Abstract
Objectives:
To conduct a pilot trial for the Fixation using Alternative Implants for the Treatment of Hip Fractures (FAITH-2) protocol to assess feasibility of a definitive trial.
Design:
Pilot trial.
Setting:
Twenty-five clinical sites across North America and Australia were initiated, but enrolment occurred in only 15 North American sites.
Patients/Participants:
Ninety-one randomized adults aged 18 to 60 years with a femoral neck fracture requiring surgical fixation.
Intervention:
Eligible patients were randomized to receive surgical treatment (sliding hip screw or cancellous screws) AND nutritional supplementation (4000 IU of vitamin D or placebo) for 6 months postfracture.
Main Outcome Measurements:
Feasibility outcomes included: clinical site initiation, participant enrolment rate, proportion of participants with complete 12-month follow-up, level of data quality, and rate of protocol adherence (number of randomization errors, crossovers between treatment groups, and daily supplementation adherence).
Results:
Eighty-six of 91 participants randomized into the pilot trial from 15 North American hospitals were deemed eligible. Four of five primary feasibility criteria were not achieved as we were unable to initiate clinical sites outside of North America and Australia due to feasibility constraints, slow participant enrolment (60 participants recruited over 36 mo), low adherence with daily nutritional supplementation at the 6-week (72.1%), 3-month (60.5%), and 6-month (54.7%) follow-up visits, and a high loss to follow-up rate of 22.1% at 12 months.
Conclusions:
Despite not meeting key feasibility criteria, we increased our knowledge on the logistics and anticipated barriers when conducting vitamin D supplementation trials in this trauma population, which can be used to inform the design and conduct of future trials on this topic.
Keywords: clinical protocols, femoral neck fractures, fracture fixation, internal, randomized controlled trial, vitamin D
1. Introduction
Pilot trials are necessary to confirm the feasibility of larger, definitive trials when there are uncertainties regarding the ability to successfully complete the trial. For example, there may be uncertainties relating to recruitment potential, randomization and blinding processes, participant adherence to trial interventions, safety of the trial drug or intervention, participant follow-up, and outcome collection.[1,2] Not only do pilot trials increase the investigators’ experience with the trial methods and interventions, but they may affirm that the trial is either feasible as written, requires minor or major protocol changes to achieve feasibility, or is not feasible at all.[1] Conducting pilot trials is also an important aspect of using limited funding dollars efficiently and may detect potential time and resource problems that may occur during the definitive trial. Ultimately, pilot trials are important for informing the design of definitive trials and can improve the likelihood of their success.[1,2]
We developed a protocol for the FAITH-2 trial; a concealed, factorial randomized controlled trial (RCT), which aimed to determine whether internal fixation with a sliding hip screw (SHS) versus cancellous screws (CS) and nutritional supplementation with vitamin D versus placebo independently lowered the risk of patient-important outcomes (reoperation, femoral head osteonecrosis, severe femoral neck malunion, and nonunion) during the 12-month postinjury follow-up period in young adults (ages 18–60) with femoral neck fractures.[3]
Prior to initiating the FAITH-2 trial, we determined it was necessary to conduct a pilot trial for several reasons. First, although nearly half of all hip fractures involve the femoral neck, the vast majority occur in elderly patients while only 3% to 10% take place in young adults below the age of 50 years.[4] Given that this is a rare injury in young patients, we determined that multinational collaboration would be imperative to meet the projected sample size requirement of 898 patients for the definitive trial.[3] Therefore, we needed to first evaluate the initiation of global sites and the rate of participant enrolment of a smaller sample to ensure that we could meet our definitive sample size in a timely manner. Second, we wanted to assess adherence with key aspects of the protocol. The FAITH trial, a predecessor elderly femoral neck fracture trial, had a crossover rate of 2.0% between treatment groups for the surgical fixation approach, and we wanted to ensure that we could achieve the same surgeon adherence with the treatment allocation in a younger population.[5] We also wanted to carefully evaluate adherence with daily dosing of the nutritional supplementation given that there is limited data for its use in adult fracture populations. Third, as prior fracture trials have struggled with participant retention and data quality, and we planned to conduct FAITH-2 globally, we wanted to confirm that we could achieve a high participant follow-up rate and obtain a complete data set for analysis.[6–9]
Therefore, the specific feasibility objectives for our pilot trial were to assess the: initiation of clinical sites globally, rate of participant enrolment, proportion of participants with complete follow-up at 12 months postfracture, level of data quality, and rate of protocol adherence (e.g., the number of errors in randomization, the number of crossovers between SHS and CS treatment groups, and adherence to the daily vitamin D supplementation). Details of the primary and secondary clinical outcomes of the FAITH-2 pilot trial will be reported separately.[10]
2. Methods
2.1. Trial design
FAITH-2 was a pilot trial conducted to determine the feasibility of a definitive 2×2 factorial design RCT comparing two alternative surgical techniques AND vitamin D supplementation versus placebo for the treatment of femoral neck fractures in young adult patients (ages 18–60). Upon providing informed consent, participants were randomized in equal proportions to 1 of 4 treatment groups: CS with 4000 IU vitamin D supplementation daily for 6 months, CS with placebo supplementation for 6 months, SHS with 4000 IU vitamin D supplementation daily for 6 months, or SHS with placebo supplementation for 6 months. Participant follow-up visits occurred postoperatively (24 hours to 14-day window), and at 6 weeks (2- to 8-week window), 3 months (2- to 4-month window), 6 months (5- to 7-month window), 9 months (7- to 11-month window), and 12 months (11 months or greater window) postfracture. A previously published protocol manuscript details the trial objectives and methods.[3] The trial was registered with ClinicalTrials.gov, number NCT01908751 and it was approved by the Hamilton Integrated Research Ethics Board (#13-807) and by all participating clinical sites’ research ethics boards/institutional review boards. The research was conducted in accordance with the Declaration of the World Medical Association (www.wma.net) and informed consent was obtained from all human subjects enrolled in the trial.
2.2. Feasibility outcomes
Feasibility outcomes for the pilot trial included: the initiation of clinical sites, rate of participant enrolment, proportion of participants with complete follow-up at 12 months postfracture, level of data quality, and rate of protocol adherence (e.g. the number of errors in randomization, the number of crossovers between SHS and CS treatment groups, and adherence to the daily vitamin D supplementation).
We determined a priori that we would consider our pilot trial a success in regard to feasibility if the following criteria were met: clinical sites initiated in North America, Europe, Asia, and Australia, 60 patients enrolled over a 12-month period, follow-up at 12 months for the proposed primary outcome of the definitive trial (patient important outcomes) achieved for at least 90% of participants, at least 90% case report form completeness with no outstanding queries at 12 months, less than 5% errors in randomization across sites, at least 90% adherence to surgical technique, and less than 5% errors in the treatment allocation of vitamin D supplementation.
Nutritional supplementation details were collected at hospital discharge and at the 6-week, 3-month, and 6-month follow-up visits. At hospital discharge, research personnel documented whether the participant received their nutritional supplement within 2 weeks of femoral neck surgery, as well as whether instructions and the importance of complying with the nutritional supplementation were discussed with the participant. At the 6-week, 3-month, and 6-month follow-up visits a nutritional assessment was conducted where the participant was asked to indicate the following: whether they missed any doses or stopped taking the nutritional supplement since their last follow-up visit, how often they took the nutritional supplement (everyday, most days, some of the days, rarely, or never), and if they were taking any additional vitamin D supplements other than the trial intervention.
2.3. Statistical analysis
2.3.1. Sample size
Since feasibility objectives in our pilot trial did not lend themselves to traditional quantitative sample size calculations, we selected a minimum sample size of 60 patients with complete follow-up to assess the feasibility of a definitive large RCT.
2.3.2. Statistical analysis of outcomes
We adopted the CONSORT extension to pilot trials in reporting the results of this pilot trial.[11]
The baseline characteristics, fracture and injury characteristics, surgical details, peri-operative care data, and feasibility outcomes were summarized using descriptive statistics reported as means (standard deviation (SD)) or medians (first quartile, third quartile) for continuous variables depending on their distribution and counts (percentage) for categorical variables. We used SPSS Version 25 to perform all analyses.
2.4. Funding sources
This trial was supported by research grants from the Canadian Institutes of Health Research (MOP-130271 and MTO-130912), McMaster Surgical Associates, and Hamilton Health Sciences (NIF 13329). Trial funders had no role in the trial design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the trial and had final responsibility for the decision to submit for publication.
3. Results
3.1. Enrolment
Fifteen trauma centers in North America enrolled 91 patients into the FAITH-2 pilot trial over approximately 3 years. Five of these enrolments were subsequently determined to be ineligible (not between the ages of 18–60 years, pathological fracture, no femoral neck fracture, did not provide informed consent (2 patients)) (Fig. 1). Therefore, 86 participants are included in this analysis. The number of excluded patients and reasons for exclusion are listed in the appendix (Table A1). Of these 86 participants, 43 were allocated to receive a SHS, 43 were allocated to receive CS, 45 were allocated to receive vitamin D, and 41 were allocated to receive placebo (Fig. 1). There were no reported deaths and 12-month follow-up was achieved for 67 participants (77.9%) (Fig. 1).
Figure 1.
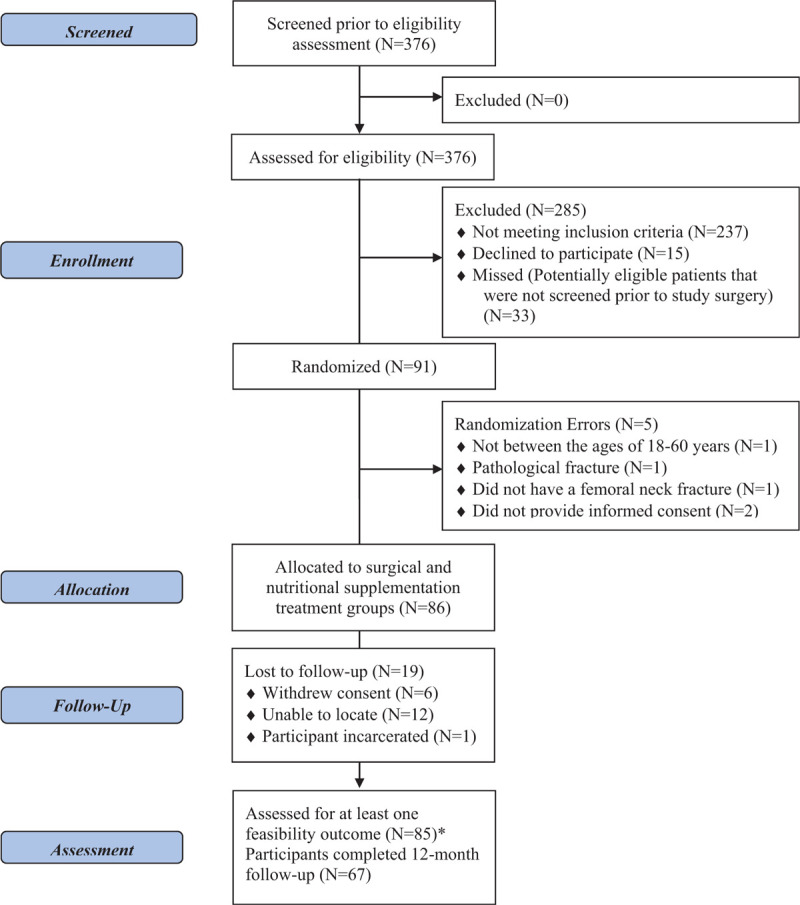
Flow diagram. ∗Feasibility outcomes assessed at hospital discharge and throughout trial follow-up period.
3.2. Participant demographics and fracture characteristics
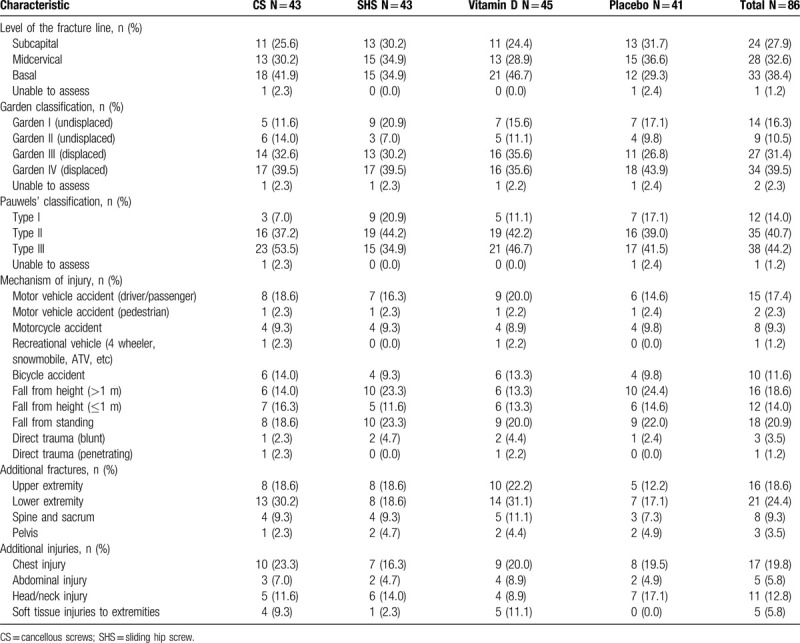
Typical participants were men (73.3%) of white/Caucasian ethnicity (79.1%) with a mean age of 41.1 (SD 12.2). The most common fracture configuration for the cohort was Pauwels Type III vertical. Additionally, most fractures sustained were displaced and resulted from a fall (Tables 1 and 2).
Table 1.
Participant demographics.
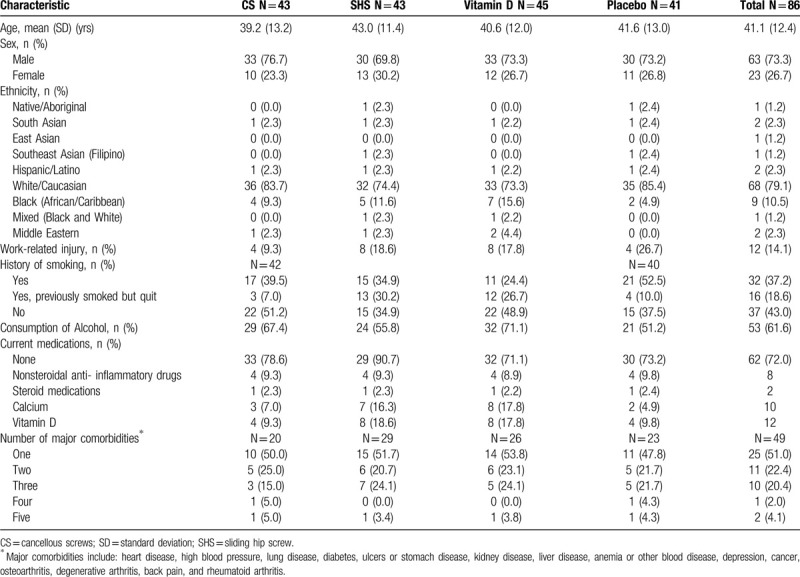
Table 2.
Fracture and injury characteristics
3.3. Feasibility outcomes
The primary feasibility criteria were not achieved due to slow initiation of clinical sites, low enrolment, poor follow-up at 12 months, and low adherence with daily nutritional supplementation (Tables 3 and 4).
Table 3.
Feasibility outcomes.
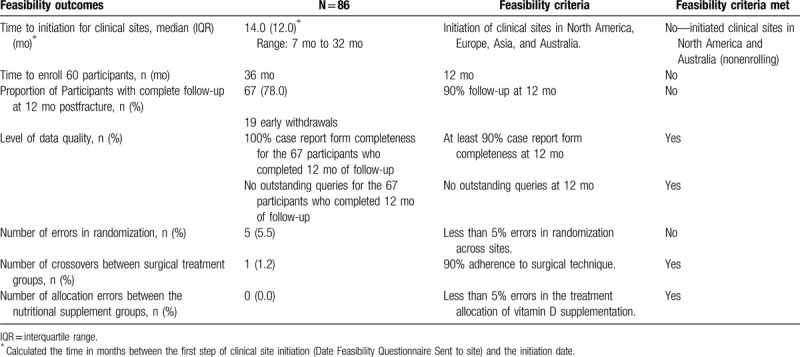
Table 4.
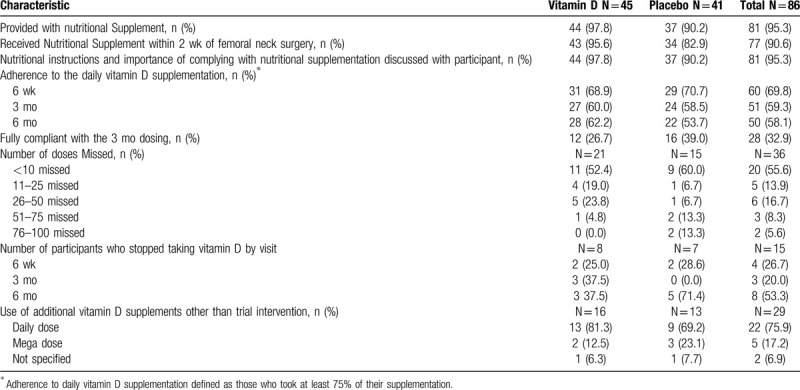
Nutritional supplementation adherence.
3.4. Initiation of clinical sites
We aimed to initiate clinical sites in North America, Europe, Asia, and Australia. Initiation of clinical sites was achieved across 25 hospitals in North America and Australia; however, the protocol could not be implemented in Europe or Asia. Of the 25 initiated clinical sites, only 15 enrolled at least 1 participant into the trial. Although 1 clinical site was initiated in Australia, the site was unable to enroll any participants. The median time to initiation of clinical sites was 14.0 months (IQR 12.0 months; range: 7–32 months). Therefore, we did not meet our clinical site initiation outcome of initiating clinical sites in North America, Europe, Asia, and Australia.
3.5. Rate of participant enrolment
We aimed to enroll 60 participants over a 12-month period. The time to enroll 60 participants was 36 months. Therefore, we did not meet our participant enrolment feasibility outcome.
3.6. Proportion of participants with complete follow-up
We aimed to have follow-up completed at 12 months for the proposed primary outcome of the definitive trial (patient important outcomes) for 90% of participants. However, only 77.9% of participants completed the final 12-month follow-up visit. Therefore, we did not meet this feasibility outcome.
3.7. Level of data quality
Our feasibility outcome for level of data quality was achieved, with 100% case report form completeness and no outstanding queries for the 67 participants who completed 12 months of follow-up.
3.8. Rate of protocol adherence
In terms of rate of protocol adherence, we aimed to achieve less than 5% of errors in randomization across sites, 90% adherence to surgical technique, and less than 5% errors in the treatment allocation of vitamin D supplementation. This outcome was attained in 2 of the 3 areas, as there were no crossovers in either the surgical treatment groups and the correct treatment allocation of the nutritional supplement was provided to each participant. However, there were 5 (5.5%) randomization errors during the trial enrolment (Fig. 1).
3.9. Nutritional supplementation details
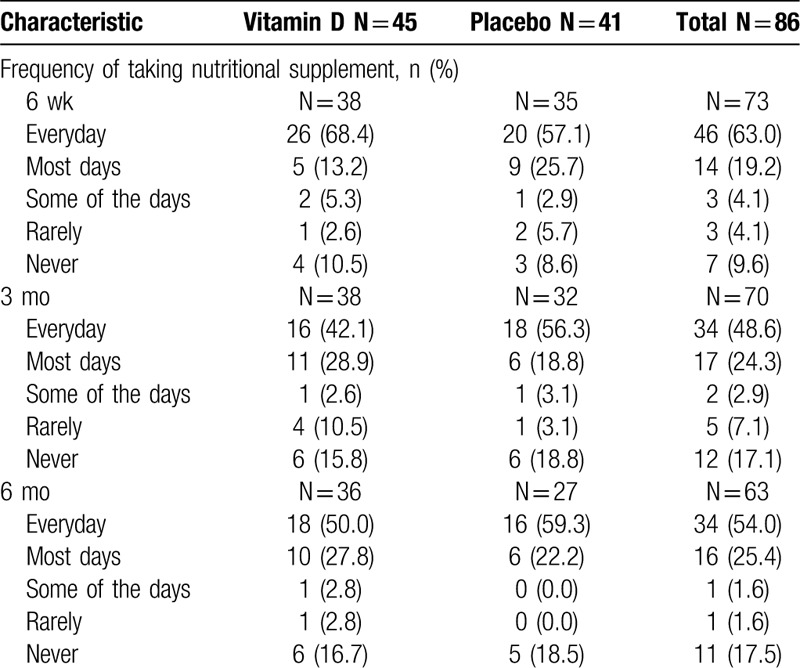
Eighty-one participants (95.3%) were provided with the 6-month's supply of the nutritional supplement. Four participants were not provided with the 6-month's supply of the nutritional supplement at the time of hospital discharge for the following reasons: 2 participants were discharged early, 1 participant opted out of taking the trial nutritional supplement for their own vitamin D, and 1 participant forgot the nutritional supplement at the hospital. For the participant who left their bottle at the hospital, a new bottle was ordered and provided to them at the 2-week postoperative appointment. The supplementation adherence data found that self-reported adherence with the daily supplementation protocol, defined as taking at least 75% of their supplementation, was not achieved. Adherence with supplementation decreased with time at the 6-week (72.1%), 3-month (60.5%), and 6-month (54.7%) follow-up visits. Only 26.7% and 39.0% of participants in the vitamin D and placebo treatment groups, respectively, reported taking their vitamin D or placebo each day for the first 3 months. Of the 36 participants who were noncompliant, 55.6% missed less than 10 doses, 13.9% missed 11 to 25 doses, 16.7% missed 26 to 50 doses, 8.3% missed 51 to 75 doses, and 5.6% missed 76 to 100 doses. A specific breakdown of patient-reported frequency of nutritional supplement use at the 6-week, 3-month, and 6-month follow-up visits is reported in Table 5. Of the 15 participants who stopped taking their supplementation, 26.7%, 20.0%, and 53.3% reported stopping at their 6-week, 3-month, and 6-month visit, respectively. Twenty-nine participants reported use of additional vitamin D supplements other than the trial intervention.
Table 5.
Participant reported frequency of nutritional supplement use
4. Discussion
The FAITH-2 pilot trial confirmed that, without substantial revisions to the trial protocol, a larger, definitive trial would not be feasible as a result of not being able to meet the following feasibility objectives: initiation of clinical sites in North America, Europe, Asia, and Australia, rate of participant enrolment, and proportion of participants with complete follow-up at 12 months postfracture. Additionally, adherence to the daily dose nutritional supplementation for the 6-months postfracture was lower than anticipated.
One of our greatest challenges when conducting the FAITH-2 pilot trial was clinical site initiation which, in turn, impacted the rate of participant enrolment. Given the rarity of femoral neck fractures in younger patients, we had aimed to initiate clinical sites globally in North America, Europe, Asia, and Australia to maximize enrolment. However, we were unable to initiate clinical sites in the Netherlands, Spain, or China due to strict criteria regulating vitamin D supplementation, difficulties with obtaining the required doses of vitamin D, and the high associated costs of conducting an investigational drug trial. The pilot trial provided a cost-effective way to assess the logistics and feasibility of initiating the FAITH-2 trial in global clinical sites. Based on the feasibility findings, we do not believe that we can overcome these barriers in a way that would allow for the conduct of a cost-efficient large definitive trial.
Ultimately, we were able to initiate 24 clinical sites across North America and 1 clinical site in Australia, but initiation times were long and only 15 sites enrolled at least 1 participant into the trial. Moreover, it took the 15 clinical sites 36 months to enroll 60 participants, not meeting our feasibility criteria of enrolling 60 participants in a 12-month period. The other 10 clinical sites (including the Australian clinical site) who did not enroll any participants into the trial, learned that a young femoral neck fracture population entering their clinics or hospitals was lacking, which negatively impacted the recruitment rate. Based on these enrolment estimates, it would take at least 16 years to complete the definitive FAITH-2 trial across 40 clinical sites.
As reported in previous fracture trials primarily involving young male patients, maintaining participant follow-up in the FAITH-2 pilot trial was challenging and resulted in a high loss to follow-up rate of 22.1%.[6–9] Of the 15 enrolling clinical sites, 8 were located in the United States, and 58 (67.4%) participants were enrolled from sites located in the United States. Despite 2 of our American clinical sites being the top enrollers for the pilot trial, they also had the highest loss to follow-up rates (39.1% and 23.5%, respectively). A trial that evaluated predictors of loss to follow-up in fracture patients discovered that participants who received treatment in the United States were more likely to be lost to follow-up than those who received treatment in other countries (odds ratio (OR)=3.56, 95% confidence interval (CI): 2.46–5.17, P < .001).[12] As mentioned, we noticed a similar trend in our data in that participants who were lost to follow-up were primarily enrolled in clinical sites located in the United States as compared with Canadian sites. Initiating more sites outside of the United States may have lowered the loss to follow-up rate; however, previous work by members of the trial team achieved similar follow-up rates for adult femoral neck fracture participants in 3 large hospitals in China.[13]
Despite 95.3% of participants being provided with a nutritional supplement bottle within 2 weeks of femoral neck surgery, as per the trial protocol, adherence to the vitamin D and placebo supplementation was low with only 58.1% of participants taking ≥75% of their daily supplement for the entire 6-month period. Little research exists on adherence to vitamin D supplementation rates in the younger fracture population, but our observation from the FAITH-2 pilot trial aligns with other published trials that report that adherence with vitamin D, as well as calcium supplementations, ranges between 20% and 60% in older adults.[14–17] Given the blinded nature of the trial, numerous participants reported stopping the use of the supplement completely and beginning consumption of their own vitamin D or using additional vitamin D supplements in combination with the trial intervention. Based on our observations in the FAITH-2 pilot trial, low adherence to the nutritional supplementation would likely also be an issue in a definitive trial unless we implemented new methods to raise adherence, such as sending reminders between trial visits to participants or using periodic loading doses of vitamin D administered at clinic visits as an alternative to daily doses delivery.
In addition to the low adherence to the daily supplementation, we experienced other challenges pertaining to the nutritional supplement intervention of the FAITH-2 pilot trial. With slow enrolment, recruitment went beyond the supplementation bottle supply expiration date, requiring replacement bottles to be sent to all clinical sites. This led to not only increased burden and costs, but required shipment of a new supplementation bottle to participants requiring a replacement between clinic visits. This was challenging due to ensuring that the temperature control requirements were fulfilled during delivery. Additionally, we were informed by the manufacturer of the nutritional supplement that some of the vitamin D being used for the trial did not maintain its 2000 IU per drop concentration stability and may have declined over time to approximately 400 IU per drop. The manufacturer of the vitamin D product confirmed that there was no safety or health risks to the trial participants due to this lack of stability, but as a preventative measure, participants whose 6-month nutritional supplement intake period had not yet ended were provided with a replacement nutritional supplement bottle that was manufactured using a new supply of vitamin D.
Despite the challenges we experienced with the nutritional supplementation component, the FAITH-2 pilot trial is still informative in terms of comparing vitamin D versus placebo and provides important data on the daily vitamin D adherence rates among participants in the young trauma population. These experiences were used to directly inform the design and conduct of the Vita-Shock trial (A Blinded Exploratory Randomized Controlled Trial to Determine Optimal Vitamin D3 Supplementation Strategies for Acute Fracture Healing) (ClinicalTrials.gov Identifier: NCT02786498). Briefly, Vita-Shock is a 4-arm phase II exploratory pilot trial designed to improve patient outcomes in tibia and femur fractures by addressing the nutritional optimization of bone health with vitamin D supplementation. The Vita-Shock trial improves upon the FAITH-2 trial by examining the effect of vitamin D in the treatment of much more prevalent fractures (i.e., tibial and femoral shaft fractures) and delivers vitamin D through 2 loading doses given within 1 week of fracture prior to hospital discharge and 6-weeks postfracture at the patient's fracture clinic visit. We hypothesize that adherence will be higher with loading doses than with daily doses of vitamin D or placebo.[18]
In summary, we experienced multiple expected and unexpected challenges with the initiation and conduct of the FAITH-2 pilot trial and were unable to meet key feasibility criteria. Despite these challenges, we maintained high data quality and completed the largest randomized comparison of our surgical and nutritional supplementation interventions in this patient population. Additionally, we learned more about the logistics and what barriers to anticipate when conducting nutritional supplement trials in a trauma population. The experiences obtained and lessons learned from conducting this trial can be applied in the planning process of future pilot trials in terms of identifying a population with a more common fracture. These experiences have directly informed the Vita-Shock trial and have improved the likelihood of successfully advancing vitamin D research for fracture healing. Finally, this experience provides an example of the importance and necessity of conducting pilot trials in general and in the area of vitamin D supplementation since the evidence on the impact of vitamin D supplementation on fracture healing and optimal dosing levels remain limited.
FAITH-2 INVESTIGATORS
Writing Committee: Sheila Sprague, Mohit Bhandari, Sofia Bzovsky, Taryn Scott, Lehana Thabane, Diane Heels-Ansdell, Robert V. O’Toole, Andrea Howe, Greg E. Gaski, Lauren C. Hill, Krista M. Brown, Darius Viskontas, Mauri Zomar, Gregory J. Della Rocca, and Gerard P. Slobogean
Steering Committee: Gerard P. Slobogean (Co-Chair, University of Maryland), Mohit Bhandari (Co-Chair, McMaster University), Sheila Sprague (McMaster University), Earl Bogoch (St. Michael's Hospital), PJ Devereaux (McMaster University), Gordon Guyatt (McMaster University), Martin J. Heetveld (Spaarne Gasthuis, Haarlem), Kyle Jeray (Greenville Health System), Susan Liew (The Alfred), Robert V. O’Toole (University of Maryland), Andrew N. Pollak (University of Maryland), Emil H. Schemitsch (University of Western Ontario), Marc Swiontkowski (University of Minnesota), Lehana Thabane (McMaster University);
Principal Investigators: Gerard P. Slobogean (Co-Chair, University of Maryland), Mohit Bhandari (Co-Chair, McMaster University),
Methods Centre: Sheila Sprague (Co-Investigator); Dale Williams (Co-Investigator); Sofia Bzovsky, Kim Madden, Alisha Garibaldi, Taryn Scott (Project Managers); Diane Heels-Ansdell (Statistical Analysis); Lisa Buckingham (Data Management); Nicole Simunovic (Grants Management) (McMaster University); Martí Bernaus (Research Fellow, Hospital Universitari Mútua Terrassa);
Data and Safety Monitoring Committee: Eleanor M. Pullenayegum (The Hospital for Sick Children), Matthew Menon (University of Alberta), David Teague (University of Oklahoma Health Sciences Center);
Adjudicator: Gregory J. Della Rocca (University of Missouri);
Clinical Sites:
Canada: Richard J. Jenkinson, Hans J. Kreder, David J.G. Stephen, Markku Nousiainen, Diane Nam, Patrick Henry, John Iazzetta, Katrina Hatzifilalithis, Katrine Milner, Monica Kunz, Aimee Theriault, Wesley Ghent, Araby Sivananthan, Fathima Adamsahib, Danyella Dias, Abeer Wasim, Ravianne Tuazon, Katerina Polihronidis (Sunnybrook Health Sciences Centre);
Emil H. Schemitsch, Aaron Nauth, Michael D. McKee, Jeremy A. Hall, Earl Bogoch, Daniel Whelan, Timothy Daniels, Sarah Ward, Henry Ahn, James Waddell, David Walmsley, Amit Atrey, Laura Parsons, Ann Dowbenka, Gitana Ramonas, Milena R. Vicente, Jennifer T. Hidy, Paril Suthar, Melanie MacNevin (St. Michael's Hospital);
Darius Viskontas, Robert McCormack, Farhad Moola, Bertrand Perey, Trevor Stone, Kelly Apostle, Dory Boyer, H. Michael Lemke, Mauri Zomar, Karyn Moon, Raely Pritchard (Moon), Brenda Chen Fan, Bindu Mohan (University of British Columbia / Fraser Health Authority – Royal Columbian Hospital);
Pierre Guy, Kelly Lefaivre, Peter O’Brien, Henry Broekhuyse, Piotr Blachut, Dean Malish, Jeffrey Potter, Raman Johal, Irene Leung, Benita Okocha, Jessica Peattie, Abdullah Mamun (University of British Columbia/Vancouver General Hospital);
Steven Papp, Wade Gofton, Allan Liew, Sherry Weir, Darren M. Roffey, Nicole Harris, Julia Foxall (The Ottawa Hospital – Civic Campus);
Andrew Furey, Keegan Au, Daniel Squire, Peter Rockwood, Ashley Buck, Karen Ryan, Garrett Wells, Zeta Hannaford, Valisha Keough, Erin Baker, Sarah Anthony (Memorial University);
USA: Mark Richardson, Thomas DiPasquale, Paul Muccino, Troy Caron, Adrienne Brandon, Krystal Swasey, Tara Moore (York Hospital);
David Hubbard, John France, Michelle A. Bramer, Brock Lindsey, Benjamin Frye, Matthew Dietz, Adam Klein, George K. Bal, E. Barry McDonough, John P. Lubicky, Scott Daffner, T. Ryan Murphy, Lisa Giblin Sutton, Sheila Rye, Nina Clovis, Sherri Davis (West Virginia University);
Robert A. Hymes, Cary C. Schwartzbach, A. Stephen Malekzadeh, Jeff E. Schulman, Michael Holzman, Anastasia Lialios-Ramfos, Lolita Ramsey, Sharon Haaser (Inova Fairfax Medical Campus);.
Gudrun Mirick Mueller, David Templeman, Andrew Schmidt, Tzivia Leviton, Jerald R. Westberg (Hennepin County Medical Center);
Saam Morshed, Eric Meinberg, Paul Toogood, David Shearer, Murat Pekmezci, Paul Knaus, Sara McFarland, Tigist Belaye, Eleni Berhaneselase, Johnathan Kwong (University of California, San Francisco);
Robert V. O’Toole, Andrew N. Pollak, Gerard P. Slobogean, Christina Boulton, Christopher LeBrun, Jason W. Nascone, Marcus F. Sciadini, Raymond Pensy, Theodore Manson, W. Andrew Eglseder Jr., Andrea L. Howe, Daniel Connelly, Katherine Ordonio, Raza Zaidi, Yasmin Degani, Merryjessica Fuerst, Carrie Schoonover, George Reahl, Peter Berger, Ryan Montalvo, Dimitrius Marinos, Daniel Mascarenhas, Joshua Rudnicki (University of Maryland);
Clifford B. Jones, Sarim Ahmed, Norman Chutkan, Michael Lucero, Jason Lowe, Russell Meldrum, Niloofar Dehghan, Ryan DiGiovanni, Pierce Johnson, Sean Karr, Sean Mitchell, Robert Walker, Debra L. Sietsema, Cyndi Ventry, Susan Mauro, Karen Walsh, Bonnie Sumner, Taylor Dykes, Michael Duran, Blake Eyberg, Joseph Walker, Collin Barber, Jessica Burns, Jill Goodwin, Ryan Shelhamer, Joshua Hustedt, Damien Richardson, Julie Robbins (The Center for Orthopedic Research and Education (CORE) Institute);
Greg E. Gaski, Todd O. McKinley, Walter W. Virkus, Laurence B. Kempton, Anthony T. Sorkin, Roman Natoli, Charles Lieder, Landon Fine, Lauren C. Hill, Krista M. Brown, Lakye Deeter (Indiana University Health Methodist Hospital);
Jennifer Hagen, André Spiguel, Kalia Sadasivan, Matthew Patrick, Melissa Johnson, Susan Beltz, Elizabeth Agustin, Chris Koenig, Sonya Brisbane (University of Florida);
Clinical Co-Investigators:
Canada: Tudor V. Tufescu and Nigar Sultana (Health Sciences Centre Winnipeg);
Ryan Bicknell, Heather Grant, Fiona Howells (Queen's University);
USA: Todd M. Oliver, Michelle Vogt, Vicki Jones, Tina Carter (Boone Hospital Center – Columbia Orthopaedic Group);
Edward Westrick and Traci Salopek (Allegheny General Hospital);
Aaron Perdue; Joel J. Gagnier, James Goulet, Mark Hake (University of Michigan);
Andrew J. Marcantonio, Michael S.H. Kain, Craig Donahue, Cynthia Carter, John S. Garfi (Lahey Hospital & Medical Center);
Australia: Susan Liew, Anne Mak, Zoe Murdoch (Alfred Health);
Supplementary Material
References
- 1.In J. Introduction of a pilot study. Korean J Anesthesiol. 2017;70:601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thabane L, Ma J, Chu R, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slobogean GP, Sprague S, Bzovsky S, et al. Fixation using alternative implants for the treatment of hip fractures (FAITH-2): design and rationale for a pilot multi-centre 2 × 2 factorial randomized controlled trial in young femoral neck fracture patients. Pilot Feasibility Stud. 2019;5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pauyo T, Drager J, Albers A, et al. Management of femoral neck fractures in the young patient: a critical analysis review. World J Orthop. 2014;5:204–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fixation using alternative implants for the treatment of hip fractures (FAITH), Investigators., fracture fixation in the operative management of hip fractures (FAITH): an international, multicentre, randomised controlled, trial. Lancet. 2017;389:1519–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray DW, Britton AR, Bulstrode CJ. Loss to follow-up matters. J Bone Jt Surg. 1997;79:254–257. [DOI] [PubMed] [Google Scholar]
- 7.Zelle BA, Bhandari M, Sanchez AI, et al. Loss of follow-up in orthopaedic trauma. J Orthop Trauma. 2013;27:177–181. [DOI] [PubMed] [Google Scholar]
- 8.Sprague S, Leece P, Bhandari M, et al. Limiting loss to follow-up in a multicenter randomized trial in orthopedic surgery. Control Clin Trials. 2003;24:719–725. [DOI] [PubMed] [Google Scholar]
- 9.Badenhorst DHS, Van der Westhuizen CA, Britz E, et al. Lost to follow-up: challenges to conducting orthopaedic research in South Africa. South African Med J. 2018;108:917–921. [DOI] [PubMed] [Google Scholar]
- 10.Slobogean GP, Sprague S, Bzovsky S, et al. Fixation using Alternative Implants for the Treatment of Hip Fractures (FAITH-2): The Clinical Outcomes of a Multi-Centre 2x2 Factorial Randomized Controlled Trial in Young Femoral Neck Fracture Patients. J Orthop Trauma. 2019;[Accepted]. [DOI] [PubMed] [Google Scholar]
- 11.Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355:i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madden K, Scott T, McKay P, et al. Predicting and preventing loss to follow-up of adult trauma patients in randomized controlled trials: an example from the FLOW trial. J Bone Jt Surg. 2017;99:1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slobogean GP, Stockton DJ, Zeng B, et al. Femoral neck shortening in adult patients under the age of 55 years is associated with worse functional outcomes: analysis of the prospective multi-center study of hip fracture outcomes in China (SHOC). Injury. 2017;48:1837–1842. [DOI] [PubMed] [Google Scholar]
- 14.Segal E, Zinman C, Raz B, et al. Low patient compliance—a major negative factor in achieving vitamin D adequacy in elderly hip fracture patients supplemented with 800IU of vitamin D3 daily. Arch Gerontol Geriatr. 2009;49:364–367. [DOI] [PubMed] [Google Scholar]
- 15.Sanfelix-Genovés J, Gil-Guillén VF, Orozco-Beltran D, et al. Determinant factors of osteoporosis patients-reported therapeutic adherence to calcium and/or vitamin D supplements. Drugs Aging. 2009;26:861–869. [DOI] [PubMed] [Google Scholar]
- 16.Castelo-Branco C, Cortés X, Ferrer M. UNICAD study investigators. Treatment persistence and compliance with a combination of calcium and vitamin D. Climacteric. 2010;13:578–584. [DOI] [PubMed] [Google Scholar]
- 17.Díez A, Carbonell C, Calaf J, et al. Observational study of treatment compliance in women initiating antiresorptive therapy with or without calcium and vitamin D supplements in Spain. Menopause J North Am Menopause Soc. 2012;19:89–95. [DOI] [PubMed] [Google Scholar]
- 18.Sprague S, Bzovsky S, Connelly D, et al. Design and rationale for an exploratory phase ii randomized controlled trial to determine optimal vitamin D3 supplementation strategies for acute fracture healing. Pilot Feasibility Stud. 2019;5:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


