Abstract
Introduction
We aimed to investigate phenotypic heterogeneity in the behavioral variant of frontotemporal dementia (bvFTD) through assessment of inhibition deficits.
Methods
We assessed occurrences of 16 behavioral inhibition deficits from video recordings of 15 bvFTD patients (early stage) and 15 healthy controls (HC) in an ecological setting. We extracted dimensions of inhibition deficit and analyzed their correlations with cognitive and clinical measures. Using these dimensions, we isolated patient clusters whose atrophy patterns were explored.
Results
After identifying two patterns of inhibition deficit (compulsive automatic behaviors and socially unconventional behaviors), we isolated three behavioral clusters with distinct atrophy patterns. BvFTD‐G0 (N = 3), an outlier group, showed severe behavioral disturbances and more severe ventromedial prefrontal cortex/orbitofrontal cortex atrophy. Compared to bvFTD‐G1 (N = 6), bvFTD‐G2 (N = 6) presented higher anxiety and depression along with less diffuse atrophy especially in midline regions.
Discussion
Identifying clinico‐anatomical profiles through behavior observation could help to stratify bvFTD patients for adapted treatments.
Keywords: compulsivity, disinhibition, ecological design, frontotemporal dementia, gray matter atrophy, subtypes
1. INTRODUCTION
Behavioral variant frontotemporal dementia (bvFTD) is a neurodegenerative disease resulting from frontotemporal lobar degeneration. 1 Because of high phenotype diversity across patients and disease stages, it is hard to understand the underlying mechanisms. 2 A precise characterization of the different clinical profiles within the bvFTD spectrum would thus help to develop a better knowledge of the pathology and its progression, and to better adapt treatments according to patients’ specific profiles.
Social disinhibition, impulsivity, and compulsivity are part of bvFTD diagnostic criteria. 3 Interestingly, because they all are “positive” (hyperactivity) symptoms they are rapidly salient to observers. From a behavioral point of view, social disinhibition refers to the lack of control of emotional, social, and more generally “overt” behaviors in a social context; 4 , 5 impulsivity involves unplanned, premature actions with diminished regard for their potential negative consequences; 3 , 6 while compulsivity relates to persistent automatic actions that have no obvious relation with an overall goal and often result from an inability to flexibly adapt behavior to the situation. 6 , 7 , 8
These three nested concepts, which share underlying mechanisms of dysfunctional inhibition of thoughts and behavior, can be hard to disentangle. Besides, the tests currently used to assess these symptoms are based on patients’ or caregivers’ reports. Consequently, they tend to be biased by the reporter's subjectivity and lack ecological validity. 9 The precise characterization of empirically observed behaviors associated with each of these symptoms is thus a first step to guarantee the accuracy of their assessment.
For this purpose, we observed and assessed our bvFTD patients’ inhibition deficits (social disinhibition, impulsivity, and compulsivity) in an ecological setting. We were then able to stratify these patients according to this behavioral assessment. Our behavioral assessment was a novel approach based on the recording and quantification, in a close‐to‐real‐life situation, of a priori defined behaviors 3 , 5 , 10 assumed to be related to a lack of inhibition in bvFTD patients and healthy controls (HC). Our first objective was to explore the dimensional structure of these selected behaviors to clarify the conceptual organization of inhibition deficit symptoms. Our second objective was to identify subgroups of bvFTD patients based on their behavioral assessment of inhibition deficit and to refine their respective profiles by exploring their neurocognitive, clinical, and neuroanatomical characteristics. To our knowledge, this is the first time that bvFTD patients have been classified according to their disinhibited behaviors evaluated through such an ecological and observational approach.
2. METHODS
2.1. Participants
A total of 15 bvFTD patients were recruited in two tertiary referral centers, at the “Institut de la Mémoire et de la Maladie d'Alzheimer” (IM2A) at the Pitié‐Salpêtrière Hospital, Paris, and at the Lariboisière Fernand‐Widal Hospital, Paris. They were diagnosed according to the International Consensus Diagnostic Criteria. 3 To respect inclusion criteria, bvFTD patients had to present a Mini‐Mental State Examination (MMSE) score above 20 and no other neurological or psychiatric disease: this allowed us to focus on the precise characterization of bvFTD at a rather early stage. Fifteen HC were recruited by public announcement. HC subjects, who were tested through the same protocol as bvFTD patients, were matched to patients for age, sex, and education level. The demographic characteristics of bvFTD patients and HC are described in File S1 in supporting information.
HIGHLIGHTS
We used an ecological approach to assess behavioral inhibition deficits.
We extracted two dimensions from measured behaviors.
Extracted dimensions were related to cognitive and clinical measures.
Using scores on these dimensions, we identified distinct profiles of behavioral variant frontotemporal dementia (bvFTD).
These behavioral profiles will contribute to early bvFTD diagnosis and targeted treatment.
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using scientific sources (e.g., PubMed). Our scientific question was inspired by publications related to behavioral variant frontotemporal dementia (bvFTD) pathophysiology, with a specific interest in the symptoms of inhibition deficit. These relevant citations are appropriately cited.
Interpretation: Using the behavioral assessment of inhibition deficit we identified bvFTD patient subgroups with specific clinical and anatomical characteristics, in agreement with a previous publication that isolated bvFTD subtypes using clustering based on gray matter atrophy.
Future directions: The article proposes a framework for the stratification of patients according to salient behavioral changes assessed at early stages through an ecological approach. This could facilitate precise diagnosis, prognosis orientation, and targeted management strategies. Additional studies should investigate this stratification strategy based on behavior observation in larger samples.
2.2. Behavioral assessment
This study is part of the ECOCAPTURE protocol (clinicaltrials.gov: NCT03272230) designed to obtain objective measures of behavioral syndromes such as apathy or disinhibition in participants undergoing a predetermined 45‐minute script. This script reproduces a close‐to‐real‐life situation in a functional exploration platform (PRISME, ICM core facility, Salpêtrière Hospital, Paris, France) transformed into a fully furnished waiting room (see File S2 in supporting information for a view of the room) and equipped with video and a sensor‐based data acquisition system that tracks the participant's behavior. Participants were asked to wait in a staff lounge before doing further tests. They had been informed previously (at the time of initial consent) that their behavior would be tracked and recorded by video cameras located in the room.
As described in Batrancourt et al., 9 participants followed a multiple‐phase scenario, starting with a first freely moving phase (7 minutes) when participants entered and discovered the room. This was followed by several different phases: (1) a second free phase (7 minutes) during which participants wore eye‐tracking glasses; (2) a phase of positive (e.g., display of a music liked by the participant with low volume from a speaker in the room) or negative (e.g., display of a crackle noise with increasing volume) environmental stimulation (7 minutes); (3) an externally guided phase consisting of filling out a simple questionnaire (e.g., question about items present or not in the room) using pens of different colors which had to be found in the room (10 minutes); (4) a second environmental stimulation, negative if the first one was positive and vice versa (7 minutes). Between these phases and at the end of the scenario, the examiner entered the room and interacted with the participant to guide them through the different steps of the scenario (see File S3 in supporting information for the description of the ECOCAPTURE scenario and of the examiner's interventions in particular). Participants were explicitly and repeatedly invited to make themselves comfortable in the room (“as if they were at home”) to promote the ecological validity of the behavior tracking context. This setting was assumed to foster the emergence of behaviors related to inhibition deficit for at least three reasons. First, this was a rather long waiting situation potentially causing impatience. Second, the scenario provided opportunities of social interactions with the examiner (and therefore opportunities of interpersonal social disinhibition). Finally, unexpected events, potentially slightly disturbing, frustrating, and/or stressful for the participant (e.g., crackle noise), were planned within the scenario.
We selected a list of behaviors related to inhibition deficits that were potentially observable in the context of the ECOCAPTURE script. Using symptom descriptions and classifications in previous literature, 3 , 5 , 10 we thus defined 16 behaviors related to compulsivity (e.g., repetitive movements, perseveration), impulsivity (e.g., emotional outburst or impulsive motor action), and social disinhibition (e.g., familiar behavior toward investigator or lack of manners; see the complete ethogram in Table 1).
TABLE 1.
–Ethogram listing the 16 behaviors and their definition
| Behavior label | Definition | Example |
|---|---|---|
| COMPULSIVITY | ||
| Utilization behavior 10 | Grasping and touching objects of the environment without any contextual reason | Opening and closing the window without any real purpose |
| Perseveration 10 | Difficulty in shifting mental set and behavioral perseveration | Repeated unsuccessful attempts to open the tap (no running water in the room) |
| Repetitive movements 3 | Repeating stereotyped, compulsive/ritualistic behaviors | Rubbing hands |
| Compulsive eating 3 | Eating excessive amounts of food in the absence of real hunger and/or inappropriate foods in the specific context | Eating canned sardines just after breakfast |
| IMPULSIVITY | ||
| Emotional outburst 5 | Persistent laughing, crying, or swearing alone in the room | Laughing at the sight of the locked box |
| Inappropriate action 5 | Doing something very unconventional and thoughtless with an object of the room | Discarding the content of a beverage in the sink |
| Singing 5 | Singing alone in the room | Singing “O Christmas Tree” without any reason |
| Dancing 5 | Dancing alone in the room | Doing a few dance steps |
| Self‐talking 5 | Speaking aloud when alone in the room | Commenting on the environment when entering the room |
| SOCIAL DISINHIBITION | ||
| Aggressive behavior toward investigator 3 | Showing hostility, verbal or physical aggressiveness toward the investigator | Yelling “Enter” with anger when the investigator knocks on the door several times |
| Familiar behavior toward investigator 3 | Showing inappropriate familiarity toward the investigator | Speaking in colloquial language |
| Nudity 3 | Exposing inappropriate parts of one's body | Removing one's trousers |
| Harsh handling of objects 3 | Handling an object of the room in a way which may cause potential damage, thus showing lack of respect for the investigator's material | Trying to break a box with a lock instead of searching for the key |
| Inappropriate gesture or posture 3 | Impolite, inappropriate physical behavior in a social context | Picking one's nose/teeth |
| Lack of decorum 3 | Failing to respect cultural norms of politeness | Yawning, sneezing, or coughing without putting hand in front of their mouth |
| Disregard for rules or investigator 3 | Lack of response to social cues, ignoring instructions given by the investigator | Not answering investigator's questions |
The video‐based behavioral data were generated by a manual video annotation tool (The Observer XT, Noldus), using the predefined ethogram. Two different examiners (DT, VG) coded the videos and calculated the intraclass correlation coefficient (ICC)–assessed intercoder reliability. All ICC scores were between 0.80 and 1, indicating a very good reliability.
2.3. Neurocognitive and clinical assessments
All participants carried out extensive cognitive and clinical assessments (e.g., the MMSE and the Frontal Assessment Battery [FAB] 11 , 12 ). Among these assessments, we selected tests of abilities and symptoms potentially related to compulsivity, impulsivity, or social disinhibition. These included the Hayling Sentence Completion Test (HSCT) to assess cognitive inhibition difficulties. 13 In the HSCT, participants are asked to complete 15 sentences using the appropriate word, as fast as possible (automatic condition, Part A), and 15 sentences using a completely unrelated word (inhibition condition, Part B). Recently, it has been demonstrated that this test is a reliable measure of cognitive inhibition impairments in pre‐symptomatic C9orf72 mutation carriers and their proximity to clinical conversion to bvFTD. 14 We used the Hayling error score (number of errors in Part B) as a measure of the difficulty to inhibit a prepotent response, as in Flanagan et al. 15 The mini‐Social Cognition & Emotional Assessment (mini‐SEA) orbitofrontal battery measured affective and emotional functions that depend on the limbic system. 16 The battery is composed of two subtests: a shortened version of the Faux Pas Test, assessing theory of mind deficits, and a facial emotion recognition test using Ekmann faces. Finally, the Dimensional Apathy Scale was used to assess apathy 17 and the Hospital Anxiety and Depression Scale (HADS) to quantify depressive and anxiety symptoms. 18
2.4. Neuroimaging data acquisition and VBM pre‐processing
Structural MRI data acquisitions were performed at CENIR (Human MRI Neuroimaging core facility, ICM, Salpêtrière hospital, Paris, France) using a 3T Siemens MRI scanner 64‐channel TIM system. The brain MRI protocol includes a 3D T1 scan allowing the study of structural abnormalities.
Structural data were analyzed with FSL‐VBM 19 (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLVBM), an optimized voxel‐based morphometry (VBM) protocol 20 carried out using FMRIB Software Library (FSL) tools. 21 First, structural images were brain‐extracted and gray matter (GM)‐segmented before being registered to the MNI 152 standard space using non‐linear registration. 22 The resulting images were averaged and flipped along the x‐axis to create a left‐right symmetric, study‐specific GM template. All native GM images were then non‐linearly registered to this study‐specific template and “modulated” to correct for local expansion (or contraction) due to the non‐linear component of the spatial transformation. The modulated GM images were then smoothed with an isotropic Gaussian kernel with a sigma of 3 mm.
2.5. Plan of analyses
All statistical analyses on demographic, behavioral, and neurocognitive data were performed using RStudio (version 1.2.5033, RStudio, Inc.). Statistical analyses on neuroimaging data were performed using FSL tools. 21 We first addressed our objective of dimensional exploration of measured behaviors of inhibition deficit. We used an exploratory factor analysis (EFA) to extract dimensions and to test their conceptual validity, we performed an analysis of the correlations between extracted dimensions and neurocognitive clinical scores. Second, using clustering driven by the extracted dimensions, we identified subgroups of bvFTD patients and analyzed their behavioral, cognitive, clinical, and neuroanatomical characteristics.
2.5.1. Dimensional analysis of inhibition deficit behaviors
We first used EFA, with a promax oblique rotation and a weighted least square (WLS) approach, to explore the factor structure of the behavioral data in both bvFTD patients and HC. This data reduction method identifies factors constituting patterns of behavior. We first assumed a three‐factor structure based on both theory (see the ethogram in Table 2 with three assumed categories of behavior) and analysis of eigenvalues. We then used an iterative process of behavioral item removal to improve construct validity of the extracted factors. 23 The internal consistency of the extracted components was assessed using Cronbach's alpha (α). Individual scores on the two extracted factors (F1 and F2) were automatically calculated using the individual's total occurrences of each behavior. These scores were compared between patients and HC using a Wilcoxon test (as data were not normally distributed).
TABLE 2.
Results of the exploratory factor analysisa
| Factor loadings | ||
|---|---|---|
| Behavior variables | F1 | F2 |
| Utilization behavior | 0.97 | –0.07 |
| Perseveration | 0.96 | –0.09 |
| Repetitive movements | 0.31 | –0.09 |
| Compulsive eating | 0.83 | –0.14 |
| Emotional outburst | 0.93 | –0.04 |
| Dancing | 0.87 | 0.27 |
| Self‐talking | 0.68 | 0.24 |
| Inappropriate action | 0.44 | 0.69 |
| Singing | –0.06 | 0.77 |
| Aggressive behavior toward investigator | –0.09 | 0.74 |
| Familiar behavior toward investigator | –0.17 | 0.67 |
| Lack of decorum | 0.05 | 0.70 |
aValues are the factor loadings of the EFA in bvFTD patients and HC (N = 30). Factor loadings represent correlation coefficients between the behavioral items and the extracted behavioral factors (or patterns) F1 and F2. Coefficients in bold denote the highest loading (among the two factors) for each item. The calculation of individual scores on F1 and F2 takes account of all the factor loadings on F1 and F2 respectively.
Abbreviations: bvFTD, behavioral variant frontotemporal dementia; EFA, exploratory factor analysis; HC, healthy controls.
Finally, Pearson correlations were tested across all 15 bvFTD patients between the calculated scores on the two factors (F1 and F2) and neurocognitive and clinical scores.
2.5.2. Characterization of bvFTD patient subgroups from inhibition deficit dimensions
We used a hierarchical clustering approach based on the individual scores calculated for each factor extracted by EFA (F1 and F2) to distinguish subgroups of bvFTD patients. We thus identified three subgroups: bvFTD‐G0 (outlier subgroup, N = 3), bvFTD‐G1 (N = 6), and bvFTD‐G2 (N = 6).
To provide a precise qualitative description of their behavioral profile, we calculated the total occurrences of each behavioral item in each bvFTD subgroup and in HC (sum of all occurrences in all the individuals of the group—see Figure 2).
FIGURE 2.
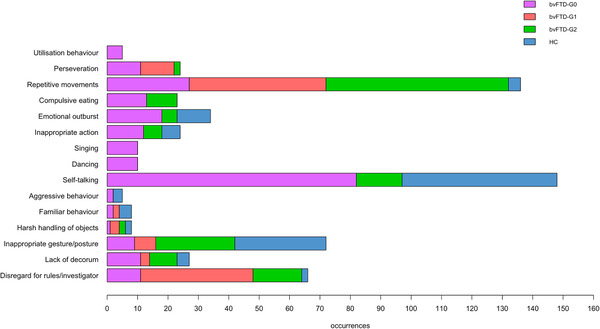
Distribution of the total occurrences of 15 behaviors relating to disinhibition among the three subgroups of behavioral variant of frontotemporal dementia (bvFTD) patients (bvFTD‐G0, N = 3/bvFTD‐G1, N = 6/bvFTD‐G2, N = 6), and healthy controls (HC, N = 15)
We then explored the cognitive and clinical scores of the two main bvFTD subgroups (bvFTD‐G1 and bvFTD‐G2) and compared them to HC through Kruskal‐Wallis tests (as data were not normally distributed) followed by post hoc comparisons using pairwise Wilcoxon tests (with correction for multiple comparisons).
Finally, controlling for age and sex, each subgroup of bvFTD patients was contrasted with HC to determine its specific pattern of atrophy. The following sets of contrasts were performed: (1) All bvFTD versus HC, (2) bvFTD‐G0 versus HC, (3) bvFTD‐G1 versus HC, and (4) bvFTD‐G2 versus HC. Voxelwise General Linear Model with threshold‐free cluster enhancement (TFCE) was applied using permutation‐based non‐parametric testing and correcting for multiple comparisons across space (controlling the family‐wise error rate at a threshold of P < .01 to emphasize differences of atrophy pattern between subgroups).
2.6. Ethical statement
This study is part of clinical trial C16‐87 sponsored by INSERM. It was granted approval by the local Ethics Committee, or “Comité de Protection des Personnes,” on May 17, 2017 and registered in a public clinical trial registry (clinicaltrials.gov: NCT03272230). All study participants gave their written informed consent to participate, in line with French ethical guidelines.
3. RESULTS
3.1. Dimensional analysis of inhibition deficit behaviors
3.1.1. EFA in bvFTD patients and HC
As shown in Table 2, we reached a final two‐dimension structure (F1 and F2) accounting for 64% of the total variance, with an inter‐factor correlation of 0.17. One behavior from the initial list (i.e., nudity) was never observed and therefore removed before analysis. Through the iterative process of behavioral item removal of the EFA, we removed a further three items (harsh handling of objects, disregard for rules/investigator, and inappropriate gesture/posture), either because they were not related to the two extracted factors or because they were equally related to the two factors (and therefore not discriminative). Cronbach's alphas of F1 and F2 were 0.64 and 0.80 respectively, indicating satisfactory internal consistency of the two behavioral factors. The three behaviors with highest loadings on F1 (i.e., utilization behavior, perseveration, and emotional outburst) were mostly categorized as compulsivity according to our ethogram and globally, behaviors with high loadings on F1 were selectively from the compulsivity and impulsivity theoretical categories. The three highest loadings on F2 (i.e., singing, aggressive behavior toward investigator, and lack of decorum) were mostly behaviors initially labeled as social disinhibition and F2 was related to behaviors from the social disinhibition and impulsivity theoretical categories exclusively. The “inappropriate action” item presented a higher loading on F2 but also a rather high cross‐loading onto F1, which means that this item was related to both extracted dimensions.
Comparisons of individual scores extracted for F1 and F2 revealed that bvFTD patients presented significantly higher scores than HC on F1 (W = 163; P = .04) but not on F2 (W = 102; P = .68).
3.1.2. Correlational analysis in bvFTD patients
The analysis of correlations between dimensions extracted by EFA and cognitive and clinical measures is presented in Table 3. Results showed that, in bvFTD patients, F1 was positively correlated to cognitive inhibition deficit measured by Hayling error score (P < .1) and positively related to anxiety (P < .1). F2 was negatively related to the capacity to recognize facial emotions assessed by mini‐SEA emotion score (P < .1) and positively associated with depression (P < .05).
TABLE 3.
Results of the correlational analysis a
| Cognitive and clinical variables | F1 | F2 |
|---|---|---|
| Hayling error | 0.44 c | 0.44 |
| Mini‐SEA Faux Pas Test | –0.19 | 0.20 |
| Mini‐SEA emotion | 0.31 | –0.45 c |
| DAS total | ‐0.06 | –0.25 |
| HADS anxiety | 0.47 c | 0.25 |
| HADS depression | 0.42 | 0.52 b |
Abbreviations: bvFTD; behavioral variant frontotemporal dementia; Hayling error, measure of cognitive disinhibition; mini‐SEA Faux Pas Test, measure of complex social cognition; mini‐SEA emotion, measure of emotion recognition; DAS, Dimensional Apathy Scale; HADS, Hospital Anxiety and Depression Scale.
Values are the Pearson correlation coefficients in bvFTD patients (N = 15).
Significant correlation at P < .05.
Significant correlation at P < .1.
3.2. Characterization of bvFTD patient subgroups from inhibition deficit dimensions
3.2.1. Clustering and identification of subgroups
The data‐driven clustering approach based on the individual scores of F1 and F2 enabled the identification of three subgroups of bvFTD patients: bvFTD‐G0 (N = 3) gathering three “unclassifiable” patients considered outliers in our study, bvFTD‐G1 (N = 6), and bvFTD‐G2 (N = 6; see Figure 1).
FIGURE 1.
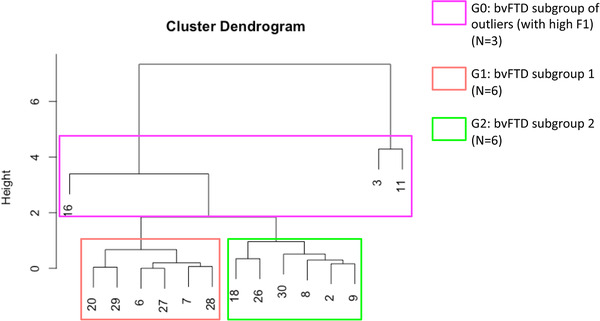
Hierarchical clustering analysis used to define subgroups of behavioral variant of frontotemporal dementia (bvFTD) patients (N = 15). After including patients with very high F1 scores into bvFTD‐G0 (N = 3), two subgroups were identified: bvFTD‐G1 (N = 6) and bvFTD‐G2 (N = 6). Vertical numbers at the bottom of the dendrogram are the study‐specific identifier codes of the participants
The three outliers were included in the same bvFTD‐G0 subgroup because they shared a very high score on F1 that was significantly higher compared to both bvFTD‐G1 (P = .04) and bvFTD‐G2 (P = .04). The bvFTD‐G0 subgroup did not differ from either bvFTD‐G1 (P = .71) or bvFTD‐G2 (P = .71) on F2. BvFTD‐G2 was very slightly superior on F1 than bvFTD‐G1 but the two subgroups were not significantly different on this dimension (P = .81). BvFTD‐G2 patients were distinguished from bvFTD‐G1 patients by their higher score on F2 (P = .02). Neither bvFTD‐G1 nor bvFTD‐G2 were significantly different from HC on F2. Therefore, in this clustering analysis, F1 allows us to identify the outliers within bvFTD‐G0 while F2 enables the distinction between bvFTD‐G1 and bvFTD‐G2, the two main subgroups of patients.
We did not find any significant difference in terms of age, sex, education level, disease duration and global cognitive performances (MMSE, FAB) between patients of the three behaviorally driven subgroups.
3.2.2. Behavioral characterization
Figure 2 summarizes the distribution of the total occurrences of 15 behaviors from the initial ethogram (as nudity was never observed) between the three subgroups of bvFTD patients and HC. Within the bvFTD‐G0 subgroup, each behavior occurred at least once, generally with a high occurrence level (especially for self‐talking): these outliers globally showed lots of behaviors related to inhibition deficit. Compared to bvFTD‐G2, bvFTD‐G1 visibly showed more occurrences of two behaviors: perseveration and disregard for rules/investigator. BvFTD‐G2 presented behaviors not observed in bvFTD‐G1 such as inappropriate actions or compulsive eating and more total occurrences of lack of decorum, inappropriate gesture/posture, and repetitive movements. BvFTD‐G2's higher score on F2 compared to bvFTD‐G1 is probably mainly due to the higher frequency of both inappropriate actions and lack of decorum.
3.2.3. Cognitive and clinical characterization
BvFTD‐G1 and bvFTD‐G2 patients had a significantly higher error score on the Hayling test (Figure 3A) and a significantly lower score on the mini‐SEA emotion recognition task compared to HC (P < .01; Figure 3B). There was no significant difference between bvFTD‐G1 and bvFTD‐G2 for any of these cognitive tests indicating that both subgroups share similar cognitive deficits. BvFTD‐G1, however, presented marginally worse social cognition skills as indicated by their significantly lower score for the mini‐SEA Faux Pas Test compared to HC (P < .01; Figure 3C).
FIGURE 3.
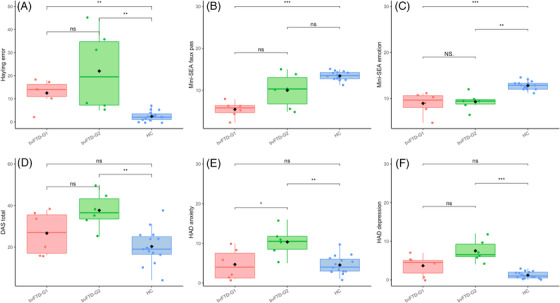
Comparisons of cognitive and clinical scores between two subgroups of behavioral variant of frontotemporal dementia (bvFTD) patients (bvFTD‐G1, N = 6 and bvFTD‐G2, N = 6) and healthy controls (HC, N = 15). Cognitive scores: (A) Hayling error: measure of cognitive disinhibition, (B) mini‐Social Cognition & Emotional Assessment (mini‐SEA) Faux Pas Test: measure of complex social cognition and (C) mini‐SEA emotion: measure of emotion recognition. Clinical scores: (D) DAS, Dimensional Apathy Scale: measure of apathy; HADS, Hospital Anxiety and Depression Scale (E) anxiety and (F) depression. Levels of significance (based on uncorrected P‐values); ns, non‐significant; *P < .05; **P < .01; ***P < .001
Concerning clinical scores, bvFTD‐G2 showed a higher apathy score (P < .01) than HC but we did not observe any difference between bvFTD‐G1 and bvFTD‐G2 (Figure 3D). BvFTD‐G2 also presented a significantly higher total HADS score (P < .01) compared to HC. More specifically on the anxiety dimension (Figure 3E), bvFTD‐G2 had a significantly higher score than HC (P = .01) and a tendency for a higher score compared to bvFTD‐G1 (P = .07). BvFTD‐G2 also had a significantly higher score than HC on the depression dimension (P = .01) and again a close‐to‐significant tendency (P = .07) to present higher levels of depression than bvFTD‐G1 (Figure 3F).
3.2.4. Neuroanatomical characterization
Three patterns of GM atrophy were identified for the three subgroups of patients, with the highest extent of atrophy found in bvFTD‐G0 and the lowest in bvFTD‐G2. Compared to HC, bvFTD‐G0 showed widespread atrophy within frontal and orbitofrontal regions (especially high in the medial frontal gyrus), the left temporal lobe, insula, and basal ganglia (caudate and putamen). Compared to HC, bvFTD‐G1 also showed rather extensive atrophy within frontal regions, the cingulate gyrus, temporal cortex, insula, and basal ganglia whereas bvFTD‐G2 revealed a more restricted pattern of GM atrophy located in frontal cortex, insula, and subcortical structures.
The overall contrast of all bvFTD patients compared to HC revealed an expected pattern of diffuse atrophy in frontal and temporal regions (data not shown). Figure 4 shows results of the following contrasts: bvFTD‐G0 versus HC, bvFTD‐G1 versus HC, and bvFTD‐G2 versus HC. File S4 in supporting information reports the detailed list of coordinates with local maximum atrophy for each patient subgroup compared to HC.
FIGURE 4.
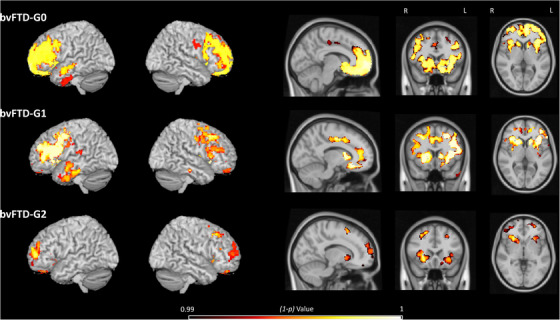
Voxel‐based morphometry–derived gray matter atrophy maps of each behavioral variant of frontotemporal dementia (bvFTD) subgroup: bvFTD‐G0 group of outliers (N = 3), bvFTD‐G1 (N = 6), and bvFTD‐G2 (N = 6). The (1‐p) value maps show the atrophy patterns compared to healthy controls (HC, N = 15) and are superimposed onto a whole‐brain Montreal Neurological Institute template. Effects are corrected for age and sex, and for family‐wise error at the whole brain level at P < .01
4. DISCUSSION
In this study, we used an original ecological approach (i.e., behavioral observation under a close‐to‐real‐life context instead of traditional clinical scales) to refine the behavioral phenotype of different kinds of inhibition deficits and subsequently identify subgroups of bvFTD patients. We found that a two‐dimension structure was the best fit to classify behaviors related to inhibition deficit. These two behavioral dimensions were related to distinct cognitive features and clinical symptoms, which supported the suggestion that they correspond to two different types of inhibition deficit. Using scores on these two dimensions, we identified three subgroups of bvFTD patients and we will discuss their behavioral, neurocognitive, clinical, and neuroanatomic characteristics.
We assumed three theoretical categories of inhibition deficits but retained only two dimensions (F1 and F2). While behaviors of the compulsivity and social disinhibition categories were segregated between F1 and F2, respectively, behaviors initially categorized as impulsivity in our ethogram were shared between the two extracted dimensions. Impulsivity is indeed a multidimensional construct that can be hard to disentangle from the overlapping concepts of social disinhibition and compulsivity. Impulsivity can sometimes be described as a subcomponent of the syndrome of disinhibition 3 , 5 or as a broad concept including disinhibition. 24 Besides, while impulsivity has previously been associated with risk seeking and compulsivity with harm avoidance, it is progressively more recognized that the two concepts are very close and share common neuropsychological mechanisms. 25 The first extracted dimension (F1) globally corresponded to a behavioral pattern of compulsive, ritualistic, automatic actions that cannot be refrained, with an important motor component (i.e., “utilization behavior,” “perseveration,” “repetitive movement,” “dancing”). The second extracted factor (F2) was related to impulsive and socially embarrassing actions in the context of our experimental setting (e.g., “singing” or discarding the content of an offered beverage as “inappropriate action”) and to actions indicating a lack of capacity to respect conventional codes (e.g., “lack of decorum,” “familiar behavior toward investigator”).
We observed a significant difference between bvFTD patients and HC regarding the first dimension associated with compulsive ritualistic behaviors but not regarding the second behavioral pattern of incompliance with social norms. This latter result was unexpected as social disinhibition is usually considered one of the most frequent and distinctive symptoms of bvFTD patients. 3 Several non‐exclusive reasons may explain this result. First, the F2 component we extracted from our behavioral measures may slightly differ from the original concept of social disinhibition described by Rascovsky et al. 3 In terms of total occurrences, the most frequently observed behaviors associated with F2 were “lack of decorum” and “inappropriate actions.” These correspond to socially unconventional behaviors, a subcategory of social disinhibition described by Rascovsky et al. (as “loss of manners or decorum”), but they do not capture the whole phenomenon. Indeed, social disinhibition includes much stronger violations of social norms such as touching/kissing strangers, verbal/physical aggressions, public nudity, urination, inappropriate sexual acts, and criminal behaviors, 3 which are obviously complex to observe within the context of any scientific research. Second, although our 45‐minute scenario provided opportunities for interactions, these periods of interaction were of short duration (maximum 2 to 3 minutes per interaction phase), which might have been insufficient for the difference between bvFTD patients and HC to be apparent on the F2 component. In particular, compared to HC, we did not detect more total occurrences in bvFTD patients of two behaviors clearly related to social disinhibition in literature: aggressive and familiar behaviors toward the investigator. These behaviors may require longer interactions to be salient in bvFTD patients. Third, the fact that participants were explicitly invited to make themselves comfortable, with the investigator making the environment feel less formal to participants, may have blunted the gap between bvFTD patients and HC. Some HC were indeed very at ease within the experimental situation and with the investigator. On the contrary, bvFTD may have inhibited their behavior a bit more than usual. Even though the ECOCAPTURE moment was presented as a waiting time between test periods, patients were generally aware that they were taking part in a day of experiments for scientific research, which may have slightly impacted their behavior.
The correlational analysis between F1/F2 and cognitive/clinical measures in patients was useful to confirm the conceptual meaning of the two extracted dimensions of inhibition deficit. In particular, F1 was found to be related to a fundamental lack of cognitive inhibition and F2 was associated with a deficit in evaluating social stimuli. This observation supports the assumption that compared to F1, F2 is a more socially related behavioral pattern, probably underpinned by a general difficulty to assess the social environment. This two‐dimension result is therefore globally consistent with a study by Paholpak et al., 5 which distinguished two subscales among the disinhibition items of the Frontal Systems Behavior Scale (FrSBe) in bvFTD: ‘‘person‐based’’ (inappropriate behaviors specific to a social context related to social cognitive processes) versus ‘‘generalized impulsivity’’ (opportunistic, general rule violations underpinned by basic loss of impulse control). Besides, F1 and F2 were not related with apathy, which is in agreement with the view that apathy and disinhibition are dissociable clinical symptoms, related to distinct neural circuitry. 26 Instead, the two behavioral dimensions of inhibition deficit tended to increase with self‐reported anxiety and depression. The dimension of automatic compulsive behaviors (F1) was more closely related with anxiety. Obsessive‐compulsive symptoms are indeed related to anxiety, at least in the general population. 27 The socially related dimension of inhibition deficit (F2) was more associated with depressive symptoms, which is in agreement with the well‐known difficulty to interpret social cognitive stimuli in patients with depressive disorders. 28
Our data‐driven approach to classification based on behavior allowed the identification of three subgroups of bvFTD patients, one being an “outlier” group. The outliers within bvFTD‐G0 were characterized by their highly pathological behavioral profile: a very high score on the dimension of compulsive automatic behaviors (F1) and many occurrences of all the behaviors suggestive of a lack of inhibition, including specific overt behaviors such as “dancing” and “singing” not seen in other subgroups. They also corresponded to those with the most severe pattern of atrophy, especially in the ventromedial prefrontal cortex (VMPFC) and the orbitofrontal cortex (OFC). This confirms the specific role of these regions in the lack of impulse control. Indeed, lesion and neuroimaging studies indicate that they are among the main regions modulating impulsivity, compulsivity, and related disorders in neurodegenerative diseases. 5 , 29 , 30 , 31 BvFTD‐G0 patients were thus clearly distinguished by their high frequency of compulsive behaviors along with their very diffuse pattern of atrophy, which suggests that high compulsivity is a clinical sign of higher severity of bvFTD.
Regarding the comparison between bvFTD‐G1 and bvFTD‐G2, these two subgroups differed in their behavioral characteristics on the socially related dimension of inhibition deficit (F2). Indeed, bvFTD‐G2 presented more total occurrences of “lack of decorum” and “inappropriate actions” than bvFTD‐G1. From the cognitive point of view, bvFTD‐G1 and bvFTD‐G2 patients shared impairments of cognitive inhibition and emotion identification. Samples in each subgroup may be too small to detect an existing difference regarding cognitive performances or the two subgroups may share some neuroanatomical characteristics explaining their similar cognitive profiles. Indeed, the two subgroups both presented atrophy in the orbitofrontal cortex, which is highly related to cognitive inhibition deficit assessed by the Hayling error score. 32 Regions typically associated with the perception of social stimuli (temporal and insular cortex) 33 , 34 , 35 were also impacted in both subgroups. Although the atrophy was globally more diffuse in bvFTD‐G1 than in bvFTD‐G2, it is possible that some core regions related to the measured cognitive scores were sufficiently affected in bvFTD‐G2 to explain their similar cognitive profile. Indeed, the severity of symptoms does not depend solely upon the severity of the GM atrophy pattern. The severity of cognitive impairment may be more closely related to specific anterior–posterior functional network disconnections that are convergent across all bvFTD subgroups. 36 Future studies should combine the investigation of behavior, GM, and structural and functional connections to characterize more precisely the phenotypical heterogeneity among bvFTD subtypes based on inhibition deficits.
In this study, the exploratory clustering driven by behavioral data has isolated subgroups of patients mostly distinguished by their clinical symptoms, not by their cognitive performances. Compared to those in bvFTD‐G1, patients in bvFTD‐G2 presented levels of self‐reported anxious and depressive symptoms that tended to be higher (P < .07 after multiple comparison correction). Though slightly superior in bvFTD‐G2, apathy was not found to be different between the two subgroups. Thus, bvFTD‐G2 was mainly characterized by high concomitant anxiety and depression. In dementia patients, depressive symptoms are strongly associated with anxiety and anxiety is one of the most common additional symptoms in depressed patients with dementia. 37 The behavioral profile of patients in bvFTD‐G2 could be defined as “agitated” or “nervous” (i.e., high quantity of repetitive movements and presence of other compulsive behaviors not observed in bvFTD‐G1 such as compulsive eating and self‐talking), which may be a physical expression of their anxiety. Induced anxiety indeed leads to more behaviors such as redundant, repetitive, and rigid hand movements, suggesting that ritualization might be an anxiety‐reducing coping strategy. 38 Besides, the respective neuroanatomical characteristics of bvFTD‐G1 and bvFTD‐G2 can contribute to explain their different clinical profiles. The less widespread atrophy of frontal regions in bvFTD‐G2 may preserve them from the high level of anosognosia generally observed in bvFTD 39 and they might therefore present a better self‐awareness than patients of bvFTD‐G1. A meta‐analysis of imaging studies concluded that cortical midline structures (including cingulate cortex) are the most consistently identified regions involved in self‐referential processing. 40 Compared to bvFTD‐G1, bvFTD‐G2's pattern of atrophy was indeed characterized by the relative sparing of these midline regions. Higher disease awareness may thus be one of the reasons patients in bvFTD‐G2 reported more anxious and depressive symptoms.
Our data‐driven classification based on behavioral assessment dissociated three patterns of atrophy that are consistent with the patterns previously described by Ranasinghe et al. 2 and obtained by clustering bvFTD patients according to GM loss (in specific regions of interest). BvFTD‐G0 and bvFTD‐G1 patterns of atrophy seem to mirror the salience network‐predominant subgroups (SN; bilateral frontoinsula and cingulate cortex) 2 while bvFTD‐G2 shares some similarities with the limbic/semantic appraisal network–predominant subgroup (SAN; temporal pole, ventral striatum, medial orbitofrontal cortex, and basolateral amygdala). 2 The SN is linked to social‐emotional‐autonomic processing and SAN elaborates semantically driven personal evaluation. In their work, Ranasinghe et al. identified two SN subgroups characterized by more widespread atrophy of frontal regions, compared to the SAN subgroup. In particular, the frontotemporal SN subgroup presented highly pronounced atrophy within medial ventral and orbitofrontal regions, which parallels our findings in bvFTD‐G0. Moreover, along with a profile of high socially unconventional behaviors and rather high frequency of some compulsive behaviors, the bvFTD‐G2 subgroup showed atrophy of frontoinsula, rostral caudate, and medial orbitofrontal cortex with a slightly right‐sided asymmetry. This result is also close to the description of the SAN subgroup made by Ranasinghe et al. 2 As in the study by Ranasinghe et al., 2 we identified bvFTD subgroups with clear distinct atrophy profiles but other phenotypical differences between subgroups were subtle. Significant overlap of symptoms across subgroups reflects the core clinical features of bvFTD syndrome. The question of the exact nature of these subgroups, in particular whether they correspond to different forms or different stages of bvFTD, still has to be addressed. Similarly to Ranasinghe et al.’s study, we did not observe any difference between subgroups relative to disease duration. This supports the suggestion that they are different forms of bvFTD. However, further longitudinal investigations would be required to confirm this hypothesis.
A first limitation of the present study is the small sample size of bvFTD patients, partially due to the heavy requirements of our protocol. All the participants of this study underwent 2 days of experimental protocol with extensive neuropsychological testing. We had very selective inclusion criteria for bvFTD patients (e.g., MMSE score >20), due to the need to include patients at a very early stage. However, the stratification of patients is necessary and useful especially in the very early stage of the disease to tailor treatments. Patient classification based on a similar method of behavioral observation should be further validated in a larger population. A second limitation is related to the design itself that we used to shed light on behaviors of inhibition deficit. The predetermined scenario probably provided opportunities for social interactions that were too short to enable the observation of frequent interpersonal social disinhibition behaviors in bvFTD patients. Results might be optimized with longer periods of social interactions included in the scenario. A third limit of this design involves the subjectivity of behavioral coding that arises from the non‐blind encoding of the videos and the labeling of individual behaviors. While the former aspects could be addressed in future studies, the latter was controlled for in the present study by the good level of intercoder reliability.
Despite these limitations, our study is the first to identify constructs of inhibition deficits based on behavioral assessment with an ecological approach. This study also suggests the possibility of categorizing bvFTD patients according to salient behavioral changes at early stages of the disease. This categorization allowed us to disentangle different neuroanatomical profiles, difficult to distinguish according to their cognitive characteristics. Thus, behavioral measures might be a powerful measure to detect bvFTD subtypes with distinct atrophy patterns. Moreover, this study suggests that classification of bvFTD patients according to their behavioral patterns of inhibition deficit may yield better prediction of patients’ specific symptoms of anxiety and depression. These preliminary findings have potential implications for clinicians by contributing to facilitate precise diagnosis, prognosis orientation, and targeted management strategies. In the future, such findings may contribute to the stratification of patients and guide the subsequent investigation of biological markers toward those associated with the vulnerability of specific cerebral regions. To further validate the assessment approach of inhibition deficits and its potential for patient stratification, future studies should continue to explore the ideal ecological conditions fostering the emergence of behaviors related to inhibition deficit. Precise guidelines to enable the evaluation of a patient's subtype according to their behaviors should also be established.
CONFLICTS OF INTEREST
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
Study supervision: Raffaella Migliaccio, Benedicte Batrancourt. Study conception and design: Valerie Godefroy, Delphine Tanguy, Benedicte Batrancourt, Raffaella Migliaccio. Data acquisition: Raffaella Migliaccio, Benedicte Batrancourt, Delphine Tanguy, Richard Levy, Carole Azuar, David Bendetowicz, Guilhem Carle, Armelle Rametti‐Lacroux, Stephanie Bombois, Emmuanuel Cognat, Johan Ferrand‐Verdejo. Analysis and interpretation of data: Valerie Godefroy, Idil Sezer, Delphine Tanguy, Richard Levy, Benedicte Batrancourt, Raffaella Migliaccio. Drafting of the manuscript: Valerie Godefroy, Delphine Tanguy, Arabella Bouzigues, Benedicte Batrancourt, Raffaella Migliaccio, Isabelle Le Ber, Richard Levy. Obtaining funding: Raffaella Migliaccio, Richard Levy, Benedicte Batrancourt. All authors critically revised the manuscript for its intellectual content.
Supporting information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
This study was funded by grant ANR‐10‐IAIHU‐06 from the program “Investissements d'avenir” by grant FRM DEQ20150331725 from the foundation “Fondation pour la recherche médicale” and by the ENEDIS company. Raffaella Migliaccio is supported by “Fondation Recherche Alzheimer” (FRA), “France Alzheimer” and “Philippe Chatrier” Foundations, and by “Rosita Gomez association”. Delphine Tanguy is supported by École Normale Supérieure Paris‐Saclay. Valérie Godefroy is supported by the Malakoff Médéric Humanis company. Arabella Bouzigues is supported by “Fondation Vaincre Alzheimer.” We sincerely acknowledge the participants and caregivers for their involvement in this study.
Godefroy V, Tanguy D, Bouzigues A, et al. Frontotemporal dementia subtypes based on behavioral inhibition deficits. Alzheimer's Dement. 2021;13:e12178. 10.1002/dad2.12178
Valérie Godefroy, Delphine Tanguy, Lara Migliaccio and Bénédicte Batrancourt have contributed equally.
Contributor Information
Valérie Godefroy, Email: valerie.godefroy@icm-institute.org.
Raffaella Migliaccio, Email: lara.migliaccio@gmail.com.
DATA AVAILABILITY STATEMENT
Anonymized data will be shared by request from any qualified investigator.
REFERENCES
- 1. Massimo L, Powers C, Moore P,, et al. Neuroanatomy of apathy and disinhibition in frontotemporal lobar degeneration. Dement Geriatr Cogn Disord. 2009;27:96‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ranasinghe KG, Rankin KP, Pressman PS, et al. Distinct subtypes of behavioral‐variant frontotemporal dementia based on patterns of network degeneration. JAMA Neurol. 2016;73:1078‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456‐2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harnishfeger KK. The development of cognitive inhibition: theories, definitions, and research evidence. In: Dempster FN & Brainerd CJ Eds. Interference and Inhibition in Cognition. San Diego, CA: Academic Press; 1995. [Google Scholar]
- 5. Paholpak P, Carr AR, Barsuglia JP, et al. Person‐based versus generalized impulsivity disinhibition in frontotemporal dementia and Alzheimer disease. J Geriatr Psychiatry Neurol. 2016;29:344‐351. [DOI] [PubMed] [Google Scholar]
- 6. Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top‐down cognitive control. Neuron 2011;69:680‐694. [DOI] [PubMed] [Google Scholar]
- 7. Fineberg NA, Apergis‐Schoute AM, Vaghi MM, et al. Mapping compulsivity in the DSM‐5 obsessive compulsive and related disorders: cognitive domains, neural circuitry, and treatment. Int J Neuropsychopharmacol. 2018;21:42‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Migliaccio R, Tanguy D, Bouzigues A, et al. Cognitive and behavioural inhibition deficits in neurodegenerative dementias. Cortex. 2020;131:265‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Batrancourt B, Lecouturier K, Ferrand‐Verdejo J, et al. Exploration deficits under ecological conditions as a marker of apathy in frontotemporal dementia. Front Neurol 2019;10:941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Snowden JS, Neary D, Mann DM. Frontotemporal dementia. Br J Psychiatry. 2002;180:140‐143. [DOI] [PubMed] [Google Scholar]
- 11. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189‐198. [DOI] [PubMed] [Google Scholar]
- 12. Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a frontal assessment battery at bedside. Neurology. 2000;69:680‐694. [DOI] [PubMed] [Google Scholar]
- 13. Burgess PW, Shallice T. Response suppression, initiation and strategy use following frontal lobe lesions. Neuropsychologia. 1996;34:263‐272. [DOI] [PubMed] [Google Scholar]
- 14. Montembeault M, Sayah S, Rinaldi D, et al. Cognitive inhibition impairments in presymptomatic C9orf72 carriers. J Neurol Neurosurg Psychiatry. 2020;91:366‐372. [DOI] [PubMed] [Google Scholar]
- 15. Flanagan EC, Wong S, Dutt A, et al. False recognition in behavioral variant frontotemporal dementia and Alzheimer's disease—Disinhibition or amnesia? Front Aging Neurosci. 2016;8:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Funkiewiez A, Bertoux M, de Souza LC, Lévy R, Dubois B. The SEA (Social Cognition and Emotional Assessment): a clinical neuropsychological tool for early diagnosis of frontal variant of frontotemporal lobar degeneration. Neuropsychology. 2012;26:81‐90. [DOI] [PubMed] [Google Scholar]
- 17. Radakovic R, Abrahams S. Developing a new apathy measurement scale: dimensional apathy scale. Psychiatry Res. 2014;219:658‐663. [DOI] [PubMed] [Google Scholar]
- 18. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361‐370. [DOI] [PubMed] [Google Scholar]
- 19. Douaud G, Smith S, Jenkinson M, et al. Anatomically related grey and white matter abnormalities in adolescent‐onset schizophrenia. Brain J Neurol. 2007;130:2375‐2386. [DOI] [PubMed] [Google Scholar]
- 20. Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel‐based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21‐36. [DOI] [PubMed] [Google Scholar]
- 21. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. [DOI] [PubMed] [Google Scholar]
- 22. Anderson JLR, Jenkinson M, Smith S. Non‐Linear Registration, Aka Spatial Normalisation FMRIB Technical Report TR07JA2. FMRIB Analysis Group of the University of Oxford. 2007;2(1). https://www.fmrib.ox.ac.uk/datasets/techrep/tr07ja2/tr07ja2.pdf. [Google Scholar]
- 23. Raubenheimer J. An item selection procedure to maximise scale reliability and validity. SA J Ind Psychol. 2004;30. [Google Scholar]
- 24. Rochat L, Billieux J, Juillerat Van der Linden A‐C, et al. A multidimensional approach to impulsivity changes in mild Alzheimer's disease and control participants: cognitive correlates. Cortex J Devoted Study Nerv Syst Behav. 2013;49:90‐100. [DOI] [PubMed] [Google Scholar]
- 25. Fineberg NA, Chamberlain SR, Goudriaan AE, et al. New developments in human neurocognition: clinical, genetic, and brain imaging correlates of impulsivity and compulsivity. CNS Spectr. 2014;19:69‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ball SL, Holland AJ, Watson PC, Huppert FA. Theoretical exploration of the neural bases of behavioural disinhibition, apathy and executive dysfunction in preclinical Alzheimer's disease in people with Down's syndrome: potential involvement of multiple frontal‐subcortical neuronal circuits. J Intellect Disabil Res. 2010;54:320‐336. [DOI] [PubMed] [Google Scholar]
- 27. Irak M, Tosun A. Exploring the role of metacognition in obsessive–compulsive and anxiety symptoms. J Anxiety Disord 2008;22:1316‐25. [DOI] [PubMed] [Google Scholar]
- 28. Weightman MJ, Air TM, Baune BT. A review of the role of social cognition in major depressive disorder. Front Psychiatry. 2014;5:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Averbeck BB, O'Sullivan SS, Djamshidian A. Impulsive and compulsive behaviors in parkinson's disease. Annu Rev Clin Psychol. 2014;10:553‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brown SM, Manuck SB, Flory JD, Hariri AR. Neural basis of individual differences in impulsivity: contributions of corticolimbic circuits for behavioral arousal and control. Emotion. 2006;6:239‐245. [DOI] [PubMed] [Google Scholar]
- 31. Matsuo K, Nicoletti M, Nemoto K, et al. A voxel‐based morphometry study of frontal gray matter correlates of impulsivity. Hum Brain Mapp. 2009;30:1188‐1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hornberger M, Geng J, Hodges JR. Convergent grey and white matter evidence of orbitofrontal cortex changes related to disinhibition in behavioural variant frontotemporal dementia. Brain. 2011;134:2502‐2512. [DOI] [PubMed] [Google Scholar]
- 33. Cerami C, Dodich A, Canessa N, et al. Neural correlates of empathic impairment in the behavioral variant of frontotemporal dementia. Alzheimers Dement. 2014;10:827‐834. [DOI] [PubMed] [Google Scholar]
- 34. Couto B, Manes F, Montañés P, et al. Structural neuroimaging of social cognition in progressive non‐fluent aphasia and behavioral variant of frontotemporal dementia. Front Hum Neurosci. 2013;7:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Adolphs R. The neurobiology of social cognition. Curr Opin Neurobiol. 2001;11:231‐239. [DOI] [PubMed] [Google Scholar]
- 36. Lee SE, Khazenzon AM, Trujillo AJ, et al. Altered network connectivity in frontotemporal dementia with C9orf72 hexanucleotide repeat expansion. Brain. 2014;137:3047‐3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Prado‐Jean A, Couratier P, Druet‐Cabanac M, et al. Specific psychological and behavioral symptoms of depression in patients with dementia. Int J Geriatr Psychiatry. 2010;25:1065‐1072. [DOI] [PubMed] [Google Scholar]
- 38. Lang M, Krátkỳ J, Shaver JH, Jerotijević D, Xygalatas D. Effects of anxiety on spontaneous ritualized behavior. Curr Biol. 2015;25:1892‐1897. [DOI] [PubMed] [Google Scholar]
- 39. Rosen HJ, Alcantar O, Zakrzewski J, Shimamura AP, Neuhaus J, Miller BL. Metacognition in the behavioral variant of frontotemporal dementia and Alzheimer's disease. Neuropsychology. 2014;28:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self‐referential processing in our brain—A meta‐analysis of imaging studies on the self. NeuroImage. 2006;31:440‐457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.


