Abstract
Hypoxia is a critical pathological element in many human diseases, including ischemic stroke, myocardial infarction, and solid tumors. Of particular significance and interest of ours are the cellular and molecular mechanisms that underlie susceptibility or tolerance to low O2. Previous studies have demonstrated that Notch signaling pathway regulates hypoxia tolerance in both Drosophila melanogaster and humans. However, the mechanisms mediating Notch-conferred hypoxia tolerance are largely unknown. In this study, we delineate the evolutionarily conserved mechanisms underlying this hypoxia tolerant phenotype. We determined the role of a group of conserved genes that were obtained from a comparative genomic analysis of hypoxia-tolerant D.melanogaster populations and human highlanders living at the high-altitude regions of the world (Tibetans, Ethiopians, and Andeans). We developed a novel dual-UAS/Gal4 system that allows us to activate Notch signaling in the Eaat1-positive glial cells, which remarkably enhances hypoxia tolerance in D.melanogaster, and, simultaneously, knock down a candidate gene in the same set of glial cells. Using this system, we discovered that the interactions between Notch signaling and bnl (fibroblast growth factor), croc (forkhead transcription factor C), or Mkk4 (mitogen-activated protein kinase kinase 4) are important for hypoxia tolerance, at least in part, through regulating neuronal development and survival under hypoxic conditions. Becausethese genetic mechanisms are evolutionarily conserved, this group of genes may serve as novel targets for developing therapeutic strategies and have a strong potential to be translated to humans to treat/prevent hypoxia-related diseases.
Keywords: Notch signaling, genetic interactions, eaat1-posive glia, Hypoxia, Drosophila melanogaster
Introduction
Hypoxia (O2 deprivation) is a common pathological factor in many human diseases, including apnea of prematurity, hypoxemia in ICU settings resulting from neurologic, hematologic, respiratory and cardiac diseases, stroke, and cancer (Sands and Owens 2015; Adler et al. 2017; Schmidt et al. 2017, 2019; Group 2018; Thille et al. 2018; Di Fiore et al. 2019). To date, strategies to treat or prevent hypoxia-induced injury are very limited. Thus, understanding the mechanisms regulating tolerance or susceptibility to hypoxia is crucial for developing effective therapeutic strategies.
A number of studies have demonstrated that Notch signaling plays an important role in regulating the cellular and molecular mechanisms underlying the responses to hypoxic stress (for selected reviews, see Andersson et al. 2011; Alberi et al. 2013; Marignol et al. 2013; Zhou and Haddad 2013; Borggrefe et al. 2016; Fearon et al. 2016; Arumugam et al. 2018). For example, (1) it has been shown that Notch intracellular domain coordinates with HIF signaling to regulate cellular response to hypoxia (Gustafsson et al. 2005; Pear and Simon 2005; Sainson and Harris 2006); (2) we have previously discovered that Notch signaling is activated and plays a critical role in regulating hypoxia tolerance in Drosophila melanogaster (i.e., D.melanogaster carrying Notch loss-of-function alleles are super-sensitive to low O2, and, in contrast, the fruit flies carrying gain-of-function alleles are remarkably resistant) (Zhou et al. 2008, 2011) (3) neuronal- or glial-specific activation of Notch rescues naïve flies from lethal degree of O2 deprivation (Zhou et al. 2011); and (4) Notch signaling is an evolutionarily conserved mechanism regulating adaptation to low O2 environments not only in Drosophila but also in humans (Jha et al. 2016). However, the molecular mechanisms underlying the role of Notch signaling in regulating hypoxia responses are still not well understood. BecauseNotch is highly pleiotropic, it is clear that specific downstream responses to Notch activation depend on cellular context and its synergic integration with other signaling pathways (Bray and Bernard 2010; Ho et al. 2018). Furthermore, due to the broad physiological role of Notch signaling in cell differentiation and metabolism, therapeutic strategies directly targeting Notch may introduce severe side-effects (Rizzo et al. 2014; Takebe et al. 2014; Mollen et al. 2018). Therefore, identifying genetic modifiers or downstream effectors that mediate the role of Notch in hypoxia are very important for developing effective therapeutic strategies.
It has long been recognized that hypoxia does not affect all organs/tissues of the body equally. Of all the organs, brain is one of the organs that are very sensitive to oxygen deprivation (Leach and Treacher 1998; Purins et al. 2012). We previously discovered that hypoxia-induced lethality can be rescued by activating Notch signaling only in the excitatory amino acid transporter 1 (Eaat1)-positive glial cells in D.melanogaster, which is likely through cell injury prevention in the central nervous system. It is remarkable that the activation of Notch in this group of glial cells alone is sufficient to enhance the organismal survival under prolonged hypoxic conditions (Zhou et al. 2011). Presumably, this group of Eaat1-positive glial cells regulates hypoxia tolerance through suppression of glutamate neurotoxicity, neuronal proliferation as well as the growth and organization of axons (Gibson 1989; Ikonomidou et al. 1989; Stacey et al. 2010). Furthermore, since we have identified a group of evolutionarily conserved genes and biological processes (including Notch signaling) between D.melanogaster and human highlanders (Jha et al. 2016) that are important for survival in hypoxic environment, some of these conserved genes may interact with Notch signaling to regulate Notch activation-conferred hypoxia tolerance. In this study, we studied the genetic interactions between Notch signaling and a group of these conserved genes [i.e.,branchless (bnl), crocodile (croc), Epidermal growth factor receptor (Egfr), grain (grn), hairy (h), invected (inv), and MAP kinase kinase 4 (Mkk4)]and identified modifiers that regulate Notch activation-conferred hypoxia tolerance in D.melanogaster.
Materials and methods
Drosophila stocks and culture conditions
The following available TRiP UAS-RNAi lines, UAS-reaper lines and Gal4 driver stocks were obtained from the Bloomington Drosophila Stock Center: [y1 sc* v1 sev21; P{TRiP.HMS01046}attP2] (bnl-RNAi, FBst0034572), [y1 sc* v1 sev21; P{TRiP.HMS01122}attP2] (croc-RNAi, FBst0034647), [y1 v1; P{TRiP.JF02283}attP2] (Egfr RNAi, FBst0036770), [y1 sc* v1 sev21; P{TRiP.HMS01085}attP2] (grn-RNAi, FBst0033746), [y1 sc* v1 sev21; P{TRiP.HMS01052}attP2] (grn-RNAi, FBst0034578), [y1 sc* v1 sev21; P{TRiP.HMS01313}attP2] (h-RNAi, FBst0034326), [y1 sc* v1 sev21; P{TRiP.HMS02209}attP2] (inv-RNAi, FBst0041675), [y1 sc* v1 sev21; P{TRiP.HMS02524}attP40] (Mkk4-RNAi, FBst0042832), [w1118; P{UAS-rpr.C}27] (FBst0005823), [w1118; P{UAS-rpr.C}14] (FBst0005824), [w*; Kr[If-1]/CyO; P{w[+mW.hs]=GAL4-da.G32}UH1] (FBst0055850, was used to derive da-Gal4) and [w*; P{w[+mC]=Eaat1-GAL4.R}2] (Eaat1-Gal4, FBst0008849). The UAS-NICD and 4XSu(H)-lacZ stocks were kindly provided by Dr. J. Posakony. BecauseUAS-NICD transgenic stock was generated on the background of w1118(Go et al. 1998), the w1118 stock (FBst0003605) was used as one of the negative controls in the dual-UAS/Gal4 experiments.
Flies were maintained in vials with Cornmeal-Molasses-Yeast medium. Flies for the hypoxia tolerance assay were prepared as follows. 20 Females of UAS-RNAi were crossed with 20 males of EN line, Eaat1-Gal4 or da-Gal4 and incubated at room air/temperature condition. After 24 hours, parents were removed. One group of the vials (n = 3 to 6 vials) containing the embryos were transferred to a computerized atmosphere chamber supplied with 5% oxygen for hypoxia treatment, and the other group of vials (n = 3 to 6 vials) were cultured in room air condition and used as controls.
Generation of dual-UAS/Gal-4 system
We have generated a unique fly line that has Notch upregulated specifically in the glial cells that produce the glutamate transporter EAAT1. The details on the strategy to generate this line are provided in Supplementary Figure S1. Briefly the UAS-NICD inserted on 3rd chromosome and Eaat1-Gal4 ([w*; P{w[+mC]=Eaat1-GAL4.R}2] (FBst0008849); stock# 8849, Bloomington, USA) were simultaneously crossed with a double balancer [w*; Cyo; TM3, Sb’](stock#2475, Bloomington, USA). The F1 flies with both Cyo and Sb phenotype from both crosses (i.e., [w; +/Cyo; UAS-NICD/TM3, Sb’]from the first cross and [w;P{Eaat1-GAL4.R}2/Cyo; +/TM3, Sb’]from the second cross) were selected and self-crossed to remove flies with Sb’ phenotype from the [w; +/Cyo; UAS-NICD/TM3, Sb’]line and Cyo phenotype from the [P{Eaat1-GAL4.R}2/Cyo;+/TM3, Sb’]lines. Subsequently, these flies were intercrossed and again select for flies with Cyo and Sb’ phenotype. Finally, they were self-crossed to obtain homozygote [w; P{Eaat1-GAL4.R}2; UAS-NICD] (i.e., the EN line).
Hypoxia treatment and survival test
Three- to five-day-old Eaat1-Gal4and EN males (n = 10) were crossed to the UAS-RNAi virgin females (n = 10) targeting specific genes. Sufficient time was given (∼3 days) for the flies to mate/cross. Simultaneously the w1118and the Gal4 line were ‘self-crossed’ and used as negative controls, the [EN] x [w1118] cross was used as a positive control. Each set of crosses were in 3 to 6 replicate vials. The vials were kept under ambient conditions for 48 hours so that the flies can lay sufficient number (50 to 100) of eggs. After 48 hours, the adults were transferred to a new vial. For the hypoxia tolerance test the original vials were then transferred to a computer-controlled hypoxia chamber, constantly maintained at 5% oxygen. The chambers were in a room with 12/12 hours light/dark cycle, and temperature ∼22°C. The adults from the new vials (i.e., from the second batch of vials) were discarded after 48 hours, and the vials with the eggs were kept at ambient oxygen conditions (∼21% O2) also with 12/12 hours light/dark cycle (temperature ∼22°C) as room air controls. After 21 days, the ratio of the empty pupae (eclosed) to the total number of pupae formed (eclosed + uneclosed) in each vial was calculated to determine the eclosion rate. Due to the fact that most of the collected embryos will be at an early larval stage, this hypoxia test was mainly to determine the survival rate from larval stage to adulthood, as representated by eclosion rate.
Immunostaining and microscopy
The third instar larval brain samples for immunostaining were prepared according to previous descriptions (Wu and Luo 2006; Zhou et al. 2011). Briefly, brains of wandering third instar larvae were dissected in PBS and fixed in 4% paraformaldehyde in PBS. Cell membranes were permeabilized with 0.3% Triton X-100 in PBS, blocked with 7% goat serum, and put in primary antibody overnight at 4°C (or for 1 hour at room temperature). This procedure was followed by washes in 0.3% Triton X-100 in PBS, incubation with secondary antibody for 90 minutes. Following staining, the brain samples were washed multiple times with PBS, mounting and microscopy. The mouse anti-NICD (undiluted in-house supernatant) and rat anti-elav (1:50) were obtained from the Developmental Studies Hybridoma Bank (DSHB); rabbit anti-repo (1:500) was a kind gift of Dr. G. Technau at the Institute of Genetics, University of Mainz, Mainz, Germany. Secondary antibodies used were goat anti-mouse; goat anti-rabbit; and goat anti-rat conjugated to Alexa 488, 546, or 647 (1:250, Invitrogen). The Prolong Gold anti-fade reagent with DAPI (Invitrogen) was used as mounting media. Confocal microscopy was performed in the University of California at San Diego Neuroscience Microscopy Shared Facility. Imaging was done on a confocal microscope (Olympus FV1000) and the images were processed with Image J.
Statistical analysis
Data were analyzed and graphed using GraphPad Prism 6 software. The differences in eclosion rate at 5% oxygen between the [EN]×[UAS-RNAi]and [Eaat1-Gal4]×[UAS-RNAi]and all the controls were assessed using ordinary one-way ANOVA with Turkey’s multiple comparison tests.
Results and discussion
The Eaat1-positive cells are a group of glia regulating glutamate metabolism and transport as well as neuronal activity in Drosophila brain (Rival et al. 2004; Peco et al. 2016). We have previously discovered that hypoxia activates Notch signaling (Zhou et al. 2008), and this activation particularly in the Eaat1-positive glial cells can significantly enhance survival of Drosophila in severely hypoxic environments (Zhou et al. 2011). We aimed in this study to dissect the potential mediators of Notch-conferred hypoxia tolerance.
Eaat1-positive glial cells are critical for Drosophila development and survival
Glutamate is both the principal excitatory neurotransmitter and a potent neurotoxin (at high concentrations) in the mammalian CNS (Krnjevic 1970; Olney and Ho 1970; Fonnum 1984; Choi 1988; Nicholls 1993). Indeed, extracellular glutamate levels are tightly regulated for precise control of neurotransmission at glutamatergic synapses, and to prevent neuronal cell death from excitotoxicity (Danbolt 2001). In Drosophila, this process is regulated by a group of cells that express Eaat1, the only Drosophila high-affinity glutamate transporter (Besson et al. 1999). Although a previous study in adult flies has shown that RNAi-mediated knocking down of Eaat1 in glial cells increased sensitivity to oxidative stress, enhanced degeneration of the brain neuropil and decreased lifespan (Rival et al. 2004), the importance of this group of glial cells in neuronal development and function still remains largely unknown.
First, we determined the developmental expression pattern of Eaat1 by crossing Eaat1-Gal4 with UAS-GFP. As shown in Figure 1, the Eaat1-Gal4 expressing cells were detected throughout all developmental stages from first instar larvae to adulthood. The Eaat1-Gal4 positive cells are a subgroup of glial cell that co-express the glial marker Repo (Figure 1C). Then, to further determine the role of Eaat1-positive glial cells in development and survival of D.melanogaster, we ablated these cells by specific expression of an apoptotic gene reaper (rpr) in the Eaat1-positive glial cells through crossing the virgin female Eaat1-GAL4 with male UAS-rpr carrying the UAS-rpr transgene on the 2nd or the X chromosome. We found that depletion of this group of cells in both males and females (in the progeny of Eaat1-Gal4 female crossed with male UAS-rpr carrying transgene on the 2nd chromosome) terminated D.melanogaster development at embryonic stage under room air condition, as no pupae and adult flies were obtained. To further confirm this phenotype, we crossed the Eaat1-Gal4 female with male UAS-rpr carrying the transgene on the X chromosome. With this cross, the rpr transgene was only expressed in the female progeny. As expected, only male progeny survived (Figure 2), demonstrating that these glial cells are essential in organismal development and survival.
Figure 1.
Distribution of Eaat1-positive glial cells during development. The Eaat1-positive glial cells were labeled by GFP in the progeny of [Eaat1-GAL4]×[UAS-GFP]crosses. The expression of glial marker (repo) and neuronal marker (elav) were labeled by immunostaining. (A–D): the distribution of Eaat1-positive glial cells at first (A), second (B), and third (C) instar larval as well as adult (D) brain. Colocalization of GFP and Repo within the same cell was highlighted in (C) (insert, image represents a Z-projection of 5–12 slices). GFP (green), Repo (red), and Elav (blue). Scale bar = 50 µm.
Figure 2.

Eaat1-positive glial cells are important for the development and survival of D.melanogaster. Eaat1-Gal4 virgin female was crossed with male UAS-rpr with transgene inserted on 2nd or X chromosome (Chr2 or ChrX) to deplete the Eaat1-positive glial cells by rpr-induced cell death. The crosses of Eaat1-Gal4 with UAS-rpr (m, Chr2) were lethal in both males and females, none of the embryos reached adult stage. In the cross between female Eaat1-Gal4 and male UAS-rpr (m, ChrX), only male flies were obtained, demonstrating that the Eaat1-positive glial cells are essential for the development and survival of D.melanogaster.
Neurons and glia are two major cell types in the nervous system of both vertebrate and invertebrate animals. Glial cells are critical for the development and function of neurons by providing neurons with survival and axonal guidance cues, electric shield, and acting as macrophages to remove injured/dead cells (Yildirim et al. 2019; Bittern et al. 2020; Hilu-Dadia and Kurant 2020). Previous studies have shown that Notch signaling plays an important and evolutionarily conserved role in regulating glial differentiation and proliferation during development or following neuronal injuries (Jacobs 2000; Hidalgo and Logan 2017; Kato et al. 2018; Bahrampour and Thor 2020). Our previous study has shown that increasing Notch activity specifically in this set of glial cells dramatically enhanced hypoxia survival, implying that these glial cells also play an important role in regulating organismal development and survival under stress conditions (Zhou et al. 2011), which, at least in part, through rebalancing hypoxia-induced dysregulation and neurotoxicity of glutamate, maintaining the function of neuropil and development of neuronal-glial system (Choi and Rothman 1990;Stacey et al. 2010; MacNamee et al. 2016). In addition, discovery of the property of Notch signaling in regulating cellular responses to hypoxic challenge further broadened the pleiotropic nature of this signaling pathway (Zhou et al. 2011; Arumugam et al. 2018).
Creation and characterization of a dual-UAS/Gal4 system
In order to determine genetic interactions that mediate the function of Notch signaling under hypoxic conditions, we created a dual-UAS/Gal4 system (Supplementary Figure S1). This system contains an Eaat1-Gal4 transgene that simultaneously drives the expression of a UAS-NICD (the functional domain of Notch receptor) transgene to activate Notch signaling and a UAS-RNAi transgene to target and knockdown the expression of the specific candidate gene of choice. In order to test the efficiency of this system, we crossed the EN-line with a UAS-LacZ transgene or a background Drosophila strain for the transgenic lines (w1118 or yw). As shown in Figure 3, the crosses containing Eaat1-Gal4, UAS-NICD, and UAS-LacZ (one copy of each transgene) showed a hypoxia tolerance similar to those containing only Eaat1-Gal4 and UAS-NICD (60%–80%), but significantly higher than those of controls (<25%) (P < 0.01), demonstrating that the presence of an additional UAS (Gal4 upstream activating sequence) did not significantly reduce the efficiency of Gal4-driven expression of UAS-NICD transgene, nor did it significantly affect the phenotype that is induced by NICD overexpression. Hence, the dosage of Eaat1-Gal4 is sufficient to simultaneously drive the expression of two UAS-transgenes.
Figure 3.

Characterization of the dual-UAS/Eaat1-Gal4 system. The EN line was crossed with UAS-LacZ or the background D.melanogaster strains (w1118 or yw) for the transgenic lines to test the efficiency of this system. No alterations on survival were observed in the crosses and controls under room air condition. A significant enhancement of hypoxia survival was detected in the [EN] × [w1118], [EN] × [yw], and [EN] × [UAS-LacZ] as compared to the controls (w1118, yw, and UAS-LacZ) under 5% O2, demonstrating that double the dosage of UAS-transgenes Eaat1-Gal4 > UAS-NICD/UAS-LacZ (the progeny of [EN] × [UAS-LacZ]) did not significantly affect the hypoxia tolerant phenotype showing in Eaat1-Gal4 > UAS-NICD (the progeny of [EN] × [w1118] or [EN] × [yw]). Bars represent mean ± SD (n = 3 vials) for each group/treatment. Ordinary one-way ANOVA [F(13, 28)= 108.3, P < 0.0001]with Turkey’s multiple comparison tests (*** P < 0.001; ns: not significant).
Evolutionarily conserved mechanisms underlying Notch-conferred hypoxia tolerance
It has been shown that combinatorial and context-dependent interactions between cellular signaling pathways regulate a wide and diverse range of biological processes for development, homeostasis, and disease. Previous studies have demonstrated that cellular context-specific integration of Notch signaling with other genes and pathways are essential for mediating the action of Notch in various developmental and pathological processes (Hurlbut et al. 2007; Bray and Bernard 2010; Borggrefe and Liefke 2012). However, the specific mechanisms by which Notch regulates hypoxia survival still largely remain uncharacterized.
To identify the genetic interactions regulating Notch-conferred hypoxia tolerance in Eaat1-positive glial cells, we tested 7 of the 23 evolutionarily conserved candidate genes [i.e., branchless (bnl), crocodile (croc), Epidermal growth factor receptor (Egfr), grain (grn), hairy (h), invected (inv), and MAP kinase kinase 4 (Mkk4)] that have available VALIUM20 UAS-RNAi transgenic lines from the Drosophila Transgenic RNAi Project (TRiP) at Harvard Medical School. These TRiP RNAi lines contain a 21 bp targeting shRNAi sequence embedded into a micro-RNA (miR-1) backbone that is very effective for knocking down the expression of a target gene in both soma and germline (Ni et al. 2011; Perkins et al. 2015). As expected, knocking down hairy[h, one of the Notch downstream genes that regulates development and hypoxia tolerance in Drosophila (Kim et al. 2000; Cui 2005; Zhou et al. 2008)] decreased Notch activation-conferred hypoxia tolerance, reducing survival rate from 86.1% to 34.7% in hypoxia (P < 0.01) (Figure 4G). Furthermore, we found that knocking down bnl, croc and Mkk4 also reduced Notch-conferred hypoxia tolerance (Figure 4, A–C). However, knocking down the other candidate genes (Egfr, grn, and inv) did not show a clear modifier effect, i.e., knocking down Egfr, grn, and inv on the background of Notch activation ([EN] × [UAS-RNAiEgfr]; [EN] × [UAS-RNAigrn] and [EN] × [UAS-RNAiinv]) showed hypoxia survival rates that were still significantly higher than those of the controls (Figure 4, D–F).
Figure 4.
Genetic interactions regulating hypoxia tolerance conferred by activating Notch in Eaat1-positive glial cells. Specific UAS-RNAi line targeting bnl, croc, Egfr, grn, h, inv or Mkk4 was crossed with the EN line to knock down the expression of the targeted genes individually on the background of Notch activation in the Eaat1-positive glial cells. The eclosion rate of the progeny under hypoxic condition (5% O2) were measured. A significant reduction of Notch activation-conferred hypoxia survival was observed in the crosses with (A) bnl knock down [Ordinary one-way ANOVA; F(4,10) = 18.25, P = 0.0001], (B) croc knock down [Ordinary one-way ANOVA; F(4,10) = 11.09, P = 0.0011], (C) Mkk4 knock down [Ordinary one-way ANOVA; F(4,10) = 13.44, P = 0.0005], or (G) h knock down [F(4,10) = 44.24, P < 0.0001]. In contrast, knock down (D) Egfr [Ordinary one-way ANOVA; F(4,10) = 37.23, P < 0.0001), (E) grn [Ordinary one-way ANOVA; F(4,10) = 23.66, P < 0.0001]or (F) inv [Ordinary one-way ANOVA; F(4,10) = 56.93, P < 0.0001]did not significantly diminish the hypoxia survival rate to control levels. Bars represent mean ± SD (n = 3 to 6 vials) for each group (*P < 0.05, ** P < 0.01, ***P < 0.001; Turkey’s multiple comparison tests).
The role of bnl, croc and Mkk4 in Drosophila neuronal development has been reported in various studies. Branchless (bnl) is a fibroblast growth factor (FGF) homolog in Drosophila that is an important regulator of tracheal and neuronal morphogenesis (Barrett et al. 2008; Muha and Muller 2013;Du et al. 2017). A neuronal expression of bnl has been detected in embryo and larval brain, which is essential for the induction of cell migration and neuronal connection (Du et al. 2017). Our results indicate that bnl is also expressed in the Eaat1-positive glial cells in addition to neurons. This pattern of expression may be important in neuronal development through regulating cell migration and axon outgrowth to create functional neuronal networks. Furthermore, the expression of bnl is highly inducible by hypoxia, and such expression of bnl is important to trigger the growth of the tracheal system to improve O2 delivery (Jarecki et al. 1999; Centanin et al. 2008). Becauseinteractions between FGF and Notch signaling has been observed in Drosophila and mammals in particular in the neuronal system (Bartlett et al. 1998; James et al. 2004; Yoon et al. 2004; Ghabrial and Krasnow 2006; Zhou and Armstrong 2007; Fujita et al. 2011; Voelkel et al. 2014), the current results suggest that bnl produced by the Eaat1-positive glial cells is essential for Notch-activation-conferred survival under severe hypoxic conditions, at least in part, by maintaining axon growth and integrity of proper neuronal-glial connectivity during development (Figure 6).
Figure 6.
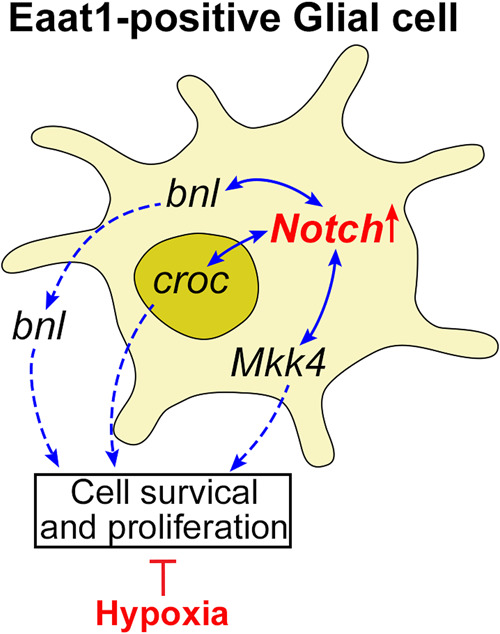
Schematic illustration of hypothetical functional output of Notch/bnl, Notch/croc and Notch/Mkk4 interactions under hypoxia. Hypoxia tolerance conferred by Notch activation in the Eaat1-positive glial cells requires functional synchronization with bnl, croc and Mkk4 to regulate cell survival and proliferation, such functional synchronization is essential for organismal survival under prolonged severe hypoxic conditions.
The crocodile (croc) gene encodes a member of the forkhead transcription factor family in Drosophila. Genes encoding this family of transcription factors are widely conserved during evolution ranging from yeast to humans (Hannenhalli and Kaestner 2009). In Drosophila, the expression of croc is mainly detected in embryo, third instar larvae and adult female flies (Lee and Frasch 2004; Lopez et al. 2017). At the embryonic stage, it is expressed in the head anlagen of the blastoderm and controls the establishment of head structures (Hacker et al. 1995). Although signaling mechanisms regulating the activity of croc are yet uncharacterized, our results indicate that the Notch/croc interaction within the Eaat1-positive glial cells is essential for organismal survival under hypoxic condition, possibly through maintaining the viability and function of these Eaat1-positive glial cells and, thus, neuronal development (Figure 6).
A number of studies have shown that Mkk4 regulates the activity of JNK pathway and neuronal development in Drosophila(Geuking et al. 2009; Rallis et al. 2010).The interactions between Notch and JNK signaling have been reported in Drosophila and mammals during development or under disease conditions. Such interactions may determine or fine-tune the final output of Notch signaling in a cell context-dependent fashion (Zecchini et al. 1999; Kim et al. 2005, 2010; Curry et al. 2006; Ho et al. 2015). It was very interesting to find that cell-specific and ubiquitous manipulation of Mkk4 exhibited opposite phenotypes in terms of hypoxia tolerance (i.e., knocking down Mkk4 specifically in Eaat1-positive glial cells led to lethality, but ubiquitous knocking down this gene enhanced hypoxia survival) (Figures 4C and 5), implying a cell context-dependent role of JNK signaling in hypoxia response. Indeed, it has been shown in tumor cells that hypoxia-induced JNK activation increases resistance to chemotherapeutic treatment (Comerford et al. 2004), but, in the pulmonary arteries, such activation leads to structural remodeling (Jin et al. 2000). Furthermore, we found that ubiquitous knocking down of several genes modulating the activity of JNK signaling can enhance hypoxia survival (Azad et al. 2012), which suggests a cell context-dependent role of JNK signaling on hypoxia response in the organism. Therefore, we hypothesize that the interaction between Mkk4 and Notch may regulate survival of glial cells under hypoxic conditions, and, hence, hypoxia tolerance in Drosophila (Figure 6).
Figure 5.

Effect of ubiquitous knocking down of candidate genes on hypoxia tolerance. Specific UAS-RNAi line targeting bnl, croc, Egfr, grn, h, inv, or Mkk4 was crossed with the da-Gal4 driver to ubiquitously knock down the expression of the targeted genes individually in D.melanogaster. The eclosion rate of the progeny under 5% O2 hypoxic condition was measured. A significant enhancement of hypoxia survival was detected in the flies with specific knocking down of grn, inv, and Mkk4. In contrast, the crosses with croc and h knocking down showed a similar survival rate with the controls. And ubiquitous knocking down of bnl or Egfr was lethal. Bars represent means ± SD (n = 3 to 6 vials) for each cross. Ordinary one-way ANOVA [F(8,48) = 31.93, P < 0.0001]with Turkey’s multiple comparison tests (**P < 0.01).
In addition, we performed an experiment with ubiquitous knocking down of these candidate genes using the da-Gal4 driver to evaluate their role in Drosophila development and hypoxia tolerance (Figure 5). We found that the progeny of [da-Gal4] × [UAS-RNAibnl] or [da-Gal4] × [UAS-RNAiEgfr] crosses were lethal under both normoxia and hypoxia conditions. In contrast, flies with da-Gal4>UAS-RNAigrn, da-Gal4>UAS-RNAiinv or da-Gal4>UAS-RNAiMkk4 exhibited significantly enhanced tolerance to hypoxia with > 2-fold eclosion rates as compared to those of the controls under hypoxia (p < 0.01). The result of Mkk4 KD is somewhat surprising: unlike specific knocking down in the Eaat1-positive glial cells (Eaat1-Gal4>UAS-RNAiMkk4) (Figure 4C), ubiquitous knockdown of Mkk4 significantly enhanced hypoxia tolerance in Drosophila, suggesting a developmental and/or cell type specific role of Mkk4 in regulating organismal response to hypoxia.
Conclusions and perspectives
In conclusion, we evaluated the interactions between Notch signaling and a group of evolutionarily conserved genes in Eaat1-positive glial cells and the role of such interactions in regulating hypoxia survival in Drosophila. The synergy of signaling between Notch and bnl, Notch and croc and between Notch and Mkk4 were found to be essential for Notch activation-conferred hypoxia tolerance. We hypothesize that the synergy between these functionally distinct signaling mechanisms regulates cell survival and neuronal development under hypoxic conditions in the Drosophila central nervous system, and this is also sufficient for the survival of the organism as a whole (Figure 6). Furthermore, we provided evidence indicating that bnl, croc, and Mkk4 are expressed in the Eaat1-positive glial cells. These conserved mechanisms have high potential to be translated into humans for developing strategies to treat hypoxia-induced injuries in the central nervous system.
Data availability
D.melanogaster strains are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables.
Supplementary material is available at G3 online.
Supplementary Material
Acknowledgments
We sincerely appreciate the comments and suggestions from Dr. James W. Posakony and thank Ms. Yu Hsin Hsiao and Ms. Ying Lu-Bo for technical supports.
Funding
Research reported in this publication was supported by National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number 1R21NS111270.
Literature cited
- Adler D, Pepin JL, Dupuis-Lozeron E, Espa-Cervena K, Merlet-Violet R, et al. 2017. Comorbidities and subgroups of patients surviving severe acute hypercapnic respiratory failure in the intensive care unit. Am J Respir Crit Care Med. 196:200–207. [DOI] [PubMed] [Google Scholar]
- Alberi L, Hoey SE, Brai E, Scotti AL, Marathe S.. 2013. Notch signaling in the brain: in good and bad times. Ageing Res Rev. 12:801–814. [DOI] [PubMed] [Google Scholar]
- Andersson ER, Sandberg R, Lendahl U.. 2011. Notch signaling: simplicity in design, versatility in function. Development 138:3593–3612. [DOI] [PubMed] [Google Scholar]
- Arumugam TV, Baik SH, Balaganapathy P, Sobey CG, Mattson MP, et al. 2018. Notch signaling and neuronal death in stroke. Prog Neurobiol. 165–167:103–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad P, Zhou D, Zarndt R, Haddad GG.. 2012. Identification of genes underlying hypoxia tolerance in Drosophila by a P-element screen. G3 (Bethesda). 2:1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrampour S, Thor S.. 2020. The five faces of notch signalling during Drosophila melanogaster embryonic CNS development. AdvExp Med Biol. 1218:39–58. [DOI] [PubMed] [Google Scholar]
- Barrett AL, Krueger S, Datta S.. 2008. Branchless and Hedgehog operate in a positive feedback loop to regulate the initiation of neuroblast division in the Drosophila larval brain. Dev Biol. 317:234–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett PF, Brooker GJ, Faux CH, Dutton R, Murphy M, et al. 1998. Regulation of neural stem cell differentiation in the forebrain. Immunol Cell Biol. 76:414–418. [DOI] [PubMed] [Google Scholar]
- Besson MT, Soustelle L, Birman S.. 1999. Identification and structural characterization of two genes encoding glutamate transporter homologues differently expressed in the nervous system of Drosophila melanogaster. FEBS Lett. 443:97–104. [DOI] [PubMed] [Google Scholar]
- Bittern J, Pogodalla N, Ohm H, Bruser L, Kottmeier R, et al. 2020. Neuron-glia interaction in the Drosophila nervous system. Dev Neurobiol. Online ahead of print. [ 10.1002/dneu.22737] [DOI] [PubMed] [Google Scholar]
- Borggrefe T, Lauth M, Zwijsen A, Huylebroeck D, Oswald F, et al. 2016. The Notch intracellular domain integrates signals from Wnt, Hedgehog, TGFbeta/BMP and hypoxia pathways. BiochimBiophysActa 1863:303–313. [DOI] [PubMed] [Google Scholar]
- Borggrefe T, Liefke R.. 2012. Fine-tuning of the intracellular canonical Notch signaling pathway. Cell Cycle 11:264–276. [DOI] [PubMed] [Google Scholar]
- Bray S, Bernard F.. 2010. Notch targets and their regulation. Curr Top DevBiol. 92:253–275. [DOI] [PubMed] [Google Scholar]
- Centanin L, Dekanty A, Romero N, Irisarri M, Gorr TA, et al. 2008. Cell autonomy of HIF effects in Drosophila: tracheal cells sense hypoxia and induce terminal branch sprouting. Dev Cell. 14:547–558. [DOI] [PubMed] [Google Scholar]
- Choi DW. 1988. Glutamate neurotoxicity and diseases of the nervous system. Neuron 1:623–634. [DOI] [PubMed] [Google Scholar]
- Choi DW, Rothman SM.. 1990. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 13:171–182. [DOI] [PubMed] [Google Scholar]
- Comerford KM, Cummins EP, Taylor CT.. 2004. c-Jun NH2-terminal kinase activation contributes to hypoxia-inducible factor 1alpha-dependent P-glycoprotein expression in hypoxia. Cancer Res. 64:9057–9061. [DOI] [PubMed] [Google Scholar]
- Cui Y. 2005. Hairy is a cell context signal controlling Notch activity. Dev Growth Differ. 47:609–625. [DOI] [PubMed] [Google Scholar]
- Curry CL, Reed LL, Nickoloff BJ, Miele L, Foreman KE.. 2006. Notch-independent regulation of Hes-1 expression by c-Jun N-terminal kinase signaling in human endothelial cells. Lab Invest. 86:842–852. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. 2001. Glutamate uptake. Prog Neurobiol. 65:1–105. [DOI] [PubMed] [Google Scholar]
- Di Fiore JM, MacFarlane PM, Martin RJ.. 2019. Intermittent hypoxemia in preterm infants. ClinPerinatol. 46:553–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Zhou A, Patel A, Rao M, Anderson K, et al. 2017. Unique patterns of organization and migration of FGF-expressing cells during Drosophila morphogenesis. Dev Biol. 427:35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon U, Canavan M, Biniecka M, Veale DJ.. 2016. Hypoxia, mitochondrial dysfunction and synovial invasiveness in rheumatoid arthritis. Nat Rev Rheumatol. 12:385–397. [DOI] [PubMed] [Google Scholar]
- Fonnum F. 1984. Glutamate: a neurotransmitter in mammalian brain. J Neurochem. 42:1–11. [DOI] [PubMed] [Google Scholar]
- Fujita K, Yasui S, Shinohara T, Ito K.. 2011. Interaction between NF-kappaB signaling and Notch signaling in gliogenesis of mouse mesencephalic neural crest cells. Mech Dev. 128:496–509. [DOI] [PubMed] [Google Scholar]
- Geuking P, Narasimamurthy R, Lemaitre B, Basler K, Leulier F.. 2009. A non-redundant role for Drosophila Mkk4 and hemipterous/Mkk7 in TAK1-mediated activation of JNK. PLoSONE 4:e7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghabrial AS, Krasnow MA.. 2006. Social interactions among epithelial cells during tracheal branching morphogenesis. Nature 441:746–749. [DOI] [PubMed] [Google Scholar]
- Gibson G. 1989. Causes of cell damage in hypoxia/ischemia, aging and Alzheimer's disease. Neurobiol Aging 10:608–609. [DOI] [PubMed] [Google Scholar]
- Go MJ, Eastman DS, Artavanis-Tsakonas S.. 1998. Cell proliferation control by Notch signaling in Drosophila development. Development 125:2031–2040. [DOI] [PubMed] [Google Scholar]
- Group ST. 2018. Hypoxemia in the ICU: prevalence, treatment, and outcome. Ann Intensive Care 8:82.[10.1186/s13613-018-0424-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, et al. 2005. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell 9:617–628. [DOI] [PubMed] [Google Scholar]
- Hacker U, Kaufmann E, Hartmann C, Jurgens G, Knochel W, et al. 1995. The Drosophila fork head domain protein crocodile is required for the establishment of head structures. EMBO J. 14:5306–5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannenhalli S, Kaestner KH.. 2009. The evolution of Fox genes and their role in development and disease. Nat Rev Genet. 10:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo A, Logan A.. 2017. Go and stop signals for glial regeneration. CurrOpin Neurobiol. 47:182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilu-Dadia R, Kurant E.. 2020. Glial phagocytosis in developing and mature Drosophila CNS: tight regulation for a healthy brain. CurrOpinImmunol. 62:62–68. [DOI] [PubMed] [Google Scholar]
- Ho DM, Guruharsha KG, Artavanis-Tsakonas S.. 2018. The Notch interactome: complexity in signaling circuitry. AdvExp Med Biol. 1066:125–140. [10.1007/978-3-319-89512-3_7] [DOI] [PubMed] [Google Scholar]
- Ho DM, Pallavi SK, Artavanis-Tsakonas S.. 2015. The Notch-mediated hyperplasia circuitry in Drosophila reveals a Src-JNK signaling axis. Elife 4:e05996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlbut GD, Kankel MW, Lake RJ, Artavanis-Tsakonas S.. 2007. Crossing paths with Notch in the hyper-network. CurrOpin Cell Biol. 19:166–175. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Mosinger JL, Salles KS, Labruyere J, Olney JW.. 1989. Sensitivity of the developing rat brain to hypobaric/ischemic damage parallels sensitivity to N-methyl-aspartate neurotoxicity. J Neurosci. 9:2809–2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JR. 2000. The midline glia of Drosophila: a molecular genetic model for the developmental functions of glia. Prog Neurobiol. 62:475–508. [DOI] [PubMed] [Google Scholar]
- James J, Das AV, Rahnenfuhrer J, Ahmad I.. 2004. Cellular and molecular characterization of early and late retinal stem cells/progenitors: differential regulation of proliferation and context dependent role of Notch signaling. J Neurobiol. 61:359–376. [DOI] [PubMed] [Google Scholar]
- Jarecki J, Johnson E, Krasnow MA.. 1999. Oxygen regulation of airway branching in Drosophila is mediated by branchless FGF. Cell 99:211–220. [DOI] [PubMed] [Google Scholar]
- Jha AR, Zhou D, Brown CD, Kreitman M, Haddad GG, et al. 2016. Shared genetic signals of hypoxia adaptation in Drosophila and in high-altitude human populations. MolBiolEvol. 33:501–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin N, Hatton N, Swartz DR, Xia X, Harrington MA, et al. 2000. Hypoxia activates jun-N-terminal kinase, extracellular signal-regulated protein kinase, and p38 kinase in pulmonary arteries. Am J Respir Cell MolBiol. 23:593–601. [DOI] [PubMed] [Google Scholar]
- Kato K, Losada-Perez M, Hidalgo A.. 2018. Gene network underlying the glial regenerative response to central nervous system injury. DevDyn. 247:85–93. [DOI] [PubMed] [Google Scholar]
- Kim J, Kerr JQ, Min GS.. 2000. Molecular heterochrony in the early development of Drosophila. ProcNatlAcadSci USA. 97:212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Kim MJ, Kim KJ, Yun HJ, Chae JS, et al. 2005. Notch interferes with the scaffold function of JNK-interacting protein 1 to inhibit the JNK signaling pathway. ProcNatlAcadSci USA. 102:14308–14313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MY, Ann EJ, Mo JS, Dajas-Bailador F, Seo MS, et al. 2010. JIP1 binding to RBP-Jk mediates cross-talk between the Notch1 and JIP1-JNK signaling pathway. Cell Death Differ. 17:1728–1738. [DOI] [PubMed] [Google Scholar]
- Krnjevic K. 1970. Glutamate and gamma-aminobutyric acid in brain. Nature 228:119–124. [DOI] [PubMed] [Google Scholar]
- Leach RM, Treacher DF.. 1998. Oxygen transport-2. Tissue hypoxia. BMJ 317:1370–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HH, Frasch M.. 2004. Survey of forkhead domain encoding genes in the Drosophila genome: classification and embryonic expression patterns. DevDyn. 229:357–366. [DOI] [PubMed] [Google Scholar]
- Lopez Y, Vandenbon A, Nose A, Nakai K.. 2017. Modeling the cis-regulatory modules of genes expressed in developmental stages of Drosophila melanogaster. PeerJ. 5:e3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamee SE, Liu KE, Gerhard S, Tran CT, Fetter RD, et al. 2016. Astrocytic glutamate transport regulates a Drosophila CNS synapse that lacks astrocyte ensheathment. J Comp Neurol. 524:1979–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marignol L, Rivera-Figueroa K, Lynch T, Hollywood D.. 2013. Hypoxia, notch signalling, and prostate cancer. Nat Rev Urol. 10:405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollen EWJ, Ient J, Tjan-Heijnen VCG, Boersma LJ, Miele L, et al. 2018. Moving breast cancer therapy up a Notch. Front Oncol. 8:518. [ 10.3389/fonc.2018.00518] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muha V, Muller HA.. 2013. Functions and mechanisms of fibroblast growth factor (FGF) signallingin Drosophila melanogaster. IntJMolSci. 14:5920–5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni JQ, Zhou R, Czech B, Liu LP, Holderbaum L, et al. 2011. A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat Methods 8:405–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG. 1993. The glutamatergic nerve terminal. Eur J Biochem. 212:613–631. [DOI] [PubMed] [Google Scholar]
- Olney JW, Ho OL.. 1970. Brain damage in infant mice following oral intake of glutamate, aspartate or cysteine. Nature 227:609–611. [DOI] [PubMed] [Google Scholar]
- Pear WS, Simon MC.. 2005. Lasting longer without oxygen: the influence of hypoxia on Notch signaling. Cancer Cell 8:435–437. [DOI] [PubMed] [Google Scholar]
- Peco E, Davla S, Camp D, Stacey SM, Landgraf M, et al. 2016. Drosophila astrocytes cover specific territories of the CNS neuropil and are instructed to differentiate by Prospero, a key effector of Notch. Development 143:1170–1181. [DOI] [PubMed] [Google Scholar]
- Perkins LA, Holderbaum L, Tao R, Hu Y, Sopko R, et al. 2015. The transgenic RNAi project at Harvard Medical School: resources and validation. Genetics 201:843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purins K, Enblad P, Wiklund L, Lewen A.. 2012. Brain tissue oxygenation and cerebral perfusion pressure thresholds of ischemia in a standardized pig brain death model. NeurocritCare 16:462–469. [DOI] [PubMed] [Google Scholar]
- Rallis A, Moore C, Ng J.. 2010. Signal strength and signal duration define two distinct aspects of JNK-regulated axon stability. Dev Biol. 339:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rival T, Soustelle L, Strambi C, Besson MT, Iche M, et al. 2004. Decreasing glutamate buffering capacity triggers oxidative stress and neuropil degeneration in the Drosophila brain. Curr Biol. 14:599–605. [DOI] [PubMed] [Google Scholar]
- Rizzo P, Mele D, Caliceti C, Pannella M, Fortini C, et al. 2014. The role of notch in the cardiovascular system: potential adverse effects of investigational notch inhibitors. Front Oncol. 4:384. [ 10.3389/fonc.2014.00384] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainson RC, Harris AL.. 2006. Hypoxia-regulated differentiation: let's step it up a Notch. Trends Mol Med. 12:141–143. [DOI] [PubMed] [Google Scholar]
- Sands SA, Owens RL.. 2015. Congestive heart failure and central sleep apnea. Crit Care Clin. 31:473–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B, Anderson PJ, Asztalos EV, Doyle LW, Grunau RE, et al. 2019. Self-reported quality of life at middle school age in survivors of very preterm birth: results from the caffeine for apnea of prematurity trial. JAMA Pediatr. 173:487–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B, Roberts RS, Anderson PJ, Asztalos EV, Costantini L, et al. 2017. Academic performance, motor function, and behavior 11 years after neonatal caffeine citrate therapy for apnea of prematurity: an 11-year follow-up of the CAP randomized clinical trial. JAMA Pediatr. 171:564–572. [DOI] [PubMed] [Google Scholar]
- Stacey SM, Muraro NI, Peco E, Labbe A, Thomas GB, et al. 2010. Drosophila glial glutamate transporter Eaat1 is regulated by fringe-mediated notch signaling and is essential for larval locomotion. J Neurosci. 30:14446–14457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe N, Nguyen D, Yang SX.. 2014. Targeting notch signaling pathway in cancer: clinical development advances and challenges. PharmacolTher. 141:140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thille AW, Cordoba-Izquierdo A, Maitre B, Boyer L, Brochard L, et al. 2018. High prevalence of sleep apnea syndrome in patients admitted to ICU for acute hypercapnic respiratory failure: a preliminary study. Intensive Care Med. 44:267–269. [DOI] [PubMed] [Google Scholar]
- Voelkel JE, Harvey JA, Adams JS, Lassiter RN, Stark MR.. 2014. FGF and Notch signaling in sensory neuron formation: a multifactorial approach to understanding signaling pathway hierarchy. Mech Dev. 134:55–66. [DOI] [PubMed] [Google Scholar]
- Wu JS, Luo L.. 2006. A protocol for dissecting Drosophila melanogaster brains for live imaging or immunostaining. Nat Protoc. 1:2110–2115. [DOI] [PubMed] [Google Scholar]
- Yildirim K, Petri J, Kottmeier R, Klambt C.. 2019. Drosophila glia: few cell types and many conserved functions. Glia 67:5–26. [DOI] [PubMed] [Google Scholar]
- Yoon K, Nery S, Rutlin ML, Radtke F, Fishell G, et al. 2004. Fibroblast growth factor receptor signaling promotes radial glial identity and interacts with Notch1 signaling in telencephalic progenitors. J Neurosci. 24:9497–9506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecchini V, Brennan K, Martinez-Arias A.. 1999. An activity of Notch regulates JNK signalling and affects dorsal closure in Drosophila. Curr Biol. 9:460–469. [DOI] [PubMed] [Google Scholar]
- Zhou D, Haddad GG.. 2013. Genetic analysis of hypoxia tolerance and susceptibility in Drosophila and humans. Annu Rev Genom Hum Genet. 14:25–43. [DOI] [PubMed] [Google Scholar]
- Zhou D, Udpa N, Gersten M, Visk DW, Bashir A, et al. 2011. Experimental selection of hypoxia-tolerant Drosophila melanogaster. ProcNatlAcadSci USA. 108:2349–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Xue J, Lai JC, Schork NJ, White KP, et al. 2008. Mechanisms underlying hypoxia tolerance in Drosophila melanogaster: hairy as a metabolic switch. PLoSGenet. 4:e1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YX, Armstrong RC.. 2007. Interaction of fibroblast growth factor 2 (FGF2) and notch signaling components in inhibition of oligodendrocyte progenitor (OP) differentiation. NeurosciLett. 421:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
D.melanogaster strains are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables.
Supplementary material is available at G3 online.




