Abstract
Elevated blood pressure (BP) and hypertension commonly occur in children and adolescents and increase the risk of cardiovascular disease in adulthood. The purpose of this review is to summarize recent research in pediatric hypertension including changes in defining hypertension, BP measurement techniques, hypertension epidemiology, risk factors, treatment, and BP-related target organ damage. Defining pediatric hypertension using the 2017 American Academy of Pediatrics’ updated Clinical Practice Guideline resulted in a larger proportion of children being classified as having elevated BP or hypertension compared with prior guidelines. Trends in the distribution of BP among US children and adolescents suggest that BP levels and the prevalence of hypertension may have increased from 2011–2014 to 2015–2018. Factors including a family history of hypertension, obesity, minority race/ethnicity, physical inactivity, high dietary intake of sodium, and poor sleep quality are associated with an increased prevalence of elevated BP and hypertension. Evidence of a linear relationship between systolic BP and target organ damage indicates that BP levels currently considered normal could increase the risk of target organ damage in childhood. Lifestyle changes, such as adhering to the Dietary Approaches to Stop Hypertension diet, are a central component of effectively reducing BP and have been shown to reduce target organ damage. Pharmacologic treatment using angiotensin-converting enzyme inhibitors and angiotensin receptor blockers is an effective and safe method for reducing BP among children with uncontrolled BP after implementing lifestyle changes. Research gaps in the prevention, detection, classification, and treatment of hypertension in children demonstrate opportunities for future study.
Keywords: antihypertensive treatment, blood pressure, blood pressure measurement, epidemiology, hypertension, lifestyle behaviors, pediatric hypertension, prevention, target organ damage
Hypertension is one of the primary modifiable risk factors for cardiovascular disease (CVD) morbidity and mortality in the United States and a public health concern worldwide.1,2 The origins of hypertensive CVD have been traced to early childhood3 with higher blood pressure (BP) levels in childhood and adolescence predicting an increased risk of hypertension in adulthood.4–6 Diagnoses of elevated BP and hypertension are increasingly common in childhood as the clinical definition of abnormal BP levels and population changes in childhood obesity have evolved.7–9 The complexity of diagnosing and treating pediatric hypertension presents several challenges for clinicians including the proper measurement of BP, defining hypertension, preventing target organ damage, supporting positive lifestyle modification, and determining when to use pharmacologic interventions to control BP. This review discusses the most recent knowledge on defining hypertension, its epidemiology, measurement, consequences, and treatment among children and adolescents.
ELEVATED BP AND HYPERTENSION DEFINITIONS
The 2017 American Academy of Pediatrics (AAP) Clinical Practice Guideline updated the definitions of elevated BP and hypertension and introduced new age-, sex-, and height-specific normative percentiles tables based on the distribution of BP among normal weight children and adolescents (Table 1).9 Compared with the prior 2004 Fourth Report guideline, which relied on normative tables that included those with overweight or obesity, the 2017 AAP guideline using tables with only healthy weight youth, classifies more children <13 years of age as having elevated BP or hypertension.10,11 Identifying children with abnormal BP levels at younger ages could increase awareness and BP control, and in turn reduce progression to higher BP levels and target organ damage. Instead of statistical, percentile-based definitions, the 2017 AAP thresholds for defining elevated BP and hypertension among adolescents ≥13 years of age align with the definitions used by the 2017 American College of Cardiology/American Heart Association adult BP guideline.12 For adolescents, elevated BP is defined as systolic BP (SBP) of 120–129 mm Hg and diastolic BP (DBP) <80 mm Hg, and hypertension is defined as SBP ≥130 mm Hg and/or DBP ≥80 mm Hg.9 This congruence of BP thresholds for older adolescents and adults relies on the strong evidence base linking BP levels and treatment at these thresholds to the risk for CVD events among adults,12 as similar data on children are not yet available. Using static BP thresholds, regardless of height and sex, also simplified the process of defining elevated BP and hypertension among adolescents for clinicians.
Table 1.
Definitions of normal blood pressure, elevated blood pressure, and hypertension among children and adolescents according to the 2017 American Academy of Pediatrics Clinical Practice Guideline
| Blood pressure categorya | Children and adolescents age 1–12 years | Adolescents age ≥13 years |
|---|---|---|
| Normal blood pressure | SBP and DBP <90th percentileb | SBP <120 mm Hg and DBP <80 mm Hg |
| Elevated blood pressurec | SBP and/or DBP ≥90th to <95th percentile or SBP ≥120 mm Hg and DBP <80 mm Hg to the 95th percentile | SBP 120–129 mm Hg and DBP <80 mm Hg |
| Stage 1 hypertension | SBP and/or DBP ≥95th percentile to <95th percentile + 12 mm Hg or SBP 130–139 mm Hg and/or DBP 80–89 mm Hg | SBP 130–139 mm Hg and/or DBP 80–89 mm Hg |
| Stage 2 hypertension | ≥95th percentile + 12 mm Hg or SBP ≥140 mm Hg and/or DBP ≥90 mm Hg | SBP ≥140 mm Hg and/or DBP ≥90 mm Hg |
Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure.
aDefinitions were obtained from the 2017 American Academy of Pediatrics Clinical Practice Guideline.
bPercentiles were determined using age-, sex-, and height-specific percentile tables from the 2017 American Academy of Pediatrics Clinical Practice Guideline.
cHigh blood pressure was defined as having either elevated blood pressure or hypertension.
EPIDEMIOLOGY
Prevalence and incidence
A recent meta-analysis that assessed the prevalence of hypertension globally among those ≤19 years of age estimated that 9.7% and 4.0% of the population had prehypertension and hypertension, respectively, using the Fourth Report guideline definitions.13 Nationally representative estimates of elevated BP and hypertension prevalence in the United States are largely limited to studies using data from the National Health and Nutrition Examination Study (NHANES). From 2013 to 2016, an estimated 7.1% of US children and adolescents 8–17 years of age had elevated BP and 3.5% had hypertension, using the 2017 AAP Clinical Practice Guideline definitions.7,10 The prevalence of elevated BP and hypertension in the United States varies across sex, racial/ethnic, and lifestyle factor groups. Having high BP, defined as having either elevated BP or hypertension, in childhood and adolescence is more common among boys (13.0%) compared with girls (8.1%) and African Americans (13.8%) and Hispanic Americans (12.8%) compared in non-Hispanic whites (9.2%).10 High BP is also more prevalent among children and adolescents who are overweight or obese (16.5%) compared with those who are normal weight (6.1%).10
Trends
A study examining trends in SBP levels over the past 20 years in the United States showed that mean SBP among children 8–12 years of age was approximately equivalent in 1999–2002 and 2015–2018, while mean SBP decreased from 1999–2002 to 2015–2016 among adolescents 13–17 years of age.14 Mean DBP decreased over the past 20 years among both children and adolescents 8–12 and 13–17 years of age.14 The age-adjusted prevalence of elevated BP was lower in 2015–2018 (5.5%) than in 1999–2002 (7.0%) among those 8–12 years of age, and the prevalence of hypertension decreased from 6.6% to 3.7% from 1999–2002 to 2015–2018 among those 13–17 years of age.14 Despite overall 20-year decreases in the prevalence of elevated BP in children and hypertension in adolescents, stable or increased BP levels and hypertension prevalence from 2011–2014 to 2015–2018 could indicated a reversal of these trends.14 Increases in obesity and severe obesity, particularly among young children, since 2013–2014 could be contributing to recent increases in elevated BP and hypertension.7,8 Awareness of the impact of the childhood obesity epidemic on BP and effective interventions are needed to reduce preventable increases in the prevalence of hypertension over time.
Risk factors for primary hypertension in childhood
Hypertension develops from a multifaceted interplay of lifestyle, behavioral, environmental, and genetic factors.15,16 Similar to adults, traditional risk factors including perinatal history, a family history of hypertension, obesity, minority race/ethnicity, physical inactivity, and a high dietary intake of sodium are associated with an increased risk of elevated BP and hypertension.9 In addition to the risk factors discussed in the 2017 AAP guideline, sleep duration of ≤8 hours/night has been associated with an increased risk of elevated BP and hypertension in youth 11–17 years of age.17 Sparano et al.18 extended these findings to younger children aged 2–9.9 years by reporting that those with shorter sleep durations had higher BP levels and a higher prevalence of having elevated BP or hypertension. Poor sleep quality, assessed by the Pittsburgh Sleep Quality Index, has also been associated with hypertension among Chinese adolescents.19 These studies indicate that improvements in sleep duration and sleep quality may be potential targets for improving BP levels in pediatric populations.
Social determinants also influence the risk of hypertension among children and adolescents. A recent study that examined factors associated with elevated BP and hypertension among children and adolescents in the United States found that children from a family with a poverty-to-income ratio ≤3.49 were more likely to have elevated BP or hypertension compared with those with a poverty-to-income ratio >3.50.14 Studies have also shown that children exposed to multiple adverse childhood experiences have greater increases in BP and a higher prevalence of hypertension in young adulthood.20,21 As advocated by the 2017 AAP guideline,9 evaluation of hypertension should include an assessment of social and psychosocial factors that could influence the risk of hypertension or white coat hypertension among children and adolescents.
BP MEASUREMENT
The 2017 AAP Clinical Practice Guideline recommends that trained healthcare professionals measure BP in the office setting annually for all children ≤3 years of age and at every healthcare encounter for children and adolescents who at increased risk for hypertension due to obesity, diabetes, renal disease, or other chronic medical conditions.9 Accurate BP measurement is essential for identifying, treating, and controlling hypertension. Measurement of BP in the office in early life is complex due to the large differences in age, weight, the white-coat effect, BP variability, and BP measurement techniques. Device related factors including limited cuff sizes and lack of standardization of algorithms for calculation of SBP and DBP from mean arterial pressure in oscillometric devices increase the difficulty of obtaining accurate measurements.9 Although an oscillometric BP measure may be suitable for screening for hypertension, to confirm an elevated office BP measurement and avoid overdiagnosis, the 2017 AAP Clinical Practice Guideline recommends averaging 2 additional BP measurements using an auscultatory technique and repeating BP measurements at an interval determined by the severity of BP elevation.9
Office and out-of-office BP measurement
Ambulatory BP monitoring (ABPM), which measures BP automatically every 15–30 minutes typically for 24 hours, with a validated device is strongly suggested by the 2017 AAP guideline as the most robust method for confirming persistently high out-of-office BP levels.9,22 Recent data suggest that the discrepancy between values obtained by office BP and ABPM is high with 7.3% of children having white coat hypertension and 8.2% of children having masked hypertension.23 White coat hypertension and masked hypertension were both more common among obese children and adolescents compared with normal weight.23 Despite the utility of ABPM in identifying a mismatch between office and out-of-office BP, there are several barriers to widespread ABPM use for hypertension screening and monitoring. ABPM can cause discomfort to patients and be poorly tolerated among children and adolescents.24 The accuracy of ABPM has been shown to be associated with patient reported tolerability, with youth who were intolerant to ABPM during wake hours having a higher prevalence of awake ambulatory hypertension.24 ABPM devices and software can cost over $3,500.25 Many insurance companies do not commonly reimburse for ABPM, and when reimbursed, the compensation is often low.25 Efforts to address these issues are needed to increase adoption of ABPM for more precise management of elevated casual BP.
Recent advances in office and out-of-office BP measurement
Recent studies have evaluated alternative methods of accurately measuring office and out-of-office BP in childhood. Data from a nonrandomized study conducted among children and adolescents suggest that using an automated oscillometric BP (AOBP) device without a physician or medical provider present results in lower office BP compared with attended measurements taken using the auscultatory method.26 Ardissino et al.27 extended the examination of AOBP measurements among children by comparing 10 unattended BP measurements, taken at 3-minute intervals using a validated AOBP device. Although the 2017 AAP guideline recommends 3 BP clinical measurements to confirm BP levels, in this study the first 3 BP measurements were significantly different from the mean, while the 4th through the 10th measurements were closer to mean value.27 These results suggest that 4 or more AOBP measurements may improve the accuracy of office BP measurement.
Home BP monitoring (HBPM) is another cost-effective, and feasible adjunct method of monitoring out-of-office BP after hypertension diagnosis.9 Recent evidence of the clinical value suggests that home BP may correlate with target organ damage as well as ABPM among children and adolescents.28 There is approximately 85% agreement between home BP and ABPM in the diagnosis of hypertension and home BP may be more reproducible than office BP.28,29 However, the agreement between home BP and office BP may differ by age. In children, evidence suggests that home BP is higher than office BP, while in adolescents home BP is equivalent to office BP.28 Future studies are needed that assess the agreement between home and office BP throughout childhood and adolescence and inform the duration and number of home BP measurements required to define HBPM. Further, to increase the viability of implementing home BP in more pediatric practices, more HBPM devices should be validated and normative data should be collected in diverse populations. As the evidence base supporting the utility and feasibility of HBPM grows, HBPM could be an important tool for assessing and understanding out-of-office BP in youth.
NOVEL METHODS FOR DEFINING HYPERTENSION
Identifying and classifying elevated BP and hypertension using age-, sex-, and height-specific normative percentile tables recommended by the 2017 AAP guideline for children <13 years of age can be complex for physicians to utilize in clinical practices with limited resources as normative tables may not be easily accessible. To simplify quick identification of those with elevated BP and hypertension, novel screening approaches using BP-to-height ratios, calculated as BP (mm Hg)/height (cm), have been proposed and compared with the standard approach of using percentile tables.30,31 In a recent meta-analysis, BP-to-height ratios consistently performed well in terms of ability to detect elevated BP and hypertension compared with percentile tables, as reflected by an area under the curve level of nearly 100% and high levels of sensitivity and specificity.31 However, this meta-analysis suggested that the positive predictive value of using BP-to-height ratio to detect hypertension in children and adolescents is low, which could result in an increased number of children being falsely identified as having hypertension and undergoing unnecessary follow-up evaluation.31 More recent studies have suggested that modifications to the BP-to-height ratio or height-based equations improve the accuracy of hypertension detection in children.30,32 A modified BP-to-height ratio (MBPHR7), calculated as , has been shown to yield better sensitivity and specificity than the unmodified BP-to-height ratio for identifying hypertension in Brazilian children aged 5–12 years.32 Yazdi et al.30 examined the ability of 3 different BP-to-height ratios to identify children with hypertension and reported that a newer equation (MBPHR3) calculated as generally performed better than MBPHR7 with a higher area under the curve and positive predicted value among young Iranian children. While BP-to-height and modified BP-to-height ratios have been increasing evaluated in epidemiologic studies, the complexity of calculating and interpreting these ratios for pediatricians may limit the utility of these tools in clinical practice.
CONSEQUENCES OF CHILDHOOD BP
BP in childhood and adolescence and target organ damage
Epidemiologic and pathophysiologic studies suggest that pediatric hypertension is associated with subclinical atherosclerosis and target organ damage in childhood, and can increased the risk of CVD in adulthood.33–35 Similar to the relationship between BP and CVD in adults,36 it has been hypothesized that a linear relationship exists between SBP in childhood and target organ damage. Results from the multicenter Study of High Blood Pressure in Pediatrics: Adult Hypertension Onset in Youth (SHIP-AHOY) cohort indicate a linear rise in left ventricular mass index with increasing ambulatory daytime SBP (Figure 1). In this cohort of youth 11–19 years of age the prevalence of left ventricular hypertrophy increased with increasing BP with 13% of the normotensive youth having left ventricular hypertrophy compared with 21% of those with SBP in the 80th to 90th percentile and 27% of youth with SBP above the 90th percentile.34 Further investigation found that youth with SBP ≥80th percentile had a significantly lower left ventricular ejection fraction and peak global longitudinal strain than those with SBP <75th percentile.37 In these studies, SBP percentiles below levels used to define clinical hypertension were associated with target organ damage and subclinical changes in ventricular function. Associations between BP levels below clinically defined treatment thresholds and target organ could challenge the archetype that considers BP reduction as only actionable above the current 2017 AAP Clinical Practice Guideline definitions. Future studies are needed to strengthen the evidence base for defining pediatric hypertension treatment thresholds that reduce target organ damage.
Figure 1.
Linear rise in left ventricular mass index with increasing ambulatory daytime systolic blood pressure, Study of High Blood Pressure in Pediatrics: Adult Hypertension Onset in Youth (SHIP-AHOY). Regression of left ventricular mass index on daytime systolic blood pressure percentile was significant after adjusting for age, sex, race, ethnicity, clinic site, body mass index z-score, diastolic blood pressure percentile, and heart rate.
Childhood BP predicts hypertension in adulthood
BP levels in childhood and adolescence have been shown to track into adulthood.38 Leveraging data from 6 cohorts included in the International Childhood Cardiovascular Cohort (i3C) Consortium, Urbina et al.39 investigated the ability of BP during childhood and adolescence to predict adult hypertension. This study found that self-reported hypertension in adulthood was significantly more likely among those with a SBP ≥90th percentile in childhood (odds ratio [OR] = 2.0; 95% confidence interval [CI]: 1.6–2.5) or adolescence (OR = 3.0; 95% CI: 2.2–4.1).39 Further, there was a graded increase in the prevalence of adult hypertension across BP category change groups with those with persistently normal BP from childhood to adolescence having a lower prevalence of hypertension in adulthood compared with those who changed BP categories or had persistently elevated BP.39 These findings were corroborated by a recent meta-analysis which estimated that an increase of 1 SD in SBP and DBP in childhood and adolescence was associated with hypertension in adulthood (OR = 1.71; 95% CI: 1.50–1.95 and OR = 1.57; 95% CI: 1.37–1.81 for SBP and DBP, respectively).38 These studies highlight the long-term implications of childhood BP on the development of hypertension in adulthood, which increases the risk of CVD.
PREVENTION AND TREATMENT
Lifestyle behaviors and BP reductions
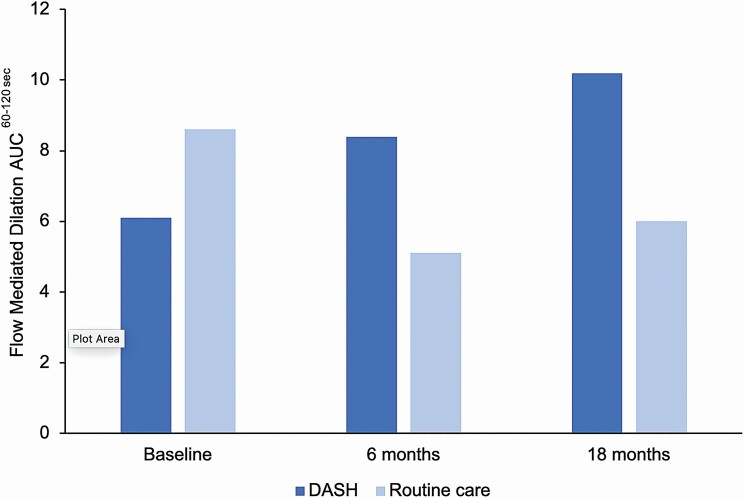
Counseling on lifestyle modifications, including adherence to the Dietary Approaches to Stop Hypertension (DASH) diet, is recommended by the 2017 AAP guideline as initial treatment for elevated BP and hypertension among children and adolescents.9 A recently published randomized control trial (n = 159) examined the impact of a 6-month DASH diet intervention on BP among youth 11–18 years of age.40 In this study, all participants received face-to-face counseling with a registered dietician at baseline and check-ins at 3 months, 6 months, and 1 year.40 The DASH intervention group was additionally encouraged to set and achieve progressive DASH compliant dietary goals weekly and were rewarded for meeting dietary goals ($2/goal met).40 The DASH diet intervention group experienced a greater reduction in SBP (2.7 mm Hg) and improvement in flow mediated dilation (2.5%) compared with the routine care group at the 6-month assessment and a greater improvement in flow mediated dilation (3.1%) at 18 months (Figure 2).40 Implementation of successful DASH diet interventions41 into clinical practice could aid in the treatment of hypertension and prevention of BP-related target organ damage in youth.
Figure 2.
Vascular function by Dietary Approaches to Stop Hypertension (DASH) diet intervention group, DASH-4-Teens Study. Abbreviation: AUC, area under the curve. Flow mediated dilation area under the curve60–120 sec = area of the response curve calculated using the Trapezoid rule where the sum of the average between flow mediated dilation 60 and 90 + average between flow mediated dilation 90 and 120 is determined.
The 2017 AAP guideline also recommends counseling to encourage increasing physical activity to 30–60 minutes of moderate to vigorous exercise at least 3 days a week.9 Compared with traditional physical activity programs, high-intensity interval training has been considered as a potentially more effective and time-efficient method of physical activity.42,43 A systematic review of high-intensity interval training interventions found 5 studies that investigated the effect of high-intensity interval training interventions on SBP and DBP in children and adolescents.42 The majority of evaluated studies concluded high-intensity interval training interventions resulted in small but significant improvements in both SBP and DBP postintervention.42 A subsequent study compared the impact of engaging in high-intensity interval training during the first 20 minutes of school physical education classes, 2 days a week for 3 months, to participation in the traditional physical education curriculum.43 School-aged children in the high interval intensity intervention group experienced a statistically significant decrease in SBP and vascular stiffness compared with the control group.43 These studies support the 2017 AAP guideline’s conclusion that any type of exercise may be beneficial for BP reduction and may suggest an option for achieving potent BP reductions with shorter durations of exercise.9
Obesity is a strong risk factor for hypertension among children and adolescents and weight loss has been shown to be beneficial for reducing BP. A randomized control trial examining the impact of engaging in a combined resistance and aerobic exercise training intervention, 3 days a week for 12 weeks found significant improvement in body fat, waist circumference and SBP among obese adolescent girls with prehypertension.44 Among children and adolescents with obesity, Seo et al.45 investigated the impact of a 16-week exercise intervention compared with the standard of care (individual counseling on diet and physical activity) on cardiometabolic risk markers. Participants in the intervention group had a lower body mass index z-score and DBP after the intervention compared with baseline.45 A recent study among obese children in Sweden, showed that reducing body mass index through lifestyle modification was an effective treatment for the majority of children with hypertensive BP levels.46 These studies suggested that treatment of obesity should be a primary avenue for treating obesity-related elevated BP and hypertension.
Pharmacologic treatment
Pharmacologic treatment with antihypertensive medication may be indicated for children and adolescents who are unsuccessful with reducing their BP with lifestyle change, based on severity of hypertension. The 2017 AAP guideline recommended that children and adolescents initiating antihypertensive treatment begin with an angiotensin-converting enzyme (ACE) inhibitor, angiotensin receptor blocker (ARB), long-acting calcium channel blocker, or a thiazide diuretic.9 However, in a recent meta-analysis of randomized control trials of antihypertensive medications with a median age of 12 years, ACE inhibitors and ARBs significantly reduced SBP and DBP more than a placebo while calcium channel blockers and a thiazide diuretic did not.47 ACE inhibitors were also significantly superior at reducing DBP than eplerenone, a mineralocorticoid receptor antagonist.47 ACE inhibitors and ARBs are also the preferred medication classes for treatment initiation among children with chronic kidney disease and diabetic nephropathy.9 These data suggest that ACE inhibitors and ARBs may be the most effective antihypertensive medication for reducing BP in those with pediatric hypertension and could simplify the choice of initial pharmacologic therapy for physicians. Girls who have reached the age of menarche should be counseled on the potential risk of ACE inhibitors and ARBs to a developing fetus during pregnancy.48
In contrast, recent evidence suggests that the best medication for each individual child may vary and a more individualized approach of selecting an initial medication may be appropriate. Samuel et al.49 used a within-patient randomized crossover trial design with an ACE inhibitor (lisinopril), diuretic (hydrochlorothiazide), and calcium channel blocker (amlodipine) to identify each individual’s preferred medication, defined as the treatment that resulted in the largest BP reduction with acceptable side effects. The ACE inhibitor, lisinopril, was the preferred medication for 49% of children treated for hypertension.49 A sizeable proportion of children preferred other classes of antihypertensive medications with 24% of children preferring the calcium channel blocker, amlodipine, and 12% of children preferring the diuretic, hydrochlorothiazide.49 Although the 2017 AAP Clinical Practice Guideline suggests that African American children may require a higher initial dose for an ACE inhibitor or respond better to a thiazide diuretic or calcium channel blocker,9 67% of African Americans in this study achieved the greatest BP reduction with lisinopril.49 Antihypertensive medication trials with larger, more diverse sample are needed to further assess the approach for selecting the most effective initial antihypertensive medications for children and adolescents.
EVIDENCE GAPS
Despite recent research focused on pediatric hypertension, there are several areas where evidence, data, and knowledge remain limited. ABPM is widely recommended for confirming office BP values.9 However, normative ABPM data among children and adolescents are currently limited to values obtained from a small, European population using only one ABPM device model.22 Ambulatory DBP values in this study showed little variation by age and height.22 Normative ABPM data collected among large, racially diverse populations and among children of young ages and short statures are needed to characterize normal ABPM in these populations and increase generalizability.9,22 Given that oscillometric BP devices determine SBP and DBP values from measured mean arterial pressure based on proprietary algorithms, data on variation in SBP and DBP measurements by device brand may also be warranted. Further, evidence for defining ABPM thresholds for elevated daytime, nighttime, and 24 hour BP as well as the associated between these measures and BP load with target organ damage is limited.50
One challenge that continues to face clinicians managing children and adolescents with hypertension is when and who to treat with pharmacologic therapy and how aggressive BP targets should be. With new data suggesting that target organ damage is occurring at a lower BP level that previously anticipated, large, longitudinal studies are needed to strengthen the evidence required to define elevated BP and hypertension in childhood based on levels of BP that lead to target organ damage in childhood and CVD in adulthood as opposed to being statistically defined. There is limited evidence that suggests controlling BP to <90th percentile results in reductions in left ventricular mass index,9 however the impact of initiating treatment at the 90th percentile on target organ damage remains unknown. To clarify the optimal level to initiate medication to reduce target organ damage and the risk for hypertension in adulthood, further studies comparing various treatment initiation and BP goal thresholds in racial diverse populations are needed.
Elevated BP and hypertension in early life increase the risk of cardiovascular target organ damage in youth and hypertension in adulthood. The 2017 AAP Clinical Practice Guideline recommends nutritional and physical activity interventions and reducing obesity as the primary avenues for treating elevated BP and hypertension in childhood. However, at what point a provider should add pharmacologic therapy to the lifestyle regimen to reduce target organ damage and the subsequent risk of CVD remains unclear. Continuing research to fill knowledge gaps in our understanding of pediatric hypertension prevention, detection, classification, and treatment is critical to informing evidence-based clinical practice guidelines.
FUNDING
Dr Hardy receives support through R01HL139716 from the National Heart, Lung, and Blood Institute. Dr Urbina receives support through 15SFRN23680000 from the American Heart Association Strategically Focused Research Network.
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD III, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA III, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, Ezzati M. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med 2009; 6:e1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Muntner P, He J, Cutler JA, Wildman RP, Whelton PK. Trends in blood pressure among children and adolescents. JAMA 2004; 291:2107–2113. [DOI] [PubMed] [Google Scholar]
- 4. Hao G, Wang X, Treiber FA, Harshfield G, Kapuku G, Su S. Blood pressure trajectories from childhood to young adulthood associated with cardiovascular risk: results from the 23-year longitudinal Georgia Stress and Heart Study. Hypertension 2017; 69:435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shen W, Zhang T, Li S, Zhang H, Xi B, Shen H, Fernandez C, Bazzano L, He J, Chen W. Race and sex differences of long-term blood pressure profiles from childhood and adult hypertension: the Bogalusa Heart Study. Hypertension 2017; 70:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Juhola J, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, Srinivasan SR, Daniels SR, Davis PH, Chen W, Kähönen M, Taittonen L, Urbina E, Viikari JSA, Dwyer T, Raitakari OT, Juonala M. Combined effects of child and adult elevated blood pressure on subclinical atherosclerosis: the International Childhood Cardiovascular Cohort Consortium. Circulation 2013; 128:217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jackson SL, Zhang Z, Wiltz JL, Loustalot F, Ritchey MD, Goodman AB, Yang Q. Hypertension among youths—United States, 2001–2016. MMWR Morb Mortal Wkly Rep 2018; 67:758–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics 2018; 141:e20173459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, de Ferranti SD, Dionne JM, Falkner B, Flinn SK, Gidding SS, Goodwin C, Leu MG, Powers ME, Rea C, Samuels J, Simasek M, Thaker VV, Urbina EM. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 2017; 140:e20171904. [DOI] [PubMed] [Google Scholar]
- 10. Kibria GMA, Swasey K, Sharmeen A, Day B. Estimated change in prevalence and trends of childhood blood pressure levels in the United States after application of the 2017 AAP Guideline. Prev Chronic Dis 2019; 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dong Y, Song Y, Zou Z, Ma J, Dong B, Prochaska JJ. Updates to pediatric hypertension guidelines: influence on classification of high blood pressure in children and adolescents. J Hypertens 2019; 37:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018; 71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 13. Song P, Zhang Y, Yu J, Zha M, Zhu Y, Rahimi K, Rudan I. Global prevalence of hypertension in children: a systematic review and meta-analysis. JAMA Pediatr 2019; 173:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hardy ST, Sakhuja S, Jaeger BC, Urbina EM, Suglia S, Feig DI, Muntner P. Trends in blood pressure and hypertension among US children and adolescents, 1999–2018. 2021, submitted for publication. [DOI] [PMC free article] [PubMed]
- 15. Zafarmand MH, Spanjer M, Nicolaou M, Wijnhoven HAH, van Schaik BDC, Uitterlinden AG, Snieder H, Vrijkotte TGM. Influence of Dietary Approaches to Stop Hypertension-type diet, known genetic variants and their interplay on blood pressure in early childhood: ABCD study. Hypertension 2020; 75:59–70. [DOI] [PubMed] [Google Scholar]
- 16. Zhang M, Mueller NT, Wang H, Hong X, Appel LJ, Wang X. Maternal exposure to ambient particulate matter ≤2.5 µm during pregnancy and the risk for high blood pressure in childhood. Hypertension 2018; 72:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bal C, Öztürk A, Çiçek B, Özdemir A, Zararsız G, Ünalan D, Ertürk Zararsız G, Korkmaz S, Göksülük D, Eldem V, İsmailoğulları S, Erdem E, Mazıcıoğlu MM, Kurtoğlu S. The relationship between blood pressure and sleep duration in Turkish children: a cross-sectional study. J Clin Res Pediatr Endocrinol 2018; 10:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sparano S, Lauria F, Ahrens W, Fraterman A, Thumann B, Iacoviello L, Marild S, Michels N, Molnar D, Moreno LA. Sleep duration and blood pressure in children: analysis of the pan-European IDEFICS cohort. J Clin Hypertens 2019; 21:572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zou Y, Xia N, Zou Y, Chen Z, Wen Y. Smartphone addiction may be associated with adolescent hypertension: a cross-sectional study among junior school students in China. BMC Pediatr 2019; 19:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Su S, Wang X, Pollock JS, Treiber FA, Xu X, Snieder H, McCall WV, Stefanek M, Harshfield GA. Adverse childhood experiences and blood pressure trajectories from childhood to young adulthood: the Georgia stress and Heart study. Circulation 2015; 131:1674–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kreatsoulas C, Fleegler EW, Kubzansky LD, McGorrian CM, Subramanian SV. Young adults and adverse childhood events: a potent measure of cardiovascular risk. Am J Med 2019; 132:605–613. [DOI] [PubMed] [Google Scholar]
- 22. Flynn JT, Daniels SR, Hayman LL, Maahs DM, McCrindle BW, Mitsnefes M, Zachariah JP, Urbina EM; American Heart Association Atherosclerosis, Hypertension and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young . Update: ambulatory blood pressure monitoring in children and adolescents: a scientific statement from the American Heart Association. Hypertension 2014; 63:1116–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lurbe E, Torró I, Álvarez J, Aguilar F, Mancia G, Redon J, Redon P. Impact of ESH and AAP hypertension guidelines for children and adolescents on office and ambulatory blood pressure-based classifications. J Hypertens 2019; 37:2414–2421. [DOI] [PubMed] [Google Scholar]
- 24. Hamdani G, Flynn JT, Daniels S, Falkner B, Hanevold C, Ingelfinger J, Lande MB, Martin LJ, Meyers KE, Mitsnefes M, Rosner B, Samuels J, Urbina EM. Ambulatory blood pressure monitoring tolerability and blood pressure status in adolescents: the SHIP AHOY study. Blood Press Monit 2019; 24:12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peterson CG, Miyashita Y. The use of ambulatory blood pressure monitoring as standard of care in pediatrics. Front Pediatr 2017; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hanevold CD, Faino AV, Flynn JT. Use of automated office blood pressure measurement in the evaluation of elevated blood pressures in children and adolescents. J Pediatr 2020; 227:204–211.e6. [DOI] [PubMed] [Google Scholar]
- 27. Ardissino G, Ghiglia S, Salice P, Perrone M, Piantanida S, De Luca FL, Di Michele S, Filippucci L, Dardi ERA, Bollani T, Mezzopane A, Tchane B, Lava SAG; SPA Project investigators . Multiple office blood pressure measurement: a novel approach to overcome the weak cornerstone of blood pressure measurement in children. Data from the SPA project. Pediatr Nephrol 2020; 35:687–693. [DOI] [PubMed] [Google Scholar]
- 28. Zeniodi ME, Ntineri A, Kollias A, Servos G, Moyssakis I, Destounis A, Harokopakis A, Vazeou A, Stergiou GS. Home and ambulatory blood pressure monitoring in children, adolescents and young adults: comparison, diagnostic agreement and association with preclinical organ damage. J Hypertens 2020; 38:1047–1055. [DOI] [PubMed] [Google Scholar]
- 29. Stergiou GS, Nasothimiou EG, Giovas PP, Rarra VC. Long-term reproducibility of home vs. office blood pressure in children and adolescents: the Arsakeion school study. Hypertens Res 2009; 32:311–315. [DOI] [PubMed] [Google Scholar]
- 30. Yazdi M, Assadi F, Daniali SS, Heshmat R, Mehrkash M, Motlagh ME, Qorbani M, Kelishadi R. Performance of modified blood pressure-to-height ratio for diagnosis of hypertension in children: the CASPIAN-V study. J Clin Hypertens 2020; 22:867–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yin X, Liu Q, Bovet P, Ma C, Xi B. Performance of blood pressure-to-height ratio as a screening tool for elevated blood pressure in pediatric population: a systematic meta-analysis. J Hum Hypertens 2016; 30:697–702. [DOI] [PubMed] [Google Scholar]
- 32. Mourato FA, Mattos SS, Lima Filho JL, Mourato MF, Nadruz W Jr. Height-based equations can improve the diagnosis of elevated blood pressure in children. Am J Hypertens 2018; 31:1059–1065. [DOI] [PubMed] [Google Scholar]
- 33. Theodore RF, Broadbent J, Nagin D, Ambler A, Hogan S, Ramrakha S, Cutfield W, Williams MJ, Harrington H, Moffitt TE, Caspi A, Milne B, Poulton R. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension 2015; 66:1108–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Urbina EM, Mendizábal B, Becker RC, Daniels SR, Falkner BE, Hamdani G, Hanevold C, Hooper SR, Ingelfinger JR, Lanade M, Martin LJ, Meyers K, Mitsnefes M, Rosner B, Samuels J, Flynn JT. Association of blood pressure level with left ventricular mass in adolescents. Hypertension 2019; 74:590–596. [DOI] [PubMed] [Google Scholar]
- 35. Yang L, Magnussen CG, Yang L, Bovet P, Xi B. Elevated blood pressure in childhood or adolescence and cardiovascular outcomes in adulthood: a systematic review. Hypertension 2020; 75:948–955. [DOI] [PubMed] [Google Scholar]
- 36. Whelton SP, McEvoy JW, Shaw L, Psaty BM, Lima JAC, Budoff M, Nasir K, Szklo M, Blumenthal RS, Blaha MJ. Association of normal systolic blood pressure level with cardiovascular disease in the absence of risk factors. JAMA Cardiol 2020; 5:1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tran AH, Flynn JT, Becker RC, Daniels SR, Falkner BE, Ferguson M, Hanevold CD, Hooper SR, Ingelfinger JR, Lande MB, Martin LJ, Meyers K, Mitsnefes M, Rosner B, Samuels JA, Urbina EM. Subclinical systolic and diastolic dysfunction is evident in youth with elevated blood pressure. Hypertension 2020; 75:1551–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang L, Sun J, Zhao M, Liang Y, Bovet P, Xi B. Elevated blood pressure in childhood and hypertension risk in adulthood: a systematic review and meta-analysis. J Hypertens 2020; 38:2346–2355. [DOI] [PubMed] [Google Scholar]
- 39. Urbina EM, Khoury PR, Bazzano L, Burns TL, Daniels S, Dwyer T, Hu T, Jacobs DR Jr, Juonala M, Prineas R, Raitakari O, Steinberger J, Venn A, Woo JG, Sinaiko A. Relation of blood pressure in childhood to self-reported hypertension in adulthood. Hypertension 2019; 73:1224–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Couch SC, Saelens BE, Khoury PR, Dart KB, Hinn K, Mitsnefes MM, Daniels SR, Urbina EM. Dietary approaches to stop hypertension dietary intervention improves blood pressure and vascular health in youth with elevated blood pressure. Hypertension 2021; 77:241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Paula Bricarello L, Poltronieri F, Fernandes R, Retondario A, de Moraes Trindade EBS, de Vasconcelos FAG. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on blood pressure, overweight and obesity in adolescents: a systematic review. Clin Nutr ESPEN 2018; 28:1–11. [DOI] [PubMed] [Google Scholar]
- 42. Eddolls WTB, McNarry MA, Stratton G, Winn CON, Mackintosh KA. High-intensity interval training interventions in children and adolescents: a systematic review. Sports Med 2017; 47:2363–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ketelhut S, Kircher E, Ketelhut SR, Wehlan E, Ketelhut K. Effectiveness of multi-activity, high-intensity interval training in school-aged children. Int J Sports Med 2020; 41:227–232. [DOI] [PubMed] [Google Scholar]
- 44. Son WM, Sung KD, Bharath LP, Choi KJ, Park SY. Combined exercise training reduces blood pressure, arterial stiffness, and insulin resistance in obese prehypertensive adolescent girls. Clin Exp Hypertens 2017; 39:546–552. [DOI] [PubMed] [Google Scholar]
- 45. Seo YG, Lim H, Kim Y, Ju YS, Lee HJ, Jang HB, Park SI, Park KH. The effect of a multidisciplinary lifestyle intervention on obesity status, body composition, physical fitness, and cardiometabolic risk markers in children and adolescents with obesity. Nutrients 2019; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hagman E, Danielsson P, Elimam A, Marcus C. The effect of weight loss and weight gain on blood pressure in children and adolescents with obesity. Int J Obes 2019; 43:1988–1994. [DOI] [PubMed] [Google Scholar]
- 47. Burrello J, Erhardt EM, Saint-Hilary G, Veglio F, Rabbia F, Mulatero P, Monticone S, D’Ascenzo F. Pharmacological treatment of arterial hypertension in children and adolescents: a network meta-analysis. Hypertension 2018; 72:306–313. [DOI] [PubMed] [Google Scholar]
- 48. Bullo M, Tschumi S, Bucher BS, Bianchetti MG, Simonetti GD. Pregnancy outcome following exposure to angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists: a systematic review. Hypertension 2012; 60:444–450. [DOI] [PubMed] [Google Scholar]
- 49. Samuel JP, Tyson JE, Green C, Bell CS, Pedroza C, Molony D, Samuels J. Treating hypertension in children with n-of-1 trials. Pediatrics 2019; 143:e20181818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hamdani G, Ferguson MA, Lande MB, Meyers K, Mitsnefes M, Samuels JA, Flynn JT, Urbina EM. Abstract 6: comparison between ambulatory BP percentile and load as predictors of target organ damage in youth. Hypertension 2020; 76:A6. [Google Scholar]