Abstract
We present an observation of depolarized scattering by silver colloids. The profile of the wavelength-dependent anisotropy of the colloidal solution of silver nanoparticles traces the plasmonic extinction spectrum. The scattering component of the colloid extinction spectrum is responsible for the depolarization effect. We believe that the presence of the orthogonal component in the silver colloid scattering can be used in dye-less sensing devices.
1. Introduction
The scattering of the light by colloidal solutions of metal nanoparticles has recently been revisited by a number of authors [1–4]. Silver sub-wavelength particles interact with light stronger than any known chromophore. This is a result of a high density of conductive electrons and imaginary dielectric constant which enable efficient excitation of the surface plasmon polariton resonances. The investigation of silver nanoparticles deposited on various substrates led to the discovery of such phenomena as luminescent blinking of the silver particles [5,6] and giant non-linear responses [7,8]. Fluorescent probes deposited on the silver surface display many-fold enhanced emission [9–12]. This extensive interest in silver nanoparticles has been stimulated by surface-enhanced Raman scattering (SERS) discovered in 1977 [13,14]. Enhancements as high as 106 to 1014 have now been reported [15], as well as detection down to single molecules [16].
The plasmon resonances in silver nanoparticles depend on two components: absorption and a near-field component that evolves into radiative far-field scattering. The relative contribution of these two components is related to the nanoparticles size [17–19]. In the case of small nanoparticles (<30 nm), absorption generally dominates the extinction spectrum. For larger nanoparticles (>50 nm), the scattering component dominates the plasmon resonance extinction spectrum. Recently, Evanoff and Chumanov [18] measured extinction, scattering, and absorption cross-sections for different sized silver colloids. Direct absorption measurements using an integrated sphere allowed them to resolve the extinction spectra into two components, absorption and scattering.
In this report we have studied the polarization properties of light scattering by silver colloids.
2. Materials and methods
2.1. Preparation of the silver colloids
Silver colloids were prepared by the reduction of silver nitrate with sodium citrate under controlled temperature/time conditions. To a stirred solution of AgNO3 (36 mg in 200 ml H2O) at 70 °C, 4 ml of trisodium citrate (34 nM) was added drop wise. The reaction mixture was warmed to 87–90 °C, and continued to be stirred for 15 min (until the color of the reaction mixture turned to yellow). The resulting mixture was then cooled in an ice-water bath for 15 min. The colloid solution was purified by centrifugation at 8000 rpm for 8 min, and the precipitate (residue) was dissolved in 1 mM trisodium citrate (8 ml).
2.2. Spectroscopic measurements
The extinction of the solution of the silver nanoparticles was measured using a Hewlett–Packard model 8543 spectrophotometer. The scattering of the silver colloids was measured using a SLM 8000 spectrofluorometer (SLM Instruments, IL, USA) with right angle excitation. The spectrofluorometer has been carefully calibrated for anisotropy measurements. Atomic Force Microscope (AFM) images were collected with an Atomic Force Microscope (TMX 2100 Explorer SPM, Veeco), equipped with AFM dry scanner, 100 μm. The AFM scanner was calibrated using the standard calibration grid as well as gold nanoparticles, 100 nm in diameter, from Ted Pella. Images were analyzed using SPMLab software.
3. Results and discussion
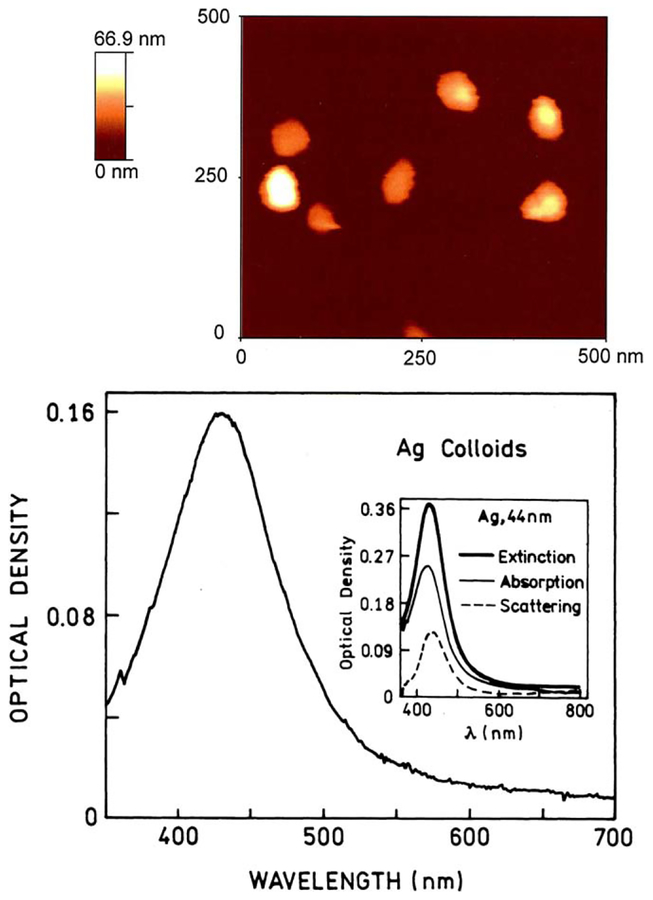
The silver colloids solution, prepared as described in Section 2, was diluted progressively to four concentrations resulting in the optical densities of 0.04, 0.08, 0.16, and 0.25. The extinction spectrum of one prepared suspension is presented in Fig. 1, bottom. The normalized extinction spectra of the three other suspensions were similar in both shape and position. The maximum for the silver nanoparticles, about 430–440 nm, corresponds to the size of about 40 nm [18]. This size was confirmed using the atomic force microscope image from the same colloids deposited onto a glass substrate Fig. 1, top. The shape of the extinction spectrum is also very similar to the shape observed by Evanoff and Chumanov [18] for a 44 nm silver nanoparticles suspension (Fig. 1, inset). The scattering component in this colloid is already significant and responsible for about 1/3 of the extinction spectrum [18].
Fig. 1.
Top: the AFM image of the silver colloids deposited on the glass slides. Bottom: extinction spectrum of the silver nanoparticles suspension. The size of the nanoparticles estimated from AFM analysis is about 40–50 nm. The maximum of extinction at 430–440 nm corresponds to the particle size of about 40 nm, as observed in [18] (inset).
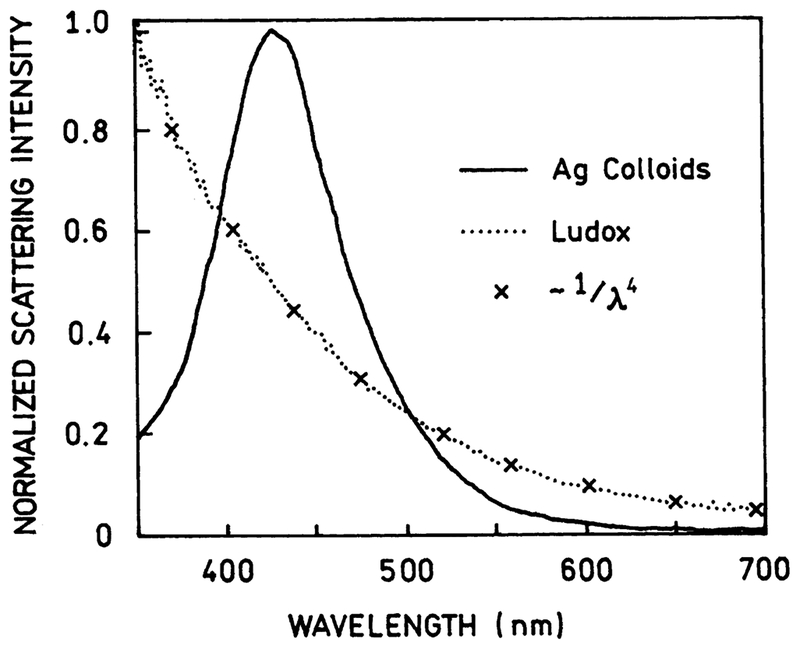
Next, we measured the scattering from the nanoparticles suspension. The excitation (white light illumination) was vertically polarized. Then, the scattering radiation was analyzed with a calibrated monochromator. First, we observed the scattering through the vertically oriented polarizer (col-linear with the excitation illumination). The scattering spectrum (Fig. 2) corresponds roughly to the extinction spectrum (Fig. 1, bottom). As a reference comparison we prepared a suspension of silica nanoparticles (Ludox) with an optical density similar to that of colloids. The observed scattering from the silica nanoparticles suspension (Fig. 2) is completely different from the scattering from silver nanoparticles suspension. The scattering from the silica nanoparticles suspension follows the 1/λ4 trend (Fig. 2), which proves the classic Raleigh process. Contrarily, the scattering from the silver nanoparticles does not match the 1/λ4 trend. Whereas the beam pathway for the silica nanoparticles is all white, for the silver nanoparticles it is blue. We have also noticed that with the top-view observation, the blue scattering from the silver colloids remains intense, while white scattering from Ludox particles is greatly reduced. This is a clear indication that the scattering from silver colloids is depolarized.
Fig. 2.
Scattering spectra of the silver colloids (——) and a suspension of silica nanoparticles, Ludox (·····). The Raleigh scattering of Ludox is being perfectly approximated by 1/λ4 trend (×).
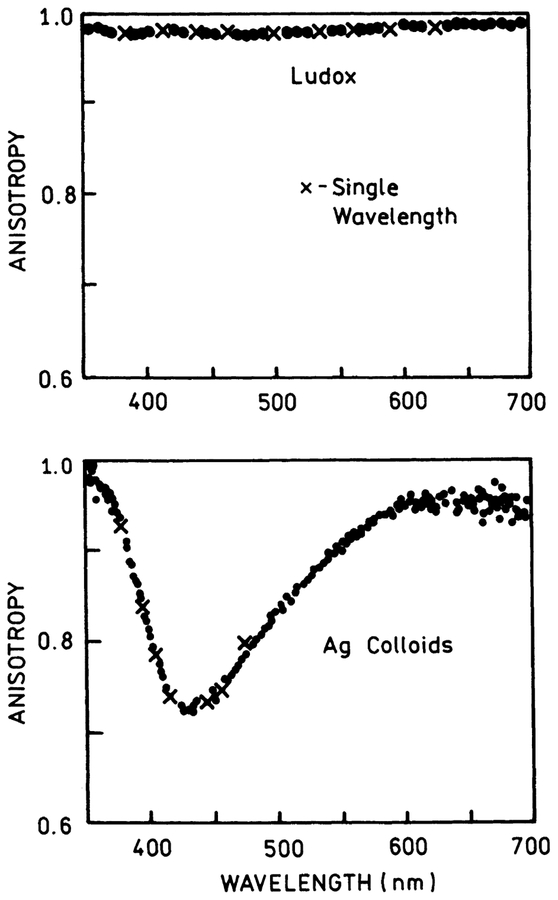
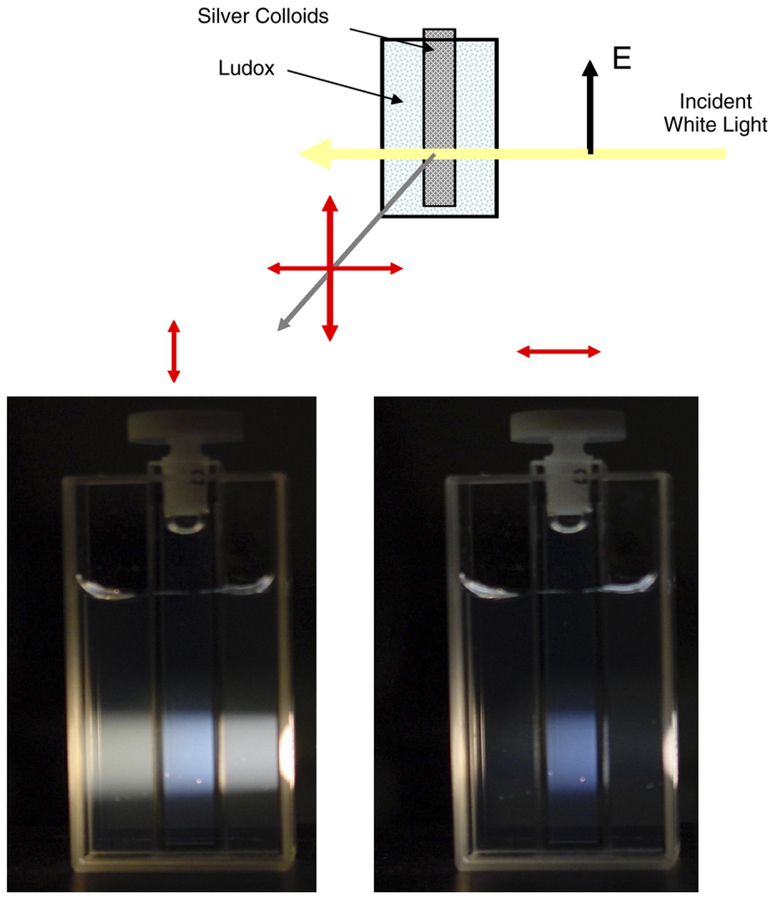
Next, we measured the anisotropy spectra of the silica and silver nanoparticles suspensions, first with white light excitation illumination, then using a single wavelength. As expected, the Ludox solution showed very high anisotropies (close to 0.9 or more), independently on the illumination/excitation wavelength (Fig. 3, top). In contrast, the anisotropy spectrum of the silver colloids showed a deep minimum (Fig. 3, bottom), corresponding to the maximum of the extinction, or more exactly, to the maximum of the scattering component in the extinction spectrum. Next we diluted (up to 16-fold) the colloid suspension and repeated the anisotropy measurements. The anisotropy profile did not change. Also we compared the anisotropy profile for freshly prepared colloids and than colloids stored overnight in the cold room. The anisotropy profile did not change at all. As it was shown in [18] and Fig. 1, bottom (inset), the maximum of the scattering component roughly corresponds to the maximum of the entire extinction spectrum. We consider an approximate 30% decrease of the anisotropy to be very significant. With a horizontal observation, the Raleigh Ludox scattering is almost completely eliminated, while silver colloid scattering is still easily seen. We have demonstrated this on the photographs in Fig. 4; here, the silver colloid solution in a small cuvette (0.5 × 0.5 cm) was placed into the larger (2 × 2 cm) cuvette filled with the Ludox solution. The white light illumination beam was vertically polarized and the photographs were taken through vertically (left) or horizontally (right) oriented polarizer. In the latter case, only scattering from the silver colloids could be seen.
Fig. 3.
The anisotropy spectra of the silica nanoparticles (top) and silver nanoparticles (bottom). The measurements were done either with the white light illumination (●) or single wavelength illumination (×). The shape of the anisotropy spectra is independent on the concentrations of either silica or silver nanoparticles.
Fig. 4.
The photograph of silica and/or silver nanoparticles scattering. A square 5 × 5 mm cuvette with the suspension of silver nanoparticles was placed in larger, 2 × 2 cm, cuvette filled with Ludox solution (suspension of silica nanoparticles). The illumination was a vertically polarized beam of the white light. The observation was either through vertically oriented polarizer (left) or horizontally oriented polarizer (right). In latter case, the scattering from silica has been eliminated, but horizontal component of silver nanoparticles remained strong.
The origin of the scattering of silver colloids is different from that of silica suspension. In the case of metal sub-wavelength particles, the plasmonic interaction with an impinging light plays a dominant role. Our observation demonstrates that the radiation from the plasmons excited in the silver nanoparticle suspension is highly depolarized.
The minimum measured anisotropy of 0.72 is equivalent [20] to a ratio of parallel-to-perpendicular scattered intensities of R = I‖/I⊥ = 9.8. This ratio is consistent with that possible for light scattering of spherical particles 14 ⩽ R ⩽ 6.25. Lower ratios of I‖/I⊥ have been observed for metallic gold rods [21,22].
We believe that by using polarized illumination on excitation and cross-polarized observation, one can selectively observe the silver colloid scattering radiation and use it for sensing and other purpose.
Acknowledgment
This research was supported by the NIH: NIBIB EB-1690, NCI CA 114460, NCRR RR-08119; and Philip Morris USA Inc. We dedicate this Letter to Professor Józef Kuśba on the occasion of his 60th birthday.
References
- [1].Yguerabide J, Yguerabide EE, Anal. Biochem 262 (2) (1998) 137. [DOI] [PubMed] [Google Scholar]
- [2].Yguerabide J, Yguerabide EE, Anal. Biochem 262 (2) (1998) 157. [DOI] [PubMed] [Google Scholar]
- [3].Malynych S, Chumanov G, J. Am. Chem. Soc 125 (10) (2003) 2896. [DOI] [PubMed] [Google Scholar]
- [4].Roll D, Malicka J, Gryczynski I, Gryczynski Z, Lakowicz JR, Anal. Chem 75 (14) (2003) 3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Peyser LA, Vinson AE, Bartko AP, Dickson RM, Science 291 (5501) (2001) 103. [DOI] [PubMed] [Google Scholar]
- [6].Geddes CD, Parfenov A, Gryczynski I, Lakowicz JR, J. Phys. Chem. B 107 (2003) 9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kim W, Safonov VP, Drachev VP, Podolskyi VA, Shalaev VM, Armstrong RL, in: Shalaev VM (Ed.), Optical Properties of Nanostructured Random Media, Topics in Applied Physics, Springer Verlag, Berlin, 2002, pp. 149–167. [Google Scholar]
- [8].Wenseleers W, Stellacci F, Meyer-Friedrichsen T, Mangel T, Marder SR, Perry JW, J. Phys. Chem. B 106 (2002) 6853. [Google Scholar]
- [9].Sokolov K, Chumanov G, Cotton TM, Anal. Chem 70 (18) (1998) 3898. [DOI] [PubMed] [Google Scholar]
- [10].Lakowicz JR, Shen Y, D’Auria S, Malicka J, Fang J, Gryczynski Z, Gryczynski I, Anal. Biochem 301 (2) (2002) 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Malicka J, Gryczynski I, Gryczynski Z, Lakowicz JR, Anal. Biochem 315 (1) (2003) 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kummerlen J, Leitner A, Brunner H, Aussenegg FR, Wokaun A, Mol. Phys 80 (1993) 1031. [Google Scholar]
- [13].Jeanmaire DL, Van Duyne RP, J. Electroanal. Chem 84 (1) (1977) 1. [Google Scholar]
- [14].Albrecht MG, Creighton AJ, J. Am. Chem. Soc 99 (1977) 5215. [Google Scholar]
- [15].Kneipp K, Kneipp H, Itzkan I, Dasari RR, Feld MS, Chem. Rev 99 (1999) 2957. [DOI] [PubMed] [Google Scholar]
- [16].Nie S, Emory SR, Science 275 (5303) (1997) 1102. [DOI] [PubMed] [Google Scholar]
- [17].Messinger BJ, von Raben KU, Chang RK, Barber PW, Phys. Rev. B 24 (2) (1981) 649. [Google Scholar]
- [18].Evanoff DD, Chumanov G, J. Phys. Chem. B 108 (37) (2004) 13957. [DOI] [PubMed] [Google Scholar]
- [19].Lakowicz JR, Anal. Biochem 337 (2) (2005) 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lakowicz JR, Principles of Fluorescence Spectroscopy, second edn., Kluwer Academic/Plenum Publishers, 1999, 698 pp. [Google Scholar]
- [21].Van de Hulst HC, Light Scattering by Small Particles, Dover Publications Inc., New York, 1957, 470 pp. [Google Scholar]
- [22].Khlebtsov NG, Melnikov AG, Bogatyrev VA, Dykman LA, Alekseeva AV, Trachuk LA, Khlebtsov BN, J. Phys. Chem. B 109(28) (2005) 13578. [DOI] [PubMed] [Google Scholar]