Abstract
Background.
Attention impairment is an under-investigated feature and diagnostic criterion of Major Depressive Disorder (MDD) that is associated with poorer outcomes. Despite increasing knowledge regarding mechanisms of attention in healthy adults, we lack a detailed characterization of attention impairments and their neural signatures in MDD.
Methods.
Here, we focus on selective attention and advance a deep multi-modal characterization of these impairments in MDD, using data acquired from n = 1008 patients and n = 336 age- and sex-matched healthy controls. Selective attention impairments were operationalized and anchored in a behavioral performance measure, assessed within a battery of cognitive tests. We sought to establish the accompanying neural signature using independent measures of functional magnetic resonance imaging (15% of the sample) and electroencephalographic recordings of oscillatory neural activity.
Results.
Greater impairment on the behavioral measure of selective attention was associated with intrinsic hypo-connectivity of the fronto-parietal attention network. Not only was this relationship specific to the fronto-parietal network unlike other large-scale networks; this hypo-connectivity was also specific to selective attention performance unlike other measures of cognition. Selective attention impairment was also associated with lower posterior alpha (8–13 Hz) power at rest and was related to more severe negative bias (frequent misidentifications of neutral faces as sad and lingering attention on sad faces), relevant to clinical features of negative attributions and brooding. Selective attention impairments were independent of overall depression severity and of worrying or sleep problems.
Conclusions.
These results provide a foundation for the clinical translational development of objective markers and targeted therapeutics for attention impairment in MDD.
Keywords: Attention, behavioral testing, depression, EEG, functional MRI
Introduction
Attention plays a critical role in both cognitive and emotional functioning. Although concentration difficulties are among the diagnostic criteria for numerous psychiatric disorders, including Major Depressive Disorder (MDD), our understanding of this domain is limited. Historical conceptualization of depression as an emotional disorder and a lack of cross-talk between clinical and cognitive neuroscience have hindered progress in understanding this key cognitive component of psychiatric illness and its interactions with emotion-related symptomatology. Given that MDD is the leading cause of disability in the United States, has over 300 million diagnoses worldwide (World Health Organization, 2017) and cognitive impairments can be among the most debilitating symptoms (Jaeger et al., 2006), understanding how attention goes awry in MDD has great potential to substantially relieve the global burden of mental illness.
Our current methods for evaluating MDD rely heavily on expert clinician characterizations of symptoms, with no currently available methods for incorporating neurobiological information such as neuroimaging or electrophysiological markers. Within this current diagnostic system, there are over 100 000 possible combinations of clinician-evaluated symptoms leading to a single diagnosis such as MDD, which means that research studies of this disorder must grapple with extreme individual heterogeneity. As a result, treatment selection in the clinic suffers from a lack of individualized metrics, such that most patients do not respond to the first-line treatment (Saveami et al., 2015) and must attempt many different treatment options before finding one that works. To counteract this problem, recent shifts in the field have emphasized the need for ‘precision psychiatry’ modeled after precision medicine for other illnesses (e.g. cancer) (Fernandes et al., 2017). In this framework, the goal becomes one of linking biological markers with well-defined symptom domains that characterize subgroups of patients and which may better guide treatment selection.
Achieving this goal will require a standardized taxonomy of links between brain and behavior and the mechanisms by which they go awry in the context of psychiatric illness. Toward this end, Williams (2016) put forward a series of testable hypotheses regarding the relationship between well-characterized large-scale neural circuitry and specific symptom profiles. Synthesizing findings across neuroimaging studies of affective disorders, Williams (2016) described six hypothesized ‘biotypes’ each anchored in dysfunction within a particular network. One such hypothesis is that patients whose symptoms include difficulty with focusing attention would show hypo-connectivity within a canonical fronto-parietal network. This network supports numerous goal-directed attention functions (Nobre et al., 1997; Corbetta and Shulman, 2002), and includes areas such as the medial prefrontal cortex, frontal eye fields, anterior insula, superior parietal lobule, and posterior parietal cortex. It appears to be critical for the executive control of attention (Ptak, 2012), with increasing involvement for more difficult tasks (Falkenberg et al., 2011). Investigations of topological organization in MDD using anatomical (Qin et al., 2014), resting-state (Luo et al., 2015), and task-based functional connectivity (He et al., 2018) found disruption to this fronto-parietal network. However, it remains to be tested whether disruptions to this network are associated with behavioral or symptom characteristics in this population.
An important sub-domain of attention that is particularly relevant to mood and therefore MDD is selective attention: the ability to selectively attend to important information while filtering out unhelpful or irrelevant distraction (Serences and Kastner, 2014). Investigations of selective attention in healthy adults have confirmed involvement of the fronto-parietal attention network (Giesbrecht et al., 2003; Prado et al., 2011). However, much of the prior research on attention in MDD has focused on negative valence biases – depressed patients’ attention tends to linger longer on negative information such as sad faces than does the attention of healthy controls (for review: Gotlib and Joormann, 2010). Although these biases toward negative information are often referred to as ‘selective attention,’ they do not always fit the definition of selective attention in cognitive neuroscience (attending to task-relevant information while ignoring distraction). Furthermore, while these findings are useful for understanding how depressed patients sample information from their environments, they do not elucidate generalized attention impairments independent of stimulus type. Here, we complement the clinical literature on negative valence biases and the cognitive neuroscience literature on selective attention by investigating non-emotional selective attention impairments in a clinical population as well as examining the relationship between these general selective attention impairments and processing of negative emotions.
To specifically probe selective attention abilities, we utilized a non-emotional Stroop color-word task. Classically, the Stroop task has been conceptualized as probing cognitive control or inhibition of prepotent responses. However, standard analyses of inhibition during this task utilize ‘interference’ scores, calculated by subtracting reaction times between the Word and Color conditions. We instead utilized independent conditions of the Stroop task as a measure of feature-based selective attention, focusing our analyses instead on reaction times in the Word and Color conditions independently. These independent reaction times in the color-word task fit the definition of a feature-based selective attention task according to the cognitive neuroscience literature, which defines selective attention as the function of selectively enhancing perceptual processing for goal-relevant features or locations while suppressing distracting sensory information (Serences and Kastner, 2014). In particular, this task involves spatially-localized stimuli (avoiding confounds with spatial selective attention) and contains two features (font color and semantic meaning) with conditions in which one feature is task-relevant while the other is distraction. Moreover, test-retest reliability is higher for individual color or word reaction times than for interference difference scores (Strauss et al., 2005). Taken together, the individual reaction time measures are most relevant for understanding selective attention impairments and their neural correlates.
Complementing the neuroimaging findings reviewed above, EEG studies have demonstrated that attention is inherently a dynamic process, involving synchronization and interplay among cortical oscillations. Studies of selective attention using EEG have demonstrated an integral role of alpha oscillations (8–13 Hz) in the suppression of distraction to support goal-relevant attention (Klimesch, 2012). Previously considered an ‘idling’ rhythm increasing in power at rest and with the eyes closed (Pfurtscheller et al., 1996), alpha has more recently been linked to attention via its role in active ignoring of task-irrelevant information (for review: Payne and Sekuler, 2014). However, it remains unknown whether increases in alpha power during eyes-closed rest is related to selective attention capabilities during active task engagement. Uncovering such a relationship in a patient population could provide a useful neural correlate of attention impairments for targeted treatment development.
Here, we provide a thorough characterization of selective attention impairments in a large cohort of un-medicated MDD patients (Williams et al., 2011). We tested the hypothesis that selective attention impairments are associated with intrinsic fronto-parietal network dysfunction measured by functional MRI (fMRI) and EEG. We leveraged clinical symptom reports to rule out alternative explanations for attention impairments (e.g. sleep difficulties), and investigated whether attention impairments may explain negative valence biases previously reported in MDD. Our multimodal approach, grounded in objective task performance rather than subjective self-report measures, enables us to define and characterize a subgroup of MDD patients into an Inattention Biotype and demonstrate construct validity of this grouping by pinpointing neural correlates of attention impairment to guide targeted treatment development.
Methods
Participants
We used multi-modal testing in a sample of un-medicated adults with a primary diagnosis of MDD (n = 1008) without co-morbid ADHD, and age- and sex-matched healthy controls (n = 336) from the iSPOT-D study (Williams et al., 2011).
Behavioral measure of attention: reaction times
Participants completed the non-emotional color-word Stroop task according to the iSPOT-D protocol (Williams et al., 2011). As described in the Introduction, we use Stroop reaction times as a measure of feature-based selective attention. We anchor the theoretical definition of our construct in a cognitive neuroscience framework (Serences and Kastner, 2014). This definition is operationalized as attending to either the color or semantic meaning of the word while ignoring its other feature. This operational definition is distinguished from an ‘interference’ scores (Color RT – Word RT) that is more typically used to operationalize response inhibition and related constructs of cognitive control. Analyses utilized reaction times (RT) in the Color (identify the color of the text) and Word (identify the semantic meaning of the text) conditions, as well as a summary score calculated according to Shilyansky et al. (2016) using both reaction times and accuracy measures on each condition (interference scores were not used as these measure response inhibition rather than selective attention). We restricted all analyses of selective attention performance to participants whose average reaction times fell within a plausible range (greater than 100 ms and less than 5000 ms). For comparisons between Stroop reaction times and performance across eight other cognitive tasks, see eMethods S1 (eFigs 1–4).
Network measure of attention: fronto-parietal hypo-connectivity
Of the full sample, 15% of participants also underwent MRI. fMRI was acquired and processed as described in Grieve et al. (2013) (see eMethods for details of fMRI methods and quantification of network dysfunction, as well as analyses of constituent connectivity measures comprising the fronto-parietal network of interest). Briefly, we used an automated meta-analysis using neurosynth.org (Yarkoni et al., 2011) with search terms ‘attention’ and ‘frontoparietal network’ (accessed 4 June 2017) of 1526 studies to define key nodes of the fronto-parietal network relevant for attention function, calculated intrinsic connectivity between these nodes, and then summarized the dysfunction by quantifying average hypo-connectivity among these measurements and normalizing based on a large sample of healthy controls. We refer to this quantification of circuit dysfunction normalized to healthy controls as a ‘fit score’ for each circuit (i.e. a higher ‘fit score’ refers to greater hypo-connectivity of the fronto-parietal attention network). Analyses of large-scale network dysfunction and selective attention impairment included 97 MDD participants with fMRI data and selective attention task reaction times in the normal range. Analyses of network dysfunction and other cognitive domains included 90 MDD participants with fMRI who had completed all cognitive tasks.
Physiological measure of attention: alpha power
EEG was recorded and preprocessed according to standardized protocols (Gatt et al., 2010; Williams et al., 2011), which include re-referencing to the average signal from the left and right mastoids (electrodes A1 and A2). We calculated the log-transformed alpha (8–13 Hz) power scores for the eyes-closed rest period (EC), eyes-open rest period (EO) and the difference between eyes-closed and eyes-open rest periods (EC-EO). Power values were analyzed at electrode ‘Pz’ and averaged over the entire 2-min rest period in each condition. Analyses of alpha power and selective attention impairment included 678 MDD participants with EEG in both eyes-closed and eyes-open rest conditions and selective attention task reaction times within the normal range.
Self-report and clinician ratings
We examined whether attention impairments in MDD participants were associated with clinical/demographic factors that have previously been demonstrated or presumed to have a relationship with attention impairments. These included Sleep Loss: reports of night-time awakenings (a measure of insomnia) assessed by the Hamilton Depression (HAM-D) Inventory given that prior research has shown a relationship between sleep and attention behavior as well as fronto-parietal circuitry (Lim et al., 2010); Symptom Severity: overall symptom severity assessed by HAM-D Inventory to test whether attention impairments occur primarily in the context of more severe mood symptoms; Worrying: using the Clinical Outcomes in Routine Evaluation (CORE) rating scale (Parker and Hadzi-Pavlovic, 1996) ‘worrying’ item to investigate whether attention impairments in MDD reflect excessive worrying that may sap attentional resources; and Subjective Concentration Difficulties: using the CORE rating scale ‘inattentiveness’ item to test the association between clinician ratings of inattentiveness and our behavioral measures of selective attention. We also investigated potential moderators of the relationship between selective attention impairment and neural dysfunction, including Age: patients’ age at first visit; Sex: reported gender identification; Years of Education: self-report; and Co-Morbid Generalized Anxiety Disorder (GAD): MINI Plus diagnoses. Analyses of clinical/demographic factors and selective attention impairment included 589–596 MDD participants with selective attention task reaction times within the normal range and the relevant clinical/demographic measures.
Negative valence biases
To determine whether selective attention performance was associated with biases toward negative information, we computed correlations between selective attention task reaction times and the differences in reaction times to sad v. neutral or sad v. happy faces (n = 963 MDD participants with Word RT data and n = 953 MDD participants with Color RT data). We also compared selective attention impairment to the number of misidentifications of neutral faces as sad in the Emotional Face Recognition task (n = 966 MDD participants and 324 healthy controls with full data for this analysis).
Statistical analysis
All statistical analyses were conducted in MATLAB. Reported correlation coefficients and corresponding p values are standard Pearson correlations. For analyses of ordered categorical clinician-ratings (i.e. Inattentiveness, HAM-D), we used analyses of variance. Moderation analyses were performed using Age, Sex, Years of Education, and Co-Morbid GAD variables coded categorically (median split) as interaction terms in linear regression models. See eMethods for more detailed analysis of the specificity of the relationships between attention impairments, posterior alpha oscillations, and fronto-parietal hypo-connectivity.
Results
Selective attention is impaired in MDD
To probe selective attention behavior, we utilized reaction times in each independent condition of a Stroop color-word task. By using reaction times from each condition, rather than the standard interference score associated with response inhibition, we isolated participants’ ability to selectively attend to relevant information (e.g. color) while ignoring distraction (e.g. semantic meaning). MDD participants performed worse overall than healthy controls in this feature-based selective attention task (Color: t(1295) = 2.924, p = 0.004, d = 0.189; Word: t(1305) = 2.286, p = 0.022, d = 0.147) (Fig. 1). It is possible that this small effect size is due to the extreme heterogeneity of symptom profiles in MDD, wherein not all individuals experiencing depression experience attention impairments. In our sample, 16.5% of MDD participants have Color reaction times that are at least one standard deviation greater than the healthy controls’ mean, and only 4.8% are at least two standard deviations greater than the healthy controls’ mean.
Fig. 1.
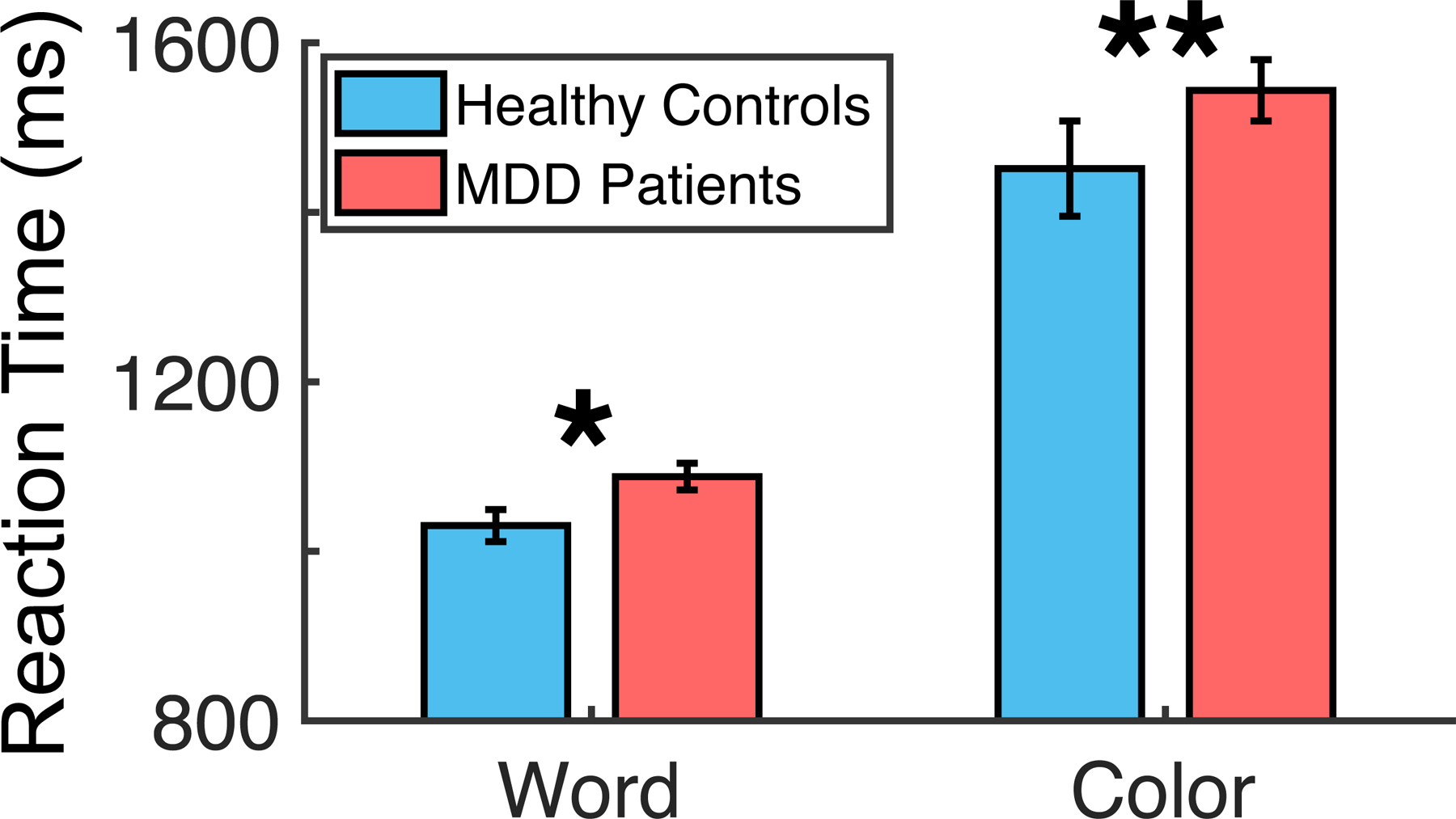
Selective attention behavior in MDD patients v. healthy controls. Reaction times to Word- and Color-naming conditions of the selective attention task. MDD, Major depressive disorder. Error bars represent bootstrapped 95% confidence intervals.
Selective attention impairments in MDD are associated with fronto-parietal network dysfunction
Consistent with our hypothesis (Williams, 2016), selective attention impairment was correlated with reduced functional connectivity within the fronto-parietal attention network in depressed individuals (Color: r(97) = 0.23, p = 0.02; Word: ns) (Fig. 2), measured using fMRI. With more stringent cutoffs for reaction time to eliminate high leverage points and retain only subjects with complete cognitive behavioral data, this association with Color RT remains at r(87) = 0.226, p = 0.049. In contrast, and highlighting the specificity of this effect, there were no significant relationships in MDD between selective attention impairment and dysfunction on six other network dysfunctions implicated in depression (all p’s>0.2), including default mode connectivity, salience network connectivity, and dysfunctions of connectivity and activation within the negative affect network evoked by sad and threatening face stimuli, within the positive affect network evoked by happy face stimuli and within the cognitive control network probed by a Go-NoGo task (Williams, 2016). Additionally, we find that fronto-parietal connectivity is specifically associated with Stroop reaction times and not with performance on any of eight other cognitive tasks assessed (Supplement S3, eFigs 5–7). Analyses of constituent connectivity measures comprising the fronto-parietal network reveal that connectivity between the anterior insula and inferior parietal lobule exhibit the strongest relationships with reaction times (eFig. 8).
Fig. 2.
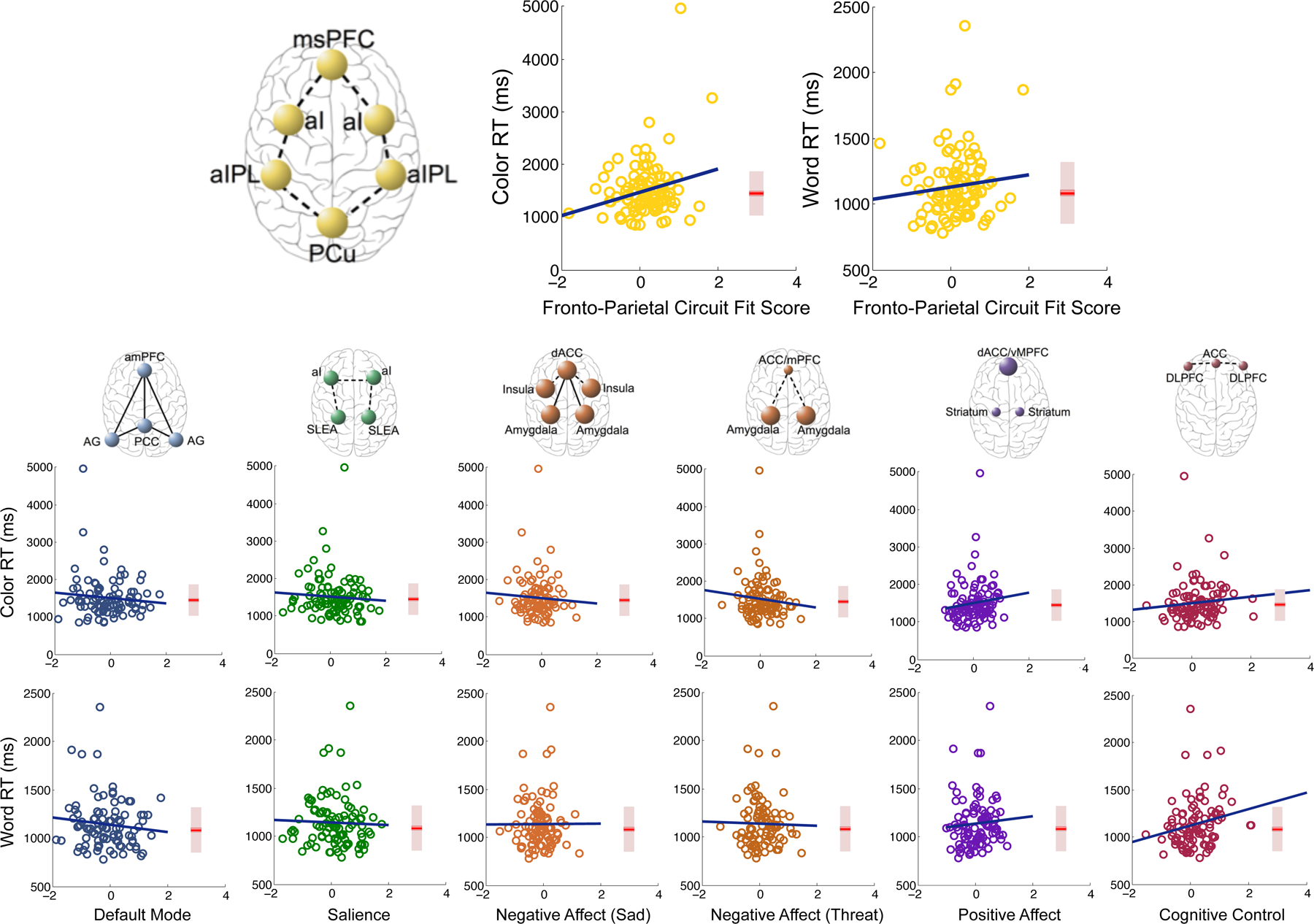
Associations of attention impairments in MDD patients with dysfunction in various large-scale networks. Circuit dysfunction fit scores are calculated independently for each circuit according to hypothesized dysfunction (e.g. fronto-parietal network hypo-connectivity) and standardized to a sample of healthy controls (see Methods and eMethods for more details on circuit fit score quantification). Box plots represent reaction time distributions for healthy controls. ACC, Anterior cingulate cortex; AG, Angular gyrus; al, Anterior insula; alPL, Anterior inferior parietal lobule; amPFC, Anterior medial prefrontal cortex; dACC, Dorsal anterior cingulate cortex; DLPFC, Dorsolateral prefrontal cortex; mPFC, Medial prefrontal cortex; msPFC, Medial superior prefrontal cortex; PCC, Posterior cingulate cortex; PCu, Precuneus; RT, Reaction time; SLEA, Sublenticular extended amygdala; vMPFC, Ventromedial prefrontal cortex.
Selective attention impairments in MDD are associated with decreased power in the alpha frequency band
Consistent with our hypothesis that selective attention capabilities are associated with alpha (8–13 Hz) oscillations at rest in MDD, we found that selective attention impairment was significantly negatively correlated with average alpha power measured by scalp EEG during a 2-min eyes-closed rest period (Color: r (678) = −0.14, p < 0.001; Word: r(678) = −0.075, p = 0.053) (Fig. 3). This relationship was strengthened when individuals’ average alpha power during an eyes-open rest period was subtracted from average alpha power during the eyes-closed rest period (Color: r(678) = −0.154, p < 0.001; Word: r(678) = −0.086, p = 0.025). Eyes-open alpha power did not appear to be related to selective attention performance (Color: r(678) = −0.039, p = 0.315; Word: r(678) = −0.017, p = 0.652). However, alpha power during rest was not significantly correlated with fronto-parietal functional connectivity (Eyes Closed: r(77) = 0.025, p = 0.832; Eyes Open: r(77) = 0.037, p = 0.748; Difference: r(77) = −0.012, p = 0.918). To test the replicability of this effect, we randomly divided our sample into five independent subsets of n = 135 participants, and found that these correlations were similar across subsamples (eTable 1).
Fig. 3.
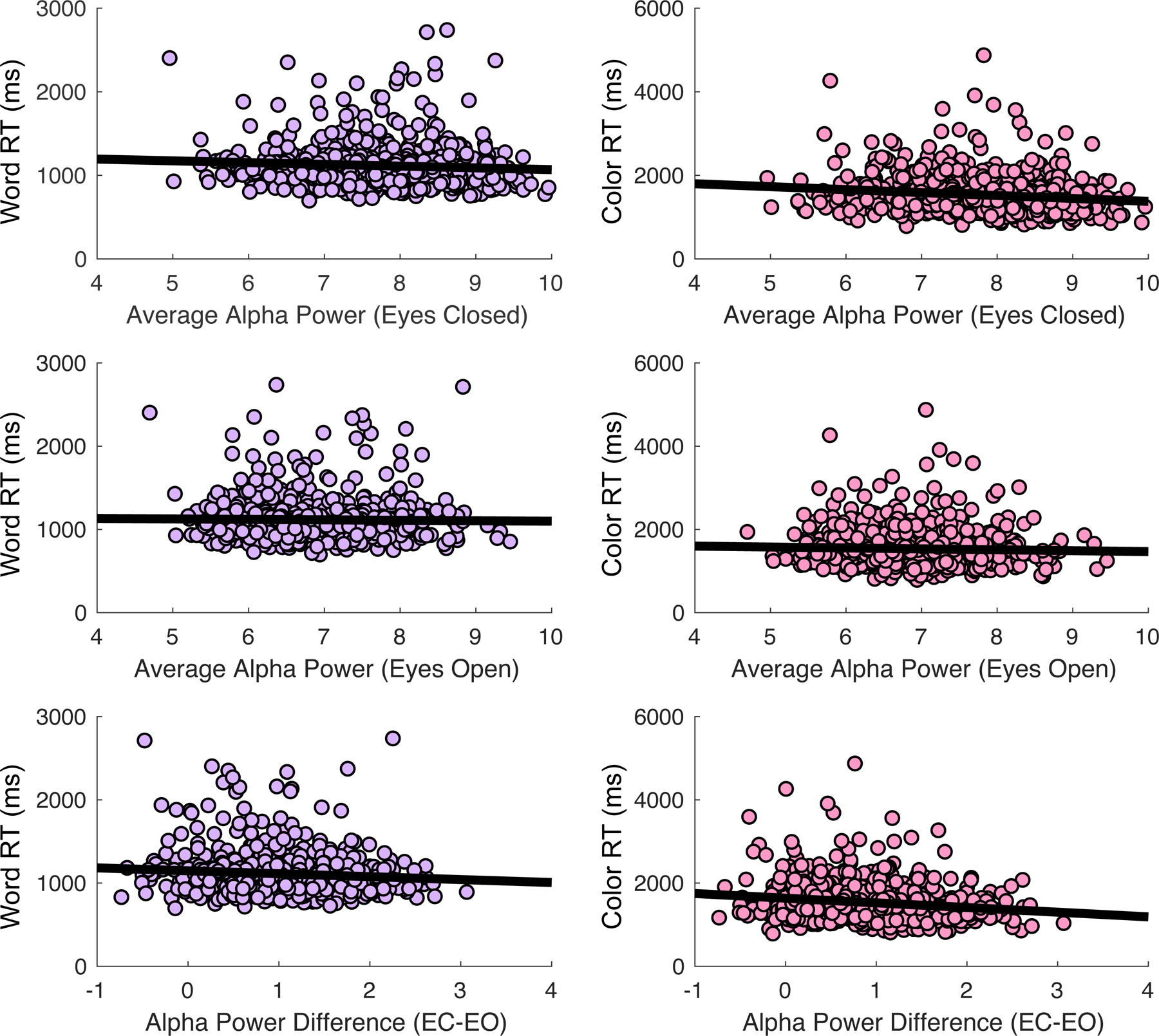
Association of selective attention impairment with average posterior alpha power (EEG) during eyes-closed and eyes-open rest periods. EC-EO, Difference between eyes-closed and eyes-open rest periods; RT, Reaction time.
Selective attention impairments in MDD are independent of other symptoms
Although sleep loss negatively impacts attention and fronto-parietal attention network activity (Lim et al., 2010), selective attention impairment was unrelated to HAM-D reported insomnia, as measured by nighttime awakenings (Color: F(2,591) = 0.83, p = 0.44, ; Word: F(2,598) = 0.9, p = 0.41, ) (eFig. 9A). Selective attention impairment was also unrelated to overall MDD symptom severity ( p’s>0.05) (eFig. 9B), or excessive worrying (Color: F(4,589) = 0.52, p = 0.72, ; Word: F(4,596) = 0.29, p = 0.89, ) (eFig. 9C), which suggests that selective attention impairments are not explained by general severity or distracting thoughts. Interestingly, observer-rated inattentiveness was also unrelated to objective performance on the selective attention task (Color: F(2,591) = 0.25, p = 0.78, ; Word: F(2,598) = 2.43, p = 0.09, ) (eFig. 9D), consistent with other work showing divergence between self-, other-, and objectively-measured cognitive functioning. The insomnia, worrying, and overall HAM-D score variables also did not eliminate the significant association between selective attention impairment and fronto-parietal network dysfunction when used as covariates in linear regression models.
Selective attention impairments may underlie negative valence biases in MDD
To determine whether top-down attention impairments predict negative valence biases, we compared selective attention performance to performance on an emotional face identification task, specifically to sad facial expressions. Selective attention performance significantly predicted the extent of negative valence bias in MDD (Sad-Neutral/Color: r(953) = 0.263, p < 0.001; Sad-Neutral/Word: r(963) = 0.194, p < 0.001; Sad-Happy/Color: r(953) = 0.278, p < 0.001; Sad-Happy/Word: r(963) = 0.206, p < 0.001) (Fig. 4a). Regression models including both difference scores and raw reaction times in the Sad condition reveal that this effect was driven by a significant relationship between reaction times during selective attention and during sad facial expression identification (Sad/Color: r(953) = 0.106, p = 0.007; Sad/Word: r(963) = 0.051, p < 0.05). Moreover, selective attention performance was related to misidentifications of neutral faces as sad faces in this task (Fig. 4b) for both MDD patients (Color: r(966) = 0.239, p < 0.001; Word: r(966) = 0.209, p < 0.001) and healthy controls (Color: r(324) = 0.243, p < 0.001; Word: r(324) = 0.117, p < 0.05).
Fig. 4.
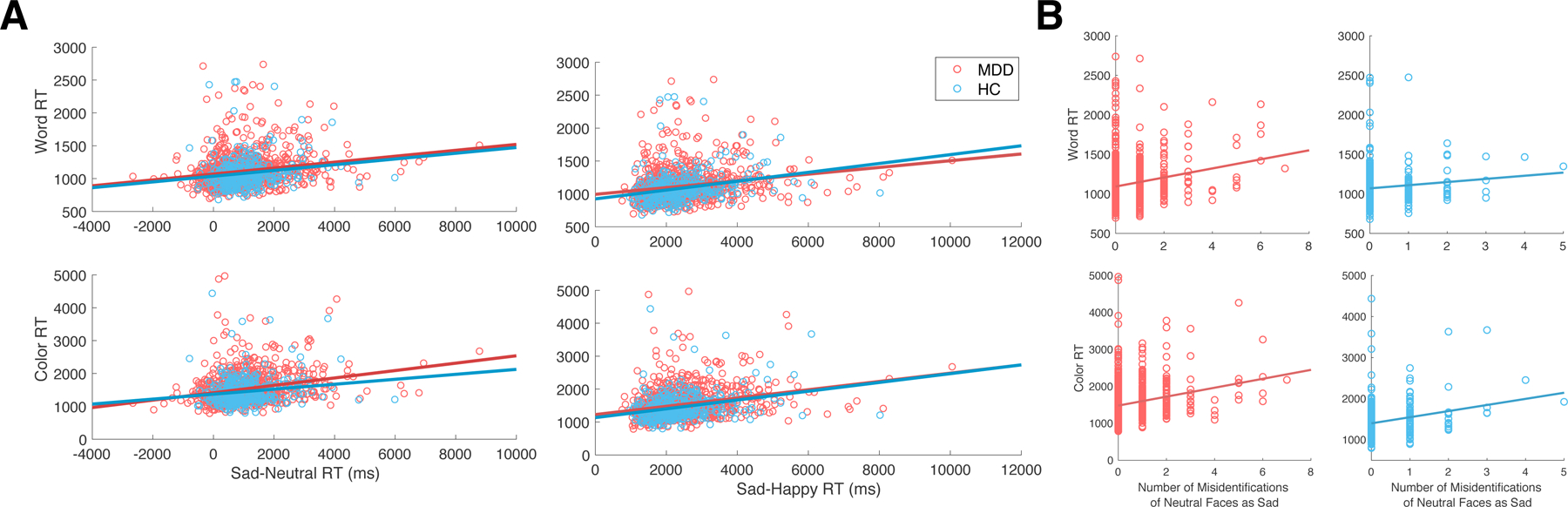
Association of selective attention performance with negative valence bias on the emotional face identification task. (a) Association of selective attention performance with the difference in reaction times between Sad and Neutral faces or Sad and Happy faces on the emotional face identification task. (b) Association of selective attention performance with misidentifications of neutral faces as sad faces. MDD, Major depressive disorder; HC, Healthy controls; RT: Reaction time.
Selective attention impairments in MDD are moderated by age and sex
To explore the role of demographic characteristics, we examined effects of Age, Sex, Years of Education, and Co-morbid GAD by including main effects and interactions with these variables in our linear regression models. Our results show that Age and Sex act as moderators in predicting selective attention impairment from network dysfunction in adults with MDD ( p’s<0.01), while Years of Education and Co-morbid GAD do not ( p’s>0.05) (Table 1). Specifically, network dysfunction was most strongly related to selective attention reaction time in older and female participants.
Table 1.
Potential moderators of associations between fronto-parietal hypo-connectivity or posterior alpha power (difference between eyes-closed and eyes-open rest) and Color reaction times.
| Moderator | Interaction | N | r | p |
|---|---|---|---|---|
| Age | ||||
| Fronto-Parietal | ||||
| Hypoconnectivity | p = 0.009 | |||
| Older Adults | 45 | 0.391 | 0.008 | |
| Younger Adults | 45 | 0.068 | 0.658 | |
| Alpha Power (EC-EO) | p = 0.0643 | |||
| Sex | ||||
| Fronto-Parietal | ||||
| Hypoconnectivity | p = 0.008 | |||
| Males | 49 | 0.021 | 0.885 | |
| Females | 41 | 0.437 | 0.004 | |
| Alpha Power (EC-EO) | p = 0.173 | |||
| Years of Education | ||||
| Fronto-Parietal | ||||
| Hypoconnectivity | p = 0.058 | |||
| Alpha Power (EC-EO) | p = 0.854 | |||
| Generalized Anxiety Disorder | ||||
| Fronto-Parietal | ||||
| Hypoconnectivity | p = 0.374 | |||
| Alpha Power (EC-EO) | p = 0.386 | |||
Abbreviation: EC-EO: the difference between eyes-closed and eyes-open rest periods.
Defining the inattention biotype
Complementing our treatment of selective attention impairment as a continuous measure, we characterized a discrete Inattention Biotype, defined as a group of depressed individuals with exceptionally poor selective attention performance. Figure 5a depicts fronto-parietal network connectivity and resting posterior alpha power in individuals with selective attention impairment substantially above the mean of 327 healthy controls. These results confirm the finding that worse selective attention impairment is associated with decreased connectivity within the fronto-parietal attention network measured by fMRI and decreased alpha power during eyes-closed rest measured by EEG. Depressed individuals in the Inattention Biotype group (>2 S.D. beyond the healthy control mean) have significantly lower connectivity of the fronto-parietal attention network ( p < 0.01), significantly lower alpha power during eyes-closed rest ( p < 0.05), and a significantly smaller difference between eyes-closed and eyes-open resting posterior alpha power ( p < 0.01) than depressed individuals not in the Inattention Biotype. Figure 5b depicts topography plots of alpha power and time-frequency transforms at the posterior electrode of interest for the extreme Inattention Biotype group compared to age- and sex-matched members of the Non-Inattention MDD group. These reveal that posterior alpha power is lower across time in the Inattention Biotype group, and demonstrate the specificity of this effect to the alpha frequency band and posterior regions as opposed to other frequency bands and other electrode locations. Additionally, we find that quality of life ratings are lower in the Inattention Biotype group compared to age- and sex-matched depressed individuals (eMethods, eTable 2) who are otherwise similar across demographic and clinical characteristics (eMethods, eTable 3).
Fig. 5.
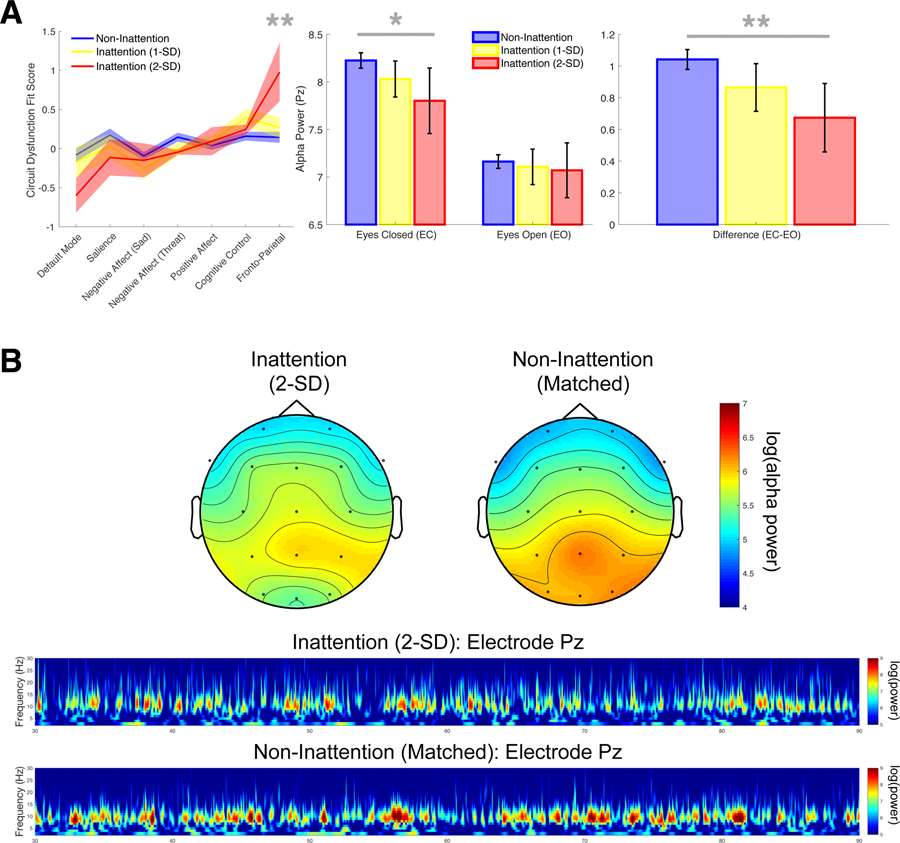
Network dysfunction and oscillatory power in an extreme Inattention Biotype group of MDD participants v. age- and sex-matched MDD participants without attention impairment. (a) Fronto-parietal network connectivity and resting posterior alpha power in participants with selective attention impairment v. those without. Error bars represent bootstrapped 95% confidence intervals. *p < 0.05, **p < 0.01. (b) Topography plots of alpha power and time-frequency transforms at the posterior electrode of interest.
Discussion
Deep phenotyping characterization of the ‘Inattention Biotype’
Deep phenotyping in the context of precision medicine has been defined as ‘the precise and comprehensive analysis of phenotypic abnormalities in which the individual components of the phenotype are observed and described’ (Robinson, 2012). Our results provide the first deep phenotyping characterization of attention impairments in MDD, revealing underlying network dysfunction and oscillatory differences associated with this behaviorally-defined construct. By leveraging multi-modal data in a large sample, we garnered evidence of distinct characteristics of attention-impaired depressed patients that we hope will provide a foothold for future research and treatment development. Our findings indicate that depressed patients show selective attention impairments in a task with neutral stimuli, which are not explained by clinical symptoms such as excessive worrying, lack of sleep, or overall depressive symptom severity. Moreover, we demonstrated a relationship between attention behavior and fronto-parietal network dysfunction in depressed patients using fMRI, consistent with prior studies in healthy adults (Prado et al., 2011). The finding that alpha power measured by scalp EEG at rest predicts selective attention impairment measured at a separate time point suggests a link between conceptualizations of alpha as a resting rhythm and as an active sensory suppression mechanism. We also showed that these selective attention impairments may underlie the valence biases previously reported in the literature (Gotlib and Joormann, 2010). Although these impairments are present on average in the MDD group, we found that a subset of patients form an Inattention Biotype that consists of particularly poor selective attention performance alongside fronto-parietal network dysfunction and decreased alpha power at rest.
Explorations of the demographic characteristics associated with inattention revealed that the relationship between network dysfunction and attention performance is moderated by age and sex, with particularly strong relationships in older adults and females. This suggests that these factors may predispose individuals toward the phenotypes we observed. However, it should be noted that MDD participants in the iSPOT-D sample are age- and sex-matched with healthy controls, so age and sex effects cannot account for the observed overall attention impairments in depressed individuals compared to controls. Moreover, the plethora of evidence supporting the role of alpha oscillations in selective attention (Klimesch, 2012; Payne and Sekuler, 2014) and the association between fronto-parietal network integrity and attention function (Giesbrecht et al., 2003; Prado et al., 2011) suggest that our results linking behavior, large-scale networks, and oscillatory activity very likely reflect an attention phenomenon moderated by age and sex rather than the converse interpretation.
Translational impact
Our findings pave the way for clinical translation in the context of precision psychiatry. Treatment of the cognitive symptoms of MDD has been challenging due to both the extreme heterogeneity in symptom profiles in individuals with MDD, and the lack of mechanistic treatment targets for cognitive domains of this disorder. Our deep-phenotyping characterization of the ‘Inattention Biotype’ could be utilized to stratify individuals with particularly poor attention function with the eventual goal of informing treatment selection tailored toward this particular domain. Our neuroimaging and electrophysiological results provide potential neural substrates for the development of more precisely targeted treatments for a specific and particularly impactful aspect of cognition: selective attention. The relationship we observed between selective attention impairments and negative biases suggests that treating attention problems may have profound impact on mood-related symptoms of this disorder as well.
These results imply that a top-down attentional control problem, in which the ability to re-focus attention on task-relevant information is disrupted, may explain the tendency of depressed individuals to linger attention specifically on negative information in the environment. Previous work has shown that such negative valence biases may perpetuate sad mood in depressed individuals (Clasen et al., 2013), which suggests that selective attention problems may fuel the cycle of recurring depressive episodes. Consequently, improving selective attention capabilities may improve mood symptomatology. Future research may therefore explore the use of more targeted pharmacological or behavioral interventions that could potentially improve attention symptoms in the context of depression. For example, dual-action antidepressants typically used as second-line or augmenting interventions for depression (e.g. buproprion), or medications typically associated with the treatment of attention symptoms of ADHD, may be more effective first-line interventions for treating depressed patients with severe attention impairments. Mindfulness meditation, which can be conceptualized as selective attention training, is known to increase alpha oscillations and improve selective attention (Kerr et al., 2011), as well as showing promising clinical results in depressed individuals (Hollon and Ponniah, 2010). Our findings suggest that improving top-down attention function may break the self-perpetuating cycle of symptoms by alleviating negative valence biases, providing a potential explanation for the effectiveness of mindfulness training in treating depression. Importantly, future studies will be necessary to quantify the intra-subject reliability of the behavioral metric used to assess selective attention (Stroop reaction times) in order to understand the reliability of this metric for individualized treatment prediction.
Conclusions and future directions
Together, our results demonstrate construct validity for the Inattention Biotype, characterized by feature-based selective attention impairments and associated with both fronto-parietal network dysfunction and decreased oscillatory power in the alpha band. Our method of anchoring in behavioral measurements has allowed us to characterize specific neuroimaging and electrophysiological correlates of selective attention impairments in this population. One strength of this method is that it has allowed us to demonstrate both convergent and divergent validity for the Inattention Biotype, using both continuous analyses and direct stratification of individuals by behavioral performance. These findings lay the groundwork for further validation of the Inattention Biotype on the path toward personalized, targeted treatments for psychiatric illness, and demonstrate one method by which to characterize cognitive biotypes in a psychiatric population.
A critical remaining question is to understand how attention problems arise, and in particular whether they are a cause or consequence of psychiatric illness. One possibility is that pathology associated with depression interferes with the attention network. Stress hyper-reactivity, which commonly occurs in depression, is associated with hypothalamic-pituitary-adrenal (HPA)-axis dysregulation and hypercortisolemia, often leading to impaired glucocorticoid signaling and subsequent neuronal atrophy (for review: Iwata et al., 2013). One of the primary areas in which atrophy occurs with stress reactivity is the medial prefrontal cortex, part of the fronto-parietal attention network, which suggests that stress reactivity may lead to attention impairments in depressed individuals who have strong HPA-axis dysfunction. Another possibility is that attention capabilities vary naturally in the population, and those who struggle with attention relative to their peers may be at increased risk of developing depression. Although longitudinal studies will be critical to disentangle these possibilities, our findings tentatively suggest that network dysfunction and alpha power may represent distinct pathways toward attention impairments given that they are each independently correlated with selective attention impairment but are uncorrelated with each other. Network dysfunction may occur as a downstream result of depression-related pathology (e.g. stress reactivity) while alpha power, known to vary naturally in the population, may be related to natural variation in attention abilities, predisposing individuals with worse attentional focus and lower alpha power to developing depression. Future investigations may also explore interactions among network-based biotypes, such as relations between fronto-parietal network and default mode network connectivity (Alarcón et al., 2018).
Supplementary Material
Acknowledgements.
We thank all the patients and volunteers who agreed to participate in this study, and the staff of the MRI facility at the Westmead Hospital, NSW, Australia. We acknowledge Brain Resource Company Operations Pty Ltd. as the sponsor for the iSPOT-D study (NCT00693849). We acknowledge the roles of Leanne Williams, Ph.D., as the cross-site academic Principal Investigator for iSPOT-D (2008–2013) and Claire Day, Ph.D., as the global trial coordinator for iSPOT-D (2008–2014) and the iSPOT-D Publication Team. We also acknowledge the hard work of the Brain Dynamics Centre iSPOT-D team at the Sydney site for their help with data collection of the presented cohort. Dr Anthony Harris is thanked for his role in supervision of clinical imaging evaluations (as PI for the Sydney site), and Dr Tim Usherwood for his role in overseeing the partnership with primary care practitioners and recruitment of patients from these primary care settings (as co-PI for the Sydney site). Dr Lavier Gomes, Ms Sheryl Foster and the Department of Radiology at Westmead are thanked for their substantial contributions to MRI data acquisition.
Financial support. L.M.W was supported by National Institutes of Health grants (R01MH101496 and UH2AG052163). A.S.K. is supported by the National Defense Science and Engineering Graduate (NDSEG) fellowship, the Stanford Center for Mind, Brain, Computation and Technology (MBCT) traineeship, and the Stanford Neurosciences PhD Program. T.M.B is supported by the National Institute of Mental Health (K23-MH113708). We acknowledge the editorial support of Jon Kilner, MS, MA (Pittsburgh, PA, USA).
Footnotes
Supplementary material. The supplementary material for this article can be found at https://doi.org/10.1017/S0033291719002290.
Trial Registration: Registration Number: CT00693849; URL: http://www.clinicaltrials.gov/ct2/show/NCT00693849.
Conflict of interest. Over the past three years, L.M.W has received scientific advisory board fees from Psyberguide of the One Mind Institute and consultancy from Humana and Blackthorn Therapeutics for projects not related to this work.
References
- Alarcón G, Pfeifer JH, Fair DA and Nagel BJ (2018) Adolescent gender differences in cognitive control performance and functional connectivity between default mode and fronto-parietal networks within a self-referential context. Frontiers in Behavioral Neuroscience 12, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasen PC, Wells TT, Ellis AJ and Beevers CJ (2013) Attentional biases and the persistence of sad mood in major depressive disorder. Journal of Abnormal Psychology 122, 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M and Shulman GL (2002) Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience 3, 201–215. [DOI] [PubMed] [Google Scholar]
- Falkenberg LE, Specht K and Westerhausen R (2011) Attention and cognitive control networks assessed in a dichotic listening fMRI study. Brain and Cognition 76, 276–285. [DOI] [PubMed] [Google Scholar]
- Fernandes BS, Williams LM, Steiner J, Leboyer M, Carvalho AF and Berk M (2017) The new field of ‘precision psychiatry’. BMC Medicine 15, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatt JM, Nemeroff CB, Schofield PR, Paul RH, Clark CR, Gordon E and Williams LM (2010) Early life stress combined with serotonin 3A receptor and brain-derived neurotrophic factor valine 66 to methionine genotypes impacts emotional brain and arousal correlates of risk for depression. Biological Psychiatry 68, 818–824. [DOI] [PubMed] [Google Scholar]
- Giesbrecht B, Woldorff MG, Song AW and Mangun GR (2003) Neural mechanisms of top-down control during spatial and feature attention. NeuroImage 19, 496–512. [DOI] [PubMed] [Google Scholar]
- Gotlib IH and Joormann J (2010) Cognition and depression: current status and future directions. Annual Reviews in Clinical Psychology 6, 285–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve SM, Korgaonkar MS, Etkin A, Harris A, Koslow SH, Wisniewski S, Schatzberg A, Nemeroff CB, Gordon E and Williams LM (2013) Brain imaging predictors and the international study to predict optimized treatment for depression: study protocol for a randomized control trial. Trials 14, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Lim S, Fortunato S, Sporns O, Zhang L, Qiu J, Xie P and Zuo XN (2018) Reconfiguration of cortical networks in MDD uncovered by multiscale community detection with fMRI. Cerebral Cortex 28, 1383–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollon SD and Ponniah K (2010) A review of empirically supported psychological therapies for mood disorders in adults. Depression and Anxiety 27, 891–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M, Ota KT and Duman RS (2013) The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behavior, and Immunity 31, 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger J, Berns S, Uzelac S and Davis-Conway S (2006) Neurocognitive deficits and disability in major depressive disorder. Psychiatry Research 145, 39–48. [DOI] [PubMed] [Google Scholar]
- Kerr CE, Jones SR, Wan Q, Pritchett DL, Wasserman RH, Wexler A, Villaneuva JJ, Shaw JR, Lazar SW, Kaptchuk TJ, Littenberg R, Hämäläinen MS and Moore CI (2011) Effects of mindfulness meditation training on anticipatory alpha modulation in primary somatosensory cortex. Brain Research Bulletin 85, 96–103. [DOI] [PubMed] [Google Scholar]
- Klimesch W (2012) Alpha-band oscillations, attention, and controlled access to stored information. Trends in Cognitive Sciences 16, 606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Tan JC, Parimal S, Dinges DF and Chee MW (2010) Sleep deprivation impairs object-selective attention: a view from the ventral visual cortex. Public Library of Science (PLoS) One 5, e9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q, Deng Z, Qin J, Wei D, Cun L, Qiu J, Hitchman G and Xie P (2015) Frequency dependent topological alterations of intrinsic functional connectome in major depressive disorder. Scientific Reports 5, 9710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre AC, Sebestyen GN, Gitelman DR, Mesulam MM, Frackowiak RSJ and Frith CD (1997) Functional localization of the system for visuospatial attention using positron emission tomography. Brain 120(pt.3), 515–533. [DOI] [PubMed] [Google Scholar]
- Parker G and Hadzi-Pavlovic D (1996) Development and structure of the CORE system. In Parker G and Hadzi-Pavlovic D (eds), Melancholia: A Disorder of Movement and Mood Cambridge, England: Cambridge University Press, pp. 223–236. [Google Scholar]
- Payne L and Sekuler R (2014) On the importance of ignoring: alpha oscillations protect selective processing. Current Directions in Psychological Science 23, 171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancák A Jr and Neuper C (1996) Event-related synchronization (ERS) in the alpha band–an electrophysiological correlate of cortical idling: a review. International Journal of Psychophysiology 24, 39–46. [DOI] [PubMed] [Google Scholar]
- Prado J, Carp J and Weissman DH (2011) Variations of response time in a selective attention task are linked to variations of functional connectivity in the attentional network. Neuroimage 54, 541–549. doi: 10.1016/j.neuroimage.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak R (2012) The frontoparietal attention network of the human brain. Neuroscientist 18, 502–515. [DOI] [PubMed] [Google Scholar]
- Qin J, Wei M, Liu H, Yan R, Luo G, Yao Z and Qing L (2014) Abnormal brain anatomical topological organization of the cognitive-emotional and the frontoparietal circuitry in major depressive disorder. Magnetic Resonance in Medicine 72, 1397–1407. [DOI] [PubMed] [Google Scholar]
- Robinson PN (2012) Deep phenotyping for precision medicine. Human Mutation 33, 777–780. [DOI] [PubMed] [Google Scholar]
- Saveami R, Etkin A, Duchemin AM, Goldstein-Piekarski A, Gyurak A, Debattista C, Schatzberg AF, Sood S, Day CV, Palmer DM, Rekshan WR, Gordon E, Rush AJ and Williams LM (2015) The international Study to Predict Optimized Treatment in Depression (iSPOT-D): outcomes from the acute phase of antidepressant treatment. Journal of Psychiatric Research 61, 1–12. [DOI] [PubMed] [Google Scholar]
- Serences JT and Kastner S (2014) A multi-level account of selective attention. In Nobre AC and Kastner S (eds), The Oxford Handbook of Attention New York, NY: Oxford University Press, pp. 76–104. [Google Scholar]
- Shilyansky C, Williams LM, Gyurak A, Harris A, Usherwood T and Etkin A (2016) Effect of antidepressant treatment on cognitive impairments associated with depression: a randomized longitudinal study. The Lancet. Psychiatry 3, 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Allen DN, Jorgensen ML and Cramer SL (2005) Test-retest reliability of standard and emotional stroop tasks: an investigation of color-word and picture-word versions. Assessment 12, 330–337. [DOI] [PubMed] [Google Scholar]
- Williams LM (2016) Precision psychiatry: a neural circuit taxonomy for depression and anxiety. The Lancet. Psychiatry 3, 472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Rush AJ, Koslow SH, Wisniewski SR, Cooper NJ, Nemeroff CB, Schatzberg AF and Gordon E (2011) International study to predict optimized treatment for depression (iSPOT-D), a randomized clinical trial: rationale and protocol. Trials 12, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2017) Depression and other common mental dis-orders: global health estimates Available at http://apps.who.int/iris/bitstream/10665/254610/1/WHO-MSD-MER-2017.2-eng.pdf?ua=1 (Accessed 15 March 2018).
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC and Wager TD (2011) NeuroSynth: a platform for large-scale automated synthesis of human functional neuroimaging data. Frontiers in Neuroinformatics Conference Abstract: 4th INCF Congress of Neuroinformatics. doi: 10.3389/conf.fninf.2011.08.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


