Highlights
-
•
A longitudinal fixel-based analysis in young brain-injured patients.
-
•
Lower fiber density and fiber cross-section in young brain-injured patients.
-
•
Restoration of left-hemispheric sensorimotor tracts following balance training.
-
•
Balance training improved patients’ postural control.
Keywords: Brain injury, Diffusion MRI, Training, Neuroplasticity, Rehabilitation
Abstract
Background and objectives
Traumatic brain injury (TBI) is one of the leading causes of death and disability in children and adolescents. Young TBI patients suffer from gross motor deficits, such as postural control deficits, which can severely compromise their daily life activities. However, little attention has been devoted to uncovering the underlying white matter changes in response to training in TBI. In this study, we used longitudinal fixel-based analysis (FBA), an advanced diffusion imaging analysis technique, to investigate the effect of a balance training program on white matter fibre density and morphology in a group of young TBI patients.
Methods
Young patients with moderate-to-severe TBI (N = 17, 10 females, mean age = 13 ± 3 years) and age-matched controls (N = 17) underwent a home-based balance training program. Diffusion MRI scans together with gross motor assessments, including the gross motor items of the Bruininks-Oseretsky Test of Motor Proficiency, the Activities-Specific Balance Confidence (ABC) Scale, and the Sensory Organization Test (SOT) were administered before and at completion of 8-weeks of training. We used FBA to compare microstructural differences in fibre density (FD), macrostructural (morphological) changes in fibre cross-section (FC), and the combined FD and FC (FDC) metric across the whole brain. We then performed a longitudinal analysis to test whether training restores the white matter in the regions found to be damaged before treatment.
Results
Whole-brain fixel-based analysis revealed lower FD and FC in TBI patients compared to the control group across several commissural tracts, association fibres and projection fibres, with FD reductions of up to 50%. Following training, TBI patients showed a significant interaction effect between Group and Time for the SOT test, as well as significant increases in macrostructural white matter (i.e., FC & FDC) in left sensorimotor tracts. The amount of change in FC and FDC over time was, however, not associated with behavioural changes.
Discussion
Our fixel-based findings identified both microstructural and macrostructural abnormalities in young TBI patients. The longitudinal results provide a deeper understanding of the neurobiological mechanisms underlying balance training, which will allow clinicians to make more effective treatment decisions in everyday clinical practice with brain-injured patients.
1. Introduction
Postural control deficits (including symptoms of dizziness, unsteadiness, or imbalance) are a common consequence of traumatic brain injury (TBI), and interfere with a patient's ability to perform activities of daily living. Between 30% and 65% of TBI patients report long-term problems with postural control (Peterson and Greenwald, 2011). Postural control deficits have been identified using clinical testing batteries or instrumented measures of gross motor performance in patients with TBI (e.g., Caeyenberghs et al., 2012, Chaplin et al., 1993, Drijkoningen et al., 2015a, Drijkoningen et al., 2015b, Drijkoningen et al., 2017, Geurts et al., 1996, King et al., 2014, Sosnoff et al., 2011). For example, using the Bruininks-Oseretsky Test of Motor Proficiency, Chaplin et al. (1993) found significant differences between TBI children and control participants, not only on fine-motor items (upper-limb speed and dexterity), but also in the gross-motor subtests of running speed, balance, strength and bilateral coordination. In addition, using a quantitative instrumented measure of postural control (based on the EquiTest System, NeuroCom International, Clackamas, OR), our previous studies (Drijkoningen et al., 2017) showed that balance performance was worse in the TBI patients compared to the healthy controls, as indicated by larger anterior/posterior body sway and less directional control over the centre of gravity.
Numerous studies have demonstrated that TBI is associated with structural white matter (WM) changes that involve sensorimotor tracts and regions that play a key role in postural control. Specifically, volume loss in TBI patients has been reported in infratentorial brain structures (i.e., brain stem and cerebellum) (e.g., Drijkoningen et al., 2015a, Drijkoningen et al., 2015b, Drijkoningen et al., 2017, Gale et al., 2005, Kim et al., 2008, Salmond et al., 2005, Sidaros et al., 2009). Decreased WM organization has been observed in sensorimotor tracts, including the cerebellar peduncles, posterior thalamic radiation, and corticospinal tract (for a meta-analysis, see Roberts et al., 2014). In addition, significant, moderate-to-high correlations between postural control and brain structure have been observed, such that increased severity of structural pathology predicts poorer postural control performance (e.g., Caeyenberghs et al., 2010, Caeyenberghs et al., 2011, Drijkoningen et al., 2015a). It is, however, still unclear whether these structural brain abnormalities can be restored with postural training.
Emerging empirical evidence indicates that postural control training may result in regional alterations in diffusion tensor imaging (DTI) metrics and/or grey matter volume in brain injured patients (Burciu et al., 2013; Prosperini et al., 2014, Sehm et al., 2014). For example, Prosperini et al. (2014) showed a reduction in radial diffusivity of the superior cerebellar peduncles following a 12-week video game balance board training conducted in multiple sclerosis patients. They interpreted these findings as a restoration of myelin sheaths on demyelinated axons of the superior cerebellar peduncles. A balance training study in patients with cerebellar degeneration by Burciu and colleagues (2013) reported a training-induced increase in grey matter density of the right dorsolateral premotor cortex, which was unaffected by the disease.
In the present study, we examined whether these structural brain changes following training are located in the same or different regions to the ones which showed alterations before training in young TBI patients. There is evidence that some heightened brain plasticity takes place in childhood and adolescence (for reviews, see Anderson et al., 2011, Noack et al., 2009, Oberman and Pascual-Leone, 2013, Spear, 2013), thereby providing an opportunity for training programs to alter the course of the disease. This is particularly important given our recent finding that TBI-related brain damage can spread in the brain in a trans-neuronal manner via the brain’s structural connectome (Poudel et al., 2020).
The objectives of this study were threefold. The first aim was to extend our previous findings of cerebellar WM alterations in a sample of young patients in the chronic stage of TBI recovery (Drijkoningen et al., 2015a). To this end, we compared the fixel-based metrics, i.e., fibre density (FD), fibre cross-section (FC) and a combined measure of fibre density and cross-section (FDC), between TBI patients and healthy controls using the fixel-based analysis (FBA) framework (Raffelt et al., 2017), where a fixel refers to a specific fibre population within a voxel. In addition, we applied a whole-brain approach instead of the region-of-interest (ROIs) approach we adopted in our previous study (Drijkoningen et al., 2015a) to examine microstructural & macrostructural WM changes, because we expected to achieve higher sensitivity within the FBA framework to identify TBI-induced changes in WM compared to the diffusion tensor model metrics (Raffelt et al., 2012, Mito et al., 2018). Based on two previous FBA studies in TBI (Verhelst et al., 2019, Wallace et al., 2020), we hypothesized lower FD/FC/FDC in multiple WM tracts in the TBI patients compared to the controls.
The second aim was to investigate whether training-induced changes in WM take place by means of neural restoration in brain regions that presented altered WM organisation before the treatment. Utilizing a tailored ‘small fixel mask correction’ approach (a more stringent variation of the small-volume correction; see Methods for details), we tested the main effect of training in WM regions that revealed significant group differences in the cross-sectional analyses. We expected to observe a recovery of FBA metrics (i.e., increased FD/FC/FDC) following balance training in regions that showed significant alterations pre-treatment.
Our third aim was to explore the association between changes in fixel-based metrics and changes in postural control, as measured by the gross motor performance changes on three postural control tests, including the gross motor items of the Bruininks-Oseretsky Test of Motor Proficiency–2nd Edition (BOT-2) (Bruininks and Bruininks, 2005), the Activities-specific Balance Confidence scale (ABC scale) (Powell and Myers, 1995), and the Sensory Organisation Test (SOT) (Drijkoningen et al., 2015a). Based on previous work (see review, Caeyenberghs et al., 2018), we anticipated a significant correlation between WM changes and postural control improvements in young patients with TBI.
2. Methods
2.1. Participants
The data in the present study overlaps with the work of Drijkoningen and colleagues (2015a), which made use of DTI to examine the WM organization of the cerebellar peduncles. For the present study, 17 moderate-to-severe TBI patients (10 females; mean age = 13 ± 3 years) and 17 typically developing participants (9 females; mean age = 14 ± 2 years) were included. Both groups were age- and sex-matched, as assessed using a t-test and a chi-squared test for age (p = 0.247) and sex (p = 0.730), respectively. The TBI patients were classified as moderate-to-severe based on the Mayo Classification System for TBI severity (Malec et al., 2007). This system classifies patients according to a combination of factors, including the length of post-traumatic amnesia, loss of consciousness duration, lowest Glasgow Coma Scale score in the first 24h, and neurological assessment of the lesions as observed in MRI or computed tomography images. A summary of the demographic and clinical data for all patients is provided in Table 1. The TBI patients’ mean age and standard deviation (SD) at the time of injury were 10.48 and 3.67 years, and the average time interval and SD between the injury and the present MRI were 3.34 and 3.29 years, respectively. All participants met MRI safety criteria (i.e., no metal implants) and patients with pre-existing developmental disorders, a recurrent TBI, significant neurological illness, intellectual disabilities, severely impaired limb function, and musculoskeletal disease were excluded. The study was approved by the ethics committee for biomedical research at KU Leuven, and the patients were recruited from several rehabilitation centres in Belgium. Written informed consent was obtained from either the participants themselves or the patients’ first-degree relatives, according to the Declaration of Helsinki.
Table 1.
Summary of demographic and injury characteristics for the TBI group.
| TBI patient #; Age (y)/Gender | Cause of injury/Age at injury (y) | Time since injury (y) | GCS/Coma duration | Lesion location/pathology based on MRI scan at PRE-session | Lesion location/pathology based on acute MRI scan within 24h after injury |
|---|---|---|---|---|---|
| TBI 01; 8.6/M | Traffic accident/7.9 | 0.7 | Coma = 5 days | Hemosiderin deposits: R semiovale centre and CC | Subdural hematoma R FL/PL/TL; cortical contusion R FL/PL; DAI in R FL |
| TBI 02; 18.1/F | Traffic accident/15.6 | 2.5 | Coma = 5 days | Small injuries surrounding drain trajectory in RH (superior frontal gyrus, head of caudate nucleus, crus anterius of internal capsule, thalamus and pons) | Subdural hematoma/hemorrhagic contusion TL/FL; Injuries R FL, thalamus, R cerebral peduncle, L mesencephalon; Cortical and subcortical hemorrhagic areas in PL/TL |
| TBI 03; 9.3/F | Traffic accident/7.9 | 1.4 | Coma = 2 weeks | Contusion: R anterior temporal pole and R orbitofrontal cortex; Injuries and atrophy in CC (body and splenium); Atrophy of R pons; Hemosiderin deposits in L cerebellar hemisphere, R nucleus lentiformis, L/R FL, L/R PL and R PL | DAI in L TL/FL, R TL/FL/PL |
| TBI 04; 16.5/F | Traffic accident/7.2 | 9.3 | NA | Injuries in R medial frontal gyrus | Epidural hematoma R FL/TL; Shift midline |
| TBI 05; 14.2/F | Traffic accident/7.7 | 6.5 | NA | Atrophy of the cerebellum; Injuries at the level of L FL, premotor cortex, L/R medial frontal gyrus, cingulum, orbitofrontal cortex (L > R); Contusion anterior temporal pole (R > L); Hemosiderin deposits in CC, L thalamus, striatum (R > L) | NA |
| TBI 06; 13.4/M | Traffic accident/12.5 | 0.8 | LOC (unknown duration) | Hemosiderin deposits: several spread out over L/R PL, R cerebellum, L superior frontal gyrus. Hemociderosis as a remnant of subdural hemorrhage | Hemorrhagic contusion L TL; brain oedema |
| TBI 07; 17.1/F | Traffic accident/12.7 | 4.4 | GCS = 3, Coma = 6 weeks | Contusion/atrophy: R superior frontal gyrus, R temporal gyrus; Injuries at the level of the R supramarginal gyrus, R angular gyrus, R precentral gyrus (M1), central sulcus, R postcentral gyrus, R medial frontal gyrus, R insula, R head and body of caudate nucleus, R globus pallidus, R putamen, anterior part of R thalamus; Hemosiderin deposits in LH (superior/inferior frontal gyrus, paraventricular WM) and several in RH | Atrophy across whole brain: R FL/TL (with hemosiderin deposits), nucleus caudatus and R nucleus lentiformis, R mesencephalon, R PL (with surrounding gliosis); cerebellum (specifically L posterior hemisphere); Hemosiderin deposits (DAI): L FL, thalamus, TL, R OL. Shift of midplane; Enlarged R lateral ventricle (with surrounding gliosis) |
| TBI 08; 19.0/F | Fall/12.5 | 6.5 | LOC (unknown duration) | Hemosiderin deposits R cerebellar vermis | Subdural hematoma L FL/TL/PL |
| TBI 09; 15.6/M | Traffic accident/12.5 | 3.2 | Coma = 10 days | Atrophy cerebellum; contusion R FL WM | DAI R TL, internal capsule, supra-orbital R FL, L FL WM (anterior corona radiata), L middle cerebellar peduncle |
| TBI 10; 13.9/M | Traffic accident/13.5 | 0.3 | GCS = 3 | Hemosiderin deposits: L FL, periventricular WM, body and genu CC, L thalamus, R external capsule, anterior TL (L > R), L/R cerebellum; limited atrophy cerebellum | DAI FL, TL, L OL (hemorrhagic injury), R TL, cerebellum, CC, external capsule, R globus pallidus, L thalamus, R cerebral peduncle, R mesencephalon |
| TBI 11; 8.5/F | Traffic accident/7.7 | 0.8 | NA | Enlarged fourth ventricle, atrophy of cerebellar vermis, contusion R cerebellar vermis, hypotrophy of middle cerebellar peduncle and L pons; contusion L TL; Hemosiderin deposits R FL, L TL, Vermis | NA |
| TBI 12; 10.9/M | Sports injury (equestrian)/7.9 | 3.1 | GCS = 4, LOC (unknown duration) | Injuries in RH: orbitofrontal cortex, inferior frontal gyrus and anterior part of medial/superior frontal gyrus; Hemosiderin deposits in L superior frontal gyrus and L cerebellar hemisphere | Hemorrhagic contusion FL/TL, subdural hematoma L FL |
| TBI 13; 11.4/M | Sports injury (equestrian)/9.8 | 1.5 | Coma (unknown duration) | Hemosiderin deposit: splenium CC | Contusion L FL/TL ; Enlarged, asymmetric ventricle (temporal horn) |
| TBI 14; 13.3/M | Traffic accident/12.1 | 1.2 | LOC = 15 min | Hemosiderin deposits L FL, genu CC | DAI in genu and splenium CC, L FL |
| TBI 15; 16.0/F | NA/NA | NA | NA | Mild atrophy in cerebellum and cerebrum, more pronounced atrophy in frontal cortices, enlarged ventricles; contusion L/R anterior temporal pole and L/R orbitofrontal cortex. Hemosiderin deposits in cerebellum, R FL | NA |
| TBI 16; 13.8/F | Object against head/3.0 | 10.8 | NA | Contusion: L anterior middle frontal gyrus and L anterior superior frontal gyrus | Hemorrhagic contusion L FL, atrophy L FL |
| TBI 17; 17.6/F | Traffic accident/17.1 | 0.5 | Coma (unknown duration) | Enlarged ventricle (occipital horn), WM loss in OL/TL junction; Injuries at the level of inferior PL, OL, medial frontal gyrus, precentral gyrus, postcentral gyrus, marginal gyrus, orbitofrontal gyrus; Contusion left cerebellar hemisphere, atrophy vermis, injuries middle cerebellar peduncle; DAI in cc, R PL | Contusion L TL/OL and contrecoup injury R FL |
Abbreviations - Non-anatomy codes: TBI = traumatic brain injury; M = male; F = female; GCS = Glasgow Coma Scale score; LOC = loss of consciousness; MRI = magnetic resonance imaging; NA = not available; Anatomy codes: WM = white matter; GM = grey matter; RH = right hemisphere; LH = left hemisphere; FL = frontal lobe; TL = temporal lobe; PL = parietal lobe; OL = occipital lobe; DAI = diffuse axonal injuries; CC = corpus callosum; R = right; L = left.
2.2. Balance training program
Both TBI patients and healthy controls underwent a home-based balance training program for 8 weeks, with 5 sessions per week (30 min per session) (Drijkoningen et al., 2015a). Specifically, two portable platforms (Fysiomed NV, Edegem, Belgium) were utilized: (i) the Pro-balance system, a portable balancer that is connected to a laptop, providing real-time visual feedback (participants could choose different games, each lasting 2 min, in which they had to move their centre of gravity in order to tilt the platform forward, backward or sideways. For example, in one of the games the participants were instructed ‘to follow a river’ by moving their centre of gravity sideways to avoid rocks); and, (ii) the Balanco system, a wobble board with three interchangeable game surfaces. These two systems are portable and cost-effective balance trainers that incorporate all necessary features for effective rehabilitation, including different levels of difficulty, storage of patients’ performance data, and real-time visual feedback to aid the patients. Each training session consisted of 14 min of exercise on the Pro-balance (seven games of 2 min) and another 14 min of exercise on the Balanco.
2.3. Gross motor tests
The gross motor items of the Bruininks-Oseretsky Test of Motor Proficiency–2nd Edition (BOT-2) (Bruininks & Bruininks, 2005), the Activities-specific Balance Confidence scale (ABC scale) (Powell and Myers, 1995), and the Sensory Organisation Test (SOT) were administered at the pre-test and post-test sessions (mean ± SD = 57 ± 2 days between the two sessions) to assess the changes in ‘objective’ and ‘subjective’ gross motor performance following the intervention in the TBI patients.
The BOT-2 is a norm-referenced validated measure used with children and youth aged 4–21 years that measures fine and gross motor skills (Bruininks and Bruininks, 2005). In the present study, we administered the following two subtests of the BOT-2: (i) ‘bilateral coordination’, measuring the motor skills involved in sports and recreational activities; and (ii) ‘balance’, consisting of nine activities to evaluate motor skills integral to posture with walking, standing, and reaching. Each subtest score was mapped in the BOT-2 score tables, which take sex and age differences into account, to generate a score ranging from 1 to 35. The two subtest scores were then summed to produce the composite ‘body coordination’ standard score, which was used in the statistical analyses.
The ABC scale is a self-report outcome measure used to assess confidence during participation in daily life activities (Powell and Myers, 1995). Participants were required to indicate their level of self-confidence on a 0–100 percent Likert scale according to the following question: “How confident are you that you will not lose your balance or become unsteady while …?” In the present study, the participants rated their confidence in the ability to complete 16 activities. The dependent variable for the ABC was the average score, calculated by summing each score for each item and dividing by 16 to obtain the mean. Higher scores on the ABC scale denoted higher balance confidence.
Objective assessment of postural control was obtained using the EquiTest System (NeuroCom International, Clackamas, Oregon). The Sensory Organisation Test (SOT) protocol systematically disrupted the sensory selection processes (i.e., somatosensory, visual inputs, or both) while measuring a subject's ability to maintain postural control (see Caeyenberghs et al., 2010, Drijkoningen et al., 2015a for more detailed information). Six sensory conditions evaluated the relative contributions of vision, vestibular, and somatosensory input in balance function. The test protocol consisted of three repetitions of each condition. An equilibrium score, expressed as percentages (0 indicating sway exceeding the limits of stability and 100 indicating perfect stability) was calculated for these 6 sensory conditions. In the present study, a composite equilibrium score, describing a person's overall level of performance during all the SOT trials was calculated, with higher scores being indicative of better balance performance.
2.4. MRI data acquisition
This study was part of a large-scale MRI study in pediatric TBI, with an overall acquisition timing for the MRI protocol of 1h. Diffusion-weighted images (DWIs) (TR/TE = 8000/91 ms, voxel size = 2.2 mm isotropic, 60 contiguous slices, b = 1000 s/mm2 at 64 diffusion gradient directions, plus one b = 0 s/mm2 volume, TA = 9:06 min:s) and T1-weighted images (TR = 2300 ms, TE = 2.98 ms, voxel size = 1 × 1 × 1.1 mm3, FOV = 256 × 240, 160 contiguous sagittal slices, TA = 7:46 min:s) were collected on a 3T Siemens Trio scanner (Erlangen, Germany) with a 12-channel matrix head coil.
2.5. MRI data processing
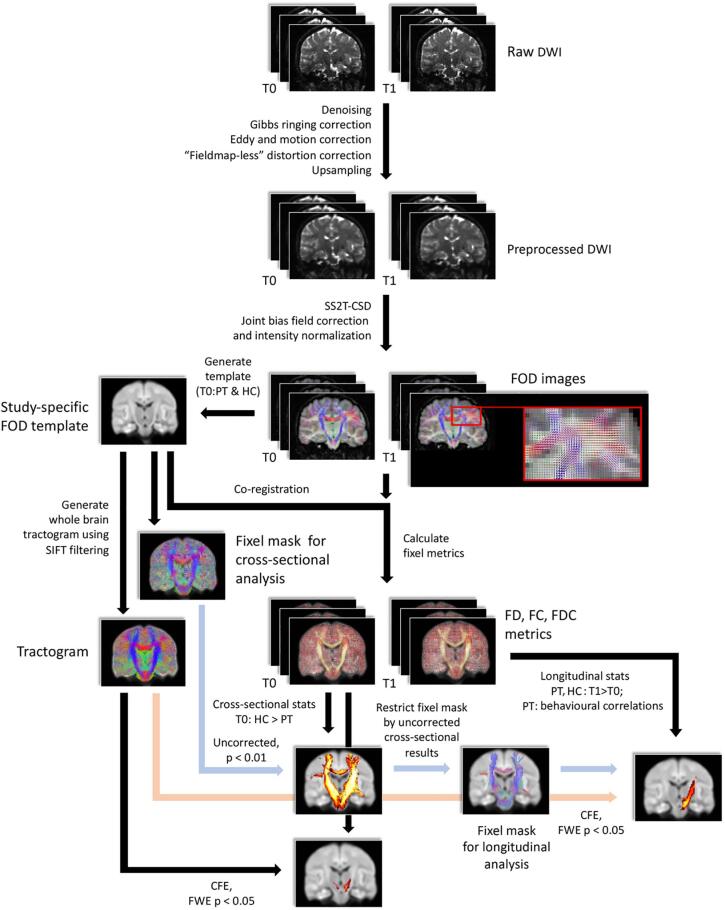
MRI data processing was performed using MRtrix3 (version RC3) (Tournier et al., 2019), mostly following the recommended procedures outlined in the MRtrix3 documentation. An overview of the data processing pipeline is shown in Fig. 1.
Fig. 1.
An overview flowchart for data processing using FBA in this study. T0: baseline; T1: follow-up; PT: TBI patients; HC: healthy controls.
DWI pre-processing – The pre-processing of each participant’s DWI data was performed using the following steps: image denoising (Veraart et al., 2016), removing Gibbs ringing artefacts (Kellner et al., 2016), intra-subject inter-volume motion and eddy current correction (Andersson and Sotiropoulos, 2016), and bias field correction (Tustison et al., 2010). Fieldmap-less susceptibility distortion correction was performed using a non-linear registration method (Wang et al., 2017) as follows: (a) T1-weighted anatomical images were registered to b = 0 images using FLIRT (FMRIB’s Linear Image Registration Tool) (Jenkinson and Smith, 2001, Jenkinson et al., 2002); (b) The b = 0 images were then registered to the aligned T1 from the previous step using a nonlinear registration approach of the Advanced Normalization Tools (ANTS) (Avants et al., 2011), yielding associated deformation fields; (c) The nonlinear warp was then subsequently applied to all the DWI data. Lastly, the pre-processed DWI data were upsampled to an isotropic voxel size of 1.3 mm for subsequent template construction and statistics of FBA (Raffelt et al., 2017).
Fixel-based analysis – From the pre-processed data, tissue-specific response functions for WM, GM, and cerebrospinal fluid (CSF) were generated using a single-shell three-tissue response function estimation approach (Dhollander and Connelly, 2016, Dhollander and Connelly, 2018). The response functions from the healthy controls were averaged to generate a unique cohort response function for each tissue type. Then, fibre orientation distributions (FODs) were reconstructed from the cohort WM and CSF response functions using the multi-tissue constrained spherical deconvolution (CSD) algorithm (Jeurissen et al., 2014). Global intensity normalization was performed to correct for intensity inhomogeneities (Raffelt et al., 2017) to enable quantitative group comparisons.
Group fixel template – A study-specific WM FOD template was created via the FOD-guided non-linear registration based on the FODs of WM from 26 participants (Raffelt et al., 2011), with 13 participants included for both healthy control and TBI groups at baseline; this ensured that the FOD template was constructed in a relatively unbiased way (as previously done in Mizuguchi et al., 2019, Rau et al., 2019). Each participant’s FODs from both sessions were then registered to the FOD template (Raffelt et al., 2011, Raffelt et al., 2012). Fixels at both template-level and individual-level were computed by performing FOD segmentation (Smith et al., 2013); fixels at the individual-level were all reoriented to the corresponding fixels of the FOD template (Raffelt et al., 2011).
Tractography – To enable connectivity-based fixel enhancement (CFE) for fixel statistics (Raffelt et al., 2015), whole-brain probabilistic tractography was performed on the FOD template. Twenty million streamlines were generated using the probabilistic iFOD2 algorithm (Tournier et al., 2010) with the following parameters: step size = 0.625 mm, maximum angle per step = 22.5°, length = 10–250 mm, FOD amplitude threshold = 0.08. The total number of streamlines was then filtered down to 2 million by applying spherical-deconvolution informed filtering of tractograms (SIFT) to reduce reconstruction biases (Smith et al., 2013).
Fixel-based metrics – Three fixel-based metrics were then computed for baseline and follow-up sessions: (i) FD: measuring fixel-wise fibre density of specific WM fibre bundles within voxels; (ii) log-FC: measuring macrostructural change of a fibre bundle in a logarithm scale; and (iii) FDC: a combined measure of FD and FC.
3. Statistical analyses
Behavioral data – Mann-Whitney U-tests were used to examine group differences at baseline in the outcomes of the BOT-2 and ABC test. To examine training-related improvements for the BOT-2 and ABC test, we conducted Wilcoxon Signed Ranks Tests within each group. For the composite equilibrium score of the SOT, a repeated measures ANOVA analysis was conducted to investigate if there was an interaction effect between Group and Time.
Baseline analyses of fixel-based metrics – Connectivity-based smoothing and statistical inference was performed using the connectivity-based fixel enhancement (CFE) approach (Raffelt et al., 2015) at the whole-brain level; non-parametric permutations tests (5000 permutations) were used to assess differences in fixel metrics between TBI patients and controls at baseline. Family-wise error (FWE)-correction was employed to control for false positives. Significant differences were defined using PFWE < 0.05, and the relevant WM tracts were identified using the JHU white-matter tractography atlas as a reference (Mori et al., 2005).
3.1. Longitudinal analyses of fixel-based metrics
We performed a ‘small fixel mask correction’ for the longitudinal analysis of each FBA metric in both TBI and healthy control groups; the small fixel masks were derived by thresholding the uncorrected p-value (fixel) map of the baseline results (using p < 0.01, retaining ~ 1/15 of the total fixels within the group FOD template). These restricted fixel masks were obtained to restrict the statistical analysis to regions that might show potential group differences (i.e., uncorrected p < 0.01) in FBA metrics at baseline. This tailored ‘small fixel mask correction’ method is similar to the traditional ‘small-volume correction’ approach, but it is more stringent as more samples (fixels in our case) are considered for statistical testing (given that we use the uncorrected p-value maps). A paired-sample t-test (using PFWE < 0.05) was then conducted to examine the effect of training on the three FBA metrics within these masks.
Considering the small-template approach might miss training effects in regions outside our analysis template, we conducted additional analyses in which we compared FBA metrics between time points at every fixel in the brain in both TBI and HC groups. Consequently, paired-sample t-tests with CFE (using PFWE < 0.05) were then conducted to examine the significance of the training effect on the three FBA metrics (i.e. FD, log-FC, and FDC) at the whole brain level in each group separately.
Correlation analysis – For the TBI group, simple regression analyses were conducted to examine the relationship between the changes in FBA metrics and the changes in the scores from the gross motor tests (BOT-2, ABC tests, and composite equilibrium scores) before and after balance training. For these analyses, the dependent variables were difference images generated for every FBA metric by subtracting baseline from follow-up images. The difference scores from the gross motor tests were the independent variable. These analyses were performed for only those variables that exhibited statistically significant improvement following training.
4. Results
4.1. Group differences and training-related changes in gross motor measures
One child in the TBI group was excluded from the BOT-2 analysis because of inability to complete the pre-test session. In addition, one child in the control group was identified as an outlier (>2SD) and therefore excluded from the analysis. The TBI group performed significantly worse on the body coordination BOT-2 subtest score (U = 55.5, p < 0.006), compared to the healthy control group (Fig. 2a). Longitudinal analyses revealed no significant change in the body coordination BOT-2 subtest score (control group: Z = -1.61, p = 0.11; TBI group: Z = -1.57, p = 0.12).
Fig. 2.
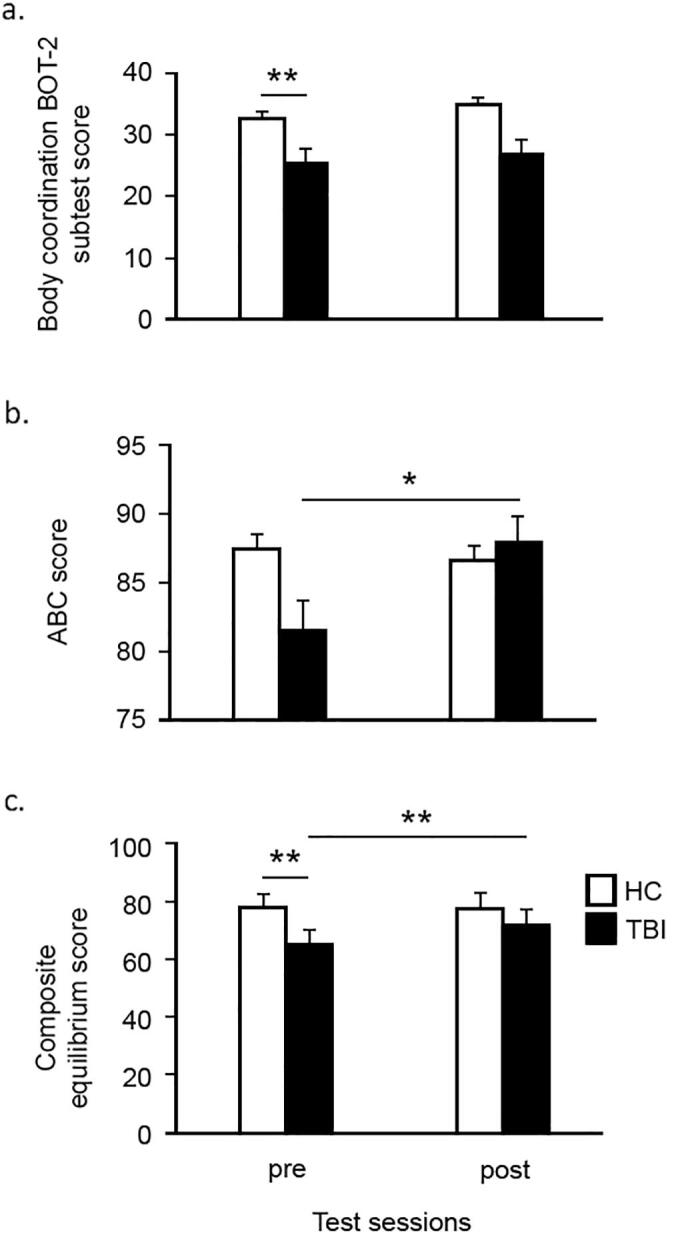
Average scores on the motor performance measures, which were administered at the pre-test and post-test session. Higher scores indicate better performance. Cross-sectional baseline analyses showed that the TBI group performed significantly worse than the controls on the body coordination BOT-2 subtest score (Fig. 2a) and the composite equilibrium score (Fig. 2c). Longitudinal analyses revealed a significant improvement for the balance confidence scale (Fig. 2b) and the composite equilibrium score (Fig. 2c). **p < 0.01, *p < 0.05.
With regards to the ABC scale, five TBI patients found it difficult to complete this questionnaire and hence the data of this scale were not collected completely in these patients. No significant group differences were found in the ABC total score (U = 65.5, p = 0.16) at pre-training. Wilcoxon Signed Ranks Tests revealed a significant improvement for this balance confidence scale for the TBI group (Z = -2.32, p < 0.021), as shown in Fig. 2b. There was no significant change in the ABC scale with training in the control group (Z = -0.05, p = 0.96).
Repeated measures ANOVA analysis of composite equilibrium scores revealed that there was a significant interaction effect, F(1,32) = 9.172, p = 0.005, between Group and Time (Fig. 2c). Post-hot tests showed a significant difference in composite equilibrium scores between HC and TBI groups at pre-test, F(1,32) = 16.411, p < 0.001; however, such a group difference was not observed after training, (F(1,32) = 2.133, p = 0.154). Also, our analysis demonstrated a significant simple effect of Time in the TBI group, F(1,16) = 11.687, p = 0.004, but not in the HC group, F(1,16) = 0.185, p = 0.673.
4.2. Cross-sectional analysis of group differences in white matter
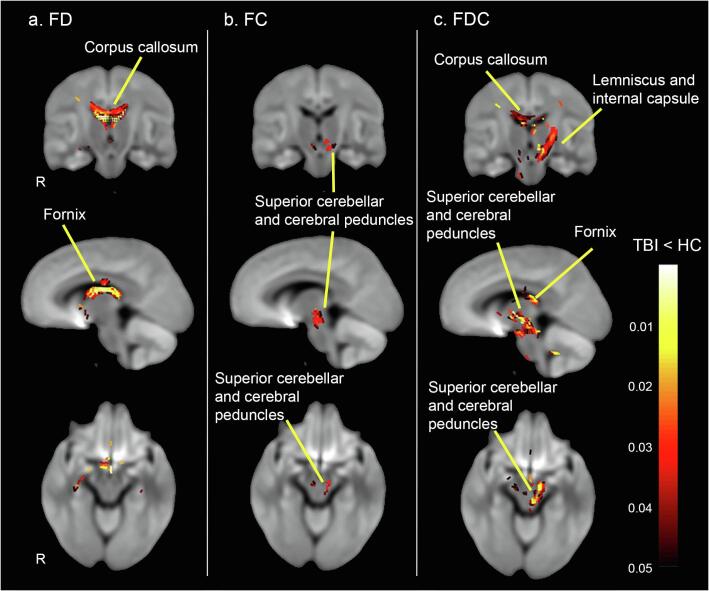
TBI patients exhibited significant decreases in all three FBA metrics, compared to controls. A substantial decrease in microstructural fibre density (FD) was observed across specific fibre pathways; most notably the fornix and the body of corpus callosum (see Fig. 3 & 4). Macrostructural decrease in fibre cross-section (log-FC) was less pronounced, but extensive in the superior cerebellar and cerebral peduncles (Fig. 3 & 4). Of note, decrease in microstructural fibre density and macrostructural fibre bundle cross-section overlapped across fibre pathways, including the left posterior limb of the internal capsule, left retrolenticular part of internal capsule and left superior corona radiata. When macrostructural and microstructural fibre differences were taken together using the FDC metric, a large decrease in FDC was observed in the body of the corpus callosum, fornix, left medial lemniscus, cerebellar and cerebral peduncles, internal capsule, external capsule, and right superior longitudinal fasciculus (Fig. 3 & 4) (all results significant at PFWE < 0.05). No significant results were found in the opposite direction (TBI > controls for FD, FC, FDC).
Fig. 3.
Significant fibre-specific white matter reductions (FWE-corrected p < 0.05) in TBI vs HC of the following three FBA metrics: (a) fibre density (FC); (b) fibre-bundle cross-section in logarithm scale (FC); and (c) the combined measure of a fibre density and cross-section (FDC) in TBI patients compared to control group at baseline (i.e. pre-test). Streamlines were color-coded by p values in TBI cohort compared to healthy controls. Of note, only selected tracts were labelled; see results section for full list of tracts.
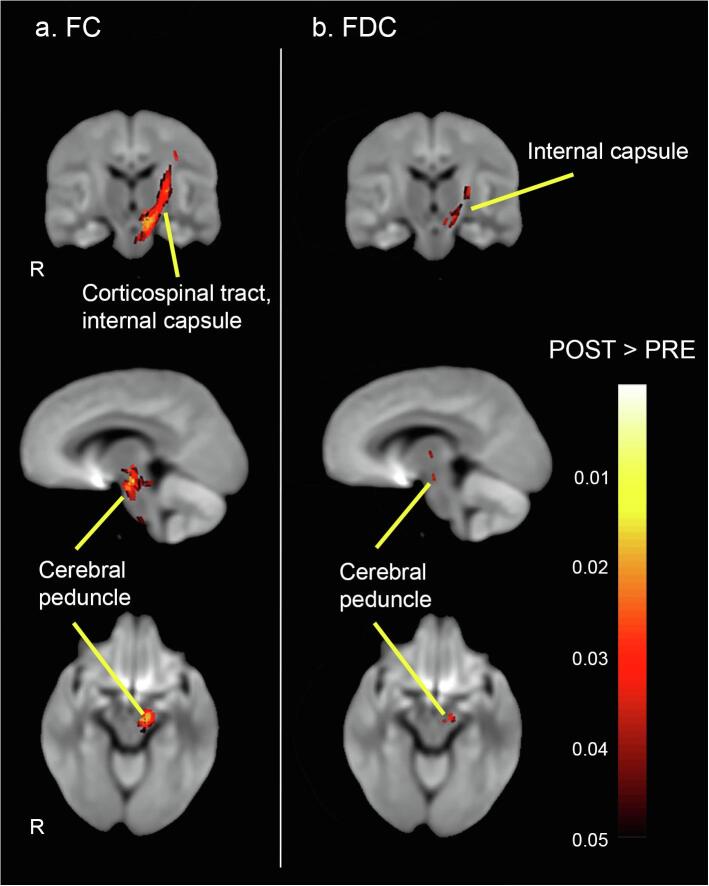
4.3. Training-related changes in white matter
Using small fixel mask correction, results of the paired t-tests revealed significantly increased (PFWE < 0.05) log-FC and FDC in TBI patients after receiving balance training (see Fig. 5). Specifically, increase in the macrostructural fibre bundle cross-section (log-FC) was found in the left corticospinal tract, left cerebral peduncle, left posterior limb of internal capsule, left retrolenticular part of internal capsule, left superior corona radiata, and left external capsule. Moreover, for the combined measure of FDC, an increase was found in the left cerebral peduncle, left posterior limb of internal capsule, and left retrolenticular part of internal capsule. Of note, no significant changes in the microstructural fibre density (FD) following balance training could be observed in the TBI patients. In addition, no significant changes were observed in the healthy control group on any metric. The whole-brain FBA analysis (PFWE < 0.05) did not show significant changes in the FBA metrics following balance training in either the TBI patients or the healthy control group (see Supplemental Material).
Fig. 5.
Significant FBA metrics increases following training (FWE-corrected p < 0.05 with small template correction): (a) Log-fc; and (b) FDC in TBI group receiving balance training comparing to pre-test. Streamlines were color-coded by p values in post-training TBI cohort compared to pre-training TBI cohort. Of note, only selected tracts were labelled, see results section for full list of tracts.
4.4. Correlations between training-induced changes in postural control and changes in fixel metrics in TBI patients
No significant associations were observed between the changes in WM and the improvement on the balance confidence scale, the body coordination BOT-2 subtest score, or the composite equilibrium score on the SOT (see Supplemental Material).
5. Discussion
In this study, we undertook a fixel-based analysis to identify the location and extent of microstructural and macrostructural alterations of WM in young moderate-to-severe TBI patients before and after balance training. Prior to training, we found lower fibre density and fibre cross-section in TBI patients compared to the control group across several commissural tracts, association fibres and projection fibres. Following training, TBI patients exhibited significant increases in macrostructural WM measures in left sensorimotor tracts. In addition, we found significant improvement on postural control, but this improvement was not associated with the observed recovery in FBA metrics.
5.1. Cross-sectional group differences and training-related changes in gross motor measures
Consistent with previous studies (e.g., Chaplin et al., 1993, Geurts et al., 1996, King et al., 2014, Sosnoff et al., 2011), we demonstrated gross motor performance impairment in young TBI patients compared with controls before training. Specifically, we observed performance on the body coordination BOT-2 subtest score was worse in the TBI patients compared to the healthy controls. With regards to training-related changes in body coordination, our results showed no significant improvements for the BOT-2 subtest score. We suggest that the nature of the motor test might have hindered the detection of changes in motor performance. Despite the fact that clinical motor test batteries include the whole movement repertoire, quantitative biomechanical measure systems (such as the Neurocom system) achieve a higher degree of validity and reliability to detect subtle changes in postural control (Caeyenberghs et al., 2010, Caeyenberghs et al., 2011), as discussed below.
Utilising the ABC scale, we examined the balance confidence of the TBI patients. The scoring of the ABC scale is not based on physical performance, but rather is determined by the patient’s self-report as to whether they feel confident in performing various activities without losing balance or experiencing a sense of unsteadiness. While improved balance confidence has been demonstrated in previous balance training studies in the elderly (e.g., Duque et al., 2013, Hafstrom et al., 2016), the psychometric properties of the ABC scale have not yet been established for paediatric populations, and there is only limited research supporting the ABC scale as a suitable measure for assessing balance confidence in adolescents with concussion (Alsalaheen et al., 2014, Alsalaheen et al., 2016). Moreover, we used a cohort of HC participants as reference group, rather than a TBI group with treatment-as-usual or a placebo treatment, which limits interpretation of the efficacy of the intervention. Further constraining interpretation, subjective measures such as the ABC scale are more vulnerable to placebo effects than objective measures (Schwarz and Büchel, 2015), and it is conceivable that a clinical population, TBI-patients in the present case, may be more susceptible to such an effect than healthy controls.
Our behavioural findings identified a significant interaction effect between Group and Time on composite equilibrium scores, showing that balance training led to significantly different behavioural outcomes between HC and TBI groups on this measure. Consistent with the finding in a previous study (Drijkoningen et al., 2015a), we found a significant increase of the composite equilibrium score in the TBI group following training; as expected, such an improvement was not observed in the HC group. Meanwhile, we found evidence of disrupted somatosensory processing in terms of a significantly decreased composite equilibrium score in TBI relative to controls at pre-test, which is consistent with previous studies (Caeyenberghs et al., 2010, Gagnon et al., 2004); results from post-hoc tests revealed this difference between HC and TBI groups did no longer exist, corroborating the efficacy of the balance training in improving balance performance. Taken together, these findings provide behavioural evidence that balance training is effective in promoting balance recovery in TBI patients.
5.2. Cross-sectional group differences in white matter
Whole-brain fixel-wise analysis revealed microstructural (FD) and macrostructural (log-FC) alterations in the cerebellar peduncles in the TBI group compared to controls. These observations corroborate findings from our previous study, where we used an ROI approach, of altered DTI measures (specifically, lower fractional anisotropy and increased mean diffusivity) in the inferior cerebellar peduncle, middle cerebellar peduncle and superior cerebellar peduncle (Drijkoningen et al., 2015a). By applying a whole-brain analysis, we were able to observe microstructural & macrostructural WM changes in multiple regions beyond the cerebellar peduncles. Specifically, our findings revealed that the TBI group had significantly lower FD, FC and FDC in several WM tracts, including the commissural tracts (corpus callosum, fornix), association fibres (e.g., superior longitudinal fasciculus), and projection fibres (e.g., internal capsule, medial lemniscus, superior corona radiata, external capsule). These results are in line with previous diffusion MRI studies of pediatric TBI (including work from our group), utilising metrics derived from DTI (for recent reviews, see Lindsey et al., 2019, Wallace et al., 2018b, Wallace et al., 2018a, Zamani et al., 2020). However, previous studies have demonstrated that FBA can offer more specific, directly interpretable, and within-voxel white matter metrics, which contrasts with other widespread diffusion imaging frameworks, like DTI, that are less specific and cannot exploit sub-voxel level information (for example, DTI cannot resolve crossing fibres and hence not all white matter voxels can be accurately modelled within the voxel-based analysis framework; see Jeurissen et al., 2013; Mito et al., 2018, Raffelt et al., 2015). FBA is therefore able to provide a more complete picture of WM damage.
The decreased values in all three FBA metrics indicate that there are both reductions in the density of axons (fewer axons) within these WM tracts and a smaller cross-sectional area that the WM tracts occupy (fewer voxels) (Raffelt et al., 2017). Future studies are needed to validate the fixel-based metrics against gold standard histological measures of fibre density and fibre cross-section for a better understanding of the cellular mechanisms underlying the fixel metrics alterations in patients with TBI.
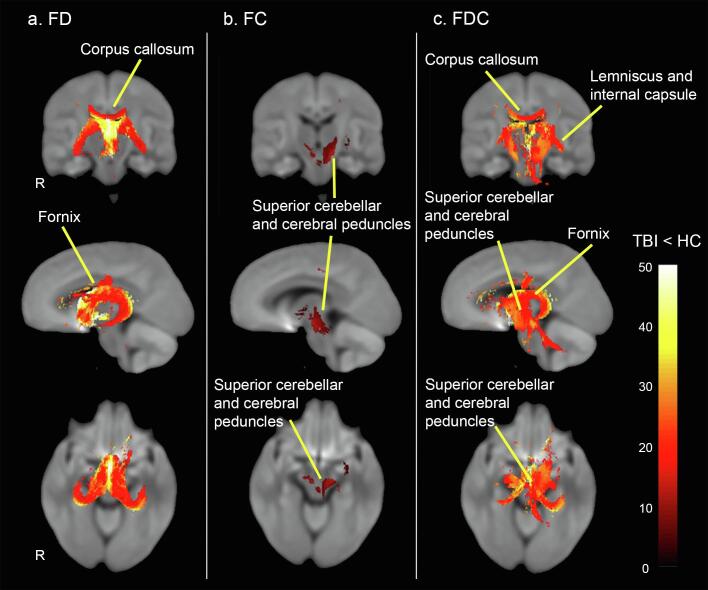
FD and FC exhibited a largely overlapping pattern of deterioration. In other words, the fixels with decreased FD overlapped with fixels presenting reduced FC, including the left posterior limb of internal capsule, left retrolenticular part of internal capsule and left superior corona radiata. However, the microstructural fibre density (FD) metric identified additional tracts, such as the fornix and the body of the corpus callosum. These findings suggest that this FBA metric may be more sensitive and specific to WM changes in patients with TBI. This suggestion is confirmed by the calculated percentage change in the fixel metrics (maximum percentage change of ~ 50% for the FD metric, ~14% for the FC metric, and ~ 40% for the FDC metric, as can be seen in Fig. 3, Fig. 4). These findings, however, should be interpreted with caution given the relatively low b-value used in the present study (see Limitations section).
Fig. 4.
Percentage (%) decreases relative to controls. Streamlines passing through significant fixels (FWE-corrected p < 0.05) were cropped from whole-brain tractogram, which were then colored by percentage (%) decrease in TBI cohort compared to healthy controls. Of note, only selected tracts were labelled; see results section for full list of tracts. Tracts are rendered in 3D overlayed on 2D slices of the brain.
Only two other FBA studies in TBI patients have been published to date (Verhelst et al., 2019, Wallace et al., 2020). Our results are in line with the study of Wallace and colleagues (2020), who tested a large TBI sample (Nmild = 133, and Nmoderate-severe = 29), and found decreased FD, FC and FDC in the moderate-to-severe TBI subgroup, with the strongest reductions in the cerebral peduncle, and internal and external capsule. Verhelst et al. (2019) only examined the FC metric due to the low diffusion weighting (b = 1200 s/mm2) in their scanning protocol, and reported an increased FC in the body of the corpus callosum and the left superior longitudinal fasciculus in their TBI patients. This increased FC was interpreted as inflammation-related swelling. The discrepancy between Verhelst et al. findings and both Wallace et al. and our results may be due to the difference in chronicity of the TBI samples (mean and SD time since injury of the present study 3.34y ± 3.29y compared to 2.3y ± 1y in the study of Verhelst).
FBA employs a brain mask in the study-specific template space to define the regions to conduct the comparisons. This mask represents the intersection of every individual’s mask in the study cohort, and therefore the template FOD field could become fragmentary if lesion masks are heterogeneous among patients. As such, it is common practice to compare whole-brain fixels between groups in the current FBA studies without masking out lesioned areas during data processing (e.g., Egorava et al., 2020). Since our TBI cohort was also comprised of patients with heterogeneous lesion locations, lesion masking was not applied in this study. Nevertheless, we consider that the obtained changes of the FBA metrics are reasonably reliable for the following reasons: (1) Family-wise error-corrected p-values were used to compute the alterations of FBA metrics for each fixel with non-parametric permutation testing (Raffelt et al., 2015), which might be able to control false positives induced by the lesion distribution. (2) For mild-to-moderate lesion severity, the multi-tissue CSD algorithm could improve the reconstruction of white matter FOD around and within a lesion (Jeurissen et al., 2014), which could potentially alleviate the impact of the lesion on, e.g., the FOD registration step (Egorava et al., 2020).
Admittedly, as lesions are not masked out for cohorts with heterogeneous lesions, it could be difficult to differentiate whether the significant differences in FBA metrics are the result of focal lesions or diffuse neurodegeneration along certain white matter pathways. This is a possible limitation of the FBA method in analyses of lesioned diffusion MRI data. While having a lesion map could potentially help to address such an issue, generating a reliable lesion map in TBI patients is not always achievable. This is because lesion delineation per se could be challenging, for instance, lesions in our moderate TBI patients may be difficult to be accurately segmented, an issue exacerbated by some injuries being only visible in some types of scans that are not always available. Development of detailed lesion mapping protocols for TBI patients may help to conduct a more thorough examination into the impact of lesions on FBA metrics.
5.3. Training-related changes in white matter
The key finding in this study was significant training-induced alterations in FC in the TBI group. Specifically, our results revealed significantly increased cross-section of fibre bundles in TBI patients following balance training in the afferent and efferent tracts, including the left corticospinal tract, left cerebral peduncle, left posterior limb of internal capsule, left retrolenticular part of internal capsule, left superior corona radiata, and left external capsule. No significant training-induced alterations in the microstructural fibre density (FD) metric could be identified with the FBA framework. These findings suggest that the balance training intervention had an effect on the cross-sectional size of the fibre bundles. Our results are in contrast with the studies of Verhelst et al., 2019, Mizuguchi et al., 2019, who found no effects. This may be explained by the insufficient effect of the training program (in Verhelst et al., 2019) or the duration of the training program (2 days of learning of the whole-body serial reaction time task in Mizuguchi et al., 2019).
Our results showed that the decreased log-FC and FDC in TBI patients was predominantly left-lateralized in brain regions at pre-test (Fig. 3&4), meanwhile the post-training analysis also indicated a lateralized restoration of white matter structures, which took place primarily within the compromised regions and/or surrounding areas (Fig. 5). The overlap between pre- and post-test findings could reflect the post-training induced white matter restoration. Specifically, the reasons for such a lateralization effect (i.e. no significant changes in the right hemisphere) include: Firstly, the lesion location of the left-hemispheric TBI patients is rather more homogeneous than the right-hemispheric cases within our cohort; the effect of lesion on WM changes could therefore be stronger surrounding the lesion site in the left hemisphere when comparing healthy controls to TBI patients at baseline (i.e. more number of fixels showed significant log-FC and FDC decrease in the left than right hemisphere; Fig. 3). However, this does suggest that a larger sample size is required to increase the statistical power to verify whether TBI causes lateralized WM degeneration. Secondly, regarding the lateralisation of the training effect (Fig. 5), our inference is that the contribution of white matter restoration is more prevalent in the ipsilateral than the contralateral hemisphere. As shown by previous studies, the restoration occurs in the first place within the nearby brain structures that likely share similar functions; if within-hemisphere repairs are not possible (e.g. for very severe cases), contralateral compensations could be triggered (Murphy and Corbett, 2009, Mohajerani et al., 2011). Therefore, the lateralized findings in the present study might be due to the fact that the compromised white matter structures had been reasonably compensated by the spared perilesional regions, without the necessity of triggering contralateral compensations, given that our cohort includes mainly moderate TBI patients.
On the other hand, the observed patterns of training-induced WM changes may be specific to young TBI patients due to the higher compensatory plasticity following TBI in childhood and adolescence (Anderson et al., 2011, Noack et al., 2009, Oberman and Pascual-Leone, 2013, Spear, 2013) and may not be directly generalizable to an older sample. Therefore, future studies need to include age group comparisons to investigate whether the pattern of training-induced WM changes in young TBI patients is more extensive than in a sample of adult TBI patients.
Interestingly, our training-related changes in FC could be identified using the small fixel mask correction approach, which is more stringent than the typical small-volume correction but still focuses analysis on areas of difference between TBI and healthy controls pre-treatment (Roiser et al., 2016). On the other hand, compared to a whole-brain level correction, this approach can provide enhanced sensitivity in detecting significant findings, which is especially useful for studies that are inherently limited by sample size, due mainly to recruitment difficulties. Note that this approach does require a reasonable hypothesis or prior knowledge to define the spatially restricted fixels-of-interest.
Finally, we sought to determine if the observed improvements in balance confidence had a linear relationship with the changes in WM. We found no significant correlations between difference scores in FBA metrics and behavioural changes. Numerous neuroplasticity studies have failed to observe relationships between neuroplastic changes and clinical changes (for a review, see Caeyenberghs et al., 2018). Our negative findings might be due to the relatively small sample size in this study, which may have led to low statistical power for the correlation analyses (Button et al., 2013). Future studies are needed to investigate the relationships between FBA metrics and behavioural measures in larger sample sizes, as well as to examine nonlinear relationships between fixel findings and clinical changes.
6. Limitations
Our study has several limitations. An important limitation is the relatively small sample size, which hindered our ability to test specific hypotheses regarding the role of specific demographic variables (e.g., age) and injury-related variables (such as age at injury and time since injury) on the gross motor performance changes and training-related structural plasticity changes. Through the ENIGMA pediatric moderate/severe TBI group (Dennis et al., 2020a, Dennis et al., 2020b), we aim to improve statistical power to address these open questions (for a paper in preprint, see Dennis et al., 2020a, Dennis et al., 2020b). Another limitation, which is shared with other current FBA studies, is the heterogeneous nature of TBI lesions. However, our FBA analysis was still able to detect cross-sectional and longitudinal effects, manifested as differences (between groups or over time) in measures of tissue micro- and macrostructure. Future studies are required to examine the impact of different lesions (large focal lesions, diffuse axonal injury, T2 hyperintensities, inflammation) on the different FBA metrics.
Another limitation is that the nature of our findings of the longitudinal analyses were mainly within-group effects as opposed to interaction effects between time and group. Although there was an effect of training, the effects of balance training on fixel metrics were not compared to an active control group of TBI patients undergoing a different (or no) training program (Thomas & Baker, 2013). In addition, future longitudinal studies will benefit from multiple follow-up assessments, which should help capture in greater detail whether the WM alterations remain after the completion of the training.
While our diffusion MRI data have been acquired using sufficient angular resolution (i.e., 64 directions), the relatively low acquired b-value (b = 1000 s/mm2) can result in incomplete suppression of extra-cellular water, thus limiting the interpretation of findings related with fibre density (Raffelt et al., 2012, Tournier et al., 2013). Future studies should employ a more suitable acquisition with higher b-values and more interleaved b-zeros. Another limitation of our scanning sequence was that the reverse-phase encoded images were not available. However, we performed field map-less correction of susceptibility induced distortions via non-linear registration of the diffusion images to the T1-weighted images.
7. Conclusion
This study provides a profile of longitudinal white matter alterations in young TBI patients following balance training. We observed reductions in white matter across several commissural tracts, association fibres and projection fibres in the TBI group. More importantly, our longitudinal analyses revealed that restoration of these white matter fibres was mainly in the left-hemispheric sensorimotor tracts following balance training, potentially resulting from the increased neural activation in these tracts. Our results provide a deeper understanding of the neurobiological mechanisms underlying training, which might assist clinicians in making more effective treatment decisions in everyday clinical practice of brain-injured patients.
CRediT authorship contribution statement
Xiaoyun Liang: Conceptualization, Methodology, Software, Formal analysis, Writing - original draft, Writing - review & editing, Visualization. Chun-Hung Yeh: Methodology, Software, Writing - review & editing. Juan F. Domínguez D: Conceptualization, Methodology, Writing - original draft, Writing - review & editing, Visualization. Govinda Poudel: Conceptualization, Methodology, Writing - original draft, Writing - review & editing. Stephan P. Swinnen: Funding acquisition, Supervision, Project administration, Conceptualization, Writing - review & editing. Karen Caeyenberghs: Funding acquisition, Investigation, Supervision, Conceptualization, Formal analysis, Writing - original draft, Writing - review & editing.
Acknowledgements
This work was supported by a grant from the Research Program of the Research Foundation Flanders (FWO) (Levenslijn G.0482.10). KC is supported by a National Health and Medical Research Council Career Development Fellowship (APP1143816). XL and GP are funded by an Australian Catholic University Research Funding (ACURF) Program Grant. CHY is grateful to the Ministry of Science and Technology of Taiwan (MOST 109-2222-E-182-001-MY3) for the support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102621.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Alsalaheen B.A., Whitney S.L., Marchetti G.F., Furman J.M., Kontos A.P., Collins M.W., Sparto P.J. Performance of high school adolescents on functional gait and balance measures. Pediatr. Phys. Ther. 2014;26(2):191–199. doi: 10.1097/PEP.0000000000000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsalaheen B.A., Whitney S.L., Marchetti G.F., Furman J.M., Kontos A.P., Collins M.W., Sparto P.J. Relationship between cognitive assessment and balance measures in adolescents referred for vestibular physical therapy after concussion. Clin. J. Sport Med. 2016;26(1):46–52. doi: 10.1097/JSM.0000000000000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson V., Spencer-Smith M., Wood A. Do children really recover better? Neurobehavioural plasticity after early brain insult. Brain. 2011;134(Pt 8):2197–2221. doi: 10.1093/brain/awr103. [DOI] [PubMed] [Google Scholar]
- Andersson J.L.R., Sotiropoulos S.N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–1078. doi: 10.1016/j.neuroimage.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B.B., Tustison N.J., Song G., Cook P.A., Klein A., Gee J.C. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54(3):2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruininks R, Bruininks B. Bruininks-Oseretsky Test of Motor Proficiency. 2nd Ed. NCS Pearson; Minneapolis, MN: 2005. [Google Scholar]
- Burciu R.G., Fritsche N., Granert O., Schmitz L., Sponemann N., Konczak J., Theysohn N., Gerwig M., van Eimeren T., Timmann D. Brain changes associated with postural training in patients with cerebellar degeneration: a voxel-based morphometry study. J. Neurosci. 2013;33(10):4594–4604. doi: 10.1523/JNEUROSCI.3381-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button K.S., Ioannidis J.P., Mokrysz C., Nosek B.A., Flint J., Robinson E.S., Munafo M.R. Confidence and precision increase with high statistical power. Nat. Rev. Neurosci. 2013;14(8):585–586. doi: 10.1038/nrn3475-c4. [DOI] [PubMed] [Google Scholar]
- Caeyenberghs K., Clemente A., Imms P., Egan G., Hocking D.R., Leemans A., Metzler-Baddeley C., Jones D.K., Wilson P.H. Evidence for training-dependent structural neuroplasticity in brain-injured patients: A critical review. Neurorehabil. Neural Repair. 2018;32(2):99–114. doi: 10.1177/1545968317753076. [DOI] [PubMed] [Google Scholar]
- Caeyenberghs K., Leemans A., De Decker C., Heitger M., Drijkoningen D., Linden C.V., Sunaert S., Swinnen S.P. Brain connectivity and postural control in young traumatic brain injury patients: A diffusion MRI based network analysis. Neuroimage Clin. 2012;1(1):106–115. doi: 10.1016/j.nicl.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caeyenberghs K., Leemans A., Geurts M., Linden C.V., Smits-Engelsman B.C., Sunaert S., Swinnen S.P. Correlations between white matter integrity and motor function in traumatic brain injury patients. Neurorehabil. Neural Repair. 2011;25(6):492–502. doi: 10.1177/1545968310394870. [DOI] [PubMed] [Google Scholar]
- Caeyenberghs K., Leemans A., Geurts M., Taymans T., Linden C.V., Smits-Engelsman B.C., Sunaert S., Swinnen S.P. Brain-behavior relationships in young traumatic brain injury patients: DTI metrics are highly correlated with postural control. Hum. Brain Mapp. 2010;31(7):992–1002. doi: 10.1002/hbm.20911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin D., Deitz J., Jaffe K.M. Motor performance in children after traumatic brain injury. Arch. Phys. Med. Rehabil. 1993;74(2):161–164. https://www.ncbi.nlm.nih.gov/pubmed/8431100 Retrieved from. [PubMed] [Google Scholar]
- Dennis E.L., Baron D., Bartnik-Olson B., Caeyenberghs K., Esopenko C., Hillary F.G., Wilde E.A. ENIGMA brain injury: Framework, challenges, and opportunities. Hum. Brain Mapp. 2020 doi: 10.1002/hbm.25046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis E.L., Caeyenberghs K., Asarnow R.F., Babikian T., Bartnik-Olson B., Bigler E.D., Wilde E.A. Challenges and opportunities for neuroimaging in young patients with traumatic brain injury: a coordinated effort towards advancing discovery from the ENIGMA pediatric moderate/severe TBI group. Brain Imaging Behav. 2020 doi: 10.1007/s11682-020-00363-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhollander, T. R., D.; Connelly, A. (2016). Unsupervised 3-tissue response function estimation from single-shell or multi-shell diffusion MR data without a co-registered T1 image. Paper presented at the ISMRM Workshop on Breaking the Barriers of Diffusion MRI, Lisbon, Portugal.
- Dhollander T.R.D., Connelly A. Paper presented at the 26th International Society of Magnetic Resonance in Medicine. 2018. Accuracy of response function estimation algorithms for 3-tissue spherical deconvolution of diverse quality diffusion MRI data. [Google Scholar]
- Drijkoningen D., Caeyenberghs K., Leunissen I., Vander Linden C., Leemans A., Sunaert S., Duysens J., Swinnen S.P. Training-induced improvements in postural control are accompanied by alterations in cerebellar white matter in brain injured patients. Neuroimage Clin. 2015;7:240–251. doi: 10.1016/j.nicl.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drijkoningen D., Caeyenberghs K., Vander Linden C., Van Herpe K., Duysens J., Swinnen S.P. Associations between Muscle Strength Asymmetry and Impairments in Gait and Posture in Young Brain-Injured Patients. J. Neurotrauma. 2015;32(17):1324–1332. doi: 10.1089/neu.2014.3787. [DOI] [PubMed] [Google Scholar]
- Drijkoningen D., Chalavi S., Sunaert S., Duysens J., Swinnen S.P., Caeyenberghs K. Regional gray matter volume loss is associated with gait impairments in young brain-injured individuals. J. Neurotrauma. 2017;34(5):1022–1034. doi: 10.1089/neu.2016.4500. [DOI] [PubMed] [Google Scholar]
- Duque G., Boersma D., Loza-Diaz G., Hassan S., Suarez H., Geisinger D., Suriyaarachchi P., Sharma A., Demontiero O. Effects of balance training using a virtual-reality system in older fallers. Clin. Interv. Aging. 2013;8:257–263. doi: 10.2147/CIA.S41453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorova N., Dhollander T., Khlif M.S., Khan W., Werden E., Brodtmann A. Pervasive white matter fiber degeneration in ischemic stroke. Stroke. 2020;51(5):1507–1513. doi: 10.1161/STROKEAHA.119.028143. [DOI] [PubMed] [Google Scholar]
- Gagnon I., Swaine B., Friedman D., Forget R. Children show decreased dynamic balance after mild traumatic brain injury. Arch Phys Med Rehabil. 2004;85(3):444–452. doi: 10.1016/j.apmr.2003.06.014. [DOI] [PubMed] [Google Scholar]
- Gale S.D., Baxter L., Roundy N., Johnson S.C. Traumatic brain injury and grey matter concentration: a preliminary voxel based morphometry study. J. Neurol. Neurosurg. Psychiatry. 2005;76(7):984–988. doi: 10.1136/jnnp.2004.036210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts A.C., Ribbers G.M., Knoop J.A., van Limbeek J. Identification of static and dynamic postural instability following traumatic brain injury. Arch. Phys. Med. Rehabil. 1996;77(7):639–644. doi: 10.1016/s0003-9993(96)90001-5. [DOI] [PubMed] [Google Scholar]
- Hafstrom A., Malmstrom E.M., Terden J., Fransson P.A., Magnusson M. Improved balance confidence and stability for elderly after 6 weeks of a multimodal self-administered balance-enhancing exercise program: a randomized single arm crossover study. Gerontol Geriatr Med. 2016;2 doi: 10.1177/2333721416644149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Smith S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jeurissen B., Leemans A., Tournier J.D., Derek J., Sijbers J. Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Human Brain Mapping. 2013;34(11):2747–2766. doi: 10.1002/hbm.22099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeurissen B., Tournier J.D., Dhollander T., Connelly A., Sijbers J. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. Neuroimage. 2014;103:411–426. doi: 10.1016/j.neuroimage.2014.07.061. [DOI] [PubMed] [Google Scholar]
- Kellner E., Dhital B., Kiselev V.G., Reisert M. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn. Reson. Med. 2016;76(5):1574–1581. doi: 10.1002/mrm.26054. [DOI] [PubMed] [Google Scholar]
- Kim J., Avants B., Patel S., Whyte J., Coslett B.H., Pluta J., Detre J.A., Gee J.C. Structural consequences of diffuse traumatic brain injury: a large deformation tensor-based morphometry study. Neuroimage. 2008;39(3):1014–1026. doi: 10.1016/j.neuroimage.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King L.A., Horak F.B., Mancini M., Pierce D., Priest K.C., Chesnutt J., Sullivan P., Chapman J.C. Instrumenting the balance error scoring system for use with patients reporting persistent balance problems after mild traumatic brain injury. Arch. Phys. Med. Rehabil. 2014;95(2):353–359. doi: 10.1016/j.apmr.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey H.M., Lalani S.J., Mietchen J., Gale S.D., Wilde E.A., Faber J., MacLeod M.C., Hunter J.V., Chu C.D., Aitken M.E., Ewing-Cobbs L., Levin H.S. Acute pediatric traumatic brain injury severity predicts long-term verbal memory performance through suppression by white matter integrity on diffusion tensor imaging. Brain Imaging Behav. 2019 doi: 10.1007/s11682-019-00093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malec J.F., Brown A.W., Leibson C.L., Flaada J.T., Mandrekar J.N., Diehl N.N., Perkins P.K. The mayo classification system for traumatic brain injury severity. J. Neurotrauma. 2007;24(9):1417–1424. doi: 10.1089/neu.2006.0245. [DOI] [PubMed] [Google Scholar]
- Mito R., Raffelt D., Dhollander T., Vaughan D.N., Tournier J.D., Salvado O., Brodtmann A., Rowe C.C., Villemagne V.L., Connelly A. Fibre-specific white matter reductions in Alzheimer’s disease and mild cognitive impairment. Brain. 2018;141(3):888–902. doi: 10.1093/brain/awx355. [DOI] [PubMed] [Google Scholar]
- Mizuguchi N., Maudrich T., Kenville R., Carius D., Maudrich D., Villringer A., Ragert P. Structural connectivity prior to whole-body sensorimotor skill learning associates with changes in resting state functional connectivity. Neuroimage. 2019;197:191–199. doi: 10.1016/j.neuroimage.2019.04.062. [DOI] [PubMed] [Google Scholar]
- Mohajerani M.H., Aminoltejari K., Murphy T.H. Targeted mini-strokes produce changes in interhemispheric sensory signal processing that are indicative of disinhibition within minutes. Proc. Natl. Acad. Sci. USA. 2011;108(22):E183–191. doi: 10.1073/pnas.1101914108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S.W., Nagae-Poetscher L.M., van Zijl P.C. Elsevier; Amsterdam The Netherlands: 2005. MRI atlas of Human White Matter. [Google Scholar]
- Murphy T.H., Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat. Rev. Neurosci. 2009;10(12):861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- Noack H., Lovden M., Schmiedek F., Lindenberger U. Cognitive plasticity in adulthood and old age: gauging the generality of cognitive intervention effects. Restor. Neurol. Neurosci. 2009;27(5):435–453. doi: 10.3233/RNN-2009-0496. [DOI] [PubMed] [Google Scholar]
- Oberman L., Pascual-Leone A. Changes in plasticity across the lifespan: cause of disease and target for intervention. Prog. Brain Res. 2013;207:91–120. doi: 10.1016/B978-0-444-63327-9.00016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson, M. G., B. D. (2011). Balance Problems after Traumatic Brain Injury. In M. S. K. T. Center (Ed.). [DOI] [PubMed]
- Poudel G.R., Dominguez D.J., Verhelst H., Vander Linden C., Deblaere K., Jones D.K., Cerin E., Vingerhoets G., Caeyenberghs K. Network diffusion modeling predicts neurodegeneration in traumatic brain injury. Ann. Clin. Transl. Neurol. 2020;7(3):270–279. doi: 10.1002/acn3.50984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell L.E., Myers A.M. The activities-specific balance confidence (ABC) Scale. J. Gerontol. A Biol. Sci. Med. Sci. 1995;50A(1):M28–34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- Prosperini L., Fanelli F., Petsas N., Sbardella E., Tona F., Raz E., Fortuna D., De Angelis F., Pozzilli C., Pantano P. Multiple sclerosis: changes in microarchitecture of white matter tracts after training with a video game balance board. Radiology. 2014;273(2):529–538. doi: 10.1148/radiol.14140168. [DOI] [PubMed] [Google Scholar]
- Raffelt D.A., Tournier J.D., Fripp J., Crozier S., Connelly A., Salvado O. Symmetric diffeomorphic registration of fibre orientation distributions. Neuroimage. 2011;56(3):1171–1180. doi: 10.1016/j.neuroimage.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Raffelt D.A., Tournier J.D., Rose S., Ridgway G.R., Henderson R., Crozier S., Salvado O., Connelly A. Apparent Fibre Density: a novel measure for the analysis of diffusion-weighted magnetic resonance images. Neuroimage. 2012;59(4):3976–3994. doi: 10.1016/j.neuroimage.2011.10.045. [DOI] [PubMed] [Google Scholar]
- Raffelt D.A., Smith R.E., Ridgway G.R., Tournier J.D., Vaughan D.N., Rose S., Henderson R., Connelly A. Connectivity-based fixel enhancement: Whole-brain statistical analysis of diffusion MRI measures in the presence of crossing fibres. Neuroimage. 2015;117:40–55. doi: 10.1016/j.neuroimage.2015.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffelt D.A., Tournier J.D., Smith R.E., Vaughan D.N., Jackson G., Ridgway G.R., Connelly A. Investigating white matter fibre density and morphology using fixel-based analysis. Neuroimage. 2017;144(Pt A):58–73. doi: 10.1016/j.neuroimage.2016.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau Y.A., Wang S.M., Tournier J.D., Lin S.H., Lu C.S., Weng Y.H., Chen Y.L., Ng S.H., Yu S.W., Wu Y.M., Tsai C.C., Wang J.J. A longitudinal fixel-based analysis of white matter alterations in patients with Parkinson’s disease. Neuroimage Clin. 2019;24 doi: 10.1016/j.nicl.2019.102098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R.M., Mathias J.L., Rose S.E. Diffusion Tensor Imaging (DTI) findings following pediatric non-penetrating TBI: a meta-analysis. Dev Neuropsychol. 2014;39(8):600–637. doi: 10.1080/87565641.2014.973958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiser J.P., Linden D.E., Gorno-Tempinin M.L., Moran R.J., Dickerson B.C., Grafton S.T. Minimum statistical standards for submissions to Neuroimage: Clinical. Neuroimage Clin. 2016;12:1045–1047. doi: 10.1016/j.nicl.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz, K. A., & Büchel, C. (2015). Cognition and the Placebo Effect - Dissociating Subjective Perception and Actual Performance. PLoS One, 10(7):e0130492. doi: 10.1371/journal.pone.0130492. eCollection 2015. [DOI] [PMC free article] [PubMed]
- Salmond C.H., Chatfield D.A., Menon D.K., Pickard J.D., Sahakian B.J. Cognitive sequelae of head injury: involvement of basal forebrain and associated structures. Brain. 2005;128(Pt 1):189–200. doi: 10.1093/brain/awh352. [DOI] [PubMed] [Google Scholar]
- Sehm B., Taubert M., Conde V., Weise D., Classen J., Dukart J., Draganski B., Villringer A., Ragert P. Structural brain plasticity in Parkinson’s disease induced by balance training. Neurobiol. Aging. 2014;35(1):232–239. doi: 10.1016/j.neurobiolaging.2013.06.021. [DOI] [PubMed] [Google Scholar]
- Sidaros A., Skimminge A., Liptrot M.G., Sidaros K., Engberg A.W., Herning M., Paulson O.B., Jernigan T.L., Rostrup E. Long-term global and regional brain volume changes following severe traumatic brain injury: a longitudinal study with clinical correlates. Neuroimage. 2009;44(1):1–8. doi: 10.1016/j.neuroimage.2008.08.030. [DOI] [PubMed] [Google Scholar]
- Smith R.E., Tournier J.D., Calamante F., Connelly A. SIFT: Spherical-deconvolution informed filtering of tractograms. Neuroimage. 2013;67:298–312. doi: 10.1016/j.neuroimage.2012.11.049. [DOI] [PubMed] [Google Scholar]
- Sosnoff J.J., Broglio S.P., Shin S., Ferrara M.S. Previous mild traumatic brain injury and postural-control dynamics. J Athl Train. 2011;46(1):85–91. doi: 10.4085/1062-6050-46.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L.P. Adolescent neurodevelopment. J. Adolesc. Health. 2013;52(2 Suppl 2):S7–13. doi: 10.1016/j.jadohealth.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C., Baker C.I. Teaching an adult brain new tricks: a critical review of evidence for training-dependent structural plasticity in humans. Neuroimage. 2013;73:225–236. doi: 10.1016/j.neuroimage.2012.03.069. [DOI] [PubMed] [Google Scholar]
- Tournier J.D., Calamante F., Connelly A. Improved probabilistic streamlines tractography by 2nd order integration over fibre orientation distributions. Proc. Intl. Soc. Mag. Reson. Med. (ISMRM) 2010;18 [Google Scholar]
- Tournier J.D., Calamante F., Connelly A. Determination of the appropriate b value and number of gradient directions for high-angular-resolution diffusion-weighted imaging. NMR Biomed. 2013;26(12):1775–1786. doi: 10.1002/nbm.3017. [DOI] [PubMed] [Google Scholar]
- Tournier J.D., Smith R., Raffelt D.A., Tabbara R., Dhollander T., Pietsch M., Christiaens D., Jeurissen B., Yeh C.H., Connelly A. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage. 2019;202 doi: 10.1016/j.neuroimage.2019.116137. [DOI] [PubMed] [Google Scholar]
- Tustison N.J., Avants B.B., Cook P.A., Zheng Y., Egan A., Yushkevich P.A., Gee J.C. N4ITK: improved N3 bias correction. IEEE Trans. Med. Imaging. 2010;29(6):1310–1320. doi: 10.1109/TMI.2010.2046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veraart J., Novikov D.S., Christiaens D., Ades-Aron B., Sijbers J., Fieremans E. Denoising of diffusion MRI using random matrix theory. Neuroimage. 2016;142:394–406. doi: 10.1016/j.neuroimage.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhelst H., Giraldo D., Vander Linden C., Vingerhoets G., Jeurissen B., Caeyenberghs K. Cognitive training in young patients with traumatic brain injury: a fixel-based analysis. Neurorehabil. Neural Repair. 2019 doi: 10.1177/1545968319868720. [DOI] [PubMed] [Google Scholar]
- Wallace E.J., Mathias J.L., Ward L. Diffusion tensor imaging changes following mild, moderate and severe adult traumatic brain injury: a meta-analysis. Brain Imaging Behav. 2018;12(6):1607–1621. doi: 10.1007/s11682-018-9823-2. [DOI] [PubMed] [Google Scholar]
- Wallace E.J., Mathias J.L., Ward L. The relationship between diffusion tensor imaging findings and cognitive outcomes following adult traumatic brain injury: A meta-analysis. Neurosci. Biobehav. Rev. 2018;92:93–103. doi: 10.1016/j.neubiorev.2018.05.023. [DOI] [PubMed] [Google Scholar]
- Wallace E.J., Mathias J.L., Ward L., Fripp J., Rose S., Pannek K. A fixel-based analysis of micro- and macro-structural changes to white matter following adult traumatic brain injury. Hum. Brain Mapp. 2020 doi: 10.1002/hbm.24939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Peterson D.J., Gatenby J.C., Li W., Grabowski T.J., Madhyastha T.M. Evaluation of Field Map and Nonlinear Registration Methods for Correction of Susceptibility Artifacts in Diffusion MRI. Frontier in Neuroinfomatics. 2017;11:1–9. doi: 10.3389/fninf.2017.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamani A., Ryan N.P., Wright D.K., Caeyenberghs K., Semple B.D. The Impact of Traumatic Injury to the Immature Human Brain: A Scoping Review with Insights from Advanced Structural Neuroimaging. J. Neurotrauma. 2020;37(5):724–738. doi: 10.1089/neu.2019.6895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.