Abstract
Spin-coated gold colloids showed a time-dependent increase in emission upon illumination at 532 nm. The emission originated from point-like locations and displayed blinking. The emission spectra of the individual spots and their spatial location remained constant, which is distinct from recent reports on silver nanoparticles. These results suggest the use of emission from gold clusters in nanophotonics devices and as luminescent probes with potential applications in medical imaging.
1. Introduction
There is presently intense interest in the optical properties of gold and silver colloids [1,2]. These colloids display bright colors due to the so-called plasmon absorption, which is in fact due to a combination of absorption and scatter [3,4]. These optical properties make metallic colloids potentially useful in nanophotonic devices [5], as well as in a wide variety of applications in biotechnology [6–10]. Several recent publications have reported emission from silver particles [11–14]. The silver emission displayed the blinking properties characteristic of single molecule fluorescence, with the additional property of different colors, with differing intensities and locations. The color of individual foci changed with time, suggesting a time and illumination dependent reorganization of the silver nanoparticles and/or clusters.
We now report a similar behavior from suspensions of gold colloids. Upon illumination there was a rapid development of spatially localized spots which emitted brightly and blinked. In contrast to silver, the color and emission spectra of the blinking spots remained the same with continuous illumination. These results suggest the use of gold colloid/cluster emission in nanotechnology and for optical probes in biotechnology and medical imaging.
2. Materials and methods
Suspensions of colloidal gold were obtained from TedPello, Inc. The colloids had a nominal diameter of 50 nm, with a reported size distribution <15%. These colloids were spin cast on a cleaned microscope cover slips (Fisher Scientific). The cover slips were initially cleaned by immersion in 30 %v/v H2O2 and 70% v/v H2SO4 for 48 h and then washed in distilled water. Specifically, 50 µl of gold colloid solution (undiluted) was dropped onto a glass microscope slide and spin coated at 3000 rpm for 30 s. The resultant gold colloid film was then allowed to dry in air for 2 min and then used.
The gold colloid-coated slides were examined using the apparatus in Fig. 1. The samples were illuminated with 0.5–25 kW/cm2 of 532 nm, light from a solid-state NdYAG laser. This light was incident on the back of a PlanNEofluor objective, either NA = 1.3 × 100, or NA = 1.2 × 40. Emission was observed in the epifluorescence mode using an Axiovert 135 TV Zeiss microscope. The emission was isolated from Rayleigh and Raman-shifted light by a combination of filters, a laser line filter 532NB3, a dichoric filter 570 DLPP and an emission filter 605DF50 (Omega Optical, Inc). Images were acquired using a cooled 12-bit high speed CCD camera, PxL37, Photometrics, and stored on a PC using V++ software from Digital Optics, Ltd., New Zealand.
Fig. 1.
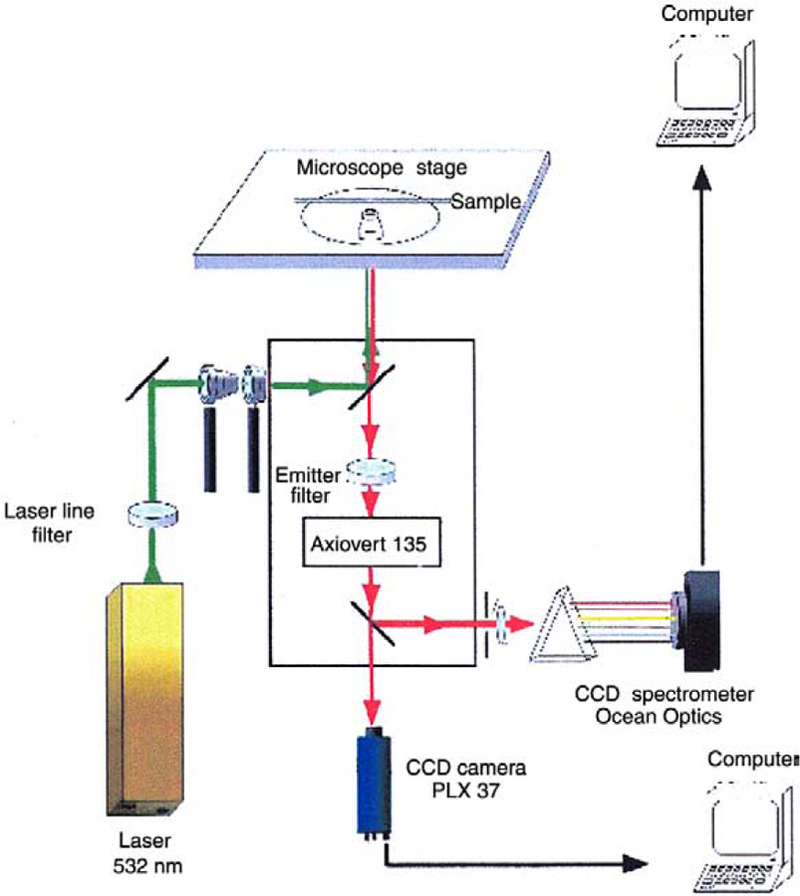
Apparatus for observation of gold emission.
3. Results
Fig. 2 shows the absorption spectrum of the gold colloids on a glass slide (left) and a TEM image (right). The absorption spectrum of the glass-bound colloids is broader and at longer wavelengths than for the same colloids in suspension (top left). This shift is thought due to colloid aggregation on the slides.
Fig. 2.
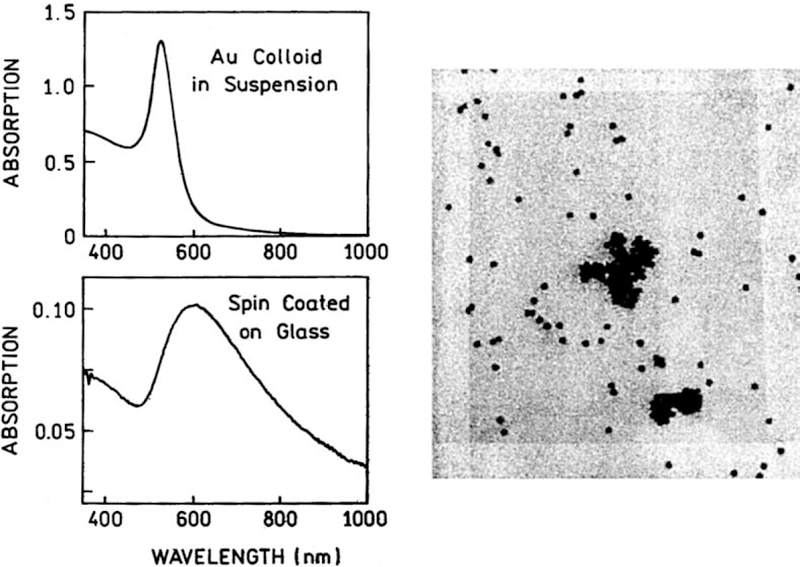
Absorption spectrum of gold colloids in suspension and deposited on a glass slide (left) and corresponding TEM image (right). The estimated size of the gold nanoparticles is about 50 nm.
The spin coated gold colloids were observed visually upon illumination at 532 nm. Within a few seconds of illumination there was a rapid appearance of highly emissive spots (Fig. 3). The number of spots increased for about a second and then remained constant. Most of the spots display blinking which persisted during minutes of observation (Fig. 3). The spots did not appear to photobleach or change color.
Fig. 3.
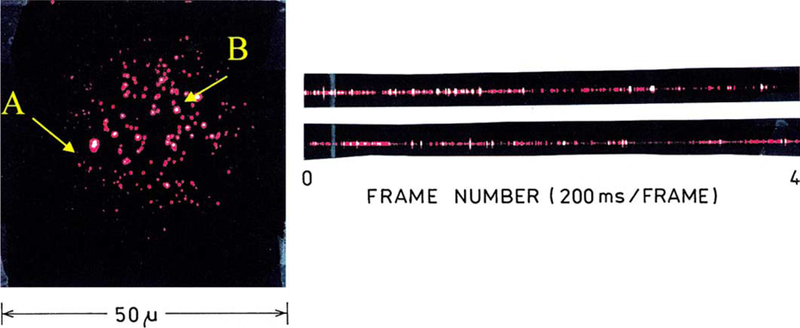
Fluorescence image of gold colloids deposited on glass slide upon excitation of 532 nm, 5 kW/cm2. For this image a water-immersion objective (40×, 1.2 NA) was used. The image was collected in a zoom time frame. Also shown is the fluorescence blinking observed simultaneously for single particles A and B (right).
We selected two spots for continuous observations (Fig. 3, left). The time-dependent emission intensities for two representative spots are shown in the traces on the right. The blinking behavior is comparable to that found for single molecule fluorescence, with the possibility of more than one emissive foci in a given spot. Similar blinking behavior was seen for other selected spots (Fig. 4).
Fig. 4.
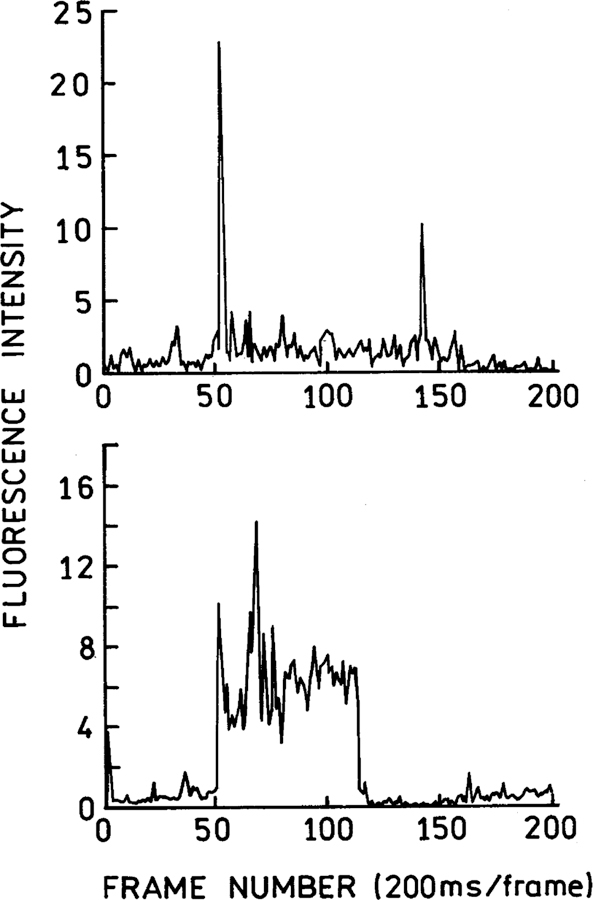
Time-dependent fluorescence blinking observed from a randomly selected single gold nanoparticle. The 532 nm illumination intensity was 5 kW/cm2.
Fig. 5 shows the emission spectra of several representative spots. Essentially the same emission spectra were observed from each spot. The emission is broad but did not change shape with continued illumination. Interestingly however, the emission intensities increased slowly for up to 5 min, after which time the emission intensity appeared constant. The blinking sites may be Raman-active, as is the case with silver particles [15–17].
Fig. 5.
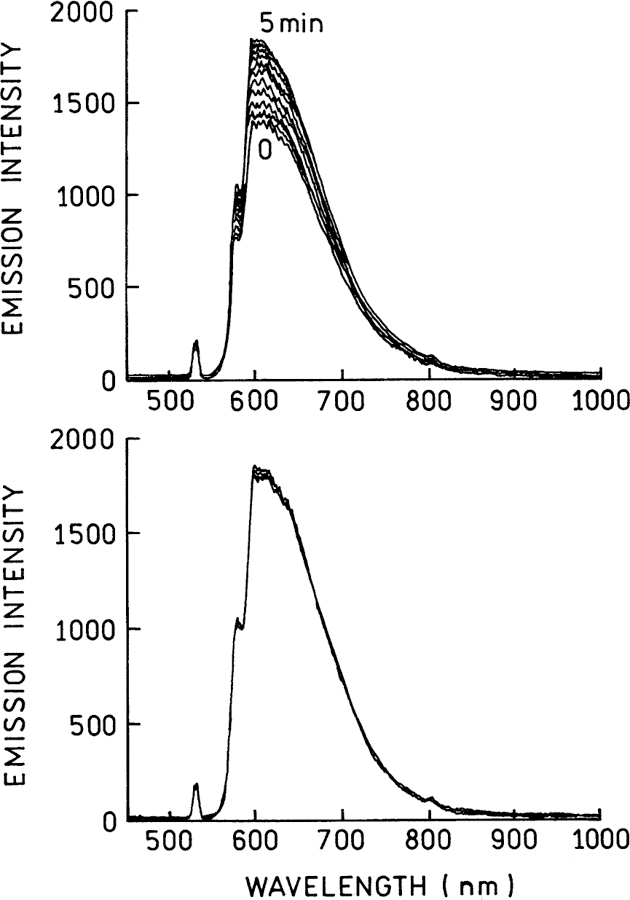
Emission spectra from the entire area (Fig. 3). Top: Recorded from 0 to 5 min illumination. Bottom: Recorded from 5 to 30 min illumination. The 532 nm illumination intensity was 5 kW/cm2.
4. Conclusions
Gold nanoparticles and/or clusters display emission and blinking, similar to that of silver particles, although the emission spectrum of gold remained constant with no shifts to different wavelengths. We believe this behavior may be useful for applications in nanotechnology. Further investigations are underway.
Acknowledgements
This work was supported by the National Center for Research Resource, RR-08119 and the National Institute of Biomedical Imaging and Bioengineering (NIBIB).
References
- [1].Kreibig U, Vollmer M, Toennies JP, Optical Properties of Metal Clusters, Springer-Verlag, Berlin, 1995. [Google Scholar]
- [2].Link S, El-Sayed MA, Int. Rev. Phys. Chem 19 (2000) 409. [Google Scholar]
- [3].Yguerabide J, Yguerabide EE, Anal. Biochem 262 (1998) 137. [DOI] [PubMed] [Google Scholar]
- [4].Yguerabide J, Yguerabide EE, Anal. Biochem 262 (1998) 157. [DOI] [PubMed] [Google Scholar]
- [5].Shen Y, Prasad PN, Appl. Phys. B 74 (2002) 641. [Google Scholar]
- [6].Stich N, Gandhum A, Matushin V, Raats J, Mayer C, Alguel Y, Schalkhammer T, J. Nanosci. Nanotechnol 2 (3/4) (2002) 375. [DOI] [PubMed] [Google Scholar]
- [7].Stich N, Gandhum A, Matushin V, Mayer C, Bauer G, Schalkhammer T, J. Nanosci. Nanotech 1 (1) (2001) 397. [DOI] [PubMed] [Google Scholar]
- [8].Storhoff JJ, Elghanian R, Mucic RC, Mirkin CA, Letsinger RL, J. Am. Chem. Soc 120 (1998) 1959. [Google Scholar]
- [9].Lakowicz JR, Anal. Biochem 298 (2001) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lakowicz JR, Shen Y, Auria SD, Malicka J, Fang J, Gryczynski Z, Gryczynski I, Anal. Biochem 301 (2002) 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lee T-H, Gonzalez JI, Dickson RM, PNAS 99 (16) (2002) 10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zheng J, Dickson RM, J. Am. Chem. Soc 124 (2002) 13982. [DOI] [PubMed] [Google Scholar]
- [13].Peyser LA, Vinson AE, Bartko AP, Dickson RM, Science 291 (2001) 103. [DOI] [PubMed] [Google Scholar]
- [14].Mihalcea C, Buchel D, Atoda N, Tominaga J, J. Am. Chem. Soc 123 (2001) 7172. [DOI] [PubMed] [Google Scholar]
- [15].Moskovits M, Tay L-L, Yang J, Haslett T, Top. Appl. Phys 82 (2002) 215. [Google Scholar]
- [16].Nie S, Emory SR, Science 275 (1997) 1102. [DOI] [PubMed] [Google Scholar]
- [17].Kneipp K, Haka AS, Kneipp H, Badizadegan K, Yoshizawa N, Boone C, Shafer-Peltier KE, Motz JT, Dasari RR, Feld MS, Appl. Spectrosc 56 (2) (2002) 150. [Google Scholar]


