Abstract
Background
Few data are available about real-life cardiotoxicity associated with s.c. versus i.v. trastuzumab treatment of early-stage, HER2-positive breast cancer, and little is known about its predisposing factors.
Patients and methods
We retrospectively reviewed data of 363 adult patients treated with adjuvant trastuzumab for HER2-positive breast cancer. Univariate statistical analysis was performed, and a multivariable logistic model was developed to identify independent risk factors of cardiac toxicity.
Results
Within 5 years, the overall incidence of events meeting our criteria was 11.8%, and an early discontinuation of trastuzumab was recorded in 20 patients (5.5%). No cases of congestive heart failure occurred, neither multiple events per patient were observed. A total of 184 patients received i.v. and 179 received s.c. trastuzumab. Compared with the s.c. formulation, a higher cardiotoxicity rate for the i.v. administration (15.2% vs 8.4%) was found, and particularly in those patients with cardiovascular risk factors (19.3% vs 8.7%), at the univariate and multivariate analyses. Although more patients with prior anthracycline-based chemotherapy experienced cardiac events, the association of this therapy with cardiac events was not significant. The incidence of cardiac events was not influenced by anthropometric data (e.g. body mass index) or a diagnosis of diabetes mellitus. 5-year event-free survival was 91.7% in the overall population; event-free survival rates were similar between the s.c. and the i.v. groups.
Conclusion
Our study shows a more favorable safety profile of s.c. versus i.v trastuzumab administration. The use of s.c. trastuzumab could be advisable in at-risk patients.
Keywords: Trastuzumab, HER2-positive, Early breast cancer, Subcutaneous, Intravenous, Cardiac toxicity
Highlights
-
•
Few data are available about cardiotoxicity associated with s.c. versus i.v. trastuzumab and its predisposing factors.
-
•
Our study shows a more favorable safety profile of s.c. versus i.v trastuzumab administration.
-
•
Thus, the use of s.c. trastuzumab could be advisable in at risk patients.
1. Introduction
The addition of intravenous (i.v.) trastuzumab to standard chemotherapy increases time to progression and improves survival in women with HER2-positive (HER2+) breast cancer [[1], [2], [3], [4]]. The downside with the use of trastuzumab is its inherent cardiotoxicity, namely decreased left ventricular ejection fraction (LVEF) and, rarely, congestive heart failure. Trastuzumab-induced cardiomyopathy is usually clinically manageable [5,6]. However, in order to decrease toxicities, trastuzumab de-escalation in early HER2+ breast cancer has also been proposed [7].
A subcutaneous (s.c.) formulation of trastuzumab has been developed to address the limitations of i.v. administration (e.g., long administration time, treatment barriers for patients requiring port-a-cath systems). S.c. trastuzumab injections take a shorter time to administer than the i.v. infusion (2–5 min vs 30–90 min, respectively) and are expected to improve treatment convenience and patient compliance [8]. Hence, s.c. trastuzumab has been approved as an alternative to i.v. trastuzumab for early-stage HER2+ breast cancer. However, approval was based on non-inferiority studies in selected populations [9,10]. Few data are available about real-life cardiotoxicity of fixed-dose s.c. trastuzumab and little is known about its predisposing factors. This study evaluates the incidence of cardiotoxicity in patients receiving fixed-dose s.c. trastuzumab versus weight-based i.v. formulation in a real-world population.
2. Patients and methods
2.1. Study population, data collection
We retrospectively collected data of all consecutive patients treated with adjuvant chemotherapy followed by trastuzumab for HER2+ breast cancer at the Humanitas Cancer Center (Rozzano, Italy), a referral center in northern Italy, from May 2009 to February 2018. All procedures were conducted in accordance with the Declaration of Helsinki and were approved by the local ethics committee. Informed consent for treatment and the use of clinical data for scientific purposes was provided by all patients.
Patients with histologically confirmed HER2+ early breast cancer (stage I–IIIA), a LVEF ≥50% determined by echocardiography and a minimum 2-year cardiological follow-up could be included. We excluded patients enrolled in clinical trials assessing anticancer treatment. Demographical and clinical variables and information on treatments were extracted from medical records.
2.2. Treatment and outcome measures
S.c. trastuzumab was administered at a fixed dose of 600 mg and trastuzumab i.v. was administered at a maintenance dose of 6 mg/kg (after the loading dose of 8 mg/kg) every 3 weeks for 1 year (18 cycles). The prescription of either s.c. or i.v. trastuzumab was based on availability and refundability.
Cardiac function was assessed just before the first administration of trastuzumab, then every 3 months until the end of the treatment, and every 1 year thereafter by evaluating LVEF, cardiac signs and symptoms. A cardiac event was defined as a decrease in LVEF by ≥ 10% from baseline, and to a value < 50%, or as the development of congestive heart failure [11].
2.3. Objectives
The main objective of the study was to compare the cardiac toxicity profile of fixed-dose trastuzumab s.c. and weight-based i.v. trastuzumab, in patients with early HER2+ breast cancer. The analysis was focused on the first 5 years of follow-up (since the first trastuzumab administration), so as to normalize observation time in the two groups. Secondary objectives included the identification of potential clinical predictors of cardiac toxicity of trastuzumab and the analysis of 5-year event-free survival (EFS) in the overall population and in the two treatment groups.
2.4. Statistical methods
Data were analyzed by descriptive statistics. Univariate statistical analysis included contingency tables with Chi-Squared or Fisher’s exact test, and nonparametric Mann-Whitney test. A multivariable logistic model was applied to identify independent risk factors of cardiac toxicity. EFS was defined as the time from the first dose of trastuzumab to local, regional or distant disease recurrence, contralateral breast cancer, death from any cause, or censoring at 5 years of follow-up (whichever occurred first). 5-year EFS was assessed using the Kaplan-Meier method for the overall, s.c. and i.v. population. The log-rank test was used to test differences between groups. All p-values were two-sided, and statistical significance was assumed at p ≤ 0.05.
Analyses were performed with SAS version 9.4 and STATA version 15.
3. Results
3.1. Study population
We evaluated data of 363 patients (362 females; median age 55 years; range: 31–85) (Table 1). Among them, 184 (50.7%) received i.v. trastuzumab and 179 (49.3%) s.c. trastuzumab. Anthracyclines were administered in 90.4% of patients (n = 328) before trastuzumab. The median cumulative anthracycline dose equivalent was 240 mg/m2 (range 189–280) in the i.v. group and 240 mg/m2 (range 60–360) in the s.c. one. The type of anthracycline was doxorubicin (n = 308) or epirubicin (n = 20). The median time interval between anthracycline administration and the start of trastuzumab was 22 days (range, 21–30) in the i.v. group and 24 days (range, 21–36) in the s.c. one. Overall, 229/363 (63.0%) patients received taxanes concomitantly to trastuzumab; among these, 194/363 (53.4%) had previously received anthracycline. In total, 284 patients (78.2%) received radiotherapy (142 on the left side, 4 bilaterally). Baseline ejection fraction (EF) was >55% (range: 55–80%) in all but five patients who had EF between 50 and 55%. Current smoking had been reported by 19.6% of patients; 11.3% of patients had quitted smoking at the first oncology visit. Overall, 37.7% of patients showed cardiac comorbidity and 29.8% used cardiologic medications regularly. Furthermore, 3.3% of patients had diabetes.
Table 1.
Patients’ characteristics in the overall population and by treatment group (subcutaneous versus intravenous trastuzumab).
| Characteristic | Entire cohort (n = 363) | Subcutaneous group (n = 179) | Intravenous group (n = 184) | p-value |
|---|---|---|---|---|
| Median age, years (range) | 55 (31–85) | 57 (31–85) | 54 (33–82) | 0.042 |
| Gender, n (%): | 0.493 | |||
|
362 (99.7) | 178 (99.4) | 184 (100.0) | |
|
1 (0.3) | 1 (0.6) | 0 (0.0) | |
| Height (m), median (range) | 1.60 (1.37–1.78) | 1.60 (1.37–1.78) | 1.60 (1.48–1.77) | 0.577 |
| Weight (kg), median (range) | 62 (42–130) | 61 (45–108) | 62 (42–130) | 0.728 |
| BMI (kg/m2) median (range) | 23.72 (15.77–50.15) | 24.09 (16.76–39.73) | 23.59 (15.77–50.15) | 0.517 |
| BMI, n (%): | 0.387 | |||
|
18 (4.9) | 7 (3.9) | 11 (6.0) | |
|
210 (57.9) | 105 (58.6) | 105 (57.1) | |
|
83(22.9) | 37 (20.7) | 46 (25.0) | |
|
52 (14.3) | 30 (16.8) | 22 (11.9) | |
| Baseline EF, n (%): | 0.675 | |||
|
358 (98.6) | 177 (98.9) | 181 (98.4) | |
|
5 (1.4) | 2 (1.1) | 3 (1.6) | |
| Radiotherapy, n (%): | 0.002 | |||
|
79 (21.8) | 24 (13.4) | 55 (29.9) | |
|
142 (39.1) | 84 (46.9) | 58 (31.5) | |
|
4 (1.1) | 2 (1.1) | 2 (1.1) | |
|
138 (38.0) | 69 (38.6) | 69 (37.5) | |
| Smokers, n (%): | 0.118 | |||
|
71 (19.6) | 37 (20.7) | 34 (18.5) | |
|
41 (11.3) | 14 (7.8) | 27 (14.7) | |
|
251 (69.1) | 128 (71.5) | 123 (66.8) | |
| Cardiovascular comorbidities, n (%): | 0.067 | |||
|
137 (37.7) | 76 (42.5) | 61 (33.1) | |
|
226 (62.3) | 103 (57.5) | 123 (66.9) | |
| Cardiological medications, n (%): | 0.025 | |||
|
108 (29.8) | 63 (35.2) | 45 (24.5) | |
|
255 (70.2) | 116 (64.8) | 139 (75.5) | |
| Diabetes, n (%): | 0.771 | |||
|
12 (3.3) | 5 (2.8) | 7 (3.8) | |
|
351 (96.7) | 174 (97.2) | 177 (96.2) | |
| Prior CT, n (%): | 0.108 | |||
|
194 (53.4) | 82 (45.8) | 112 (60.9) | |
|
134 (37.0) | 81 (45.3) | 53 (28.8) | |
|
35 (9.6) | 16 (8.9) | 19 (10.3) |
BMI: Body mass index; EF: Ejection fraction; CT: Chemotherapy.
3.2. Cardiac toxicity
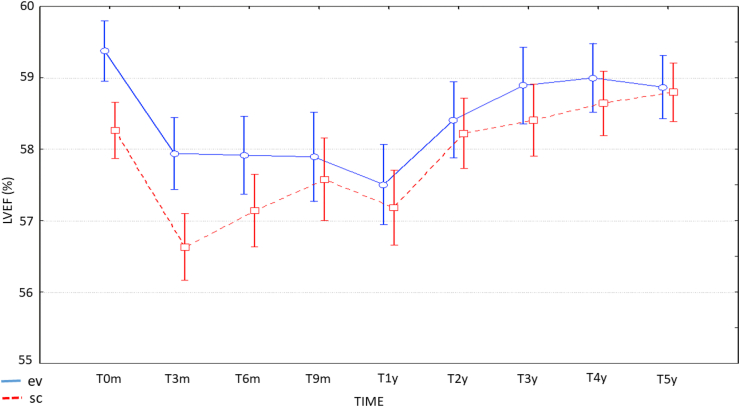
Within the predefined period of 5 years of observation, the incidence of events meeting our criteria in the entire cohort was 11.8% (n = 43). All patients showed LVEF decrease, with a median reduction of 12% (range: 6–30) (Fig. 1). LVEF values were not statistically different at each timepoint in s.c. or i.v. group between the two groups (interaction test, p = 0.38).
Fig. 1.
LVEF measures over time (mean ± SD) at baseline, at 3, 6, 9 and 12 months and at 2, 3, 4 and 5 years.
Almost all cardiac events occurred during trastuzumab administration, except for two cases (5 and 22 months after the end of treatment). The majority of events had reversed (24/43, 55.8%) at the data cut-off. No cases of congestive heart failure occurred, neither multiple events per patient were observed. In total, 343 patients completed the planned trastuzumab treatment, with an early discontinuation in 20 patients (5.5%), of whom eight received s.c trastuzumab and 12 i.v. treatment. Early discontinuation was due to persistent cardiotoxicity (LVEF decline for >8 weeks) in 19/20 cases.
Risk factors for cardiac events were cardiological therapy (cardiac events in patients with vs without cardiac medications: 19.3% vs 8.7%, p = 0.004) and cardiac comorbidity (18.2% vs 8.0%, p = 0.0033). These two risk factors were also strongly associated (p < 0.0001). Previous smoking (21.9%) was a further risk factor for cardiotoxicity compared to active smoking (4.2%) and never smoking (12.7%) (p = 0.02), even if former smokers did not have a higher incidence of cardiac comorbidities (11/41 [26.8%] vs 27/71 [38.0%] vs 99/251 [39.4%], in former, present and never smokers, respectively; p = 0.30). Previous treatment with anthracyclines was not a risk factor for developing events, although we recorded a higher number of events in anthracycline-treated patients compared with others (12.5% vs 5.0%, p = 0.40). In addition, baseline EF (p = 0.47), radiotherapy on the left side (p = 0.41), BMI classes (p = 0.54) and diabetes (p = 0.43) had no impact on the occurrence of cardiac events.
3.3. Clinical predictors of cardiac toxicity: administration route and concomitant cardiac treatment
At the univariate analysis, the administration route had an impact on cardiac toxicity, with a higher frequency in patients in the i.v. group (15.2% in i.v. and 8.4% in s.c. group, p = 0.044). In order to assess whether the different cardiac toxicity profiles of the administration route of trastuzumab (s.c. vs i.v.) were influenced by different distributions of potential risk factors (e.g., cardiac comorbidities, smoking, previous anthracycline therapy) in the two groups, we analyzed the relative number of each variable in s.c. versus i.v. trastuzumab samples (Table 1). The s.c. group differed from the i.v. group by a slightly higher median age (57 vs 54 years; p = 0.042), more concomitant cardiological treatments (p = 0.025) and higher exposure to left radiation therapy (p = 0.002). However, the last two risk factors were represented more in the s.c. group, reporting less cardiac toxicity events. Furthermore, the higher number of patients on concomitant cardiological medications in the s.c. group was maintained for each drug class (angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, β-blockers, diuretics and lipid-lowering agents).
We then confirmed the role of administration route by a multiple logistic regression analysis. Of all the parameters used in the model (administration route, cardiac concomitant medications, previous anthracycline-based treatment, radiation therapy, age and BMI), regression analysis showed that patients receiving i.v. trastuzumab were 2.41-times more likely, while patients without concomitant cardiac treatments were 1.96-times significantly less likely, to develop cardiac toxicity (p = 0.014; Table 2).
Table 2.
Multiple logistic regression analysis for predicting cardiac toxicity in HER2-positive breast cancer patients.
| Variables | Odds ratio | 95% CI |
|---|---|---|
| Trastuzumab (i.v. vs s.c.) | 2.41 | 1.19–4.84 |
| BMI | 0.96 | 0.89–1.03 |
| RT | 1.21 | 0.61–2.39 |
| Anthracyclines | 2.75 | 0.62–12.26 |
| Cardiac concomitant treatments (no vs yes) | 0.29 | 0.14–0.63 |
| Age | 0.99 | 0.96–1.03 |
BMI: Body mass index; CI: Confidence interval; i.v.: Intravenous; RT: Radiation therapy; s.c.: Subcutaneous.
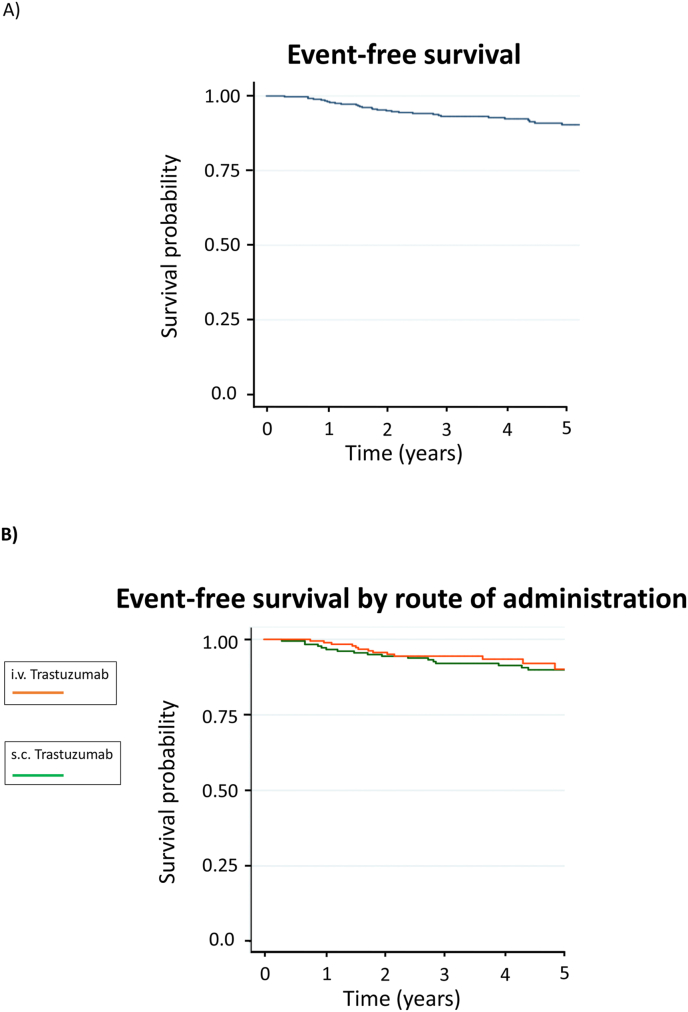
3.4. 5-Year event-free survival analysis
In the overall population, 5-year EFS was 91.7%; in the s.c. and i.v. trastuzumab groups, the 5-year EFS were 90.5% and 92.9%, respectively (Fig. 2). No differences between the two treatment groups were observed (p = 0.30). At 5 years, 30/363 patients (8.3%) had an EFS event: 7/363 (1.9%) had a local (5 s.c., 2 i.v.), 2/363 (0.5%) a regional (1 s.c., 1 i.v.) and 19/363 (5.2%) a distant recurrence (10 s.c.,9 i.v.) and 2/363 (0.6%) had contralateral breast cancer (1 s.c., 1 i.v.).
Fig. 2.
Event-free survival in the overall population (A) and in the i.v. versus s.c. group (B).
4. Discussion
To our knowledge, our study is the largest monocentric study comparing cardiac toxicity of s.c. versus i.v. trastuzumab in HER2+ breast cancer patients in an adjuvant real-world setting, aiming to define potential predictors of cardiac toxicity (Table S1).
In our population, the overall incidence of cardiac dysfunction was 11.8%, consistently with previous reports [9,10]. Our long-term safety analysis showed that most cardiac events (mainly LVEF reduction) occurred during treatment and were reversible. Reversibility of cardiac toxicity in our patients was lower than reported in the adjuvant trastuzumab trials [[1], [2], [3], [4]]. The principal explanation for this discrepancy could rely on slight inconsistency in the definition of cardiac recovery in the medical literature, as consensus on the definition for cardiotoxicity is still lacking [[11], [12], [13]]. The adjuvant trastuzumab trials themselves used slightly different definitions of cardiac recovery [3,14,15]. In our study, a full recovery of LVEF was defined as the return of LVEF to at least its normal value.
A further reason for our lower reversibility rate could be the stricter inclusion criteria used in RCT. As a matter of fact, the proportion of patients who recovered in our study is closer to the one described in the real-world study OHERA (67.3%; “patients with significant LVEF drop who achieved resolution” in Table 2). Furthermore, a recent cohort study reported that one-third of the population who developed cardiac toxicity after trastuzumab had long-term impaired cardiac function [12].
Most patients received anthracycline-based chemotherapy (90.4%) before trastuzumab administration, without differences between i.v. and s.c. group (89.7% in i.v. and 91.1% in s.c.). This percentage is in line with those reported in the HERmione study (75% of patients, all receiving s.c. trastuzumab) [16] and in the OHERA trial (90.2%, all receiving i.v. trastuzumab) [17]. In all these studies (including ours), the incidence of cardiac toxicity was not higher in patients who had received prior anthracycline therapy. However, the relatively small number of patients not receiving an anthracycline-based regimen does not permit to rule out a potential impact of the previous regimen on the trastuzumab-induced cardiotoxicity. The hypothesis of an increased risk of cardiac dysfunction when trastuzumab is administered after anthracyclines was raised by randomized clinical trials [1,2,18,19] and by a Chinese observational study [20]. The reason for these findings could rely on the different mechanisms of cardiac toxicity of trastuzumab and anthracyclines. Anthracyclines have a direct cytotoxic effect mediated by calcium channels and reactive oxygen species (ROS) [21], while trastuzumab is mainly responsible for alterations in cellular metabolic pathways in cardiomyocytes [22]. However, cardiac dysfunction associated with trastuzumab after the use of adjuvant anthracyclines is relatively transient [2,18,19].
Remarkably, we enrolled patients with mild to moderate cardiac comorbidities excluding only patients with a severe cardiac dysfunction (according to good clinical practice), while interventional trials preferentially enrolled patients with few cardiac comorbidities. The recent prospective real-life HERmione study extended the analysis to patients with cardiac comorbidities; however, it only assessed s.c. trastuzumab [16]. Another study including patients with pre-existing cardiac conditions was the real-world OHERA study; however, it only analyzed i.v. trastuzumab [17]. In our study, cardiac comorbidities and cardiovascular medication at baseline were associated with higher risk of cardiac toxicity. Since they were strongly associated (p < 0.0001), we only included concomitant cardiological medications in the multivariable analysis. Surprisingly, concomitant cardiological treatments were more frequent in patients receiving s.c. trastuzumab (the subgroup with a lower incidence of cardiac events) than in the i.v. group. Furthermore, each class of cardiological drugs was more frequent in the s.c. group. This latter observation prevents the speculation that some special classes could have a very high cardioprotective activity and be responsible for the reduced risk of patients in the s.c. group.
Previous smoking was associated with higher risk of cardiac toxicity while active and no smoking did not, although in our sample active smoking and not-smoking patients presented a higher incidence of cardiac comorbidities. One possible explanation is that subjects with a familiar higher risk of either cancer or cardiovascular disease quitted smoking to prevent future illness [23,24].
i.v. administration was associated with a higher cardiac toxicity than the s.c. one (15.2% vs 8.4%, respectively), both at univariate and multivariate analysis. The final analysis of the HannaH trial showed a comparable efficacy and safety of s.c. and intravenous i.v. trastuzumab, in apparent contrast with our findings [25]. Interestingly, the incidence of cardiac adverse events reported in the HannaH trial (44 of 297 [14.8%] and 42 of 298 [14.1%] in s.c. and i.v. arms, respectively) was similar to the incidence of cardiac adverse events we observed in our i.v. population (15.2%). On the contrary, our s.c. population showed a significantly lower incidence of cardiac adverse events (8.4%). Of note, the HannaH trial was a prospective, randomized trial, while our study is a retrospective evaluation, and possible confounding factors might have played a role in the comparison. Considering each administration route of trastuzumab separately, the s.c. trastuzumab cardiac safety profile is consistent with the known profile of s.c. trastuzumab in clinical trials [9,10,[26], [27], [28]] without new safety concerns, while the cardiac safety profile of i.v. trastuzumab, although is similar to that reported in randomized controlled trials like the HannaH trial) [10], was higher if compared to real-life data on thousands of patients in the OHERA study (7.6%) [17]. Of note, cardiological treatments were more frequently used in the s.c. group (35.2% in s.c. group vs 24.5% in i.v. group), regardless of drug class, although more cardiac events were found in patients using cardiological treatment in the i.v. group (26.7% in i.v. vs 14.3% in s.c.). This suggests a link between cardiac toxicity and cardiological treatments rather than a spurious association. Moreover, cardiological therapies (especially, beta-blockers and angiotensin-converting enzyme inhibitors) had already been prescribed as an active treatment for a pre-existing cardiovascular condition and not as a prevention of the potential cardiotoxicity of trastuzumab [29]. 5-year EFS was similar between the two administration routes, consistently with previous data [1,27].
Furthermore, our study evaluated the potential influence of bodyweight on the safety of fixed-dose s.c. trastuzumab. Multiple logistic regression analysis of HannaH study reported that bodyweight did not influence the rates of severe or serious adverse events [25]. In the phase III SafeHer study, an exploratory analysis evaluating safety of s.c. trastuzumab by bodyweight found that the lower bodyweight subgroups had comparable incidence of adverse events to the overall population [28]. These data deriving from subgroup analyses of phase III trials are confirmed by our pre-planned analysis in a real-world setting, thus contributing to rule out the theoretical concern of a potential s.c. trastuzumab overtreatment in patients with a bodyweight <59 kg.
Limitations of our study are those inherent to retrospective research, and also the small number of patients with a baseline LVEF 50–55%. On the other hand, a monocentric study has the advantage to be uniform in the evaluation of patients, by both cardiologists and oncologists. In particular, treatment schedules (including time interval between anthracycline administration and the start of trastuzumab, cumulative anthracycline dose) as well as number of follow-up visits were highly homogeneous and adherent to international and to our internal guidelines, thus limiting the influence that established variables of cardiotoxicity for trastuzumab might have influenced our findings. An additional limitation could not be avoided due to the real-life setting of the study: echocardiograms for LVEF assessment were not performed by a single cardiologist but by several cardiologists of the Cardiology Unit of our Hospital, according to the current clinical practice. Of note, all cardiologists were part of the internal cardio-oncology multidisciplinary team.
Furthermore, the duration of the follow-up is adequate, considering that late chronic cardiotoxicity can appear after 1 year, with no evidence of a further increase in cardiac events over time [30].
To date, the phase III randomized HannaH and PrefHer trials showed that the s.c. formulation was not inferior to the i.v. one and was preferred by the majority of early breast cancer patients, respectively [25,27]. On the other hand, nowadays, there is a great diffusion of biosimilar use in oncology, mainly for pharmacoeconomic issues and several biosimilars of trastuzumab have been introduced in the clinical practice but they are all administered by i.v. infusion. On the contrary, the evolving paradigm of breast cancer care is focused on effectiveness, but also on non-invasive and time-saving procedures. Indeed, very recently, the US Food and Drug Administration (FDA) has approved the fixed-dose combination of pertuzumab and trastuzumab, both administered by s.c. injection, following the phase III FeDeriCa study [31].
In conclusion, our results showed that the route of administration of trastuzumab and patients’ concomitant medications are independent predictive factors of trastuzumab-induced cardiac toxicity, thus suggesting that they are two key elements when planning adjuvant strategy for HER2+ breast cancer patients at risk of developing cardiac toxicity.
Author contributions
Conceptualization and study design: AS, RDS. Data curation: RDS, LG. Investigation: All. Writing: RDS, AS. Reviewing: All. All Authors have read and approved the final version of the manuscript.
Funding
None.
Declaration of competing interest
Armando Santoro has received honoraria from BMS, AstraZeneca, MSD, Lilly, Bayer, Takeda, Roche, Mundipharma, Novartis, Servier, Amgen, ArQule, Celgene, Incyte, AbbVie, Gilead, Pfizer, Daiichi, Sandoz, and Sanofi. Rita De Sanctis has received honoraria form EISAI, Kyowa Kirin and Novartis. The other authors have no funding and conflicts of interests to disclose.
Acknowledgments
Editorial assistance was provided by Luca Giacomelli, PhD, and Aashni Shah (Polistudium SRL, Milan, Italy). This assistance was supported by internal funds.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2021.03.004.
Ethical approval
All procedures were conducted in accordance with the Declaration of Helsinki and were approved by the local ethics committee. Informed consent for treatment and the use of clinical data for scientific purposes was provided by all patients.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Slamon D., Eiermann W., Robert N., Breast Cancer International Research Group Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piccart-Gebhart M.J., Procter M., Leyland-Jones B. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 3.Cameron D., Piccart-Gebhart M.J., Gelber R.D., Procter M. Herceptin Adjuvant (HERA) Trial Study Team. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389:1195–1205. doi: 10.1016/S0140-6736(16)32616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niraula S., Gyawali B. Optimal duration of adjuvant trastuzumab in treatment of early breast cancer: a meta-analysis of randomized controlled trials. Breast Canc Res Treat. 2019;173:103–109. doi: 10.1007/s10549-018-4967-8. [DOI] [PubMed] [Google Scholar]
- 5.Nemeth B.T., Varga Z.V., Wu W.J., Pacher P. Trastuzumab cardiotoxicity: from clinical trials to experimental studies. Br J Pharmacol. 2017;174:3727–3748. doi: 10.1111/bph.13643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menna P., Minotti G., Salvatorelli E. Cardiotoxicity of targeted cancer drugs: concerns, "the cart before the horse," and lessons from trastuzumab. Curr Cardiol Rep. 2019;21:33. doi: 10.1007/s11886-019-1121-0. [DOI] [PubMed] [Google Scholar]
- 7.Eiger D., Franzoi M.A., Pondé N. Cardiotoxicity of trastuzumab given for 12 months compared to shorter treatment periods: a systematic review and meta-analysis of six clinical trials. ESMO Open. 2020;5 doi: 10.1136/esmoopen-2019-000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pivot X., Gligorov J., Müller V. Preference for subcutaneous or intravenous administration of trastuzumab in patients with HER2-positive early breast cancer (PrefHer): an open-label randomised study. Lancet Oncol. 2013;14(10):962–970. doi: 10.1016/S1470-2045(13)70383-8. [DOI] [PubMed] [Google Scholar]
- 9.Ismael G., Hegg R., Muehlbauer S. Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I-III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial. Lancet Oncol. 2012;13:869–878. doi: 10.1016/S1470-2045(12)70329-7. [DOI] [PubMed] [Google Scholar]
- 10.Jackisch C., Hegg R., Stroyakovskiy D. HannaH phase III randomised study: association of total pathological complete response with event-free survival in HER2-positive early breast cancer treated with neoadjuvant-adjuvant trastuzumab after 2 years of treatment-free follow-up. Eur J Canc. 2016;62:62–75. doi: 10.1016/j.ejca.2016.03.087. [DOI] [PubMed] [Google Scholar]
- 11.Curigliano G., Cardinale D., Suter T. ESMO Guidelines Working Group. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2012;23(Suppl 7) doi: 10.1093/annonc/mds293. vii155–vii166. [DOI] [PubMed] [Google Scholar]
- 12.Jacobse J.N., Schaapveld M., Boekel N.B. Risk of heart failure after systemic treatment for early breast cancer: results of a cohort study. Breast Canc Res Treat. 2021;185(1):205–214. doi: 10.1007/s10549-020-05930-w. [DOI] [PubMed] [Google Scholar]
- 13.Yoon H.J., Kim K.H., Kim H.Y. Impacts of non-recovery of trastuzumab-induced cardiomyopathy on clinical outcomes in patients with breast cancer. Clin Res Cardiol. 2019;108(8):892–900. doi: 10.1007/s00392-019-01417-x. [DOI] [PubMed] [Google Scholar]
- 14.Perez E.A., Romond E.H., Suman V.J. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32(33):3744–3752. doi: 10.1200/JCO.2014.55.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romond E.H., Perez E.A., Bryant J. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 16.Jacquin J.P., Uwer L., Savignoni A. Safety profile of subcutaneous trastuzumab in patients with HER2-positive early breast cancer: the French HERmione non-interventional prospective study. Breast. 2020;49:1–7. doi: 10.1016/j.breast.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lidbrink E., Chmielowska E., Otremba B. A real-world study of cardiac events in > 3700 patients with HER2-positive early breast cancer treated with trastuzumab: final analysis of the OHERA study. Breast Canc Res Treat. 2019;174:187–196. doi: 10.1007/s10549-018-5058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romond E.H., Perez E.A., Bryant J. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 19.Smith I., Procter M., Gelber R.D. 2-Year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 20.Xue J., Jiang Z., Qi F. Risk of trastuzumab-related cardiotoxicity in early breast cancer patients: a prospective observational study. J Breast Cancer. 2014;17:363–369. doi: 10.4048/jbc.2014.17.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwardson W., Narendrula R., Chewchuk S. Role of drug metabolism in the cytotoxicity and clinical efficacy of anthracyclines. Curr Drug Metabol. 2015;16:412–426. doi: 10.2174/1389200216888150915112039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitani T., Ong S.G., Lam C.K. Human-induced pluripotent stem cell model of trastuzumab-induced cardiac dysfunction in patients with breast cancer. Circulation. 2019;139:2451–2465. doi: 10.1161/CIRCULATIONAHA.118.037357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haug U., Riedel O., Cholmakow-Bodechtel C., Olsson L. First-degree relatives of cancer patients: a target group for primary prevention? A cross-sectional study. Br J Canc. 2018;118:1255–1261. doi: 10.1038/s41416-018-0057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iacoviello L., Bonaccio M., de Gaetano G., Donati M.B. Epidemiology of breast cancer, a paradigm of the "common soil" hypothesis. Semin Canc Biol. 2020 doi: 10.1016/j.semcancer.2020.02.010. pii: S1044-579X(20)30043-2. [DOI] [PubMed] [Google Scholar]
- 25.Jackisch C., Stroyakovskiy D., Pivot X. Subcutaneous vs intravenous trastuzumab for patients with ERBB2-positive early breast cancer: final analysis of the HannaH Phase 3 randomized clinical trial. JAMA Oncol. 2019;5 doi: 10.1001/jamaoncol.2019.0339. e190339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackisch C., Kim S.B., Semiglazov V. Subcutaneous versus intravenous formulation of trastuzumab for HER2-positive early breast cancer: updated results from the phase III HannaH study. Ann Oncol. 2015;26:320–325. doi: 10.1093/annonc/mdu524. [DOI] [PubMed] [Google Scholar]
- 27.Pivot X., Verma S., Fallowfield L., PrefHer Study Group Efficacy and safety of subcutaneous trastuzumab and intravenous trastuzumab as part of adjuvant therapy for HER2-positive early breast cancer: final analysis of the randomised, two-cohort PrefHer study. Eur J Canc. 2017;86:82–90. doi: 10.1016/j.ejca.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 28.Gligorov J., Ataseven B., Verrill M., SafeHer Study Group Safety and tolerability of subcutaneous trastuzumab for the adjuvant treatment of human epidermal growth factor receptor 2-positive early breast cancer: SafeHer phase III study’s primary analysis of 2573 patients. Eur J Canc. 2017;82:237–246. doi: 10.1016/j.ejca.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Blanter J.B., Frishman W.H. The preventive role of angiotensin converting enzyme inhibitors/angiotensin-II receptor blockers and β-adrenergic blockers in anthracycline- and trastuzumab-induced cardiotoxicity. Cardiol Rev. 2019;27:256–259. doi: 10.1097/CRD.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 30.Perez E.A., Suman V.J., Davidson N.E. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol. 2008;26:1231–1238. doi: 10.1200/JCO.2007.13.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan A. Presented at SABCS; San Antonio, Texas: 2019. Subcutaneous administration of the fixed-dose combination of trastuzumab and pertuzumab in combination with chemotherapy in HER2-positive early breast cancer: primary analysis of the phase III, multicenter, randomized, open-label, two-arm FeDeriCa study. Abstract #PD4-07. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.