Abstract
Objective.
A prolonged QTc (LQT) is a surrogate for the risk of Torsade-de-Pointes (TdP). QTc interval duration is influenced by sex hormones: estradiol prolongs and testosterone shortens QTc. Drugs used in the treatment of breast cancer have divergent effects on hormonal status.
Methods.
We performed a disproportionality analysis using the European database of suspected adverse drug reaction (ADR) reports to evaluate the reporting odds-ratio (ROR chi-square) of LQT, TdP and ventricular arrhythmias associated to selective estrogen receptor modulators (SERMs: tamoxifen and toremifene) as opposed to aromatase inhibitors (AIs: anastrozole, exemestane and letrozole). When the proportion of an ADR is greater in patients exposed to a drug (SERMs) compared to patients exposed to control drug (AIs), this suggests an association between the specific drug and the reaction and is a potential signal for safety. Clinical and demographical characterization of patients with SERMs-induced LQT and ventricular arrhythmias was performed.
Results.
SERMs were associated with higher proportion of LQT reports vs. AIs (26/8318 vs. 11/14851, ROR: 4.2[2.11–8.55], p<0.001). SERMs were also associated with higher proportion of TdP and ventricular arrhythmias reports vs. AIs (6/8318 vs. 2/14851, ROR:5.4[1.29–26.15], p:0.02; 16/8318 vs. 12/14851, ROR:2.38[1.15–4.94], p:0.02, respectively). Mortality was 38% in patients presenting ventricular arrhythmias associated to SERMs.
Conclusions.
SERMs are associated with more reports of drug-induced LQT, TdP and ventricular arrhythmias as compared to AIs. This finding is consistent with estradiol-like properties of SERMs on the heart as opposed to effects of estrogen deprivation and testosterone increase induced by AIs.
Keywords: Long QT syndrome, torsade de pointes, ventricular arrhythmias, selective estrogen receptor modulators, aromatase inhibitors, sex steroid hormones
Background.
Cardiac repolarization, measured by QT interval duration corrected for heart rate (QTc) on surface electrocardiogram,1 is influenced by sex steroid hormones.2–4 Long QT (LQT) is a surrogate for the risk of Torsade de Pointes (TdP).2 In women, estradiol is associated with QTc increase while testosterone is associated with QTc shortening.2 It has been shown that contraceptive pills might influence QTc depending on their anti-androgenic properties.5 Selective estrogen receptor modulators (SERMs) and aromatase inhibitors (AIs) are cornerstone endocrine drugs used for hormone sensitive breast cancer treatment.6 SERMs used in clinical practice are mainly tamoxifen and, toremifene to a lesser degree.6,7 SERMs have variable agonists/antagonists effects depending on the target tissue, being antagonists for breast and agonists for metabolism and cardiovascular organs.6,8 AIs block peripheral conversion of testosterone into estradiol, leading to decreased estradiol and increased testosterone levels.6,9 AIs, i.e anastrozole, letrozole and exemestane are used in post-menopausal women first line or when there is a disease progression following tamoxifen therapy.6,9 Therefore, due to their peculiar pharmacology, SERMs are expected to increase LQT and TdP as compared to AIs.
Methods.
We performed a disproportionality analysis using the European-database of suspected adverse drug reaction (ADR) reports to evaluate the reporting odds-ratio (ROR) of LQT, TdP and ventricular arrhythmias as a function of SERMs or AIs use. European-database of suspected ADR reports is a publicly accessible portal designed to search data on suspected ADR for authorized medicinal products in the European Economic Area (http://www.adrreports.eu/fr/search_subst.html#). Case/non-case analyses were performed for SERMs (tamoxifen and toremifene) vs. AIs (anastrozole, exemestane and letrozole). This method compares the proportion of specific ADR reported for these two groups. Reactions are based on the medical dictionary for regulatory activities classification dictionary of terms for side effects. Disproportionality was estimated by calculating the ROR Chi-square (Table 1). If the proportion of an ADR is greater in patients exposed to a drug (SERMs) than in patients not exposed to this drug (AIs), this suggests an association between the specific drug and the reaction and is a potential signal for safety.10–12 We searched for ADR related to suspected drug-induced LQT, TdP and ventricular arrhythmias. Selected medical dictionary for regulatory activities classification Terms used for definition of ventricular arrhythmias were: ventricular arrhythmias, ventricular tachycardia, ventricular fibrillation and TdP. Selected medical dictionary for regulatory activities classification terms used for definition of LQT were: Long QT syndrome, Electrocardiogram QT prolonged or Electrocardiogram QT abnormal.
Table 1.
Calculation of the reporting Odds-Ratios (ROR).
| Reports with the suspected ADR: i.e LQT or TdP or Ventricular arrhythmias |
Reports without the suspected ADR |
|
|---|---|---|
| Reports with suspected drugs (exposed: SERMs) | A | B |
| Reports with control drugs (non-exposed: AIs) | C | D |
A: Number of reports of drug-induced arrhythmias (LQT or TdP or ventricular arrhythmias) associated with given drugs (SERMs).
B: Number of reports of other adverse drug reactions associated with given drugs (SERMs).
C: Number of reports of drug-induced arrhythmias associated with control drugs (AIs).
D: Number of reports of other adverse drug reactions associated with control drugs (AIs).
ROR = (A/C)/(B/D) = AD/BC. (1)
Abbreviations: ADR, adverse drug reaction; AIs, aromatase inhibitors; LQT, long QT; SERMs, selective estrogen receptor modulators; TdP, Torsade-de-pointes
Clinical and demographical characterization of the cohort of patients with suspected SERMs-induced LQT, TdP and ventricular arrhythmias was performed. Details were extracted from VigiBase,13 where data from European-database of suspected ADR reports are usually transferred electronically on a daily basis. VigiBase is the international pharmacovigilance database managed by the World Health Organization’s Uppsala Monitoring Center. Complementary information and relevant unreported cases to European-database of suspected ADR reports or VigiBase were searched in Pubmed, last accessed December 2017.14
The use of confidential electronically processed patient data was approved by the French National Commission for Data Protection and Liberties (Commission Nationale de l’Informatique et des Libertés; reference:1922081).
Results.
Analysis of European-database of suspected ADR reports from 12/2001 to 08/2017 revealed 23169 individual safety case reports (ISCRs) of suspected ADR for SERMs (n:8318) and AIs (n:14851). These ISCRs mainly involved adult (18–64yo:50.3%, 65–85yo:44.7%, >85yo:4.4%, <18yo:0.6%) women (97.7%). Adult women on SERMs were younger as compared to women on AIs (18–64yo:54.3% vs. 48.3%, p<0.0001). For SERMs, most of these ISCR were associated with tamoxifen (95.2%). For AIs, repartition of ISCRs by molecule was: letrozole (44.4%), anastrozole (33.1%) and exemestane (22.5%). For ISCRs reported on SERMs, 26 were LQT (24 on Tamoxifen), 6 were TdP (5 on Tamoxifen), and 16 were ventricular arrhythmias (14 on Tamoxifen). For ISCRs reported on AIs, 11 were LQT (9 on letrozole and 2 on exemestane), 2 were TdP (1 on exemestane and 1 on anastrozole), and 12 were ventricular arrhythmias (6 on letrozole, 5 on anastrozole and 1 on exemestane).
SERMs were associated with higher proportion of LQT as compared to AIs (26/8318 vs. 11/14851, ROR:4.2[2.11–8.55], p<0.001; Figure 1). SERMs were also associated with higher proportion of TdP and ventricular arrhythmias as compared to AIs (6/8318 vs. 2/14851, ROR:5.4[1.29–26.15], p:0.02; 16/8318 vs. 12/14851, ROR:2.38[1.15–4.94], p:0.02; respectively; Figure 1). More details concerning specific numbers of ISCR as a function of medical dictionary for regulatory activities classification terms and molecules are detailed in Table 2.
Figure 1.
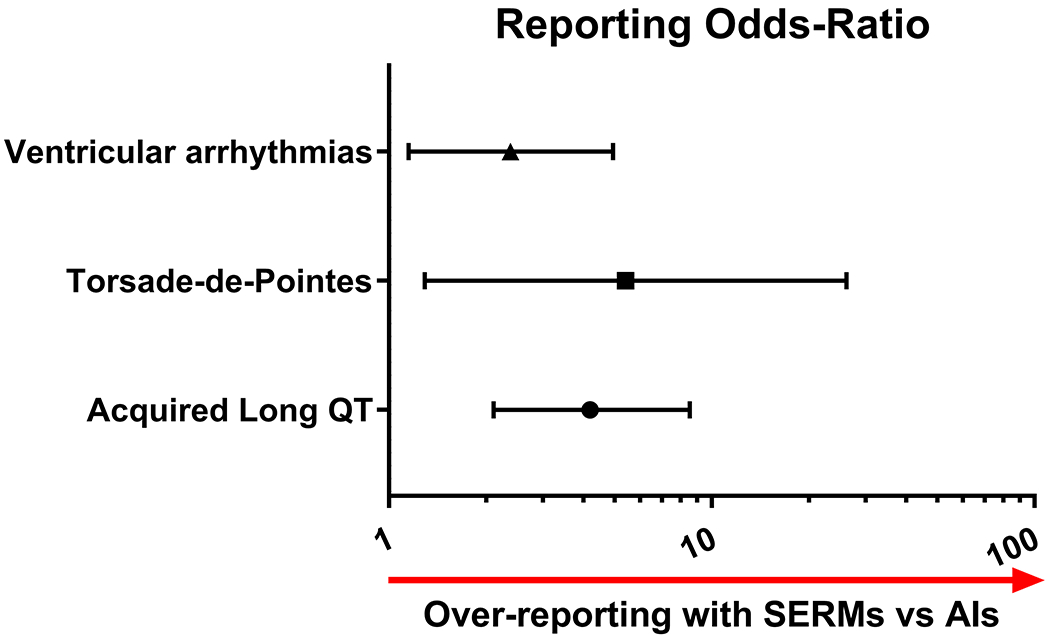
Forest plot detailing reporting Odds-Ratio (ROR) of suspected adverse drug reactions (ADR) related to acquired long QT, Torsade-de-Pointes, or ventricular arrhythmias between selective estrogen receptor modulators (SERMs) and aromatase inhibitors (AIs) collected from the European database of suspected ADR reports accessed in 08/2017.
Table 2.
Details concerning data collected from the European database of suspected adverse drug reaction reports (last accessed in 08/2017) concerning number and type (Medical dictionary for regulatory activities classification) of declared individual safety case reports (ISCRs) in patients receiving Selective Estrogen Receptor Modulators (SERMs: Tamoxifen, toremifene) and Aromatase inhibitors (anastrozole, exemestane, letrozole).
| Total number of ISCRs | Long QT syndrome or Electrocardiogram QT prolonged | Electrocardiogram QT interval abnormal | Total: QT prolonged | Torsade de Pointes | Ventricular Tachycardia | Ventricular Fibrillation | Ventricular arrhythmia | Total: Ventricular arrhythmias | |
|---|---|---|---|---|---|---|---|---|---|
| Tamoxifene | 7915 | 24 | 0 | 24 | 5 | 3 | 6 | 0 | 14 |
| Toremifene | 403 | 2 | 0 | 2 | 1 | 0 | 1 | 0 | 2 |
| SERMs | 8318 | 26 | 0 | 26 | 6 | 3 | 7 | 0 | 16 |
| Anastrozole | 4928 | 0 | 0 | 0 | 1 | 3 | 0 | 1 | 5 |
| Exemestane | 3337 | 2 | 0 | 2 | 1 | 0 | 0 | 0 | 1 |
| Letrozole | 6586 | 8 | 1 | 9 | 0 | 5 | 1 | 0 | 6 |
| Aromatase inhibitors | 14851 | 10 | 1 | 11 | 2 | 8 | 1 | 1 | 12 |
Clinico-demographic details concerning ISCRs of LQT (n=30) and ventricular arrhythmias (n=21) associated to SERMs extracted from the international pharmacovigilance database (where information from EMA are transferred) and PubMed (through 12/2017) are detailed in Tables 3 and 4. LQT and ventricular arrhythmias overwhelmingly affected women with breast cancer on tamoxifen taking standard dose (10–30 mg/day, oral) with no other culprit drugs or liable conditions (Tables 3 and 4). Time to onset between SERM intake and LQT apparition could be as fast as 24 hours and the median time was of a week. Information concerning LQT evolution after SERM withdrawal was available in 10 patients. In these latter, QTc duration normalized after stopping SERM. Recurrence of LQT after SERM rechallenge was reported in one women. In two women with breast cancer, SERM was switched to an AI with subsequent normalization of QT duration. In one women, genetic study of enzymes involved in metabolism of tamoxifen identified a poor metabolizer status for CYP2D6, CYP3A, UGT2B7 and UGT1A1. In two other women, it was hypothesized that tamoxifen induced LQT resulted from a pharmacokinetic interaction leading to CYP3A4 inhibition by an interacting drug (acitretine, norfloxacin). Of note, SERMs induced ventricular arrhythmias lead to life threatening situations with recovery in 24% and death in 38% of patients.
Table 3.
Details concerning patients with selective estrogen receptor modulators (SERMs) associated long QT syndrome with no ventricular arrhythmias (n=30) collected from VigiBase (last accessed in 09/2017) and Pubmed (last accessed in 12/2017)
| Characteristic | Number (%) | Number with available data |
|---|---|---|
| Female gender | 19 (66%) | 29 |
| Age | ||
| <18y | 3 (11%) | 27 |
| 18-64y | 15 (56%) | |
| 65–85y | 8 (29%) | |
| >85y | 1 (4%) | |
| Indication | ||
| Breast cancer | 11 (55%) | 20 |
| Meningioma, glioma | 3 (15%) | |
| Other | 6 (30%) | |
| Region of reporting | ||
| Americas | 9 (36%) | 25 |
| Europe | 13 (52%) | |
| Asia | 2 (8%) | |
| Other concomitant reported suspected medications* | ||
| None (only SERMs) | 18 (60%) | 30 |
| 1 other | 10 (33%) | |
| 2 others | 2 (7%) | |
| Regimen of culprit drug | ||
| Tamoxifen (10-30 mg/day; Oral) | 17 (57%) | 30 |
| Tamoxifen (100-300 mg/day; Oral) | 4 (13%) | |
| Tamoxifen (dose unknown) | 4 (13%) | |
| Toremifene (300 mg/day; Oral) | 5 (17%) | |
| Magnitude of QTc prolongation | ||
| QTc (470-500 msec) | 1 (10%) | 10 |
| QTc > 500msec | 9 (90%) | |
| Concurrent reported condition favoring LQT syndrome | ||
| Hypokalemia, hypomagnesemia | 1 (3%) | 30 |
| Cardiac conditions (ischemia, heart failure) | 2 (6%) | |
| Bradycardia | 2 (6%) | |
| Atrial fibrillation | 2 (6%) | |
| Reporting year | ||
| 1990 – 2004 | 6 (20%) | 30 |
| 2005 – 2010 | 4 (13%) | |
| 2011 – 2017 | 20 (67%) | |
| Reporter Qualification | ||
| Health Professional | 25 (86%) | 29 |
| Consumer (Non health professional) | 4 (14%) | |
| Time to onset (median, [interquartile range]), days | 7 [5, 66.5] days (1, 1415) days |
21 |
Other concomitant reported suspected medications were hydrochlorothiazide (n=1), glycopyrronium (n=1), anti-depressant drugs (n=3), class III anti-arrhythmic drugs (n=2), fluoroquinolone (n=2), acitretine (n=1), angiotensin-converting-enzyme inhibitor (n=1) and anticancer drugs (n=3).
Table 4.
Characteristics of patients with selective estrogen receptor modulators (SERMs) associated ventricular arrhythmias (n=21, of which n=6 TdP) collected from VigiBase (last accessed in 09/2017) and Pubmed (last accessed in 12/2017)
| Characteristic | Number (%) | Number with available data |
|---|---|---|
| Female gender | 18 (95%) | 19 |
| Age | ||
| <18y | 0 (0%) | 18 |
| 18-64y | 5 (28%) | |
| 65-85y | 11 (61%) | |
| >85y | 2 (11%) | |
| Indication | ||
| Breast cancer | 5 (84%) | 6 |
| Other | 1 (16%) | |
| Region of reporting | ||
| Americas | 12 (57%) | 21 |
| Europe | 6 (29%) | |
| Asia | 3 (14%) | |
| Other concomitant reported suspected medications* | ||
| None (only SERMs) | 14 (66%) | 21 |
| 1 other | 1 (5%) | |
| ≥2 others | 6 (29%) | |
| Regimen of culprit drug | ||
| Tamoxifen (10-20 mg/day; Oral) | 10 (47%) | 21 |
| Tamoxifen (200 mg/day; Oral) | 1 (5%) | |
| Tamoxifen (dose unknown) | 7 (33%) | |
| Toremifene (40-120 mg/day; Oral) | 2 (10%) | |
| Toremifene (dose unknown) | 1 (5%) | |
| Concurrent reported condition and outcome | ||
| Cardiac conditions (ischemia, heart failure) | 3 (14%) | 21 |
| Atrial fibrillation | 1 (5%) | |
| Life threatening (circulatory collapse) recovered | 5 (24%) | |
| Mortality | 8 (38%) | |
| Reporting year | ||
| 1983 – 2000 | 9 (42%) | 21 |
| 2000 – 2010 | 6 (29%) | |
| 2011 – 2017 | 6 (29%) | |
| Reporter Qualification | ||
| Health Professional | 11 (100%) | 11 |
| Time to onset (median, [interquartile range]), days (min, max), days | 156 [61, 633] days (10, 2967) days |
14 |
Other concomitant reported suspected medications were furosemide (n=1), anti-asthmatic drugs (n=1), neurotropic drugs (n=4), anti-arrhythmic drugs (n=2), azithromycin (n=1), amlodipine (n=1), and anticancer drugs (n=4).
Discussion
The main finding of this study is that SERMs were associated with higher proportion of ADR reports related to LQT, TdP and ventricular arrhythmias as compared to AIs in European-database of suspected ADR reports (Figure 2). Almost all these reports on SERMs were observed with standard oral dose of tamoxifen, which is much more widely used than toremifene.6,7 However, the overall number of events were small but, in an era where treatment for hormone positive breast cancer is moving toward combination therapy where SERMs and AIs are combined with other therapies (such as kinase inhibitors) which have their own effects of QT, these results can be more significant for the cancer population.
Figure 2.

Synthetic representation of the main study results.
Tamoxifen was approved before the systematic requirement for dedicated thorough QTc studies and there is also no information on QTc measurements from large clinical trials evaluating the efficacy of tamoxifen in breast cancer.7 To date, tamoxifen is often considered by tertiary drug information sources as a drug with intermediate effects on QTc interval. United States Food and Drug Administration label does not mention any warning, precaution of use in population at risk or particular electrocardiographic monitoring while using tamoxifen. Our finding reveals the potential for tamoxifen to induce ventricular arrhythmias, particularly TdP. TdP induced by tamoxifen has rarely been reported in the literature. Our finding is in line with three case reports of acquired LQT on tamoxifen used at standard dose (20mg/day).14 Two phase I trials investigating use of high dose tamoxifen (80–680mg/m2/day) in advanced epithelial tumors (n:53) and pediatric malignant gliomas (n:14) have shown that up to 40% of patients had a significant QT prolongation (≥10%) and about 10% had very marked QTc increase (prolongation ≥20%).15,16 In these trials, one patient had sudden death associated with QTc prolongation and premature ventricular contractions. 15,16 Development of tamoxifen in these indications was not pursued. A thorough QT study conducted in men showed that toremifene (differing from tamoxifen for one chlorine atom) prolongs the QTc interval in a dose- and concentration-related manner.7
The influence of tamoxifen and/or its active metabolite (endoxifen) on ventricular repolarization have been studied in several animal models.17–21 Data mainly showed an IKr (rapidly activating delayed rectifier potassium channel) inhibition, a mechanism also involved in estradiol-induced2,22 and in most drug-induced LQT (Figure 2).2,23 In human embryonic kidney cells expressing IKr currents, tamoxifen and endoxifen inhibited IKr currents by direct channel blockage and by disruption of channel trafficking to the plasma membrane in a concentration-dependent manner.19 IKr blockage induced by tamoxifen was also found in Xenopus laevis oocytes expressing IKr channels.20 However, it has also been shown in ventricular myocytes that tamoxifen inhibited sodium (in rat)17 and L-type calcium (canine18 and rabbit21) currents, beyond potassium currents inhibition. This multi-channel blockade induced by tamoxifen might counter balance effects of isolated IKr inhibition17,18,21 and, therefore result in a mild ventricular repolarization prolongation and explain a modest QTc prolongation in clinical settings when there is no other liable conditions potentiating LQT.
Extrapolating preclinical models to humans is tricky, in part due to potential differences in the composition of cardiac ionic channels among the different species.24 In addition, given the large number of women with breast cancer requiring SERMs for a period ranging from 5 to 10 years[5], it can be expected that these patients will take a number of other drugs at risk for TdP.23
Our findings are further supported by previous data showing that AIs increase testosterone and decrease estradiol levels resulting in ventricular repolarization shortening (Figure 2).2,22 Accordingly, Kurokawa et al. recently showed that ablation of circulating levels of estradiol in aromatase knockout mice blunted the QT prolonging effect of E-4031, an IKr blocker.22 Moreover, it has been shown that high dose anastrozole lead to QT shortening in dogs.25
There are limitations to our work based on EMA and VigiBase reports. The sources are non-homogeneous, and there is limited possibility of verification of the rhythm abnormalities by means of electrocardiographic tracings, nor completeness of reporting for comorbid conditions and concomitant drugs. The exact denominator and clinical characteristics of patients exposed to SERMs or AIs can’t be evaluated, but in the present situation it was mainly breast cancer women patients. Total number of ISCRs for each drug is used as denominator for this kind of disproportionality analysis in pharmacovigilance databases for signal detection.10–12 The volume of reports for a particular medicinal product may be influenced by the extent of use of the product, publicity, the nature of the reactions and other factors such as competition bias. Even though magnitude of disproportionality (ROR) for LQT and TdP have been associated to extent of IKr drug-induced blockade using VigiBase;11 or to identification of over-reporting for LQT and TdP on anti-androgenics vs. testosterone using EMA;4 there is still a risk that comparisons of disproportionality between medicinal products in pharmacovigilance databases may be misleading. Older age is expected to contribute to higher pro-arrhythmia risk.2,26 Therefore, the age difference found between AIs vs SERMs users is unlikely to bias our results, but status for other risk factors for TdP between AIs vs SERMs users were unknown.
In conclusion, LQT and TdP were more likely to be reported on SERMs as compared to AIs. Further investigation may be needed to better understand risk of LQT or TdP in high-risk patients such as carrier of congenital LQT syndrome or when SERMs are used in combination with other QT prolonging drugs, such as new anticancer drugs developed for breast cancer. In addition, AIs appear to be a therapeutic alternative in breast cancer patients developing LQT and TdP on SERMs. Electrocardiographic monitoring should be considered in patients on SERMs with a risk of TdP.
Keypoints.
What is already known about this subject?
Cardiac repolarization assessed by QTc interval duration is influenced by sex steroid hormones: estradiol prolongs QTc and testosterone shortens it. Drugs used in the treatment of breast cancer have divergent effects on hormonal status.
What does this study add?
The effects of selective estrogen receptor modulators (SERMs) compared to aromatase inhibitors (AIs) on QT duration and torsade de pointes (TdP) risk are unknown. We showed that SERMs are associated with more reports of drug-induced long QT (reporting Odds-Ratio:4.2, p<0.001), TdP (reporting Odds-Ratio:5.4, p:0.02) and ventricular arrhythmias (reporting Odds-Ratio:2.38, p:0.02) as compared to AIs. This finding is consistent with estradiol-like properties of SERMs on the heart as opposed to effects of estrogen deprivation and testosterone increase induced by AIs.
How might this impact on clinical practice?
AIs might be a better alternative to SERMs in breast cancer patients at risk for long QT and TdP. This issue will be more critical as endocrine therapy is combined with other therapies for the treatment of breast cancer. Electrocardiographic monitoring should be considered in patients on SERMs with a risk of TdP.
Acknowledgments.
This study was supported by Philippe Foundation and Fondation Recherche Medicale “SPE20170336816”. The information does not represent the opinion of the European Medicines Agency or World Health Organization.
Footnotes
Disclosure Statement: The authors have nothing to disclose. The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in HEART editions and any other BMJPGL products to exploit all subsidiary rights
NCT: NCT03259711
References.
- 1.Salem JE, Germain M, Hulot JS, et al. GENomE wide analysis of sotalol-induced IKr inhibition during ventricular REPOLarization, “GENEREPOL study”: Lack of common variants with large effect sizes. PLoS One 2017;12(8):e0181875. doi: 10.1371/journal.pone.0181875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salem JE, Alexandre J, Bachelot A, et al. Influence of steroid hormones on ventricular repolarization. Pharmacol Ther 2016;167:38–47. doi: 10.1016/j.pharmthera.2016.07.005 [DOI] [PubMed] [Google Scholar]
- 3.Abehsira G, Bachelot A, Badilini F, et al. Complex Influence of Gonadotropins and Sex Steroid Hormones on QT Interval Duration. J Clin Endocrinol Metab 2016;101(7):2776–84. doi: 10.1210/jc.2016-1877 [DOI] [PubMed] [Google Scholar]
- 4.Salem JE, Waintraub X, Courtillot C, et al. Hypogonadism as a reversible cause of Torsade de Pointes in men. Circulation 2018;In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sedlak T, Shufelt C, Iribarren C, et al. Oral contraceptive use and the ECG: evidence of an adverse QT effect on corrected QT interval. Ann Noninvasive Electrocardiol 2013;18(4):389–98. doi: 10.1111/anec.12050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tryfonidis K, Zardavas D, Katzenellenbogen BS, et al. Endocrine treatment in breast cancer: Cure, resistance and beyond. Cancer Treat Rev 2016;50:68–81. doi: 10.1016/j.ctrv.2016.08.008 [DOI] [PubMed] [Google Scholar]
- 7.Vogel CL, Johnston MA, Capers C, et al. Toremifene for breast cancer: a review of 20 years of data. Clin Breast Cancer 2014;14(1):1–9. doi: 10.1016/j.clbc.2013.10.014 [DOI] [PubMed] [Google Scholar]
- 8.Christodoulakos GE, Lambrinoudaki IV, Botsis DC. The cardiovascular effects of selective estrogen receptor modulators. Ann N Y Acad Sci 2006;1092:374–84. doi: 10.1196/annals.1365.034 [DOI] [PubMed] [Google Scholar]
- 9.van Londen GJ, Perera S, Vujevich K, et al. The impact of an aromatase inhibitor on body composition and gonadal hormone levels in women with breast cancer. Breast Cancer Res Treat 2011;125(2):441–6. doi: 10.1007/s10549-010-1223-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Puijenbroek E, Diemont W, van Grootheest K. Application of quantitative signal detection in the Dutch spontaneous reporting system for adverse drug reactions. Drug Saf 2003;26(5):293–301. [DOI] [PubMed] [Google Scholar]
- 11.De Bruin ML, Pettersson M, Meyboom RH, et al. Anti-HERG activity and the risk of drug-induced arrhythmias and sudden death. Eur Heart J 2005;26(6):590–7. doi: 10.1093/eurheartj/ehi092 [DOI] [PubMed] [Google Scholar]
- 12.Rothman KJ, Lanes S, Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf 2004;13(8):519–23. doi: 10.1002/pds.1001 [DOI] [PubMed] [Google Scholar]
- 13.Lindquist M VigiBase, the WHO Global ICSR Database System: Basic Facts. Drug Information Journal 2008;42(5):409–19. doi: 10.1177/009286150804200501 [DOI] [Google Scholar]
- 14.Fung K, Imeson J, Cusano F. The clinical significance of QT prolongation associated with tamoxifen: A review of the literature. J Oncol Pharm Pract 2017:1078155217720006. doi: 10.1177/1078155217720006 [DOI] [PubMed] [Google Scholar]
- 15.Pollack IF, DaRosso RC, Robertson PL, et al. A phase I study of high-dose tamoxifen for the treatment of refractory malignant gliomas of childhood. Clin Cancer Res 1997;3(7):1109–15. [PubMed] [Google Scholar]
- 16.Trump DL, Smith DC, Ellis PG, et al. High-dose oral tamoxifen, a potential multidrug-resistance-reversal agent: phase I trial in combination with vinblastine. J Natl Cancer Inst 1992;84(23):1811–6. [DOI] [PubMed] [Google Scholar]
- 17.He J, Kargacin ME, Kargacin GJ, et al. Tamoxifen inhibits Na+ and K+ currents in rat ventricular myocytes. Am J Physiol Heart Circ Physiol 2003;285(2):H661–8. doi: 10.1152/ajpheart.00686.2002 [DOI] [PubMed] [Google Scholar]
- 18.Dick GM, Kong ID, Sanders KM. Effects of anion channel antagonists in canine colonic myocytes: comparative pharmacology of Cl-, Ca2+ and K+ currents. Br J Pharmacol 1999;127(8):1819–31. doi: 10.1038/sj.bjp.0702730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chae YJ, Lee KJ, Lee HJ, et al. Endoxifen, the active metabolite of tamoxifen, inhibits cloned hERG potassium channels. Eur J Pharmacol 2015;752:1–7. doi: 10.1016/j.ejphar.2015.01.048 [DOI] [PubMed] [Google Scholar]
- 20.Thomas D, Gut B, Karsai S, et al. Inhibition of cloned HERG potassium channels by the antiestrogen tamoxifen. Naunyn Schmiedebergs Arch Pharmacol 2003;368(1):41–8. doi: 10.1007/s00210-003-0766-8 [DOI] [PubMed] [Google Scholar]
- 21.Liu XK, Katchman A, Ebert SN, et al. The antiestrogen tamoxifen blocks the delayed rectifier potassium current, IKr, in rabbit ventricular myocytes. J Pharmacol Exp Ther 1998;287(3):877–83. [PubMed] [Google Scholar]
- 22.Kurokawa J, Sasano T, Kodama M, et al. Aromatase knockout mice reveal an impact of estrogen on drug-induced alternation of murine electrocardiography parameters. J Toxicol Sci 2015;40(3):339–48. doi: 10.2131/jts.40.339 [DOI] [PubMed] [Google Scholar]
- 23.Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med 2004;350(10):1013–22. doi: 10.1056/NEJMra032426 [DOI] [PubMed] [Google Scholar]
- 24.O’Hara T, Rudy Y. Quantitative comparison of cardiac ventricular myocyte electrophysiology and response to drugs in human and nonhuman species. Am J Physiol Heart Circ Physiol 2012;302(5):H1023–30. doi: 10.1152/ajpheart.00785.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plourde PV, Dyroff M, Dukes M. Arimidex: a potent and selective fourth-generation aromatase inhibitor. Breast Cancer Res Treat 1994;30(1):103–11. [DOI] [PubMed] [Google Scholar]
- 26.Rautaharju PM, Mason JW, Akiyama T. New age- and sex-specific criteria for QT prolongation based on rate correction formulas that minimize bias at the upper normal limits. Int J Cardiol 2014;174(3):535–40. doi: 10.1016/j.ijcard.2014.04.133 [DOI] [PubMed] [Google Scholar]


