Infant-Led Positive Affect | x | x | |
| Infant-Led Vocalizations | x | x | |
Mullen Scales of Early Learning (MSEL; Mullen, 1995).
The MSEL measures developmental competencies in children aged 0 to 68 months across five key scales: gross motor, fine motor, visual reception, receptive language, and expressive language. Scale scores were used in the BSRC outcome classification algorithm to determine outcome group membership (Ozonoff et al., 2014).
Autism Diagnostic Observation Scales, Second Edition (ADOS-2; Lord et al., 2012).
The ADOS-2 is a semi-structured standardized diagnostic tool to measure symptoms of ASD in the social communication and restricted and repetitive behavior domains. At children’s laboratory visits, a trained clinician administered and scored the ADOS-2. ADOS-2 severity scores were included to determine children’s outcome classification, using BSRC criteria (Ozonoff et al., 2014). See Table 1 for mean calibrated severity scores.
Analytic Plan
Of the 175 infants, the majority completed the parent-child play task (administered at the 6, 9, and 12 month visits) at two of the three time points (High-risk M = 2.00, SD = .78; Low-risk M = 2.15, SD = .80). To better account for the longitudinal nature of the dyadic data and missing data patterns, risk and outcome group differences across the three composites were conducted using nonparametric multilevel models in Statistical Analysis Software (SAS) Version 9.4. See supplemental material for additional missing data details.
All data were checked for normality prior to analyses and cleaned using IBM Statisitical Package for the Social Sciences Version 24. Multilevel models included fixed effects for group membership, visit (6, 9, 12), infant sex (male = 1), maternal education in years, and the interaction between group membership and visit age. Dyadic synchrony and responsiveness composites were modeled as continuous variables. For any significant main effects of group or visit, estimated marginal means were conducted to determine at which visit age(s) significant group differences were apparent. Dyadic synchrony and responsiveness composite means at 6, 9, and 12-months (stratified by risk and outcome status) are provided in Tables 3 and 4. Significant differences were interpreted if p < .05. To limit Type I errors, the Benjamini and Hochberg Procedure (1995) was used to control for false discovery rates by risk and outcome at α = 0.20 level.
Table 3.
Dyadic synchrony and responsiveness composite frequencies at 6, 9, and 12 months stratified by risk
| 6 months | 9 months | 12 months | ||||
|---|---|---|---|---|---|---|
| High-risk | Low-risk | High-risk | Low-risk | High-risk | Low-risk | |
| n | 53 | 43 | 78 | 56 | 77 | 54 |
| Mean(SE) | ||||||
| Dyadic Synchrony | 51.3 (2.9) | 54.8 (3.1) | 63.0 (3.0) | 67.0 (3.4) | 72.5 (3.3) | 82.9 (3.9) |
| Infant Responsiveness | 14.7 (1.1) | 15.0 (1.1) | 17.3 (1.0) | 18.9 (1.2) | 20.7 (1.3) | 25.3 (1.5) |
| Maternal Responsiveness | 23.3 (1.7) | 27.7 (1.8) | 29.9 (1.8) | 30.6 (1.8) | 35.2 (1.6) | 39.1 (1.9) |
Table 4.
Dyadic synchrony and responsiveness composite frequencies at 6, 9, and 12 months stratified by outcome
| 6 months | 9 months | 12 months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ASD | Non-TD | TD | ASD | Non-TD | TD | ASD | Non-TD | TD | |
| n | 10 | 12 | 60 | 14 | 14 | 90 | 12 | 15 | 94 |
| Mean(SE) | |||||||||
| Dyadic Synchrony | 56.4 (7.6) | 53.3 (6.6) | 54.6 (2.6) | 69.2 (8.8) | 57.1 (6.3) | 65.4 (2.5) | 73.1 (8.3) | 63.4 (6.2) | 79.0 (3.0) |
| Infant Responsiveness | 17.0 (2.8) | 14.8 (2.3) | 15.3 (1.0) | 20.7 (3.1) | 15.6 (2.1) | 17.9 (0.9) | 18.5 (2.9) | 16.6 (2.3) | 23.7 (1.2) |
| Maternal Responsiveness | 24.9 (4.5) | 25.2 (3.6) | 26.5 (1.5) | 31.5 (3.9) | 26.7 (3.9) | 30.5 (1.4) | 36.9 (4.5) | 30.9 (3.6) | 37.8 (1.4) |
Results
Aim 1
Nonparametric multilevel models by risk status revealed a significant main effect of visit for dyadic synchrony, infant responsiveness, and maternal responsiveness, respectively. To determine at which visit age(s) risk status differences were evident, estimated marginal means for risk status X visit interaction revealed significant differences at 12 months of age for dyadic synchrony and infant responsiveness. No significant covariates were observed. Results are summarized in Table 5 and illustrated in Figure 1.
Table 5.
Linear model parameter estimates for dyadic synchrony and responsiveness by risk status
| Dyadic Synchrony |
Infant Responsiveness |
Maternal Responsiveness |
||||
|---|---|---|---|---|---|---|
| Fixed Effects | Estimate (SE) | t | Estimate (SE) | t | Estimate (SE) | t |
| Risk Groupa | 5.77(10.5) | 0.55 | 4.51(4.0) | 1.13 | −3.02(5.4) | −0.56 |
| Visit | 4.64(0.8) | 5.61** | 1.66(0.3) | 5.31** | 1.92(0.4) | 4.57** |
| Group x Visit | −1.22(1.1) | −1.11 | −0.72(0.4) | −1.73 | 0.03(0.6) | 0.06 |
| Infant Sexb | 3.25(2.7) | 1.19 | 1.38(1.0) | 1.34 | 2.74(1.5) | 1.86 |
| Maternal Education (yrs) | 0.79(0.6) | 1.34 | 0.36(0.2) | 1.61 | 0.18(0.3) | 0.57 |
| Estimated Marginal Meansc | ||||||
| Group x 6M Visit | 2.06(5.1) | 0.41 | 0.10(1.9) | 0.05 | 3.40(2.6) | 1.29 |
| Group x 9M Visit | 4.42(4.3) | 1.02 | 1.51(1.6) | 0.92 | 1.83(2.3) | 0.81 |
| Group x 12M Visit | 9.26(4.4) | 2.11* | 4.39(1.7) | 2.64* | 3.11(2.3) | 1.36 |
Note:
Dichotomized as High-risk = 1; Low-risk = 0.
Dichotomized as Male = 1; Female = 0.
Separate models were specified for marginal means treating visit as a categorical variable to identify when age differences emerged.
p values < .05
p values < .001
Figure 1.
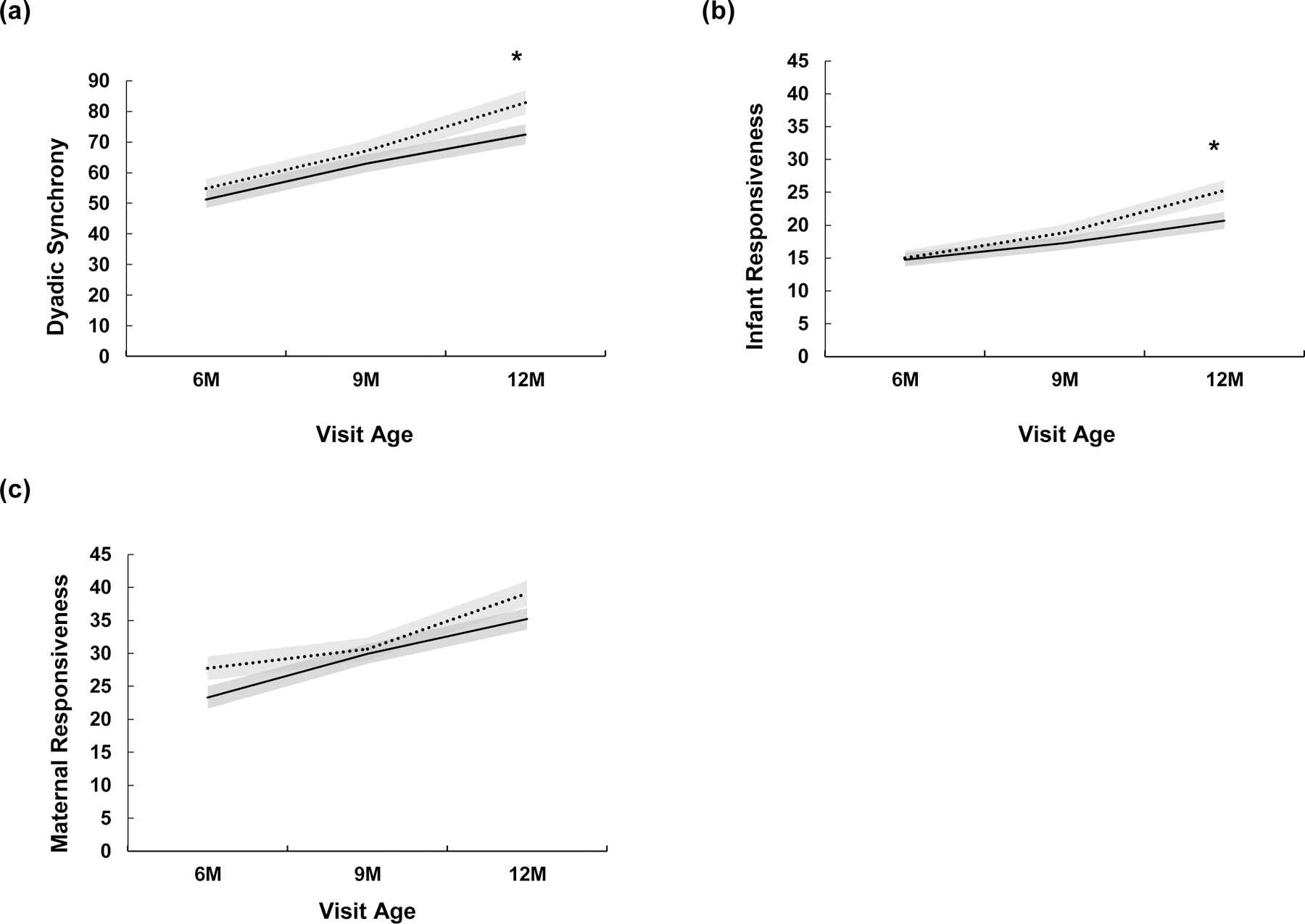
Dyadic synchrony and responsiveness at 6, 9, and 12 months stratified by risk status
Aim 2
Nonparametric multilevel models by outcome classification revealed a significant main effect of infant responsiveness. Further inspection revealed a significant difference between TD and Non-TD outcome groups at 12 months of age, such that children later classified with Non-TD outcomes were significantly less responsive to their mothers than the TD group. Maternal education was a significant covariate for infant responsiveness, such that years of education were positively associated with higher levels of infant responsiveness. Results are summarized in Table 6.
Table 6.
Linear model parameter estimates for dyadic synchrony and responsiveness by outcome
| Dyadic Synchrony |
Infant Responsiveness |
Maternal Responsiveness |
||||
|---|---|---|---|---|---|---|
| Fixed Effects | Estimate (SE) | t | Estimate (SE) | t | Estimate (SE) | t |
| Outcome Groupa | −9.24(8.4) | −1.10 | −6.51(3.1) | −2.08* | −1.35(4.3) | −0.31 |
| Visit | 0.50(2.4) | 0.21 | −0.92(0.9) | −1.03 | 1.10(1.2) | 0.90 |
| Group x Visit | 1.18(0.9) | 1.34 | 0.78(0.3) | 2.38* | 0.26(0.5) | 0.59 |
| Infant Sexb | 3.48(3.0) | 1.17 | 1.62(1.1) | 1.49 | 2.87(1.6) | 1.74 |
| Maternal Education (yrs) | 1.13(0.6) | 1.77 | 0.60(0.2) | 2.58* | 0.20(0.4) | 0.56 |
| Estimated Marginal Meansc | ||||||
| Group x 6M Visit | ||||||
| ASD x Non-TD | 2.51(4.0) | 0.63 | ||||
| ASD x TD | 2.43(3.2) | 0.77 | ||||
| Non-TD x TD | −0.08(2.9) | −0.03 | ||||
| Group x 9M Visit | ||||||
| ASD x Non-TD | 4.75(3.5) | 1.36 | ||||
| ASD x TD | 2.43(2.7) | 0.91 | ||||
| Non-TD x TD | −2.32(2.7) | −0.87 | ||||
| Group x 12M Visit | ||||||
| ASD x Non-TD | 1.83(3.6) | 0.51 | ||||
| ASD x TD | −5.14(2.8) | −1.81 | ||||
| Non-TD x TD | −6.97(2.6) | −2.71* | ||||
Note:
Coded as ASD = 1; Non-TD = 2; TD = 3.
Dichotomized as Male = 1; Female = 0.
Separate models were specified for marginal means treating visit as a categorical variable to identify when differences emerged.
p values < .05
Post-hoc analyses
To aid in our interpretation of the 12-month risk and outcome results, multinomial regression models were conducted in SPSS Version 24 to determine whether the dyadic synchrony and responsiveness composites at 12 months were associated with children’s developmental functioning, as indexed by the MSEL and ADOS calibrated severity scores, at their final laboratory visit. Infant sex and maternal education in years were included as covariates. Results are summarized in Table 7.
Table 7.
Synchrony and Responsiveness at 12 months predicting observed developmental functioning at 36 months
| Overall | 12 Month | |||||||
|---|---|---|---|---|---|---|---|---|
| EL (n = 101) |
RL (n = 101) |
VR (n = 101) |
CSS (n = 108) |
|||||
| Mean (SD) | 49.1 (9.7) | 49.1 (11.2) | 53.7 (13.2) | 2.21 (2.1) | ||||
| β | t | β | t | β | t | β | t | |
| Dyadic Synchrony | .27 | 2.93 e * | .27 | 2.82 e * | .06 | 0.56 | −.21 | −2.17 * |
| Infant Responsiveness | .23 | 2.42 e * | .26 | 2.64 * | .08 | 0.77 | −.28 | −2.94 * |
| Maternal Responsiveness | .21 | 2.25 e * | .23 | 2.40 e * | .05 | 0.52 | −.16 | −1.73 |
| ASD-specific | 12 Month | |||||||
| EL (n = 7) |
RL (n = 7) |
VR (n = 8) |
CSS (n = 11) |
|||||
| 40.4 (14.8) | 36.9 (14.3) | 41.6 (13.6) | 6.91 (2.5) | |||||
| β | t | β | t | β | t | β | t | |
| Dyadic Synchrony | .17 | 0.38 | .57 | 1.56 | .53 | 1.52 | −.58 | −2.11 |
| Infant Responsiveness | .01 | 0.03 * | .45 | 1.14 | .41 | 1.08 | −.61 | −2.33 * |
| Maternal Responsiveness | .21 | 0.48 | .58 | 1.58 | .71 | 2.45 * | −.32 | −1.01 |
| Non-TD specific | 12 Month | |||||||
| EL (n = 13) |
RL (n = 13) |
VR (n = 12) |
CSS (n = 15) |
|||||
| 42.2 (11.3) | 42.5 (15.0) | 43.2 (13.3) | 3.1 (2.0) | |||||
| β | t | β | t | β | t | β | T | |
| Dyadic Synchrony | −.08 | −0.22 | −.10 | −0.31 | −.68 | −2.01 | −.13 | −0.46 |
| Infant Responsiveness | −.29 | −0.79 | −.00 | −0.01 | −.71 | −1.88 | −.08 | −0.28 |
| Maternal Responsiveness | −.03 | −0.10 | −.05 | −0.19 | −.35 | −1.19 | −.28 | −1.06 |
Notes: Infants with valid parent-child play data and MSEL domain or CSS by respective outcome group were included in the models. EL = expressive language scores on the Mullen Scales of Early Learning (MSEL). RL = receptive language scores on the MSEL. VR = visual reception scores on the MSEL. CSS = ADOS Calibrated Severity Scores.
maternal education is significant covariate. Infants with valid parent-child play data and MSEL domain or CSS by respective outcome group were included in the models.
p < 0.05
Regardless of outcome classification, higher dyadic synchrony, infant responsiveness, and maternal responsiveness at 12 months was positively associated with significantly higher MSEL receptive and expressive language scores. In addition, higher dyadic synchrony and infant responsiveness composites were associated with lower ADOS calibrated severity scores. Maternal education was a significant covariate on select models, such that higher expressive and receptive language scores were associated with more years of maternal education.
To tease apart potential contributions of respected outcome differences at 12 months on developmental progress, an additional set of multinomial regression models were conducted for the ASD and Non-TD groups, respectively. For the ASD group, more infant responsiveness was positively associated with higher expressive language scores. Higher maternal responsiveness at 12 months was positively associated with higher receptive language scores. In addition, infant responsiveness at 12 months was negatively associated with ADOS severity scores at infants’ final visit, such that lower infant responsiveness was associated with higher symptom severity. No significant associations between 12 month dyadic composites and developmental progress indices were observed for the Non-TD group.
Discussion
Consistent with previous research, dyadic behaviors during the first year did distinguish between familial-risk status and outcome classifications by 12 months, and dyadic behaviors were associated with language skills, regardless of outcome status (e.g., Young et al., 2009). In general, more synchronous and responsiveness dyads at 12 months had children who received higher receptive and expressive language scores by 36 months. For children with ASD, dyads coded as more responsive at 12 months had children with higher expressive language scores by 36 months.
This result is in line with previous research on the effect of maternal responsiveness on child language skills, both in typically developing children and children with ASD. Specifically, in typically developing samples, maternal responsiveness to their infants’ expressive behaviors are associated with later language skills (Nicely et al., 1999) and language milestones (Tamis-LeMonda et al., 2001). Similarly, for children with ASD, more maternal use of responsive communication strategies was associated with higher language scores (e.g., Haebig, McDuffie, & Weismer, 2013; Siller & Sigman, 2002, 2008). The findings of the present study highlight mother-infant synchrony and responsiveness as a mechanism through which interventions may promote language development in children at elevated risk for ASD.
Consistent with previous studies, behavioral differences among children who receive an ASD diagnosis are not readily apparent prior to 12 months of age (Gangi et al., 2018; Ozonoff et al., 2010; Rozga et al., 2011). For example, Ozonoff et al. (2010), a study which followed a very similar paradigm in a distinct sample, reported that dyadic/social differences (i.e., gaze to face) were not significantly different for children with ASD until 12 months of age or later (when compared to typically developing peers). For a detailed presentation of several early social/behavioral signs of ASD see reviews by Jones et al. (2014), Szatmari et al. (2016), and Iverson (2018). In the present study, low infant responsiveness within the ASD group at 12 months was associated with higher autism symptomology by infants’ final visit. In addition, infants in the ASD group with highly responsive mothers at 12 months demonstrated higher cognitive skills by their final laboratory assessment. With replication, the findings of the present study highlight mother-infant responsiveness as a mechanism through which interventions may promote cognitive development in children at elevated risk for ASD.
Intriguingly, the lowest synchrony composite scores across all three time points were for the children in the non-TD group. Given the small size of this group (n = 15), this finding should not be overinterpreted, but it does draw attention to the developmental importance of parent-child interactions in at risk development. Many of the children in the non-TD group had language concerns and this finding may be an extension of the relations between dyadic synchrony and language development. In Yirmiya et al (2006) a small sample (n = 5) of children classified at non-TD demonstrated a similar pattern with lower synchrony scores at 4 months of age. Other studies have also noted fewer/lower prosocial behavior in children classified as non-TD (sometimes referred to/further classified as exhibiting the broader autism phenotype; BAP). Examples include lower frequencies of information seeking during a social task (Cornew, Dobkins, Akshoomoff, McCleery, & Carver, 2012) and descriptive reports of fewer joint attention bids (Sullivan et al., 2007).
Infant sibling prospective designs are well suited for exploring dyadic contexts in ASD and the BAP. As highlighted in the introduction, recent studies have demonstrated that beyond the approximately 20% familial risk of a subsequent ASD diagnosis (Ozonoff et al., 2010), at least a fifth of the remaining 80% exhibit some features or characteristics of an ASD by 12 months of age (Messenger et al., 2013; Georgiades et al., 2013). Observations like these have likely contributed to the growing research base on elevated-risk interventions (prior to known diagnostic outcomes). To continue providing families support, while also minimizing unnecessary distress, developmental monitoring studies should examine difficulties in non-TD outcomes within a social context to provide a foundational understanding of observable similarities and differences between non-TD, ASD, and TD groups respectively, that may directly benefit elevated-risk intervention designs (e.g., Green et al., 2017).
Limitations and Future Direction
These results are not without limitations. Prospective infant sibling designs frequently struggle to maintain robust sample size distributions from risk status to developmental outcome classifications (i.e., TD, Non-TD, ASD). In the current study, 16 children received an ASD classification by their outcome visit; however, only a subset of those children had completed the mother-infant play interaction task at the qualifying visit ages. Thus, this study may have been underpowered to find outcome group differences. To account for these sampling constraints, more multisite collaborations, would allow researchers to aggregate data in order to robustly examine group differences across various risk and outcome classifications.
The lack of significant ASD outcome group differences in this study may also reflect our decision to examine behaviors in the first year of life, consistent with the neurotypical development of dyadic synchrony (Feldman, 2007), which occurs before most diverging paths related to ASD. Future research could build on this study by assessing synchrony and responsiveness growth curves that extend beyond 12 months of age. In addition, the present study and the majority of previous investigations have focused on mean-level indices of play behaviors. However, it is widely recognized that dyadic interactions are complex and mean-level indices may not adequately capture complexity or temporal processes within the interaction (e.g., Messinger et al., 2017; Chow, Haltigan, & Messinger, 2010). Future research may consider applying nonlinear trajectory modeling and/or dynamic systems approaches to existing dyadic data in order to better capture the unfolding and bidirectional nature of dyadic interactions.
Supplementary Material
Acknowledgements:
The authors would like to thank the participating families for their time and commitment to this prospective monitoring project. We would also like to thank the team of trained behavioral coders for their hard-work and contributions to the current study.
Footnotes
Conflict of Interest Statement:
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Contributor Information
A. M. Kellerman, Purdue University, West Lafayette, IN
A. J. Schwichtenberg, Purdue University, West Lafayette, IN
R. Abu-Zhaya, Hebrew University of Jerusalem, Jerusalem, Israel
M. Miller, University of California, Davis – M.I.N.D. Institute
G. S. Young, University of California, Davis – M.I.N.D. Institute
S. Ozonoff, University of California, Davis – M.I.N.D. Institute
References
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B Methodological, 57, 289–300. doi: 10.2307/2346101 [DOI] [Google Scholar]
- Bornstein MH, & Tamis-Lemonda CS (1997). Maternal responsiveness and infant mental abilities: Specific predictive relations. Infant Behavior and Development, 20, 283–296. doi: 10.1016/S0163-6383(97)90001-1 [DOI] [Google Scholar]
- Bruner J (1985). Child’s talk: Learning to use language. Child Language Teaching and Therapy, 1, 111–114. [Google Scholar]
- Campbell SB, Leezenbaum NB, Mahoney AS, Day TN, & Schmidt EN (2016). Social engagement with parents in 11-month-old siblings at high and low genetic risk for autism spectrum disorder. Autism, 19, 915–924. doi: 10.1177/1362361314555146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SB, Leezenbaum NB, Mahoney AS, Moore EL, & Brownell CA (2016). Pretend play and social engagement in toddlers at high and low genetic risk for autism spectrum disorder. Journal of Autism and Developmental Disorders, 46, 2305–2316. doi: 10.1007/s10803-016-2764-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman T, Baron-Cohen S, Swettenham J, Baird G, Drew A, & Cox A (2009). Predicting language outcome in infants with autism and pervasive developmental disorder. International Journal of Language and Communication Disorders, 38, 265–285. doi: 10.1080/136820310000104830 [DOI] [PubMed] [Google Scholar]
- Charman T, Young GS, Brian J, Carter A, Carver LJ, Chawarska K, … Zwaigenbaum L (2017). Non-ASD outcomes at 36 months in siblings at familial risk for autism spectrum disorder (ASD): A baby siblings research consortium (BSRC) study. Autism Research, 10, 169–178. doi: 10.1002/aur.1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow S, Haltigan JD, Messinger DS (2010). Dynamic infant-parent affect coupling during the Face-to-Face/Still-Face. Emotion, 10, 101–114. doi: 10.1037/a0017824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornew L, Dobkins KR, Akshoomoff N, McCleery JP, Carver LJ (2012). Atypical social referencing in infant siblings of children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 42, 2611–2621. doi: 10.1007/s10803-012-1518-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denham SA (1993). Maternal emotional responsiveness and toddlers’ social-emotional competence. Journal of Child Psychology and Psychiatry, 34, 715–728. doi: 10.1111/j.1469-7610.1993.tb01066.x [DOI] [PubMed] [Google Scholar]
- Feldman R (2007). Parent–infant synchrony: Biological foundations and developmental outcomes. Current Directions in Psychological Science, 16, 340–345. doi: 10.1111/j.1467-8721.2007.00532.x [DOI] [Google Scholar]
- Feldman R (2015). The adaptive human parental brain: Implications for children’s social development. Trends in Neurosciences, 38, 387–399. doi: 10.1016/j.tins.2015.04.004 [DOI] [PubMed] [Google Scholar]
- Feldman R, & Greenbaum CW (1997). Affect regulation and synchrony in mother—infant play as precursors to the development of symbolic competence. Infant Mental Health Journal, 18, 4–23. doi: [DOI] [Google Scholar]
- Feldman R, Greenbaum CW, Yirmiya N, & Mayes LC (1996). Relations between cyclicity and regulation in mother-infant interaction at 3 and 9 months and cognition at 2 years. Journal of Applied Developmental Psychology, 17, 347–365. doi: 10.1016/S0193-3973(96)90031-3 [DOI] [Google Scholar]
- Fogel A (1993). Two principles of communication: Co-regulation and framing. In Nadel J & Camaioni L (Eds.), New perspective in early communicative development (pp. 9–22). London: Routledge. [Google Scholar]
- Gangi DN, Schwichtenberg A, Iosif AM, Young GS, Baguio F, & Ozonoff S (2018). Gaze to faces across interactive contexts in infants at heightened risk for autism. Autism, 22, 763–768. doi: 10.1177/1362361317704421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiades S, Szatmari P, Zwaigenbaum L, Bryson S, Brian J, Roberts W … Garon N (2013). A prospective study of autistic-like traits in unaffected siblings of probands with autism spectrum disorder. Journal of the American Medical Association Psychiatry, 70, 42–48. doi: 10.1001/2013.jamapsychiatry.1 [DOI] [PubMed] [Google Scholar]
- Gotham K, Pickles A, & Lord C (2009). Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism and Developmental Disorders, 39, 693–705. doi: 10.1007/s10803-008-0674-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J, Pickles A, Pasco G, Bedford R, Wan MW, Elsabbagh M, et al. (2017). Randomised trial of a parent-mediated intervention for infants at high risk for autism: Longitudinal outcomes to age 3 years. Journal of Child Psychology and Psychiatry, 58, 1330–1340. doi: 10.1111/jcpp.12728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haebig E, McDuffie A, & Weismer ES (2013). The contribution of two categories of parent verbal responsiveness to later language for toddlers and preschoolers on the autism spectrum. American Journal of Speech Language Pathology, 22, 57–70. doi: 10.1044/1058-0360(2012/11-0004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker CM, Ibañez LV, Nguyen TP, Messinger DS, & Stone WL (2016). The effect of parenting style on social smiling in infants at high and low risk for ASD. Journal of Autism and Developmental Disorders, 46, 2399–2407. doi: 10.1007/s10803-016-2772-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutman T, Chela MK, Gillespie-Lynch K, & Sigman M (2012). Selective visual attention at twelve months: Signs of autism in early social interactions. Journal of Autism and Developmental Disorders, 42, 487–498. doi: 10.007/s10803-011-1262-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson J (2018). Early motor and communicative development in infants with an older sibling with autism spectrum disorder. Journal of Speech, Language, and Hearing Research, 61, 2673–2684. doi: 10.1044/2018_JSLHR-L-RSAUT-18-0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowski KP, & Iverson JM (2018). Look at mommy: An exploratory study of attention-related communication in mothers of toddlers at risk for autism. Language Learning and Development, 15, 126–137. doi: 10.1080/15475441.2018.1544074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe J, Beebe B, Feldstein S, Crown CL, Jasnow MD, Rochat P, & Stern DN (2001). Rhythms of dialogue in infancy: Coordinated timing in development. Monographs of the society for research in child development, 66, i–149. [PubMed] [Google Scholar]
- Jones E, Gliga T, Bedford R, Charman T, & Johnson MH (2014). Developmental pathways to autism: A review of prospective studies of infants at risk. Neuroscience and Biobehavioral Reviews, 39, 1–33. doi: 10.1016/j.neubiorev.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasari C, Sigman M, Mundy P, & Yirmiya N (1988). Caregiver interactions with autistic children. Journal of Abnormal Child Psychology, 16, 45–56. doi: 10.1007/BF00910499 [DOI] [PubMed] [Google Scholar]
- Kellerman AM, Schwichtenberg AJ, Tonnsen BL, Posada G, & Lane SP (2019). Dyadic interactions in children exhibiting the broader autism phenotype: Is the broader autism phenotype distinguishable from typical development? Autism Research, 12, 469–481. doi: 10.1002/aur.2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert-Brown BL, McDonald NM, Mattson WI, Martin KB, Ibañez LV, Stone WL, & Messinger DS (2015). Positive emotional engagement and autism risk. Developmental Psychology, 51, 848–855. doi: 10.1037/a0039182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leezenbaum NB, Campbell SB, Butler D, & Iverson JM (2014). Maternal verbal responses to communication of infants at low and heightened risk of autism. Autism, 18, 694–703. doi: 10.1177/1362361313491327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey EW, Cremeens PR, Colwell MJ, & Caldera YM (2009). The structure of parent–child dyadic synchrony in toddlerhood and children’s communication competence and Self-control. Social Development, 18, 375–396. doi: 10.1111/j.1467-9507.2008.00489.x [DOI] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, & Bishop SL (2012). Autism diagnostic observation schedule manual (2nd ed.). Torrance, CA: Western Psychological Services. [Google Scholar]
- McFadden KE, & Tamis-Lemonda CS (2013). Maternal responsiveness, intrusiveness, and negativity during play with infants: Contextual associations and infant cognitive status in a low-income sample. Infant Mental Health Journal, 34, 80–92. doi: 10.1002/imhj.21376 [DOI] [Google Scholar]
- Messinger D, Young GS, Ozonoff S, Dobkins K, Carter A, Zwaigenbaum L, …Sigman M (2013). Beyond autism: A baby siblings research consortium study of high-risk children at three years of age. Journal of the American Academy of Child & Adolescent Psychiatry, 52, 300–308. doi: 10.1016/j.jaac.2012.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinger DS, Mattson WI, Todd JT, Gangi DN, Myers ND, & Bahrick LE (2017). Temporal dependency and the structure of early looking. PLoS ONE, 12, e0169458. doi: 10.1371/journal.pone.0169458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Young GS, Hutman T, Johnson S, Schwichtenberg AJ, & Ozonoff S (2015). Early pragmatic language difficulties in siblings of children with autism: implications for DSM-5 social communication disorder?. Journal of Child Psychology and Psychiatry, 56, 774–781. doi: 10.1111/jcpp.12342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Iosif AM, Young GS, Hill M, Hanzel EP, Hutman T, … Ozonoff S (2016). School-age outcomes of infants at risk for autism spectrum disorder. Autism Research, 9, 632–642. doi: 10.1002/aur.1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen EM (1995). Mullen scales of early learning (AGS ed.), Circle Pines, MN: American Guidance Service Inc. [Google Scholar]
- Nichols CM, Ibañez LV, Foss-Feig JH, & Stone WL (2014). Social smiling and its components in high-risk infant siblings without later ASD symptomatology. Journal of Autism and Developmental Disorders, 44, 894–902. doi: 10.1007/s10803-013-1944-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicely P, Tamis-LeMonda CS, & Bornstein MH (1999). Mothers’ attuned responses to infant affect expressivity promote earlier achievement of language milestones. Infant Behavior and Development, 22, 557–568. doi: 10.1016/S01636383(00)00023-0 [DOI] [Google Scholar]
- Northrup JB, & Iverson JM (2015). Vocal coordination during early parent–infant interactions predicts language outcome in infant siblings of children with autism spectrum disorder. Infancy, 20, 523–547. doi: 10.1111/infa.12090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Iosif AM, Baguio F, Cook IC, Hill MM, Hutman T, … Young GS (2010). A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child and Adolescent Psychiatry, 49, 256–266. doi: 10.1016/j.jaac.2009.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, … Stone WL (2011). Recurrence risk for autism spectrum disorders: A baby siblings research consortium study. Pediatrics, 128, e488–e495. doi: 10.1542/peds.2010-2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Belding A, Hill M, Hill A, Hutman T, … Iosif AM (2014). The broader autism phenotype in infancy: When does it emerge? Journal of the American Academy of Child & Adolescent Psychiatry, 53, 398–407. doi: 10.1016/j.jaac.2013.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavola L, Kunnari S, & Moilanen I (2005). Maternal responsiveness and infant intentional communication: Implications for the early communicative and linguistic development. Child: Care, Health and Development, 31, 727–735. doi: 10.1111/j.1365-2214.2005.00566.x [DOI] [PubMed] [Google Scholar]
- Paul R, Fuerst Y, Ramsay G, Chawarska K, & Klin A (2011). Out of the mouths of babes: Vocal production in infant siblings of children with ASD. Journal of Child Psychology and Psychiatry, 52, 588–598. doi: 10.1111/j.1469-7610.2010.02332.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon KK, Watson LR, Baranek GT, & Poe MD (2012). To what extent do joint attention, imitation, and object play behaviors in infancy predict later communication and intellectual functioning in ASD? Journal of Autism and Developmental Disorders, 42, 1064–1074. doi: 10.1007/s10803-011-1349-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozga A, Hutman T, Young GS, Rogers SJ, Ozonoff S, Dapretto M, & Sigman M (2011). Behavioral profiles of affected and unaffected siblings of children with autism: Contribution of measures of mother-infant interaction and nonverbal communication. Journal of Autism and Developmental Disorders, 41, 287–301. doi: 10.1007/s10803-010-1051-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siller M & Sigman M (2002). The behaviors of parents of children with autism predict the subsequent development of their children’s communication. Journal of Autism and Developmental Disorders, 32, 77–89. doi: 10.1023/A:1014884404276 [DOI] [PubMed] [Google Scholar]
- Siller M, & Sigman M (2008). Modeling longitudinal change in the language abilities of children with autism: Parent behaviors and child characteristics as predictors of change. Developmental Psychology, 44, 1691–1704. doi: 10.1037/a0013771 [DOI] [PubMed] [Google Scholar]
- Schwichtenberg AJ, Kellerman AM, Young GS, Miller M, & Ozonoff S (2019). Mothers of children with autism spectrum disorders: Play behaviors with infant siblings and social responsiveness. Autism, 23, 821–833. doi: 10.1177/1362361318782220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard E, Milosavljevic B, Pasco G, Jones EJH, Gliga T, Happe F, Johnson MH, Charman T, & BASIS Team. (2016). Mid-childhood outcomes of infant siblings at familial high-risk of autism spectrum disorder. Autism Research, 10, 546–557. doi: 10.1002/aur.1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner AM, Gengoux GW, Smith A, & Chawarska K (2018). Parent-child interaction synchrony for infants at-risk for autism spectrum disorder. Journal of Autism and Developmental Disorders, 48, 3562–3572. doi: 10.1007/s10803-018-3624-8 [DOI] [PubMed] [Google Scholar]
- Sullivan M, Finelli J, Marvin A, Garrett-Mayer E, Bauman M, & Landa R (2007). Response to joint attention in toddlers at risk for autism spectrum disorder: A prospective study. Journal of Autism and Developmental Disorders, 37, 37–48. doi: 10.1007/s10803-006-0335-3 [DOI] [PubMed] [Google Scholar]
- Swanson MR, Donovan K, Paterson S, Wolff JJ, Parish-Morris J, Meera SS, … IBIS Network. (2019). Early language exposure supports later language skills in infants with and without autism. Autism Research, epub ahead of print. doi: 10.1002/aur.2163 [DOI] [PMC free article] [PubMed]
- Szatmari P, Chawarska K, Dawson G, Georgiades S, Landa R, Lord C, … Halladay A (2016). Prospective longitudinal studies of infant siblings of children with autism: Lessons learned and future directions. Journal of the American Academy of Child & Adolescent Psychiatry, 55, 179–187. doi: 10.1016/j.jaac.2015.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott MR, Nelson CA, & Tager-Flusberg H (2015). Maternal gesture use and language development in infant siblings of children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 45, 4–14. doi: 10.1007/s10803-013-1820-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott MR, Nelson CA, & Tager-Flusberg H (2016). Maternal vocal feedback to 9-month-old infant siblings of children with ASD. Autism Research, 9, 460–470. doi: 10.1002/aur.1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamis-LeMonda CS, Bornstein MH, Baumwell L, & Melstein Damast A (1996). Responsive parenting in the second year: Specific influences on children’s language and play. Early Development and Parenting, 5, 173–183. doi: [DOI] [Google Scholar]
- Tamis-LeMonda CS, Bornstein MH, & Baumwell L (2001). Maternal responsiveness and children’s achievement of language milestones. Child development, 72, 748–767. doi: 10.1111/1467-8624.00313 [DOI] [PubMed] [Google Scholar]
- Tronick EZ (1989). Emotions and emotional communication in infants. American psychologist, 44, 112–119. doi: 10.1037//0003-066x.44.2.112 [DOI] [PubMed] [Google Scholar]
- Tronick E, & Cohn JF (1989). Infant-mother face-to-face interaction: Age and gender differences in coordination and the occurrence of miscoordination. Child Development, 60, 85–92. doi: 10.1111/j.1467-8624.1989.tb02698.x [DOI] [PubMed] [Google Scholar]
- Wan MW, Green J, Elsabbagh M, Johnson M, Charman T, & Plummer F (2012). Parent-infant interaction in infant siblings at risk of autism. Research in Developmental Disabilities, 33, 924–932. doi: 10.1016/j.ridd.2011.12.011 [DOI] [PubMed] [Google Scholar]
- Wan MW, Green J, Elsabbagh M, Johnson M, Charman T, Plummer F (2013). Quality of interaction between at-risk infants and caregiver at 12–15 months is associated with 3-year autism outcome. Journal of Child Psychology and Psychiatry, 54, 763–771. doi: 10.1111/jcpp.12032 [DOI] [PubMed] [Google Scholar]
- Yirmiya N, Gamliel I, Pilowsky T, Feldman R, Baron-Cohen S, & Sigman M (2006). The development of siblings of children with autism at 4 and 14 months: Social engagement, communication, and cognition. Journal of Child Psychology and Psychiatry, 47, 511–523. doi: 10.1111/j.1469-7610.2005.01528.x [DOI] [PubMed] [Google Scholar]
- Young GS, Merin N, Rogers S, & Ozonoff S (2009). Gaze behavior and affect at 6 months: Predicting clinical outcomes and language development in typically developing infants and infants at risk for autism. Developmental Science, 12, 798–814. doi: 10.1111/j.1467-7687.2009.00833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


