Abstract
Dimensionality reduction is a common tool for visualization and inference of population structure from genotypes, but popular methods either return too many dimensions for easy plotting (PCA) or fail to preserve global geometry (t-SNE and UMAP). Here we explore the utility of variational autoencoders (VAEs)—generative machine learning models in which a pair of neural networks seek to first compress and then recreate the input data—for visualizing population genetic variation. VAEs incorporate nonlinear relationships, allow users to define the dimensionality of the latent space, and in our tests preserve global geometry better than t-SNE and UMAP. Our implementation, which we call popvae, is available as a command-line python program at github.com/kr-colab/popvae. The approach yields latent embeddings that capture subtle aspects of population structure in humans and Anopheles mosquitoes, and can generate artificial genotypes characteristic of a given sample or population.
Keywords: population structure, population genetics, data visualization, pca, variational autoencoder, deep learning, machine learning, neural network
Introduction
As we trace the genealogy of a population forward in time, branching inherent in the genealogical process leads to hierarchical relationships among individuals that can be thought of as clades. Much of the genetic variation among individuals in a species thus reflects the history of isolation and migration of their ancestors. Describing this population structure is itself a major goal in biogeography, systematics, and human genetics; wherein one might attempt to infer the number of genotypic clusters supported by the data (Holsinger and Weir 2009), estimate relative rates of migration (Petkova et al. 2016), or observe turnover in the ancestry of people living in a geographic region (Antonio et al. 2019).
Estimation of population structure is also critical for our ability to accurately link genetic variation to phenotypic variation, because population structure is a major confounding factor in genome-wide association studies (GWAS) (Lander and Schork 1994; Pritchard and Donnelly 2001; Freedman et al. 2004; Marchini et al. 2004). Downstream studies that use GWAS information can themselves be compromised by inadequate controls for structure, for instance in recent work trying to identify the effects of natural selection on complex traits (Mathieson and McVean 2012; Berg et al. 2019; Sohail et al. 2019). Dimensionality reduction via principal components analysis (PCA) has been an important tool for geneticists in this regard, and is now commonly used both to control for the effects of population structure in GWAS (Patterson et al. 2006; Price et al. 2006) as well as for visualization of genetic variation.
As a visualization tool however, PCA scatterplots can be difficult to interpret because information about genetic variation is split across many axes, while efficient plotting is restricted to two dimensions. Though techniques like plotting marginal distributions as stacked density plots can aid interpretation, these require binning samples into “populations” prior to visualization, are rarely used in practice, and remain difficult to interpret in complex cases. Recently two techniques from the machine learning community—t-SNE (van der Maaten and Hinton 2008) and UMAP (McInnes et al. 2018)—have shown promising performance in producing two-dimensional visualizations of high-dimensional biological data. In the case of UMAP, Diaz-Papkovich et al. (2019) recently showed that running the algorithm on a large set of principal component axes allows visualization of subtle aspects of population structure in three human genotyping datasets.
However, interpreting UMAP and t-SNE plots is also complicated by a lack of so-called global structure. Though these methods perform well in clustering similar samples, distances between groups are not always meaningful—two clusters separated by a large distance in a t-SNE plot can be more similar to each other than either is to their immediate neighbors (Becht et al. 2019). The degree to which initialization and hyperparameter tuning can alleviate this issue remains an open question in the literature (Kobak and Linderman 2019).
To create meaningful and interpretable visualizations of population genetic data, we would like a method that encodes as much information as possible into just two dimensions while maintaining global structure. One way of achieving this is with a variational autoencoder (VAE).
VAEs consist of a pair of deep neural networks in which the first network (the encoder) encodes input data as a probability distribution in a latent space and the second (the decoder) seeks to recreate the input given a set of latent coordinates (Kingma and Welling 2013). Thus a VAE has as its target the input data itself. The loss function for a VAE is the sum of reconstruction error (how different the generated data is from the input) and KL divergence between a sample’s distribution in latent space and a reference distribution which acts as a prior on the latent space [here we use a standard multivariate normal, but see Davidson et al. (2018) for an alternative design with a hyperspherical latent space]. The KL term of the loss function incentivizes the encoder to generate latent distributions with meaningful distances among samples, while the reconstruction error term helps to achieve good local clustering and data generation. VAE’s have been used extensively in image generation (Larsen et al. 2015; Gulrajani et al. 2016; Hou et al. 2016) and several recent studies have applied them to dimensionality reduction and classification of single-cell RNAseq data (Grønbech et al. 2018; Lafarge et al. 2019; Wang and Gu 2018; Hu and Greene 2019). At deeper timescales than we test here, Derkarabetian et al. (2019) recently explored the use of VAEs in species delimitation.
In population genetics two recent studies have studied the utility of generative deep neural networks for creating simulated genotypes. Montserrat et al. (2019) use a class-conditional VAE to generate artificial human genotypes, while Yelmen et al. (2019) use a restricted Boltzman machine and provide an in-depth assessment of the population genetic characteristics of their artificial genotypes. These studies found that such generative methods can produce short stretches of artificial genotypes that are difficult to distinguish from real data, but performance was improved by using a generative adversarial network—either in combination with a VAE as in Montserrat et al. (2019) or as a standalone method in Yelmen et al. (2019). In this study we focus not on generation of simulated genotypes, but instead on the learned latent space representations of genotypes produced by a VAE, and study when and how they can best be used for visualizing population structure.
We introduce a new method, popvae (for population VAE), a command-line python program that takes as input a set of unphased genotypes and outputs sample coordinates in a low-dimensional latent space. We test popvae with simulated data and demonstrate its utility in empirical datasets of humans and Anopheles mosquitoes. In general, popvae is most useful for complex samples for which PCA projects important aspects of structure across many axes. Relative to t-SNE and UMAP, the approach appears to better preserve global geometry at the cost of less pronounced clustering of individual sample localities. However, we show that hyperparameter tuning and stochasticity associated with train/test splits and parameter initialization are ongoing challenges for a VAE-based method, and the approach is much more computationally intensive than PCA.
Methods
Model
In this manuscript, we describe the application of a VAE to population genetic data for clustering and visualization (Kingma and Welling, 2013). Formally let X be our dataset consisting of N observations (i.e., individual genotypes) such that , and let the probability of those data with some set of parameters θ be . For VAEs we are interested in representing the data with a latent model, assigning some latent process parameters z, such that we can write a generative latent process as , where is the prior distribution on z. The last conditional probability here is often referred to as the decoder, as it maps from latent space to data space.
For VAEs we also define a so-called encoder model , where represents the parameters of the encoding (the mapping of x to the latent space z), and we seek to optimize the encoder such that . In practice the parameters represent the weights and biases of the encoding neural network. We thus step from data space by using:
| (1) |
| (2) |
The complete VAE information flow then has three steps: the encoder estimates sample distributions in latent space as , we sample from the prior on the latent space using , and finally decode back to data space using . Training is then performed by optimizing the evidence lower bound or ELBO which has parameters of the encoder and decoder within it such that:
| (3) |
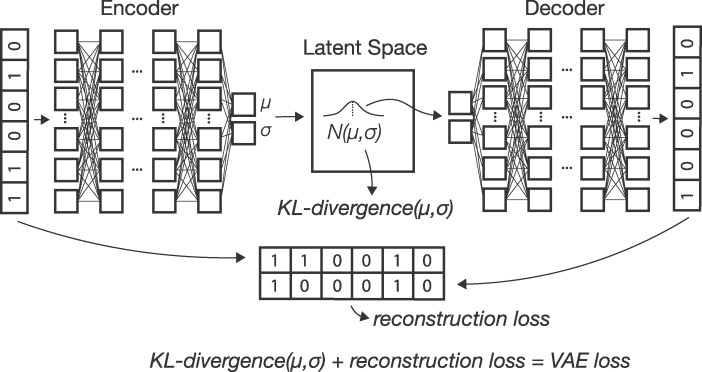
Optimization of the ELBO here leads to simultaneous fitting of the parameters of the encoder, , and the decoder, θ. In practice we use binary cross-entropy between true and generated sequences for the first term, and KL divergence of sample latent distributions (relative to a standard normal ) for the second term of Equation (3). A graphical depiction of this computational flow can be seen in Figure 1.
Figure 1.
A schematic of the VAE architecture. Input allele counts are passed to an encoder network which outputs parameters describing a sample’s location as a multivariate normal in latent space. Samples from this distribution are then passed to a decoder network which generates a new genotype vector. The loss function used to update weights and biases of both networks is the sum of reconstruction error (from comparing true and generated genotypes) and KL divergence between sample latent distributions and .
Implementation
We implemented this model in python 3 using the tensorflow and keras libraries (Abadi et al. 2015; Chollet et al. 2015), with preprocessing relying on numpy, pandas, and scikit-allel (Oliphant 2006; McKinney 2010; Miles and Harding 2017). popvae reads in genotypes from VCFs, Zarr files (https://zarr.readthedocs.io/en/stable/), or a bespoke hdf5 file format. Genotypes are first filtered to remove singletons and nonbiallelic sites, and missing data is filled by taking two draws from a binomial distribution with probability equal to the allele frequency across all samples [a binned version of the common practice of filling missing genotypes with the mean allele frequency (Jombart 2008; Dray and Josse 2015)]. Filtered genotpes are then encoded with 0/0.5/1 representing homozygous ancestral, heterozygous, and homozygous derived states, respectively.
Samples are split into training and validation sets before model training. We also experimented with using all samples for training and a fixed number of epochs but found this generally led to poor performance (Appendix 1, Supplementary Figure S1). Training samples are used to optimize weights and biases of the neural network, while validation samples are used to measure validation loss after each training epoch (a complete pass through the data), which in turn tunes hyperparameters of the optimizer. By default we use a random 90% of samples for training. However we found considerable variation in latent representations of some datasets when using different sets of training and validation samples (see, e.g., Supplementary Figure S2), so we encourage users to compare multiple training runs with different starting seeds when interpreting plots.
Popvae’s encoder and decoder networks are fully connected feed-forward networks whose size is controlled by two parameters—“width,” which sets the number of hidden units per layer, and “depth,” which sets the number of hidden layers. We experimented with a range of network sizes and set defaults to depth 6 and width 128, which performed well on the empirical analyses described here (Supplementary Table S1 and Figure S3A). However we also include a grid search function by which popvae will conduct short training runs across a user-defined range of network sizes and then fit a final model using the network size with minimum validation loss.
We use a linear activation on the input layers to both networks and a sigmoid activation on the output of the decoder [this produces numeric values bound by (0, 1)]. We interpret the sigmoid decoder outputs as the probability of observing a derived allele at a site, consistent with our 0/0.5/1 encoding of the input genotypes. All other layers use “elu” activations (Clevert et al. 2015), a modification of the more common “relu” activation which avoids the “stuck neuron” problem by returning small but nonzero values with negative inputs.
We use the Adam optimizer (Kingma and Ba 2014) and continue model training until validation loss has not improved for p epochs, where p is a user-adjustable “patience” parameter. We also set a learning rate scheduler to decrease the learning rate of the optimizer by half when validation loss has not improved for epochs. This is intended to force the optimizer to take small steps when close to the final solution, which increases training time but in our experience leads to better fit models. Users can adjust many hyperparameters from the command line, and modifying our network architectures is straightforward for those familiar with the Keras library.
To evaluate model training popvae returns plots of training and validation loss by epoch (e.g., Supplementary Figure S4), and also outputs estimated latent coordinates for validation samples given the encoder parameters at the end of each epoch. These can then be plotted to observe how the model changes over the course of training, which can sometimes help to diagnose overfitting. We also include an interactive plotting function which generates a scatter plot of the latent space and allows users to mouse-over points to view metadata (Supplementary Figure S5). This is intended to allow users to quickly iterate through models while adjusting hyperparameters. In Appendix 1, we discuss alternate approaches to network design and optimization tested while developing popvae.
Data availability
popvae is available at https://github.com/kr-colab/popvae, and scripts for reproducing plots and analyses in this manuscript are available at https://github.com/cjbattey/popvae_analysis_scripts. HGDP genotypes used in this paper are available at ftp://ngs.sanger.ac.uk/production/hgdp, AG1000G genotypes at https://www.malariagen.net/data/ag1000g-phase-2-ar1, and 1000 genomes phase 3 data at https://www.internationalgenome.org/category/phase-3/.
Supplementary material is available at figshare: https://doi.org/10.25387/g3.13311539. .
Results
Latent spaces reflect human migration history
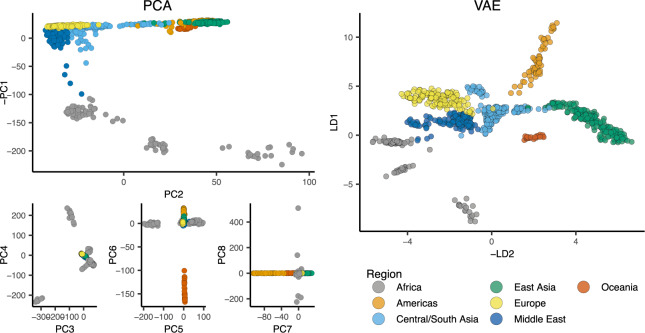
We first applied popvae to 100,000 SNPs from chromosome 1 in the Human Genetic Diversity Project [HGDP; Bergström et al. (2020)], a sample of global modern human diversity. The resulting latent space reflects geography from the point of view of human demographic history (Figures 2 and 4, Supplementary Figure S6). Sub-Saharan African and South American populations are placed on opposite ends of one latent dimension, and north African (Mozabite) and east Asian samples are on opposite ends of the second; mirroring the geography of Africa and Eurasia. Samples from the Americas are roughly centered among Eurasian samples on latent dimension (LD) 2, consistent with recent demographic modeling studies suggesting a mix of Eurasian ancestries in ancestral American populations (Posth et al. 2018; Flegontov et al. 2019). Indeed the closest American samples to the European cluster are Maya individuals who were found to have low levels of recent European admixture in previous analyses (Rosenberg et al. 2002; Bergström et al. 2020) (Supplementary Figure S6), suggesting popvae is picking up on the signal of gene flow associated with European colonization of the Americas.
Figure 2.
PCA axes 1–8 (left) and popvae run at default settings (right) for 100,000 random SNPs from chromosome 1 of the HGDP data. Axes are flipped to approximate geography.
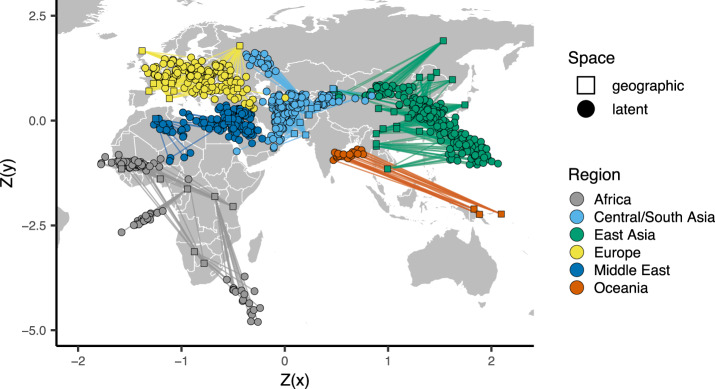
Figure 4.
Comparing the VAE latent space with the geography of sampling localities in non-American HGDP samples (see Supplementary Figure S8 for a plot including the Americas). Circles show z-normalized sample locations in latent space and squares show the corresponding location in geographic space.
These patterns are similar to those seen in PCA, but many aspects of ancestry that are difficult to see on the first two PC axes are conveniently summarized in popvae’s latent space. For example, differentiation within the Americas and Oceania is not visible until PC6 and PC7, respectively, but is clear in the 2D VAE latent space. This shows adjacent clusters for the islands of Bougainville and Papua New Guinea, and a cline in Eurasian ancestry from North through South America (Supplementary Figure S6).
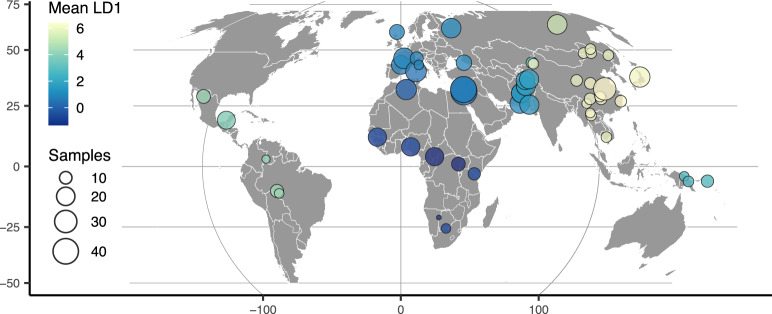
To highlight the flexibility of the VAE approach, we also trained a model with a 1D latent space and used this to scale colors on a sampling map (Figure 3). This results in a single latent dimension that approximates the diagonal of our 2D model, with African and East Asian samples on either end of the spectrum. A comparison using PCA but summarizing only the first principal component emphasizes diversity within Africa (Supplementary Figure S7) and provides little resolution for out-of-Africa groups.
Figure 3.
HGDP population locations with color scaled to the mean latent coordinate of a 1D popvae latent space.
Finally, to emphasize the correspondence of the VAE latent space with geography, we can also directly compare geographic and latent spaces by rescaling both sets of coordinates with a z-normalization and plotting them together on a map (Figure 4). As can be seen, the visual correspondence between geographic and latent coordinates is striking in this case.
Inversions and population structure in Anopheles mosquitoes
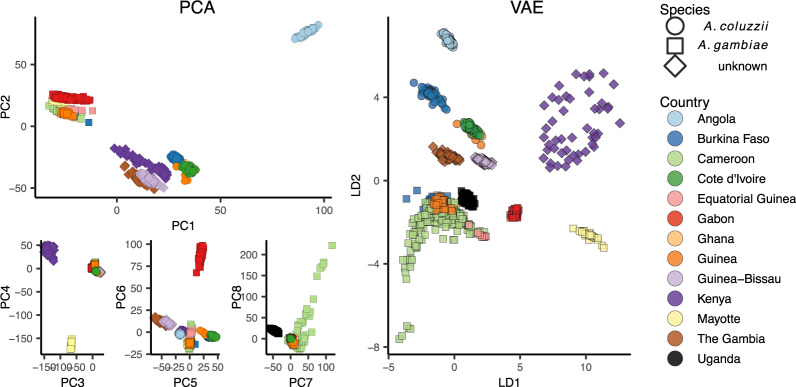
We next applied popvae to DNA sequenced from the Anopheles gambiae/coluzzii complex across sub-Saharan African by the AG1000G project (Miles et al. 2017; AG1000G Consortium 2020) (Figure 5). Using 100,000 randomly selected SNPs from chromosome 3R we again find that the VAE captures elements of population structure that are not apparent by visualizing two PC axes at a time. For example, samples from Kenya and the island of Mayotte off East Africa are highly differentiated (Fst > 0.18 relative to all other groups), but are placed between clusters of primarily west-African coluzzii and gambiae samples on a plot of PC1/2. The VAE instead places these populations on the opposite end of one latent dimension from all other groups and closest to Ugandan samples—similar to their relative geographic position and positions on PC3/4. The VAE also captures the relatively high differentiation of samples from Gabon and significant variation within Cameroon, which are not visible until PC6 and PC8, respectively. Further details of population structure in this species complex are discussed in AG1000G Consortium (2020).
Figure 5.
PCA (left) and VAE (right) run on 100,000 random SNPs from chromosome 3R of the AG1000G phase 2 data.
A. gambiae/coluzzii genomes are characterized by a series of well-studied inversions on chromosomes 2L and 2R (Coluzzi et al. 2002) which segregate within all populations and are associated with both malaria susceptibility and ecological niche variation (Riehle et al. 2017). The large 2La inversion contains at least one locus for insecticide resistance (Rdl), and has experienced multiple hard sweeps and introgression events in its recent history (Grau-Bové et al. 2020). Inversions have significant effects on local PCA (Li and Ralph 2019) which often lead to samples clustering by inversion karyotype rather than geography on the first two PC axes (Ma and Amos 2012).
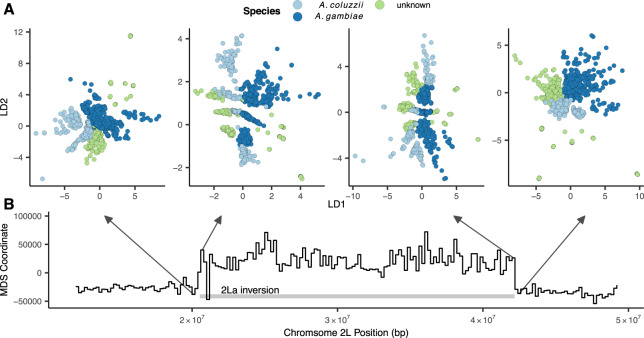
To test how our VAE responds to inversions we fit models to SNPs extracted from 200,000 bp nonoverlapping windows across the 2LA inversion in the AG1000G phase 2 data (Figure 6, Supplementary Figure S11). We took an approach similar to Li and Ralph (2019) to summarize differences in latent spaces across windows while accounting for axis rotation and scaling. Latent dimensions were first scaled to 0–1 and the pairwise Euclidean distance matrix among individuals was calculated for each window to generate rotation- and scale-invariant representations of the latent space. We then calculated Euclidean distances among all pairs of per-window distance matrices, giving us a matrix representing relative differences in latent spaces across windows. Last, we used multidimensional scaling to compress this distance matrix to a single dimension, and plotted this value against genomic position across the 2La inversion region.
Figure 6.
Latent spaces reflect inversion karyotypes at the 2La inversion in A. gambiae/coluzzii. (A) VAE latent spaces for AG1000G phase 2 samples from windows near the 2La inversion breakpoints, colored by species. (B) Multidimensional scaling values showing difference in the relative position of individuals in latent space across windows—high values reflect windows in which samples cluster by inversion karyotype, and low values by species.
This analysis found two clear classes of latent spaces inside and outside the inversion (Figure 6). Outside the inversion samples generally cluster by species and geography, while inside the inversion samples form three clusters corresponding to the homozygous and heterozygous inversion karyotypes, similar to results found with PCA (Riehle et al. 2017; Grau-Bové et al. 2020). Interestingly the VAE retains geographic and species clustering within inversion classes, but loads these aspects of structure on a different latent dimension than the karyotype clusters (e.g., LD1 reflects species clusters while LD2 reflects inversion karyotypes in the windows shown in Figure 6). Unlike PCA, latent dimensions from a VAE are not ranked by variance explained and nothing in the loss function incentivizes splitting particular aspects of variation onto separate axes, so we found this pattern of partitioning geographic and karyotypic signals somewhat surprising.
Simulations and sensitivity tests
In general a method’s ability to detect population structure in a sample of genotypes scales with the degree of differentiation and the size of the genotype matrix. Patterson et al. (2006) found that there is a “phase change” phenomenon by which methods like PCA transition from showing no evidence of structure to strong evidence of structure when , where n is the number of genotyped SNPs and m is the number of sampled individuals.
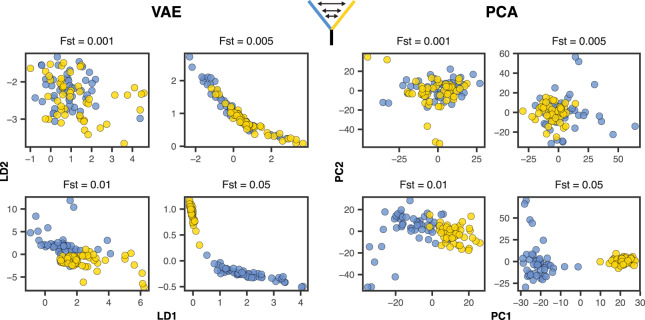
To compare the performance of PCA and VAE around this threshold, we ran a series of two-population, isolation with migration model coalescent simulations in msprime (Kelleher et al. 2016) while varying the symmetric migration rate to produce an expected equilibrium Fst ranging from 0.0001 to 0.05. We sampled 50 diploid genomes from each population and downsampled the resulting genotype matrix to 10,000 SNPs. Given this sample size we expect the threshold for detecting structure to be approximately Fst = 0.001.
With tuned hyperparameters the VAE appeared slightly more sensitive to weak structure than the first two axes of a PCA (Figure 7). Both popvae and PCA reflect some population structure at (though this is clearer in the VAE) but none at , consistent with Patterson et al. (2006)’s “phase change” suggestion. However the VAE’s performance was highly sensitive to hyperparameter tuning on this dataset. At default settings popvae latent spaces reflect no clear structure until Fst = 0.05 (Supplementary Figures S12 and S13). In particular we found that increasing the “patience” parameter to 500 was necessary for even marginal performance in this case, and running a grid search across network sizes was needed to match PCA’s sensitivity to weak structure.
Figure 7.
VAE latent spaces and PCA run on two-population coalescent simulations with Fst varying from 0.0001 to 0.05. Points are colored by population. popvae was run with tuned hyperparameters and patience set to 500. See Supplementary Figure S12 for (much worse) performance with default settings.
Comparison with UMAP and t-SNE
In addition to PCA we also compared the VAE’s latent spaces to t-SNE (van der Maaten and Hinton 2008) and UMAP (Diaz-Papkovich et al. 2019) (Supplementary Figures S14 and S15), both of which have been used recently for population genetic visualization. We first ran both methods on the top 15 PC axes [following Diaz-Papkovich et al. (2019)] with default settings on the human and Anopheles datasets and used the R packages “umap” (Konopka 2019) and “tsne” (Donaldson 2016) as our reference implementations.
For HGDP data both UMAP and t-SNE produce latent spaces that roughly correspond to continental regions (Supplementary Figure S14). Running both methods at default settings, UMAP’s latent space was much more tightly clustered—for example grouping all samples from Africa into a single small region. Similar patterns were seen in the AG1000G data (Supplementary Figure S15)—both t-SNE and UMAP produce latent spaces that strongly cluster sample localities and species. However, global geometry appeared to be poorly preserved in t-SNE and UMAP latent spaces. That is, though clusters in latent space correspond to sampling localities, distances among clusters do not appear to meaningfully reflect geography or genetic differentiation.
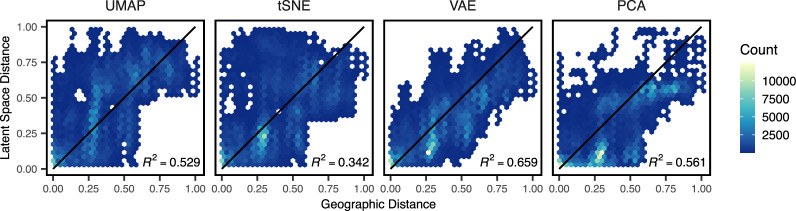
To compare how well different methods reflect geography, we compared pairwise distances among individuals in latent and geographic space for Eurasian human samples (HGDP regions Europe, Central/South Asia, the Middle East, and East Asia). Geographic distances were great-circle distance calculated on a WGS84 ellipse with the R package “sp” (Pebesma and Bivand 2012). Distances were scaled to 0–1 for this analysis, and we calculated the coefficient of determination (R2) across geographic and latent-space distance for each method as a metric. VAE latent space distances have the strongest correlation with geographic distance (Figure 8; ), followed by PCA (), UMAP (), and t-SNE ().
Figure 8.
Comparing pairwise distances in geographic and latent space for Eurasian human genotypes across four dimensionality reduction methods run at default settings. All distances are scaled to 0–1. Black lines show a 1:1 relationship.
Finally to test how parameter tuning of tSNE and UMAP impacts our results, we reproduced our analysis of HGDP data using double and triple the default values for n_neighbors (UMAP) and perplexity (tSNE). Though scatter plots are visually similar at these settings (Supplementary Figure S16) the correlation between latent-space and geographic distances of Eurasian samples is improved in both methods at double default settings (t-SNE: , UMAP: ; Supplementary Figure S17). At triple default settings we observed slightly better performance for tSNE and slightly worse for UMAP (Supplementary Figures S18 and S19).
Run times and computational resources
We compared popvae’s run times to PCA, UMAP, and t-SNE using sets of 100,000 and 10,000 SNPs from the HGDP as described above. popvae was run using default settings (i.e., fitting a single network rather than running a grid search over network sizes) using a consumer GPU (Nvidia GeForce RTX 2070). PCAs were run in the python package scikit-allel (Miles and Harding 2017), which in turn relies on singular-value decomposition functions from the numpy library (Oliphant 2006).
popvae was much slower than PCA or UMAP, but comparable to running t-SNE on PC coordinates. However, for datasets of the size we tested here none of these run times present significant challenges—all methods return sample latent coordinates in less than five minutes. We have not conducted exhaustive tests on CPU training times for popvae, but in general find these to require at least twice as much time as GPU runs.
However for larger datasets we expect popvae’s run time performance would suffer further in comparison to PCA and UMAP. The major computational bottleneck is loading tensors holding weights for the input and output layers of the encoder and decoder networks into GPU memory. These tensors have dimensions n_snps x network_width so they become extremely large when running on large genotype matrices. Our development machine has 8GB GPU RAM and can process up to roughly 700,000 SNPs in a single analysis using a 128-unit-wide network. Throughout this study we have limited our analysis to relatively small subsets of genome-wide SNPs to allow us to explore a range of network sizes in reasonable time. Scaling up to a single model fit to all genome-wide SNPs—on the order of 107 for datasets like the HGDP—would require access to specialized hardware with very large GPU memory pools.
Generating genotypes
The VAE framework also allows us to generate genotypes characteristic of a given population by sampling from the latent space of a trained model. Simulated genotypes generated by process-based models like the coalescent are a key tool in population genetics, because they allow us to explore the impact of various generative processes—demography, selection, etc.—on observed genetic variation (Adrion et al. 2020a). In contrast popvae’s generative model provides essentially no mechanistic insight beyond the strong observed correlation of latent and geographic spaces. However, if the VAE accurately reproduces characteristics of real genotypes it could be a fast alternative to simulation that does not require parameterizing a custom demographic model.
We compared these approaches by analyzing empirical data from European (CEU), Han (CHB), and Yaruban (YRI) human genotypes in the 1000 Genomes Project data (Consortium et al. 2015). We first subset 50 samples from each population and then fit a 2D popvae model to all SNPs from chromosome 22. To generate genotypes, we drew a sample from the latent distribution of each individual and passed these coordinates to the trained decoder network. We interpret the sigmoid activation output of our decoder as the probability of observing a derived allele at each site, and generate derived allele counts by taking two draws from a binomial distribution with where is the decoder output for individual i at site j.
As a baseline comparison we used coalescent simulations from the standardpopsim library (Adrion et al. 2020a) of the three-population out-of-Africa model (OutOfAfrica_3G09)—a rigorously tested implementation of the demographic model fit to the joint site frequency spectrum in Gutenkunst et al. (2009) using the msprime coalescent simulator (Kelleher et al. 2016). For this comparison, we changed standardpopsim’s default human mutation rate of – to match the rate used in Gutenkunst et al. (2009), used the HapMapII_GRCh37 recombination map for chromosome 22, and sampled 100 haploid chromosomes from each population.
Last, we examined three facets of population genetic variation on real, VAE-generated, and simulated genotype matrices: the site frequency spectrum, the decay of linkage disequilibrium with distance along the chromosome, and embeddings from a PCA. These analyses were conducted in scikit-allel (Miles and Harding 2017) after masking genotypes to retain only sites with the most stringent site accessibility filter (“P”) in the 1000 genome project’s phase 3 site accessibility masks. LD statistics were calculated only for YRI samples using SNPs between positions and in the hg18 reference genome and summarized by calculating the mean LD for all pairs of alleles in 25 distance bins (similar results in three different genomic windows are shown in Supplementary Figure S20). Results are plotted in Figure 9.
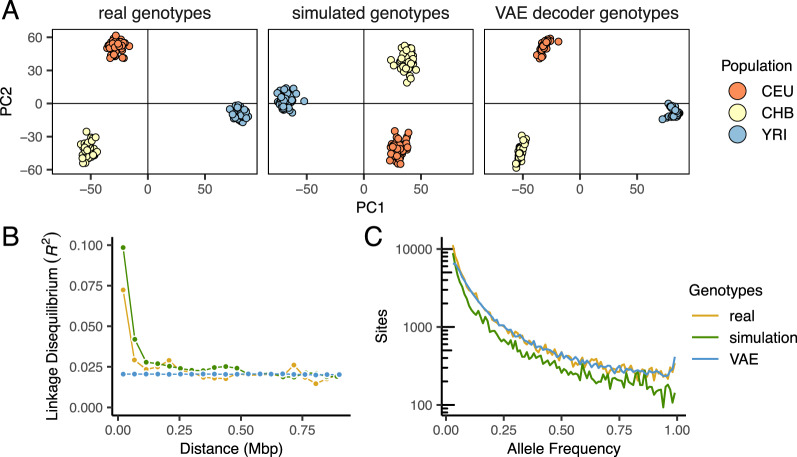
Figure 9.
Comparing real, VAE-generated, and simulated genotype matrices for three populations from the 1000 genomes project. The VAE decoder and coalescent simulation produce similar results in genotype PCA (A), but the VAE fails to reproduce the decay of LD with distance along the chromosome seen in real data (B). The site frequency spectrum is very similar for real and VAE-generated genotypes, but suffers from scaling issues in the coalescent simulation (C).
In general we found all methods produce similar results in a plot of the first two PC axes, suggesting they capture broad patterns of allele frequency variation created by population structure. The site frequency spectrum is also very similar for the VAE and real data, while the simulated genotypes suffer from a scaling issue. This could reflect differences in the input data—Gutenkunst et al. (2009) fit models to an SFS calculated from a set of sanger-sequenced loci in 1000 genomes samples, rather than the short-read resequenced SNPs from the 1000 Genomes project we use—or an inaccuracy in one of the constants used to convert scaled demographic model parameters to real values (accessible genome size, generation time, or mutation rate). LD decay shows the largest difference among methods. Simulation and real data both reflect higher LD among nearby SNPs which decays with distance, while the VAE genotypes produced no correlation between distance along a chromosome and pairwise LD.
These differences reflect the strengths and weaknesses of each method. The VAE decoder does not require a pre-defined demographic model and by design exactly fits the matrix size of input empirical data, so it should not suffer from the scaling issues that frequently impact population genetic models. But the lack of mechanistic biological knowledge in its design means it misses obvious and important features of real sequence data like the decline of LD with distance. In this case, the lack of LD decay in VAE decoder sequences means this implementation should not be used for testing properties of analyses like GWAS, in which LD among a subset of sequenced loci and an unknown number of truly causal loci is a crucial parameter. Though other network designs [e.g., a convolutional neural network (Flagel et al. 2019) or a recurrent neural network (Adrion et al. 2020b)] could potentially address the specific shortcoming of LD decay, the general problem of a nonmechanistic generator failing to mimic features of the data produced by well-understood processes seems intrinsic to the machine learning approach.
Discussion
Dimensionality reduction of genotypic variation is a key analytic tool in modern genomics and their visualizations are often the central figure of a genetic study. For example, Antonio et al. (2019) studied a 10,000-year transect of genotypes from Rome and extensively used PCA to visualize changes in ancestry in the city over time. In cases like this producing informative plots of population structure is a requisite step for the analysis and can shape the way data is interpreted both by authors and readers.
In this study we demonstrate how VAEs can be used for visualization and low dimensional summaries of genotype data. VAEs have at least two attractive properties for genetic data: they allow users to define the output dimensionality, and they preserve global geometry (i.e., relative positions in latent space) better than competing methods. As we have shown in humans and mosquitoes, this allows users to generate visualizations that summarize relationships among samples without either comparing across several panels (as with PCA) or attempting to ignore possibly spurious patterns of global structure (as with t-SNE and UMAP).
VAEs are also generative models. That is to say that VAEs allow us to create genotypes that capture aspects of population genetic variation characteristic of the training set. Though in theory this could be used as an alternative to simulation, our implementation fails to replicate at least one important aspect of real genomes—the decay of linkage disequilibrium with distance along a chromosome—and thus offers limited utility for tasks such as boosting GWAS sample sizes or as a substitute for simulation. We point researchers interested in generating genotypes via deep learning approaches to recent work by Yelmen et al. (2019) and Montserrat et al. (2019), which describe similar, deep learning based methods more tightly aimed at generating realistic genotypes.
There are also several significant limitations of our method as a visualization tool. First, we lack a principled understanding of how the VAE output maps to parameters of idealized population models like the coalescent (Kingman 1982). This is in contrast to PCA, which was first applied to population genetic data without strong connections to theory (Menozzi et al. 1978) but is now fairly well characterized in reference to population genetic models (Novembre and Stephens 2008; McVean 2009).
Hyperparameter tuning is another challenge. As we showed, popvae has many hyperparameters that significantly affect the output latent space and no principled way to set them a priori. Though we include a grid-search function for network sizes, this is slow and is still dependent on other hyperparameters—like the patience used for early stopping, or the learning rate of the optimizer—which we have set to defaults that may not be optimal for all datasets. This is not a unique issue to VAEs; hyperperameters of methods like t-SNE and UMAP can significantly affect embeddings (Kobak and Linderman 2019), and preprocessing choices such as how to scale allele counts prior to PCA dramatically vary the appearance of final plots (Patterson et al. 2006). However it does require extra work on the part of users interested in exploring the full parameter space.
A parallel issue is stochasticity in the output. Stochasticity is introduced by the random test/train split, parameter initialization states, and even the execution order of operations run in parallel on GPU during model training. Though all but the last of these can be fixed by setting a random seed, which itself could be (unfortunately) seen as a hyperparameter, there is no obvious way to compare models fit to different validation sets in a world of limited training examples. This introduces noise which could potentially allow users to cherry-pick a preferred latent space.
For example, one run of our best-performing network architecture on the HGDP data produced a latent space in which samples Papua New Guinea and Bougainville are separated by roughly the same distance as samples from north Africa and East Asia. In contrast all other fits of the same network architecture cluster these samples (Supplementary Figure S2, see the top middle panel). We chose a latent space for the main text that lacked this feature because it occurred in only one training run, but acknowledge this procedure is suboptimal. Developing a method to summarize across multiple latent spaces, perhaps via ensemble learning approaches, would be useful for postprocessing VAE output when latent spaces vary.
The last major shortcoming is computational effort. Popvae is much slower and more computationally intensive than PCA, and requires specialized and expensive GPU or TPU hardware to run on large sets of SNPs (Table 1).
Table 1.
Run times for VAE, PCA, UMAP, and t-SNE HGDP data
| Run time (s) | SNPs | Method |
|---|---|---|
| 204.4 | 100,000 | VAE |
| 3.6 | PCA | |
| 6 | UMAP | |
| 124.8 | t-SNE | |
| 78.8 | 10,000 | VAE |
| 0.5 | PCA | |
| 2.7 | UMAP | |
| 119.5 | t-SNE |
UMAP and t-SNE were run on the top 20 PC axes (run times thus include running the PCA).
One important question we did not explore in this study is whether VAE latent space coordinates offer any improvement over PCA when used as covariates to correct for population structure in GWAS (Price et al. 2006). UMAP and t-SNE are generally thought to be inappropriate for this use because of their failure to preserve global geometry (Diaz-Papkovich et al. 2019), but because the VAE appears to strongly reflect geography in humans it may be useful for this task. Testing this aspect of the VAE could be done in simulation but would benefit from empirical investigations in large human datasets—a task which is beyond the scope of the present study, but perhaps fruitful for further investigation.
Here we have shown that our implementation of a VAE, popvae, can produce informative visualizations of population genetic variation and offers some benefits relative to competing methods. However our approach is just one implementation of a huge class of potential models falling under the VAE umbrella. Altering the prior on the latent space (Davidson et al. 2018), the weighting of the loss function (Higgins et al. 2017), or the type of neural network used in either the encoder or decoder all offer avenues for further research and potential improvement (see also Appendix 1, where we briefly describe alternate approaches we experimented with). Entirely different methods of visualizing population structure which focus on genetic variants rather than individuals, like that proposed in Biddanda et al. (2020), also offer a complementary perspective on the nature of genetic differentiation. As population genetic data becomes increasingly common across evolutionary biology, we anticipate visualization techniques will receive increased attention from researchers in many areas, and believe VAEs offer a promising avenue for research.
Acknowledgments
We thank Peter Ralph for the suggestion to use binomial sampling to bin the decoder output, and other members of the Kern-Ralph co-lab for comments on the software and manuscript. Thanks to the reviewers and editors for your comments. Audry Gill developed a successful pilot of this project early after its inception.
Funding
C.J.B. was supported by NIH awards R01GM117241 and F32GM136123. A.D.K. was supported under NIH awards R01GM117241 and R01HG010774.
Conflicts of interest: None declared.
Appendix 1: Other things we tried that did not work
We tried a bunch of things while developing popvae. Here we document some of our dead-ends in the hope they may be useful to others developing similar methods.
A convolutional neural network
We first developed popvae using convolutional neural networks (CNNs) for both the encoder and decoder. The feed-forward network we use here was originally intended as a naive baseline for comparing our CNN performance, but it turned out to be faster and more accurate (i.e., lower validation loss), and had much lower memory requirements than any CNN we tried. These included 2D CNNs run on phased haplotypes, 1D CNNs run on unphased genotype counts, hybrid CNN+feed-forward networks stacking convolutional and dense layers in succession, and restricting the CNN to either the encoder or the decoder.
A recurrent neural network
We also tested recurrent neural networks [using the cudnnGRU() layer in keras] as one or both of the encoder/decoder pair. Due to memory limitations we were only able to test relatively small, shallow networks with this approach (width 32, depth up to 3). Like the CNNs these were slower, less accurate, and more resource-intensive than the dense network we describe in the main text.
Skipping the validation set
It would be nice to not need a validation set. The train/test split introduces extra stochasticity and you have to ignore some hard-earned data in training.
Unfortunately we could not find a good way of setting the learning rate scheduler or establishing a good stopping time for model training without a validation set. Training on all samples leads to constantly decreasing loss so all training runs go to the maximum number of epochs. Examining the progress of latent spaces through model training for these runs, the encoder seems to quickly identify and then refine structure in the input samples, but eventually samples begin to cluster in a ring around the origin at 0,0. This appears to reflect the Gaussian prior on the latent space dominating the loss function as the reconstruction error approaches some lower bound. In runs with validation sets we observed that validation loss typically increases once points begin circling the origin (Supplementary Figure S1), suggesting it reflects a typical overfitting behavior. Unfortunately the number of epochs needed for this to occur is different for every dataset and we found no general solution for estimating its location other than a validation set.
So we recommend using a validation set of at least 10%, and comparing latent spaces from runs with multiple starting seeds (and so different train/validation splits). In a pinch, users can set –train_prop 1 to train on all samples and heuristically examine latent spaces output during training to figure out a good stopping point.
Batch normalization
Putting a batch normalization layer anywhere in either the encoder or decoder made validation loss worse in all of our tests.
Dropout
As above, dropout layers either made no difference or yielded slightly higher validation losses no matter where we put it.
Reweighting the loss function
Higgins et al. (2017) proposed a modification of the standard VAE loss function which amounts to multiplying the KL divergence by a factor β. This puts extra weight on the normal prior of the latent space and on the MNIST dataset delivered more clustered and interpretable latent spaces. Unfortunately the only suggested method for estimating β in a truly unsupervised setting like ours is heuristic examination of model output. We experimented with several values and found no consistent benefits either in latent space or validation loss relative to our baseline approach. However this seems like a productive area for further investigation and we plan to continue experimenting along these lines (and encourage others to do so as well).
Literature cited
- 1000 Genomes Project Consortium, et al. 2015. A global reference for human genetic variation. Nature 526:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abadi M, Agarwal A, Barham P, Brevdo E, Chen Z, et al. 2015. TensorFlow: large-scale machine learning on heterogeneous systems. Software available from tensorflow.org. (Accessed: 2020 October)
- Adrion JR, Cole CB, Dukler N, Galloway JG, Gladstein AL, et al. 2020a. A community-maintained standard library of population genetic models. eLife. 9:e54967. 10.7554/eLife.54967. [DOI] [PMC free article] [PubMed]
- Adrion JR, Galloway JG, Kern AD.. 2020b. Predicting the landscape of recombination using deep learning. Mole Biol Evol. 37:1790–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AG1000G Consortium. 2020. Genome variation and population structure among 1142 mosquitoes of the African malaria vector species anopheles gambiae and anopheles coluzzii. Genome Res. 30: 1533-1546. doi: 10.1101/gr.262790.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonio ML, Gao Z, Moots HM, Lucci M, Candilio F, et al. 2019. Ancient rome: a genetic crossroads of europe and the mediterranean. Science. 366:708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becht E, McInnes L, Healy J, Dutertre C-A, Kwok IW, et al. 2019. Dimensionality reduction for visualizing single-cell data using umap. Nat Biotechnol. 37:38–44. [DOI] [PubMed] [Google Scholar]
- Berg JJ, Harpak A, Sinnott-Armstrong N, Joergensen AM, Mostafavi H, et al. 2019. Reduced signal for polygenic adaptation of height in uk biobank. ELife. 8:e39725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström A, McCarthy SA, Hui R, Almarri MA, Ayub Q, et al. 2020. Insights into human genetic variation and population history from 929 diverse genomes. Science. 367:eaay5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddanda DA,, Rice P, Novembre J.. 2020. Geographic patterns of human allele frequency variation: a variant-centric perspective. eLife. 9:e60107. https://elifesciences.org/articles/60107 [DOI] [PMC free article] [PubMed]
- Chollet F, et al. 2015. Keras. https://github.com/fchollet/keras. (Accessed: 2020 October)
- Clevert D-A, Unterthiner T, Hochreiter S.. 2015. Fast and accurate deep network learning by exponential linear units (ELUs). arXiv Preprint arXiv. 1511.07289.
- Coluzzi M, Sabatini A,, TorreMaria D, Di Deco A, Petrarca V.. 2002. A polytene chromosome analysis of the anopheles gambiae species complex. Science. 298:1415–1418. [DOI] [PubMed] [Google Scholar]
- Davidson TR, Falorsi L, Cao ND, Kipf T, Tomczak JM.. 2018. Hyperspherical variational auto-encoders. arXiv Preprint arXiv. 1804.00891.
- Derkarabetian S, Castillo S, Koo PK, Ovchinnikov S, Hedin M.. 2019. A demonstration of unsupervised machine learning in species delimitation. Mol Phylogenet Evol. 139:106562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Papkovich A, Anderson-Trocmé L, Ben-Eghan C, Gravel S.. 2019. Umap reveals cryptic population structure and phenotype heterogeneity in large genomic cohorts. PLoS Genet. 15:e1008432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson J. 2016. tsne: T-distributed stochastic neighbor embedding for R (t-SNE), R package version 0.1-3, 2016. https://CRAN.R-project.org/package=tsne. (Accessed: 2020 October).
- Dray S, Josse J.. 2015. Principal component analysis with missing values: a comparative survey of methods. Plant Ecol. 216:657–667. [Google Scholar]
- Flagel L, Brandvain Y, Schrider DR.. 2019. The unreasonable effectiveness of convolutional neural networks in population genetic inference. Mol Biol Evol. 36:220–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegontov P, Alt In Iş Ik NE, Changmai P, Rohland N, Mallick S, et al. 2019. Palaeo-Eskimo genetic ancestry and the peopling of Chukotka and North America. Nature. 570:236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman ML, Reich D, Penney KL, McDonald GJ, Mignault AA, et al. 2004. Assessing the impact of population stratification on genetic association studies. Nat Genet. 36:388–393. [DOI] [PubMed] [Google Scholar]
- Grau-Bové X, Tomlinson S, O’Reilly AO, Harding NJ, Miles A, et al. 2020. Evolution of the insecticide target rdl in African anopheles is driven by interspecific and interkaryotypic introgression. Mole Biol and Evol. 37:2900–2917. 10.1093/molbev/msaa128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grønbech CH, Vording MF, Timshel PN, Sønderby CK.. Tune Hannes Pers, and Ole Winther. 2018. scvae: Variational . auto-encoders for single-cell gene expression data. Bioinformatics. 36:4415–4422. 10.1093/bioinformatics/btaa293 [DOI] [PubMed] [Google Scholar]
- Gulrajani I, Kumar K, Ahmed F, Taiga AA, Visin F, et al. 2016. Pixelvae: a latent variable model for natural images. arXiv Preprint arXiv. 1611.05013.
- Gutenkunst RN, Hernandez RD, Williamson SH, Bustamante CD.. 2009. Inferring the joint demographic history of multiple populations from multidimensional SNP frequency data. PLoS Genet. 5:e1000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins I, Matthey L, Pal A, Burgess C, Glorot X, et al. 2017. beta-VAE: learning basic visual concepts with a constrained variational framework. ICLR. 2:6. [Google Scholar]
- Holsinger KE, Weir BS.. 2009. Genetics in geographically structured populations: defining, estimating and interpreting FST. Nat Rev Genet. 10:639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X, Shen L, Sun K, Qiu G.. 2016. Deep feature consistent variational autoencoder. arXiv Preprint arXiv. 1610.00291v1
- Hu Q, Greene CS.. 2019. Parameter tuning is a key part of dimensionality reduction via deep variational autoencoders for single cell RNA transcriptomics. In: PSB. World Scientific. p. 362–373. [PMC free article] [PubMed] [Google Scholar]
- Jombart T. 2008. adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics 24:1403–1405. [DOI] [PubMed] [Google Scholar]
- Kelleher J, Etheridge AM, McVean G.. 2016. Efficient coalescent simulation and genealogical analysis for large sample sizes. PLoS Comput Biol. 12:e1004842-22.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingma DP, Ba J.. 2014. Adam: a method for stochastic optimization. arXiv Preprint arXiv. 1412.6980.
- Kingma DP, Welling M.. 2013. Auto-encoding variational bayes. arXiv Preprint arXiv. 1312.6114.
- Kingman FJC. 1982. The coalescent. Stochast Process Appl. 13:235–248. [Google Scholar]
- Kobak D, Linderman GC.. 2019. Umap does not preserve global structure any better than t-SNE when using the same initialization. bioRxiv.
- Konopka T. 2019. umap: Uniform Manifold Approximation and Projection, R package version 0.2.3.1. https://CRAN.R-project.org/package=umap. (Accessed: 2020 October).
- Lafarge MW, Caicedo JC, Carpenter AE, Pluim JP, Singh S, et al. 2019. Capturing single-cell phenotypic variation via unsupervised representation learning. Proceedings of The 2nd International Conference on Medical Imaging with Deep Learning, in PMLR. 102:315–325 [PMC free article] [PubMed]
- Lander ES, Schork NJ.. 1994. Genetic dissection of complex traits. Science. 265:2037–2048. [DOI] [PubMed] [Google Scholar]
- Larsen ABL, Sønderby SK, Larochelle H, Winther O.. 2015. Autoencoding beyond pixels using a learned similarity metric. arXiv Preprint arXiv. 1512.09300v2.
- Li H, Ralph P.. 2019. Local PCA shows how the effect of population structure differs along the genome. Genetics. 211:289–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Amos CI.. 2012. Investigation of inversion polymorphisms in the human genome using principal components analysis. PLoS One 7:e40224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchini J, Cardon LR, Phillips MS, Donnelly P.. 2004. The effects of human population structure on large genetic association studies. Nat Genet. 36:512–517. [DOI] [PubMed] [Google Scholar]
- Mathieson I, McVean G.. 2012. Differential confounding of rare and common variants in spatially structured populations. Nat Genet. 44:243–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes L, Healy J, Melville J.. 2018. Umap: uniform manifold approximation and projection for dimension reduction. arXiv Preprint arXiv. 1802.03426.
- McKinney W. 2010. Data structures for statistical computing in python. In: van der Walt, S, Millman, J editors. Austin, Texas: Proceedings of the 9th Python in Science Conference. p. 51–56.
- McVean G. 2009. A genealogical interpretation of principal components analysis. PLoS Genet. 5:e1000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menozzi P, Piazza A, Cavalli-Sforza L.. 1978. Synthetic maps of human gene frequencies in europeans. Science. 201:786–792. [DOI] [PubMed] [Google Scholar]
- Miles A, Harding N.. 2017. cggh/scikit-allel: v1.1.8, July 2017. 10.5281/zenodo.822784. (Accessed: 2020 October). [DOI]
- Miles A, Harding NJ, the AG1000G Consortium. 2017. Genetic diversity of the African malaria vector Anopheles gambiae. Nature. 552:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montserrat DM, Bustamante C, Ioannidis A.. 2019. Class-conditional vae-gan for local-ancestry simulation. arXiv Preprint arXiv. 1911.13220
- Novembre J, Stephens M.. 2008. Interpreting principal component analyses of spatial population genetic variation. Nat Genet. 40:646–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliphant T. 2006. NumPy: A Guide to NumPy. USA: Trelgol Publishing. (Accessed 2019 December). http://www.numpy.org/. [Google Scholar]
- Patterson N, Price AL, Reich D.. 2006. Population structure and eigenanalysis. PLoS Genet. 2:e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pebesma E, Bivand R.. 2012. The comprehensive R archive network. https://cran.r-project.org/.
- Petkova D, Novembre J, Stephens M.. 2016. Visualizing spatial population structure with estimated effective migration surfaces. Nat Genet. 48:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posth C, Nakatsuka N, Lazaridis I, Skoglund P, Mallick S, et al. 2018. Reconstructing the deep population history of Central and South America. Cell. 175:1185–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, et al. 2006. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 38:904–909. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Donnelly P.. 2001. Case–control studies of association in structured or admixed populations. Theor Popul Biol. 60:227–237. [DOI] [PubMed] [Google Scholar]
- Riehle MM, Bukhari T, Gneme A, Guelbeogo WM, Coulibaly B, et al. 2017. The anopheles gambiae 2la chromosome inversion is associated with susceptibility to Plasmodium falciparum in Africa. Elife. 6:e25813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg NA, Pritchard JK, Weber JL, Cann HM, Kidd KK, et al. 2002. Genetic structure of human populations. Science. 298:2381–2385. [DOI] [PubMed] [Google Scholar]
- Sohail M, Maier RM, Ganna A, Bloemendal A, Martin AR, et al. 2019. Polygenic adaptation on height is overestimated due to uncorrected stratification in genome-wide association studies. Elife. 8:e39702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Maaten L, Hinton G.. 2008. Visualizing data using t-SNE. J Mach Learn Res. 9:2579–2605. [Google Scholar]
- Wang D, Gu J.. 2018. Vasc: dimension reduction and visualization of single-cell RNA-seq data by deep variational autoencoder. Genom Proteom Bioinform. 16:320–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelmen B, Decelle A, Ongaro L, Marnetto D, Tallec C, et al. 2019. Creating artificial human genomes using generative models. bioRxiv. doi: 10.1101/769091. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
popvae is available at https://github.com/kr-colab/popvae, and scripts for reproducing plots and analyses in this manuscript are available at https://github.com/cjbattey/popvae_analysis_scripts. HGDP genotypes used in this paper are available at ftp://ngs.sanger.ac.uk/production/hgdp, AG1000G genotypes at https://www.malariagen.net/data/ag1000g-phase-2-ar1, and 1000 genomes phase 3 data at https://www.internationalgenome.org/category/phase-3/.
Supplementary material is available at figshare: https://doi.org/10.25387/g3.13311539. .