Abstract
Background & Aims:
Chronic hepatitis B (CHB) can lead to hepatocellular carcinoma (HCC). While both tenofovir disoproxil (TDF) and entecavir (ETV) have been shown to reduce the risk of HCC, their comparative effectiveness is unclear. We estimated the comparative effectiveness of these two agents in reducing the risk of HCC in patients with CHB, through a systematic review and meta-analysis.
Approach & Results:
We searched multiple electronic databases from Jan 1, 1998 to October 31, 2019, for randomized controlled trials (RCTs) and observational comparative effectiveness studies in adults with CHB treated with ETV compared to TDF, reporting the incidence of HCC (minimum follow-up 12 months). Primary outcome was incidence of HCC, calculated as incidence rate ratio (IRR) with 95% CI (unadjusted analysis) and hazard ratio (HR) with 95% CI (adjusted analysis, where reported). Of 1971 records identified, 14 studies (263,947 person-years) were included for quantitative analysis. On unadjusted meta-analysis of 14 studies, the risk of HCC was not statistically different between ETV and TDF (IRR 1.28; 95% CI, 0.99–1.66). When utilizing available adjusted data (multivariate or propensity-matched data), the risk of HCC among patients treated with ETV was 27% higher when compared to TDF (7 studies; 95% CI, 1.01–1.60, p=0.04). Additional analysis of adjusted data when separately reported among patients with cirrhosis demonstrated an adjusted HR of 0.90 (95% CI, 0.66 – 1.23), suggesting no difference between ETV and TDF-treated groups. The overall confidence in estimates was very low (observational studies, high heterogeneity).
Conclusions:
Tenofovir may be associated with lower risk of HCC when compared to entecavir.
Keywords: chronic hepatitis B, nucleos(t)ide analogues, tenofovir, entecavir, hepatocellular carcinoma, antivirals, meta-analysis, systematic review
Chronic hepatitis B virus infection affects more than 250 million people around the world, causing nearly 1 million deaths per year(1). Chronic hepatitis B (CHB) is the leading cause of hepatocellular carcinoma (HCC) worldwide, which can occur even in the absence of cirrhosis in a subset of patients(2). HCC is the third-most common cause of cancer-related death in the world(3). Improved understanding of Hepatitis B (HBV)-related HCC may help reduce the burden of morbidity and mortality due to HCC.
Over the past two decades, researchers have identified several modifiable and non-modifiable risk factors for HBV-related HCC including Hepatitis B e-antigen (HBeAg) status (4), cirrhosis (5), and serum HBV DNA levels (6). This recognition has led to achieving a virologic response with undetectable HBV DNA as one of the key treatment endpoints in patients with chronic HBV infection (7). The currently approved oral treatment regimens for chronic HBV infection are the nucleos(t)ide analogues lamivudine, adefovir, entecavir, tenofovir disoproxil or alafenamide, and telbivudine. These antiviral agents achieve biochemical and virologic response with varying efficacy. American Association for the Study of Liver Diseases (AASLD) guidelines recommend entecavir or tenofovir as first-line nucleos(t)ide analogues owing to their potency and high genetic barrier to resistance, particularly in nucleos(t)ide-naïve patients (8).
A pairwise meta-analysis in 2013, focusing on head-to-head comparisons, failed to discern a difference between oral anti-viral agents in reducing the risk of HCC in patients with CHB (9). Recent observational studies published in the last two years have yielded conflicting results on which of these two agents may confer a higher degree of protection from HCC. Choi et al. (10), using a South Korean national cohort, observed a significantly lower risk of HCC in tenofovir-treated patients compared to entecavir-treated patients. Data from a Hong Kong-wide cohort (11) also suggested a lower risk of HCC in the tenofovir-treated group when compared to entecavir-treated patients. On the other hand, a more recent multicenter observational study from South Korea failed to demonstrate a difference in hepatocarcinogenesis between entecavir and tenofovir (12). A separate multinational study (13) did not observe a difference in risk of hepatocarcinogenesis between tenofovir and entecavir in patients with CHB.
Therefore, to inform the comparative efficacy of entecavir (ETV) and tenofovir disoproxil (TDF) in modifying the risk of HCC in patients with CHB, we performed a systematic review and meta-analysis. We critically appraised the certainty of evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology.
MATERIALS & METHODS
This meta-analysis was initially conducted following an a priori established protocol (PROSPERO registry: CRD42018118027), with planned pairwise and network meta-analysis comparing the effectiveness of all oral anti-viral agents for chronic hepatitis B in preventing risk of HCC. Based on feedback from editorial and peer-review, we modified this protocol to focus on comparing ETV vs. TDF as a pairwise meta-analysis. Since our study synthesized previously published literature, informed consent and ethics-board approval were not required.
Study Selection
Studies eligible for this meta-analysis included randomized controlled trials (RCTs) and observational studies that met the following inclusion criteria: (a) Patients: adults (age >18 years) with chronic HBV infection (generally defined as hepatitis B surface antigen (HBsAg) persisting for at least six months; (b) intervention: entecavir (c) control: tenofovir disoproxil; (d) outcome: risk of HCC. Due to a paucity of randomized trials addressing the comparative efficacy of the two antiviral agents for reducing risk of HCC (related to short follow-up duration, small sample size, and low event rate), we a priori opted to include observational studies in this meta-analysis.
We excluded studies with (a) mean or median follow-up of cohort less than 12 months, or where total person-year, mean or median follow-up of cohort was not reported (unable to ascertain incidence of HCC), (b) HIV or hepatitis C co-infected patients, (c) cohorts that included pregnant women or children, (d) patients with pre-existing HCC or other malignancies, (e) patients with organ transplantation or on immunosuppressive agents, (f) non-comparative studies (single-arm studies), (g) studies designed as crossover studies, and (h) studies in which patients were treated with agents other than ETV or TDF. If multiple studies were conducted with overlapping cohorts, primary analysis included all studies if some aspects of cohort were non-overlapping; sensitivity analyses including only one cohort at a time were also conducted.
Data Sources & Searches
The search strategy was designed and conducted by an experienced librarian with input from the study’s principal investigator. We updated a previously conducted systematic review (previously from January 1, 1998 to September 16, 2014) for the AASLD clinical guidelines for management of HBV infection, through October 30th, 2018 (14) by the same librarian and literature review team. The databases included Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, and Daily, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials (CENTRAL), Ovid Cochrane Database of Systematic Reviews, and Scopus. Controlled vocabulary supplemented with keywords were used to search for comparative studies of antiviral agents for chronic HBV. The actual search strategy is available in the supplementary appendix. Conference proceedings from the annual Liver Meeting and the International Liver Congress (2014–19) were also searched manually. Following peer review, a focused updated literature search of Medline was performed through October 31, 2019. Two investigators reviewed the study title and abstract and identified studies for inclusion. In case of discrepancy, the article was re-reviewed in conjunction with another investigator.
Data Abstraction and Risk of Bias Assessment
Two investigators independently abstracted data on study-, participant-, disease-, and treatment-related characteristics and outcomes from each study using a pre-designed template. The following data were extracted: article reference; type of study (trial design); number of patients; follow-up period; geographical region; average age; number of HBeAg positive patients; number of patients with cirrhosis; incidence of virologic, biochemical and serologic response; and incidence of HCC. Specifically, to ascertain incidence of HCC, we used total person-year follow-up with each drug. If this was not reported, we estimated follow-up by multiplying the number of patients on intervention by the mean or median follow-up of cohort. HCCs that occurred within six months of study/antiviral initiation were excluded. In addition, we also abstracted data on adjusted analyses as reported in individual studies, including analytical approach (Cox proportional hazard analysis, propensity score matched or adjusted analysis), along with the list of adjusted variables. Discrepancies between reviewers were resolved by referencing to the original article, with any further disagreement arbitrated by senior investigator.
Two investigators independently assessed the risk of bias in included studies. Any discrepancies were resolved through consensus, in conjunction with a senior investigator. For RCTs and quasi-randomized trials, the updated Cochrane tool (https://www.riskofbias.info/) for assessing risk-of-bias was used to determine whether there is high, low or unclear risk of bias in the following domains: random sequence generation, allocation concealment, blinding or participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting bias, and other sources of bias. For observational studies, the Quality In Prognosis Studies tool (QUIPS) (15) was used to evaluate validity and bias in studies of prognostic factors across six domains: participation, study attrition, prognostic factor measurement, confounding measurement and account, outcome measurement, and analysis and reporting.
Outcomes
The primary outcome of interest was incidence rate of HCC per person-year (unadjusted analysis). To ascertain comparative effectiveness of the ETV vs. TDF, we reported incidence rate ratio. We also performed a secondary analysis focusing only on studies that reported analysis adjusted for key covariates, utilizing maximally adjusted effect estimates reported in individually studies (Cox proportional hazard analysis, propensity score matched- or adjusted- analysis).
Statistical Analysis
We estimated incidence rate ratio (IRR), hazard ratio (HR) and 95% confidence intervals (CI), using the DerSimonian-Liard random-effects model. Statistical heterogeneity was assessed using the I2 statistic, with values over 50% suggesting substantial heterogeneity. Small study effects were assessed using funnel plots (16). Comparisons were performed using Comprehensive Meta-Analysis, v2.0. For univariate analysis (IRR), we evaluated the stability of association and identified source of heterogeneity through subgroup analysis based on study-level meta-regression based on mean age, proportion of patients who were HBeAg positive, proportion of patients with cirrhosis, mean HBV DNA, and mean baseline ALT. In case of multiple overlapping cohorts, sensitivity analyses including only one cohort at a time was also conducted. We also conducted sensitivity analysis by excluding studies that enrolled treatment-experienced patients. Publication bias was assessed graphically using Funnel plot, and statistically using Egger’s test.
We evaluated the certainty in effect estimates using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework for meta-analysis. In this approach, direct evidence from RCTs starts at high confidence, and can be rated down based on risk of bias, indirectness, imprecision, inconsistency (or heterogeneity) and/or publication bias, to levels of moderate, low and very low confidence. Direct evidence from observational studies starts at low confidence, and can be rated down for previously mentioned factors, or rated up if the magnitude of effect is large, dose-response effect is observed or all plausible confounding would reduce a demonstrated effect; where evidence was derived from both RCTs and observational studies, we conservatively attributed certainty to the lower level of evidence.
RESULTS
With our electronic search strategy, we identified 1971 unique studies in addition to the previously identified studies as part of the aforementioned AASLD 2016 systematic review (14). An additional seven studies were added by manual searches. After applying the inclusion and exclusion criteria, a total of 14 studies (10–13, 17–26) met inclusion criteria. The study selection flow diagram is shown in Figure 1.
Figure 1:
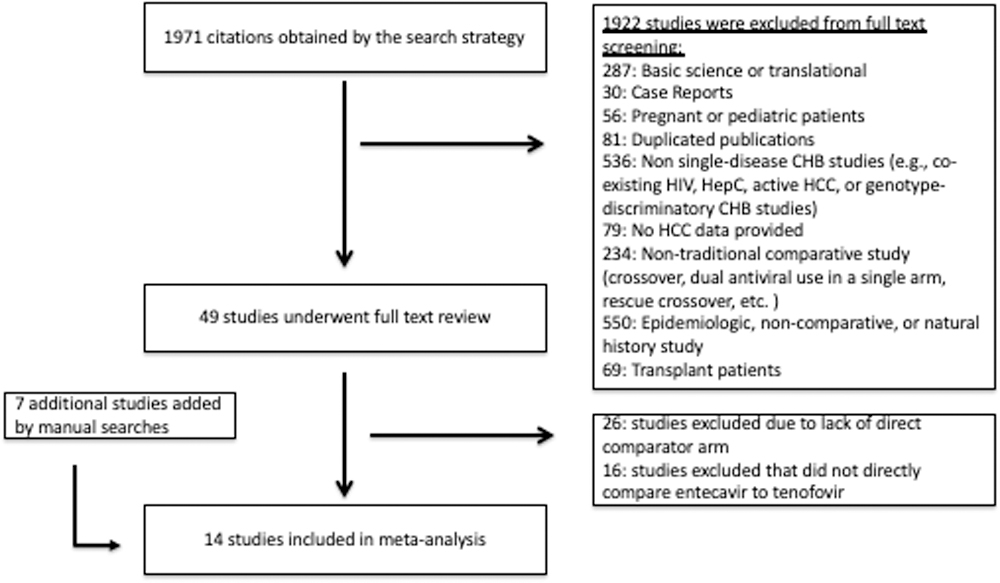
Study identification and selection
Characteristics of Studies and Patients
Out of the 14 included studies, 13 were observational studies and one was an RCT. Together, the studies included a total of 263,947 person-years. The length of follow-up ranged from 18 months to 66 months. The mean follow-up of the 14 studies was 45.61 months. The mean of median ages of the patients was 46.7, ranging from 30 to 53. Three studies excluded patients with cirrhosis; one study did not have available data on percentage of patients with cirrhosis, stratified by antiviral used. Among studies that included patients with cirrhosis, the calculated mean for the percentage of patients with cirrhosis was 27%. Mean baseline HBV DNA, available in all but one study, was 6.28 log IU/mL. The mean baseline ALT, available in all but three studies, was 118 U/L. The majority of the studies only included treatment-naïve patients, with a few exceptions. Observational studies by Kim YM et al. (27), Gordon et al. (19) and Riveiro-Barciela et al. (24) explicitly included all-comers, including nucleos(t)ide-experienced patients. Although not by design, a small minority of patients in the Choi et al. (10) national cohort were nucleos(t)ide-experienced patients. Characteristics of individual studies and participants is shown in Table 1.
Table 1.
Characteristics of studies that met our inclusion criteria
| Author, Year | Location | Study Type | Antiviral | Patients (N) | Follow-up (months) | HCC (N) | Baseline Age (Years) | Baseline % cirrhosis | HBeAg positive (N) | HBeAg positive (%) †† | Baseline HBV DNA (log10 IU/mL) | Baseline ALT (IU/mL) | Virologic Response (%) | Biochemical Response (%) | HBeAg loss (N) | Incident HCC (%) over time (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Batirel et al 2014 | Turkey | Observational | ETV | 90 | 27.2 | 0 | 43.3 ± 12.9 | 0 | 29 | 32 | 8.28 | 116.7 ± 92.6 | 86 | 98 | 7 | n/a |
| TDF | 105 | 33 | 0 | 42 ± 11.2 | 0 | 36 | 34 | 8.34 | 120 ± 96.6 | 86 | 99 | 16 | n/a | |||
| Wu et al 2017 | Taiwan | Observational | ETV | 313 | 49 | 21 | 47 ± 12.3 | 30 | 172 | 55 | 7.18 (0.74) | 316 | 88 | 82 | 57 | 6.7 (48) |
| TDF | 106 | 37.9 | 8 | 47.1 ± 12.1 | 27 | 50 | 47 | 7.36 (0.7) | 303 | 91 | 84 | 148 | 7.7 (48) | |||
| Riveiro-Barciela et al 2017 | Spain | Observational | ETV | 187 | 55 ± 22 | 3 | 50 ± 13 | 34 | 34 | 18 | 4.9 | 138 ± 367 | 100 | 92 | 11 | n/a |
| TDF | 424 | 49 ± 29 | 11 | 50 ± 13 | 31 | 67 | 16 | 3.8 | 68 ± 139 | 98 | 88 | 16 | n/a | |||
| Choi et al 2018 | South Korea | Observational | ETV | 11464 | 51 (40.4, 55.4) | 590 | 49.3 ± 9.8 | 26 | NA | NA | 6.7 (5.6, 7.9) | 32 (21, 54) | NA | NA | NA | 5.1† (48) |
| TDF | 12692 | 36 (29.3, 42.1) | 394 | 48.6 ± 9.8 | 27 | NA | NA | 6.4 (5.4, 7.6) | 35 (24, 58) | NA | NA | NA | 3 † (48) | |||
| Kim BG et al 2018 | South Korea | Observational | ETV | 721 | 66 | 40 | 52 ± 11 | 48 | 430 | 60 | 6.4 (1.4) | 143.1 ± 172.4 | 98 | NA | NA | 5.1 (48) |
| TDF | 604 | 33 | 14 | 50 ± 11 | 44 | 376 | 63 | 6.0 (1.6) | 136.5 ± 228.1 | 95 | NA | NA | 4.0 (48) | |||
| Yu et al 2018 | South Korea | Observational | ETV | 406 | 69.9 | 31 | 53 ± NA | 36 | 212 | 52 | 6.1 | 93 | 82 | NA | NA | 4.0 (48) |
| TDF | 176 | 33.6 | 7 | 49 ± NA | 44 | 104 | 59 | 6.93 | 100 | 83 | NA | NA | 5.0 (48) | |||
| Kayaaslan et al 2018 | Turkey | Observational | ETV | 166 | 48 | 0 | 43 ± NA | 0 | 56 | 34 | 6.92 | 89 | 97 | 92 | 10 | n/a |
| TDF | 86 | 18 | 0 | 42 ± NA | 0 | 41 | 48 | 6.6 | 92 | 69 | 83 | 5 | n/a | |||
| Kim YM et al 2018 | South Korea | Observational | TDF | 112 | 38.5 ± 9.2 | 3 | 49.3 ± 10.9 | 26.8 | 62 | 55 | 6.0 ± 1.5 | 67 (36–145) | 91.1 | NA | NA | n/a |
| ETV | 191 | 66.6 ± 26.8 | 13 | 47.7 ± 12.3 | 27.8 | 116 | 61 | 6.3 ± 1.2 | 124.5 (71–246) | 94.2 | NA | NA | n/a | |||
| Cai et al 2019 | China | RCT | TDF | 157 | 33.2 | 0 | 30.8 ± 8.7 | 0 | 157 | 100 | 7.57 ± 0.93 | NA | 92 | 86.3 | 51 | n/a |
| ETV | 158 | 33.2 | 0 | 30.9 ± 8.4 | 0 | 158 | 100 | 7.69 ± 0.98 | NA | 87 | 87.7 | 55 | n/a | |||
| Kim SU et al 2019 | South Korea | Observational | TDF | 1413 | 59.2 | 102 | 48.8 ± 12 | 29.1 | 694 | 49 | 5.4 ± 2.1 | NA | 87.5 | NA | NA | 7.7 (60) |
| ETV | 1484 | 59.2 | 138 | 48.2 ± 11.5 | 33.6 | 758 | 51 | 5.7 ± 2.1 | NA | 86.5 | NA | NA | 9.3 (60) | |||
| Yip et al 2019 | Hong Kong | Observational | TDF | 1309 | 33.6 | 8 | 43.2 ± 13.1 | 3 | 723 | 55 | 4.8 | 43 | 72 | 58 | 248 | 1.1 (60) |
| ETV | 28041 | 44.4 | 1386 | 53.4 ± 13.0 | 13 | 8306 | 30 | 5.3 | 62 | 75 | 69 | 287 | 7.0 (60) | |||
| Lee at al 2019 | South Korea | Observational | TDF | 1439 | 36.4 | 50 | 47.29 ± 11.16 | 34 | 823 | 57 | 6.41 (5.34, 7.49) | 94 (51, 194) | 97.7 | 86.7 | NA | 5.6 (60) |
| ETV | 1583 | 60 | 84 | 46.66 ± 11.76 | 36 | 974 | 62 | 6.49 (5.28, 7.67) | 98 (53, 201) | 91.4 | 91.6 | NA | 5.5 (60) | |||
| Gordon et al 2019††† | USA | Observational | TDF | 407 | 48 | 13 | 48 | NA | NA | NA | NA | NA | NA | NA | NA | n/a |
| ETV | 415 | 66 | 18 | 51 | NA | NA | NA | NA | NA | NA | NA | NA | n/a | |||
| Hsu et al 2019 | Multinational | Observational | ETV | 4387 | 60 (39.6–60) | 285 | 50.81 ± 0.17 | 27.8 | 1537 | 35 | 5.48 ± 0.03 | 200 ±5.65 | NA | NA | NA | 7.3 (60) |
| TDF | 700 | 38.7 (23.8–56.2) | 13 | 45.74 ± 0.47 | 18.7 | 208 | 30 | 4.99 ± 0.09 | 147 ± 11.28 | NA | NA | NA | 3.2 (60) |
ETV = Entecavir; TDF = tenofovir disoproxil; HCC = hepatocellular carcinoma; HBeAg = Hepatitis B e Antigen; HBV = hepatitis B virus; ALT = alanine transaminase
n/a = not available or not-applicable
= estimated based on time-to-event graph
= % less accurate than N, due to lack of universal HBeAg testing
= abstract only
The largest number of HCCs were detected in the Yip et al. (11, 28) Hong-Kong cohort, with a total of 1386 cases in the ETV group and eight in the TDF group. The second largest number of HCCs were detected by Choi et al. (10), with a total of 590 cases in the ETV group and 394 in the TDF group.
Primary outcome
The total HCC events in the entire pool were 3,232 cases in 263,947 person-years. The ETV group had 2609 HCC events in 201,754 person-years, whereas the TDF group had 623 HCC events in 62,192 person-years. The overall pooled incidence rate ratio was 1.28 (95% CI; 0.99 – 1.66, p=0.06), with considerable heterogeneity (I2=68%); (Figure 2). Sensitivity analysis excluding Choi et al. (10) demonstrated no change in point estimate of 1.30 (95% CI; 0.91 – 1.84, p=0.15). Study-level meta-regression analysis demonstrated that heterogeneity in risk ratio can be explained by age (p=0.004), proportion eAg positive (p=0.003) and HBV DNA (p=0.025), but not by proportion with cirrhosis (p=0.17) or baseline ALT (p=0.60) (Figure 3). There was no evidence of publication bias (supplementary appendix, Egger’s test p=0.92). Sensitivity analysis by excluding studies that enrolled treatment-experienced patients (19, 21, 24) did not significantly change the point estimate (IRR, 1.40; 95% CI, 0.93–2.12).
Figure 2:
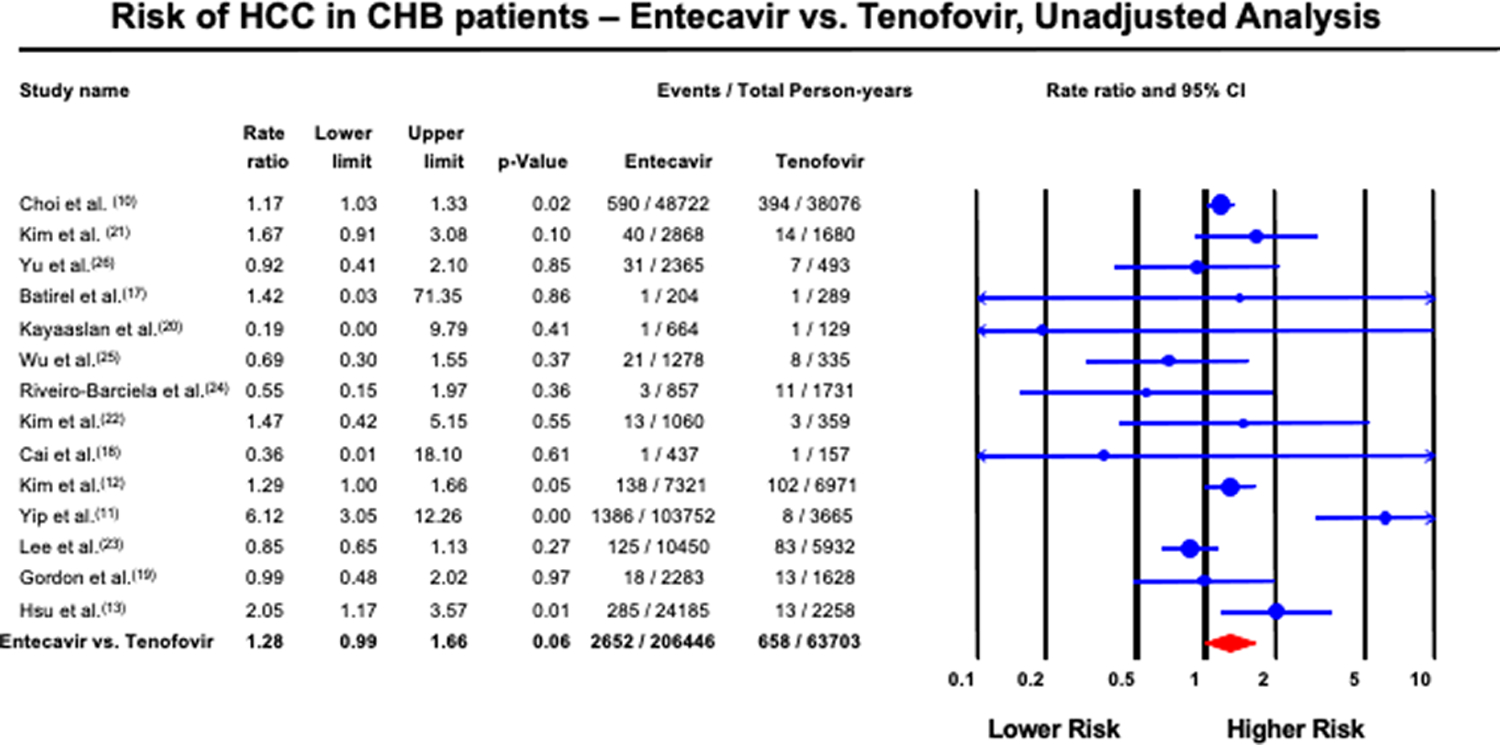
Forest plot of incidence of HCC in CHB patients treated with ETV and TDF, unadjusted analysis
Figure 3:
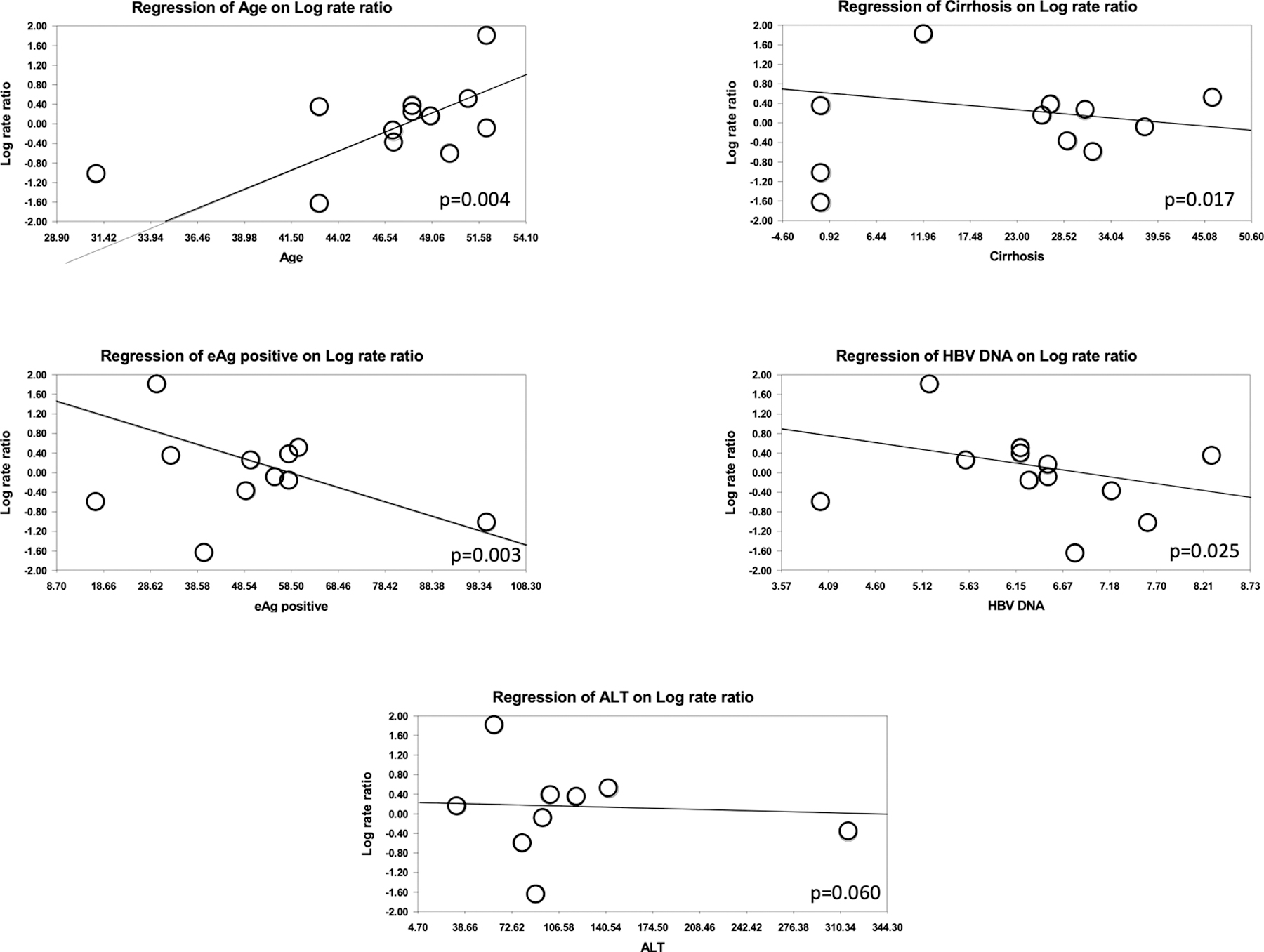
Meta-regression of relative risk of HCC among entecavir vs. tenofovir groups
Seven studies provided hazard ratios adjusted via multivariate analysis or propensity-score matched analysis. There was slight variation in the adjusted covariates, but all of these studies accounted for age, sex, and presence of cirrhosis. Further details are provided in Table 2. Among these studies, the risk of HCC was 27% higher with ETV vs. TDF (95% CI; 1.01 – 1.60, p=0.04), with moderate heterogeneity (I2 = 58%) (Figure 4). Sensitivity analysis by including Choi et al., but excluding all other South Korean studies (12, 21, 23, 26, 27), did not change the point estimate (HR 1.48; 95% CI, 1.13 – 1.93, p<0.01). Sensitivity analysis by excluding each South Korean study at a time (12, 21, 23, 26, 27) did not significantly change the overall summary estimate (HR 1.16; 95% CI, 0.94 – 1.44, p=0.17). Sensitivity analysis by excluding Gordon et al., the only study with adjusted data that enrolled treatment-experienced patients (19), did not change the overall summary estimate (HR 1.26; 95% CI 1.00–1.59). We further conduced subgroup-analysis using adjusted or propensity score matched data in the subset of patients with cirrhosis, when data were available (10, 12, 13, 21, 23). This demonstrated an adjusted HR of 0.90 (95% CI, 0.66 – 1.23), albeit with moderate-to-high heterogeneity (I2 = 64%). Notably, three studies explicitly excluded patients with cirrhosis (17, 18, 20).
Table 2.
Classification of adjusted data availability among included studies
| Author, Year | Location | Study Type | Adjusted analysis for HCC | Adjusted Co-variates |
|---|---|---|---|---|
| Choi et al 2018 | South Korea | Observational | PSM | Age, sex, socioeconomic status, level of health-care, cirrhosis, ascites, varices, diabetes, hypertension, smoking, drinking, BMI, and ALT |
| Kim BG et al 2018 | South Korea | Observational | PSM | Age, sex, HBeAg positivity, cirrhosis, serum levels of HBV DNA, ALT, AST, albumin, total bilirubin, creatinine and alpha‐fetoprotein, international normalized ratio, platelet count, diabetes mellitus, hypertension |
| Kim SU et al 2019 | South Korea | Observational | PSM | Age, gender, diabetes, hypertension, BMI, compensated cirrhosis, HBeAg positivity, serum levels of HBV DNA, international normalized ratio, platelet count, bilirubin, albumin, CPT score, MELD score |
| Yip et al 2019 | Hong Kong | Observational | PSM | Age, sex, cirrhosis, diabetes, hypertension, HbeAg positivity, serum levels of HBV DNA, international normalized ratio, platelet count, bilirubin, albumin, ALT, Creatinine, Follow-up duration, calendar year of initiation |
| Lee at al 2019 | South Korea | Observational | PSM | Age, sex, severity of underlying liver disease, APRI, FIB-4 index, diabetes mellitus, hypertension, BMI, alcohol drinking, esophageal varix, AST, ALT, total bilirubin, albumin, creatinine, gamma-glutamyl transferase, prothrombin time, platelet count, Child-Pugh score, HBeAg positivity, HBV DNA and alpha‐fetoprotein |
| Gordon et al 2019 | USA | Observational | PSM | Age, sex, race, insurance, HBV viral load. Fibrosis-4 score, cirrhosis |
| Hsu et al 2019 | Multinational | Observational | PSM | Age, sex, Asian countries vs. US, HbeAg positivity, serum levels of HBV DNA, AST, ALT, bilirubin, albumin, international normalized ratio, Creatinine, platelets, alpha‐fetoprotein, cirrhosis, decompensation, diabetes, BMI, Fib-4 index |
PSM = propensity score matching; HBV = hepatitis B virus; HBeAg = Hepatitis B e Antigen; AST = aspartate transaminase; ALT = alanine transaminase
Figure 4:
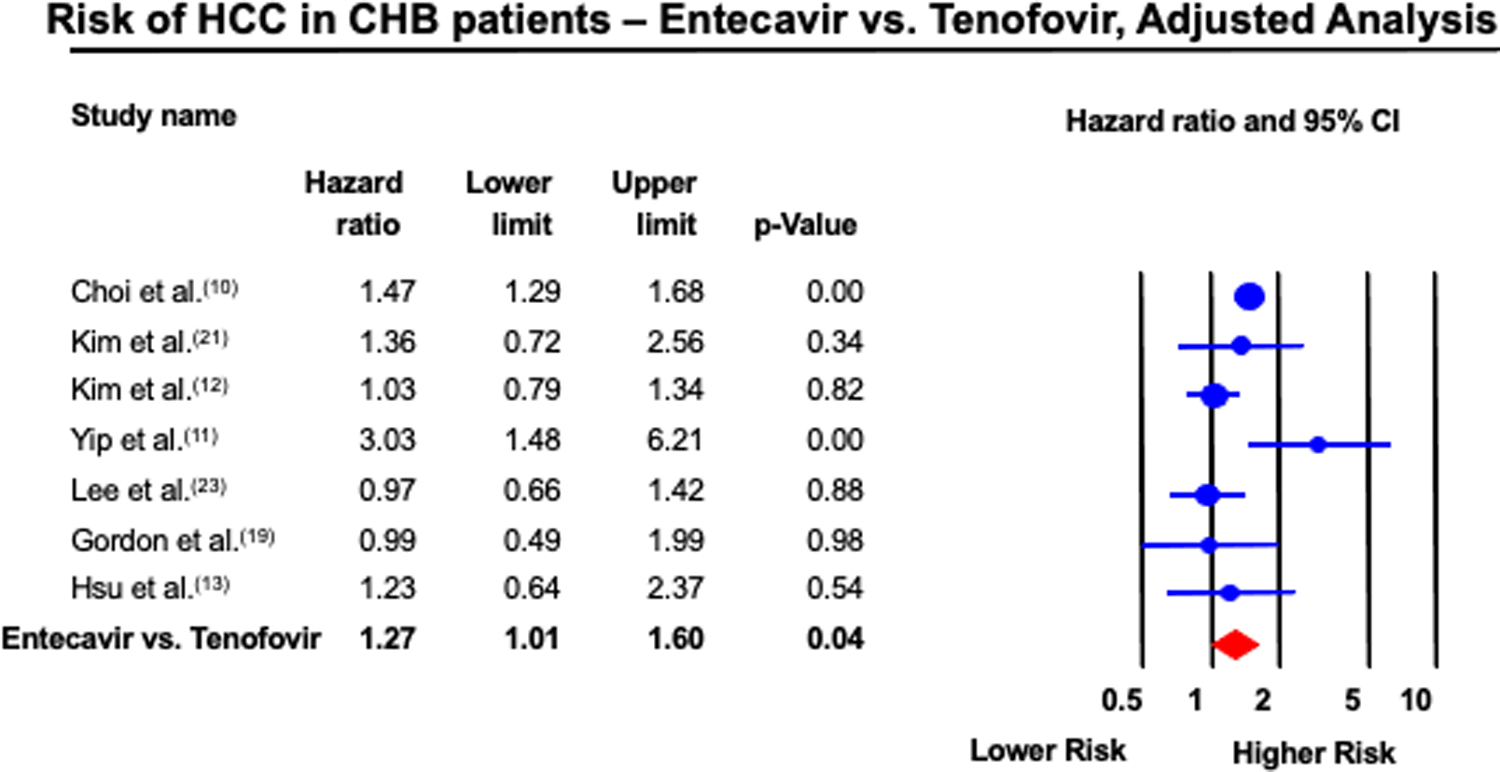
Forest plot of incidence of HCC in CHB patients treated with ETV and TDF, adjusted analysis
Risk of Bias Assessment and Certainty of Evidence
The risk of bias assessment for included studies is presented in the supplementary appendix. Overall, there was moderate to high risk of bias among all observational studies, particularly in statistical analysis and reporting of study attrition. The single included RCT had zero HCC events. Using GRADE, the overall certainty of evidence supporting the use of TDF over ETV for reducing the risk of HCC in patients with CHB was rated as very low (evidence derived from observational studies, further rated down due to heterogeneity).
DISCUSSION
Several oral antiviral agents are available for the management of CHB; current guidelines recommend the use of TDF (or TAF) and ETV due to their high efficacy and tolerability. In this systematic review and meta-analysis of 14 studies, we observed several important findings. Firstly, when unadjusted data are used, there is no statistically significant difference in the incidence of HCC among patients treated with ETV vs. TDF (p=0.06), with moderate heterogeneity. Meta-regression analysis suggests that heterogeneity can be explained by differences in baseline age, HbeAg status, and HBV DNA levels, confirming prior studies (6). On meta-analysis of adjusted data, entecavir may be associated with a 27% higher risk of HCC when compared to tenofovir (p=0.04). Moderate heterogeneity was observed in these estimates drawn from primarily observational studies, and overall certainty in effect estimates was very low.
Due to a dearth of long-term RCTs, we relied almost exclusively on observational studies in this study. Observational studies lack the random allocation of the intervention necessary to optimally test exposure-outcome hypotheses. We conservatively estimated certainty of evidence derived from the observational studies. Additionally, the full 14-study meta-analysis pools unadjusted effect estimates, which can contribute to high risk of bias since baseline patient characteristics differed between studies. Some studies included patients with cirrhosis, where others exclusively studied patients with cirrhosis. There was also variation in baseline age, HBV DNA level, and ALT levels. We performed study-level meta-regression analysis to help account for these differences, though this approach is frequently underpowered to detect important differences. We also separately analyzed the pooled adjusted hazard ratios among studies, when available, on multivariate or propensity-score matched estimates. These studies hazard ratios that were adjusted for age, sex, cirrhosis, and HBV viral load, among other parameters. These data demonstrated that the risk of HCC among patients treated with ETV was 27% higher when compared to TDF. However, a separate analysis of studies where adjusted data were discreetly reported among patients with cirrhosis vs. no cirrhosis, suggested no difference between ETV and TDF-treated groups, although with high heterogeneity.
Our study has several limitations. One source of confounding is that while we excluded all studies designed as crossover studies, not all studies specifically excluded treatment-experienced patients. We performed sensitivity analysis excluding studies that sought to enroll treatment-experienced patients, which reduced the power of the study and resulted in wider confidence intervals. Another possible source of confounding is that tenofovir is preferred over entecavir among pregnant patients or patients who may become pregnant (8). Therefore, it is conceivable that younger women may preferentially be administered tenofovir over entecavir. However, adjusted analysis for age and sex may mitigate some of this confounding. Furthermore, it has also been postulated (19) that variation in hepatocarcinogenesis may have a racial/ethnic association. This was not explored in our study; notably, eight of 14 studies were conducted in predominantly Asian populations. We also note that several of the included studies were conducted in South Korea, with varying degree of overlap of cohorts. Sensitivity analyses by including non-overlapping studies did not significantly change the overall summary estimate. Finally, HCC is a time-related event. Due to scarcity of readily comparable temporal data in a head-to-head fashion, we were unable to perform pooled time-to-event analysis of HCC; however these heterogenic granular data have been reported in Table 1. Another limitation to this study is that many of the older studies were not specifically designed to compare the comparative incidence of HCC between ETV and TDF, whereas the later studies were designed to identify this difference.
In summary, based on a meta-analysis of 14 studies in patients with CHB, we observed that tenofovir may be associated with a lower risk of HCC when compared to entecavir. This may have implications on clinical practice, such as preferentially using tenofovir in patients at highest risk of HCC. However, the overall quality of evidence favoring this assertion is low, due to the heavy reliance on observational studies.
Supplementary Material
Acknowledgments
Financial support
none
List of abbreviations
- CHB
Chronic hepatitis B
- HCC
Hepatocellular carcinoma
- TDF
Tenofovir disoproxil
- ETV
Entecavir
- RCT
Randomized controlled trials
- IRR
Incidence Rate Ratio
- HR
Hazard Ratio
- HBV
Hepatitis B virus
- HBeAg
Hepatitis B e-antigen
- AASLD
American Association for the Study of Liver Diseases
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- PROSPERO
International prospective register of systematic reviews
- QUIPS
Quality In Prognosis Studies tool
REFERENCES
- 1.Organization WH. Hepatitis B. In: Organization WH, editor. Fact sheets: World Health Organization; 2018. [Google Scholar]
- 2.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142:1264–1273.e1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcellin P, Kutala BK. Liver diseases: A major, neglected global public health problem requiring urgent actions and large-scale screening. Liver International 2018;38:2–6. [DOI] [PubMed] [Google Scholar]
- 4.Yang H-I, Lu S-N, Liaw Y-F, You S-L, Sun C-A, Wang L-Y, Hsiao CK, et al. Hepatitis B e Antigen and the Risk of Hepatocellular Carcinoma. New England Journal of Medicine 2002;347:168–174. [DOI] [PubMed] [Google Scholar]
- 5.Papatheodoridis GV, Lampertico P, Manolakopoulos S, Lok A. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: A systematic review. Journal of Hepatology 2010;53:348–356. [DOI] [PubMed] [Google Scholar]
- 6.Chen CJ, Yang Hi Fau - Su J, Su J Fau - Jen C-L, Jen Cl Fau - You S-L, You Sl Fau - Lu S-N, Lu Sn Fau - Huang G-T, Huang Gt Fau - Iloeje UH, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA - Journal of the American Medical Association. [DOI] [PubMed]
- 7.Lampertico P, Agarwal K, Berg T, Buti M, Janssen HLA, Papatheodoridis G, Zoulim F, et al. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. Journal of Hepatology 2017;67:370–398. [DOI] [PubMed] [Google Scholar]
- 8.Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016;63:261–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singal AK, Salameh H, Kuo YF, Fontana RJ. Meta-analysis: the impact of oral anti-viral agents on the incidence of hepatocellular carcinoma in chronic hepatitis B. Aliment Pharmacol Ther 2013;38:98–106. [DOI] [PubMed] [Google Scholar]
- 10.Choi J, Kim HJ, Lee J, Cho S, Ko MJ, Lim YS. Risk of Hepatocellular Carcinoma in Patients Treated with Entecavir vs Tenofovir for Chronic Hepatitis B: A Korean Nationwide Cohort Study. JAMA Oncology. 2018. [DOI] [PMC free article] [PubMed]
- 11.Cheuk-Fung Yip T, Wai-Sun Wong V, Lik-Yuen Chan H, Tse Y-K, Chung-Yan Lui G, Lai-Hung Wong G. Tenofovir is Associated With Lower Risk of Hepatocellular Carcinoma Than Entecavir in Patients With Chronic HBV Infection in China. Gastroenterology 2019. [DOI] [PubMed]
- 12.Kim SU, Seo YS, Lee HA, Kim MN, Lee YR, Lee HW, Park JY, et al. A multicenter study of entecavir vs. tenofovir on prognosis of treatment-naïve chronic hepatitis B in South Korea. Journal of Hepatology 2019;71:456–464. [DOI] [PubMed] [Google Scholar]
- 13.Hsu YC, Wong GL, Chen CH, Peng CY, Yeh ML, Cheung KS, Toyoda H, et al. Tenofovir Versus Entecavir for Hepatocellular Carcinoma Prevention in an International Consortium of Chronic Hepatitis B. Am J Gastroenterol 2019. [DOI] [PubMed]
- 14.Lok ASF, McMahon BJ, Brown RS Jr., Wong JB, Ahmed AT, Farah W, Almasri J, et al. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis. Hepatology 2016;63:284–306. [DOI] [PubMed] [Google Scholar]
- 15.Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 2006;144:427–437. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batirel A, Guclu E, Arslan F, Kocak F, Karabay O, Ozer S, Turanli M, et al. Comparable efficacy of tenofovir versus entecavir and predictors of response in treatment-naive patients with chronic hepatitis B: a multicenter real-life study. International Journal of Infectious Diseases 2014;28:153–159. [DOI] [PubMed] [Google Scholar]
- 18.Cai D, Pan C, Yu W, Dang S, Li J, Wu S, Jiang N, et al. Comparison of the long-term efficacy of tenofovir and entecavir in nucleos(t)ide analogue-naïve HBeAg-positive patients with chronic hepatitis B: A large, multicentre, randomized controlled trials. Medicine 2019;98:e13983–e13983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon SC, Zhou Y, Li J, Rupp LB, Boscarino JA, Daida YG, Schmidt MA, et al. LBP-13-Effect of treatment of hepatitis B patients with tenofovir disoproxil or entecavir on risk of hepatocellular cancer death in a U.S. Cohort. Journal of Hepatology 2019;70:e147. [Google Scholar]
- 20.Kayaaslan B, Akinci E, Ari A, Tufan ZK, Alpat SN, Gunal O, Tosun S, et al. A long-term multicenter study: Entecavir versus Tenofovir in treatment of nucleos(t)ide analogue-naive chronic hepatitis B patients. Clinics and Research in Hepatology and Gastroenterology 2018;42:40–47. [DOI] [PubMed] [Google Scholar]
- 21.Kim BG, Park NH, Lee SB, Lee H, Lee BU, Park JH, Jung SW, et al. Mortality, liver transplantation and hepatic complications in patients with treatment-naïve chronic hepatitis B treated with entecavir vs tenofovir. Journal of Viral Hepatitis 2018;25:1565–1575. [DOI] [PubMed] [Google Scholar]
- 22.Kim Y, Shin H, Lee J, Joo K, Cha J, Jeon J, Yoon J, et al. Real-world single-center experience with entecavir and tenofovir disoproxil fumarate in treatment-naive and experienced patients with chronic hepatitis B. Saudi Journal of Gastroenterology 2018;24:326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SW, Kwon JH, Lee HL, Yoo SH, Nam HC, Sung PS, Nam SW, et al. Comparison of tenofovir and entecavir on the risk of hepatocellular carcinoma and mortality in treatment-naïve patients with chronic hepatitis B in Korea: a large-scale, propensity score analysis. Gut 2019:gutjnl-2019–318947. [DOI] [PMC free article] [PubMed]
- 24.Riveiro-Barciela M, Tabernero D, Calleja JL, Lens S, Manzano ML, Rodriguez FG, Crespo J, et al. Effectiveness and Safety of Entecavir or Tenofovir in a Spanish Cohort of Chronic Hepatitis B Patients: Validation of the Page-B Score to Predict Hepatocellular Carcinoma. Digestive Diseases & Sciences 2017;62:784–793. [DOI] [PubMed] [Google Scholar]
- 25.Wu IT, Hu TH, Hung CH, Lu SN, Wang JH, Lee CM, Chen CH. Comparison of the efficacy and safety of entecavir and tenofovir in nucleos(t)ide analogue-naive chronic hepatitis B patients with high viraemia: a retrospective cohort study. Clinical Microbiology & Infection 2017;23:464–469. [DOI] [PubMed] [Google Scholar]
- 26.Yu JH, Jin YJ, Lee JW, Lee DH. Remaining hepatocellular carcinoma risk in chronic hepatitis B patients receiving entecavir/tenofovir in South Korea. Hepatol Res 2018;48:862–871. [DOI] [PubMed] [Google Scholar]
- 27.Kim Y, Shin H, Lee J, Joo K, Cha J, Jeon J, Yoon J, et al. Real-world single-center experience with entecavir and tenofovir disoproxil fumarate in treatment-naïve and experienced patients with chronic hepatitis B. Saudi Journal of Gastroenterology 2018;.24: 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yip TCF, Wong VW-S, Tse Y-K, Chan H, Wong GL-H. LBO-03-Tenofovir treatment has lower risk of hepatocellular carcinoma than entecavir treatment in patients with chronic hepatitis B. Journal of Hepatology 2019;70:e128. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


