Abstract
Objective:
Temporalis fascia is a commonly used graft material in tympanoplasty; however, little is known about how the histological structure of fascia remodels postimplantation. Herein, we aim to quantify the pre- and postoperative micro-structure of temporalis fascia and compare histological findings to the native tympanic membrane (TM).
Methods:
Temporal bone specimens having undergone successful subtotal or total drum replacement using temporalis fascia were identified (n = 3). Surgically prepared preimplantation temporalis fascia (PreTF, n = 4) and normal TMs (n = 5) were used as controls. Multiple measurements of thickness of PreTF and of normal and fascia reconstructed TMs at the meso-tympanum and hypotympanum were obtained. Collagen fiber patterns of normal and reconstructed TMs were histologically described.
Results:
In cases of fascia tympanoplasty, the mean time of surgery to death was 16 years (range 8–28 years). All cases contained an aerated middle ear without residual perforation. There was no significant difference between the thickness of PreTF and fascia of reconstructed TMs (234.9 ± 144.9 μm vs. 162.9 ± 71.9 μm, P = 0.1). The lamina propria and total thicknesses of controls (59.8 ± 39.3 μm and 83.7 ± 42.4 μm, respectively) were thinner than the PreTF and fascia-reconstructed TMs, respectively, in all cases (P ≤ 0.001, P ≤ 0.001). Reconstructed TMs contained a thick, longitudinal fiber structure that was qualitatively similar to PreTF.
Conclusion:
Based on human temporal bone specimens, temporalis fascia does not significantly remodel, change thickness, or change fibrous structure following successful tympanoplasty. Results have implications for selection and surgical preparation of graft materials in TM reconstruction.
Keywords: Tympanoplasty, temporalis fascia, otopathology
INTRODUCTION
The goal of tympanoplasty is to effectively reconstruct the tympanic membrane (TM), thereby reestablishing a barrier to the middle ear while also improving conductive hearing. Since the introduction of medial and lateral graft tympanoplasty 60 years ago,1,2 techniques in the surgical reconstruction of the TM have not significantly changed. Autologous materials, such as temporalis muscle fascia, perichondrium, and cartilage, are routinely used as tissue grafts to reconstruct the TM. Despite being the standard of care, tympanoplasty with autologous grafts has variable outcomes and may fail in up to 20% of patients, leaving them with a persistent perforation.3–5 Hearing outcomes after tympanoplasty are also variable. In two recent large reviews of type I tympanoplasty outcomes, persistent conductive hearing loss greater than 10 decibels (dB) was found in 60% of patients postoperatively.5,6 Although the cause of graft failure and persistent conductive hearing loss in tympanoplasty is multifactorial, the structure of the reconstructed TM at the time of surgery is known to play a critical role in the postoperative healing and hearing result.6
The structural characteristics of the native TM are critical for its successful performance. The normal TM is 50 to 70 μm thin and composed of radial and circumferential collagen fibers that provide both mechanical stability and effective sound conduction.7,8 Thin TMs are also known to be acoustically favorable to thick TMs given that sound-induced vibration is negatively affected by thickening the eardrum.9 Taken together, the goal of TM reconstruction should be to, as best possible, recapitulate the structural properties of the healthy human TM in both fiber arrangement and thickness parameters. This requires both preoperative attention to graft structural characteristics and consideration of postoperative remodeling of TM graft materials.
Temporalis fascia has been the primary autologous graft material used in medial and lateral graft tympanoplasty given its accessibility relative to the surgical site and relative ease of manipulation for surgical placement. The architecture and collagen composition of temporalis fascia has been extensively studied ex vivo,10–13 demonstrating thick, linear, type I collagen fibers14 within both the superficial and deep temporalis fascia. Following tympanoplasty, autologous graft materials theoretically become incorporated into the remnant native TM.15 Animal studies have shown that autologous fascia incorporates into the lamina propria of the TM, with relative preservation of the pre-implanted fascia structure at 1 year.15
Despite being the standard of care for more than half a century, little is known about how temporalis fascia grafts incorporate and remodel following tympanoplasty in humans. In this study, we investigate otopathologic cases of successful temporalis fascia tympanoplasty to understand how autologous tissue grafts incorporate postimplantation. We hypothesize that the preoperative structure of temporalis fascia grafts will not significantly change in thickness or fibrous structure following implantation. A knowledge of how grafts remodel postoperatively may help surgeons better prepare grafts before implantation and may also influence the design of tissue-engineered TM replacements.
MATERIALS AND METHODS
A search of the National Temporal Bone, Hearing and Balance Pathology Resource Registry16 was performed to identify cases of patients having undergone temporalis fascia tympanoplasty for subtotal or total perforations during life (fascia-reconstructed TMs). Inclusion criteria was successful fascia tympanoplasty with an aerated middle ear. Successful was defined as closure of the perforation postoperatively, with an intact TM at the time of death. Cases were excluded if there was evidence of external ear canal removal (canal wall down mastoidectomy), perforation of the TM, middle ear effusion, cholesteatoma, clinical history of persistent otorrhea, or if the graft material utilized was not solely temporalis fascia. Temporal bone specimens in which the TM had not been entirely preserved, either due to removal or preparation artifact, were also excluded. Fascia reconstructed cases were compared to 1) age-matched temporal bone specimens without a history of middle ear disease or tympanic membrane surgery (normal TM controls); and 2) temporalis fascia grafts, which were harvested from human patients and surgically prepared but not implanted (PreTF controls).
Temporal bones were removed fresh at autopsy and prepared according to methods previously described.17 In brief, the histopathologic process included fixation (in 10% buffered formalin), decalcification in ethylenediaminetetraacetic acid, embedment in celloidin, sectioning in the axial plane at 20 μm thickness, and then staining every tenth section with hematoxylin and eosin (H&E). Temporalis fascia (PreTF controls) was harvested from surgical patients, flattened in a fascia press, and prepared as if to be used as a TM graft—but instead was embedded in paraffin, sectioned, and stained with H&E. All specimens are publicly available for review through the National Temporal Bone Registry (tbregistry.org).
Analysis of Structure and Thickness
Specimens were evaluated by light microscopy, with special attention to the fibrous structure, thickness, and composition of the lamina propria within fascia reconstructed and normal TMs. Identical evaluation of the collagen structure was performed in PreTF controls. Image J software (http://rsbweb.nih.gov/ij/) was used to measure the thickness of the epithelial layer (distance from basement membrane of epithelium to ear canal air/specimen interface) and mucosal layer (distance from inner base of cuboidal mucosal cells to middle ear air/specimen interface) of fascia reconstructed and normal TMs, as well as the total thickness of all specimens. To objectively and reproducibly measure TM thickness, two measurements each were taken on the anterior and posterior TM, located 0.5 to 1 mm from the junction of the bony ear canal and TM in the mesotympanic and hypotympanic TM (Fig. 1). This provided a total of four measurements in both reconstructed and normal TMs. Four consecutive measurements at defined locations were made on PreTF controls.
Fig. 1.
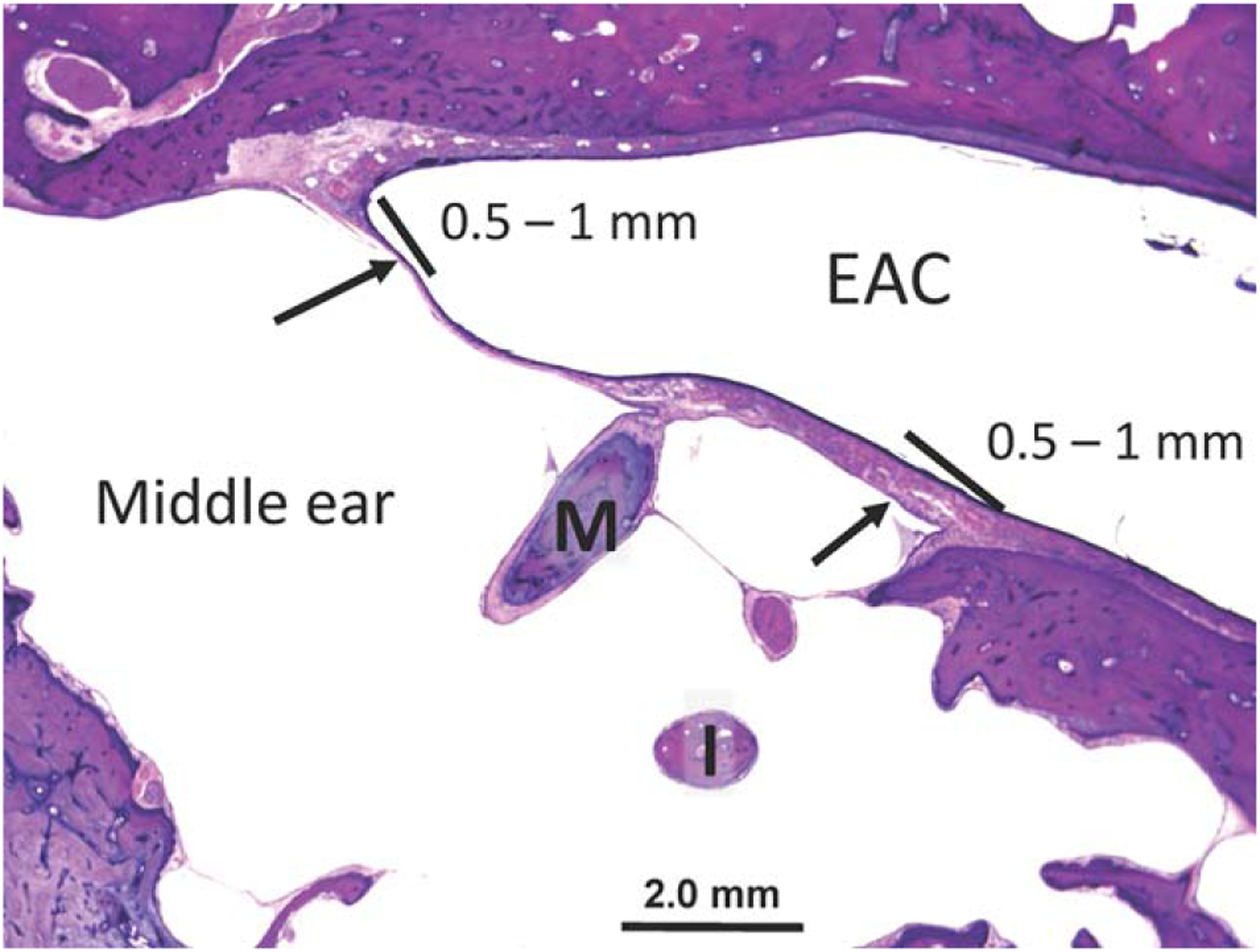
Normal tympanic membrane. The thickness of both native and reconstructed TMs was measured in the anterior and posterior portion of the TM, located 0.5 to 1 mm from the junction with the bony ear canal (arrows). Each of these measurements was taken within the mesotympanic (shown here) and hypotympanic sections of the TM for a total of four measurements.
EAC = external auditory canal; I = incus; M = malleus; TM = tympanic membrane.
Statistical Analysis
All values are reported as the mean and standard deviation (SD). Student t test (two-tailed, unpaired) was used to compare measurements between two groups with significance set at P < 0.05. Correlations between quantitative variables were analyzed with Pearson’s correlation coefficient and bivariate analysis.
A post-hoc power analysis was performed using a clinically significant difference in TM thickness of at least 50 μm. This value is based on previous finite element models of TM thickness, which show that increasing the TM from 50 μm to 100 μm results in a significant decrease in stapes velocity.18 Significance level was set at an α = 0.05, and power was set at β = 0.8. A minimum of seven total samples was deemed necessary to detect clinically significant changes in TM thickness of reconstructed eardrums.
RESULTS
Review of the temporal bone bank demonstrated 16 patients who had undergone tympanoplasty in life. Of these, 13 were excluded for nonaerated middle ear, failure of graft, nontemporalis fascia graft, or canal wall down procedure, leaving three ears for analysis. Each underwent a successful temporalis fascia tympanoplasty and possessed an aerated middle ear and mastoid at the time of otopathologic analysis. Demographic information about the patients at the time of surgery and death is available in Table I.
TABLE I.
Patient and Tympanic Membrane Characteristics for All Specimens.
| Patient no. | Laterality | Age at Tympanoplasty (yr) | Age at Death (yr) | Duration of Reconstructed TM (yr) | Mean Specimen Thickness (μm) | Standard Deviation (μm) |
|---|---|---|---|---|---|---|
| Temporalis Fascia Reconstructed Tympanic Membrane Cases | ||||||
| 1 | Left | 11 | 19 | 8 | 170.7* | 52.5 |
| 2 | Left | 59 | 87 | 28 | 142.5 | 76.0 |
| 3 | Right | 50 | 62 | 12 | 266.0 | 5.8 |
| Mean | 16 | 195.1 | 74.8 | |||
| Preimplantation Temporalis Fascia Controls | ||||||
| 1 | 219.5 | 61.2 | ||||
| 2 | 144.5 | 40.6 | ||||
| 3 | 467.7 | 171.7 | ||||
| 4 | 166.3 | 35.7 | ||||
| Mean | 234.9 | 144.9 | ||||
| Normal Tympanic Membrane Controls | ||||||
| 1 | Left | 30 | 46.8 | 3.0 | ||
| 2 | Right | 90 | 93.5 | 53.8 | ||
| 3 | Right | 89 | 67.2 | 13.2 | ||
| 4 | Right | 77 | 84.5 | 56.8 | ||
| 5 | Left | 77 | 126.3 | 19.3 | ||
| Mean | 83.7 | 42.4 | ||||
In patient 1, the anterior angle within the mesotympanic section of the reconstructed TM was significantly thickened, consistent with postoperative blunting. Therefore, measurements from this area of the TM were excluded so as not to bias the results.
TM = tympanic membrane.
Temporalis Fascia Reconstructed Tympanic Membrane Cases
All three cases underwent a subtotal or total drum replacement using temporalis fascia, allowing measurement of the reconstructed TM in all quadrants using the above described techniques. Given the available clinical history, there was no mention of ongoing otologic problems following tympanoplasty for included fascia reconstructed cases. The mean duration that the patients lived with their reconstructed TMs was 16 years (range 8–28 years).
Fascia Reconstructed TM Case 1
The specimen is from a 19-year-old male who suffered from recurrent bilateral acute otitis media and hearing loss during childhood. Audiometric testing at age 10 showed speech reception thresholds (SRT) of 70 dB on the right and 88 dB on the left, and otoscopic examination revealed perforations of both TMs. One year later, the patient underwent bilateral type I tympanoplasty with subtotal underlay of temporalis fascia grafts. Follow-up testing ~5 years later showed mixed hearing loss on the right and sensorineural hearing loss on the left, with no significant changes in SRTs.
Histological analysis of the specimens showed a fully reconstructed and healed left TM (Fig. 2A), but there was a persistent perforation on the right ear. Only the left ear was included in this study. The left fascia-reconstructed TM appeared significantly thickened at the anterior tympanomeatal angle, consistent with postoperative blunting; however, it was also thickened posterior to the malleus. Figure 2B shows a higher magnification image of the temporalis fascia graft material comprising the lamina propria of the reconstructed TM. The average thickness of this reconstructed TM was 170.7 μm, with a SD of 52.5 μm. The majority of the TM was comprised of the fascia graft (79% ± 6% of thickness of TM). A uniform, single monolayer of linear fibers is observed within the lamina propria. The average epithelial layer thickness was 19.0 ± 9.5 μm (11% ± 6% of thickness of TM), and the average mucosal layer thickness was 17.3 ± 5.5 μm (10% ± 3% of thickness of TM).
Fig. 2.
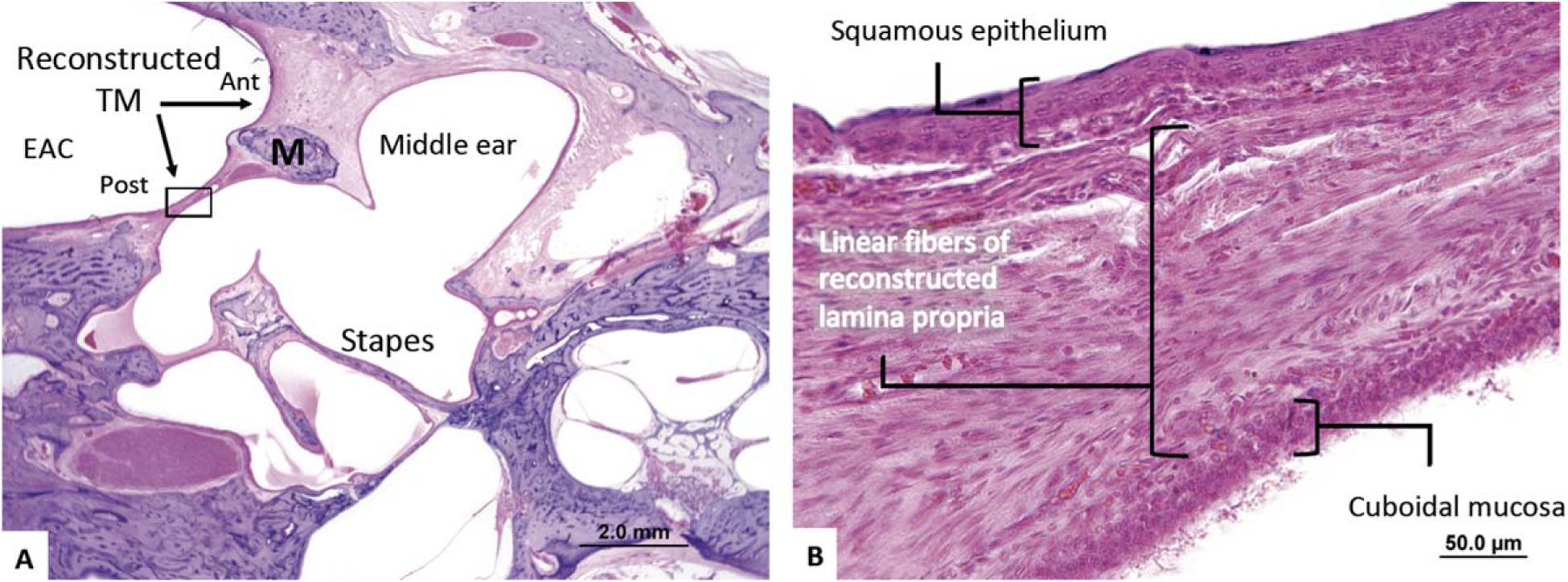
A 19-year-old patient underwent a successful left temporalis fascia total drum replacement. (A) The TM is blunted anteriorly and is thickened posteriorly where linear fibers representing the temporalis fascia graft can be visualized. (B) The reconstructed lamina propria is thickened and contains exclusively linear fibers.
Ant = anterior; EAC = external auditory canal; M = malleus; Post = posterior; TM = tympanic membrane.
Fascia Reconstructed TM Case 2
The specimen comes from an 87-year-old female with progressive bilateral hearing loss that started in her 50s. Otoscopic examination revealed retracted TMs bilaterally, and pretreatment audiometry showed air conduction thresholds of 40 to 55 dB on the right and 55 to 60 dB on the left. The patient underwent left-sided type III, minor columella tympanoplasty at 59 years of age, which included incus repositioning and subtotal underlay of a temporalis fascia graft. A similar procedure was subsequently performed on the right side. Postoperative audiometry demonstrated improvements in air conduction thresholds of 15 to 30 dB on the left and 5 to 10 dB on the right.
Histological analysis showed intact TMs bilaterally, with a nonaerated tympanomastoid compartment on the right and a well-aerated compartment on the left (Fig. 3A). The left side was included in this study. The fascia-reconstructed TM demonstrates mild retraction throughout the grafted pars tensa and pars flaccida. Multiple areas of the native, unreconstructed TM (anterior) have become thin and dimeric, whereas the reconstructed TM (posterior) is thickened and contains a monolayer lamina propria with linear fibrous appearance. The manubrium is intact, whereas the incus has been surgically removed and replaced with an autograft ossicle strut between the stapes head and the grafted TM. A high-resolution image of the fascia-reconstructed TM (Fig. 3B) shows the graft forming the lamina propria with a preserved monolayer of linear fibers. The average thickness of this reconstructed drum was 142.5 μm, with a SD of 76.0 μm; the majority of the TM was comprised of the fascia graft (76% ± 7% of thickness of TM), with an average epithelial layer thickness of 16.8 ± 4.0 μm (12% ± 3% of thickness of TM) and an average mucosal layer thickness of 14.0 ± 6.4 μm (10% ± 4% of thickness of TM).
Fig. 3.
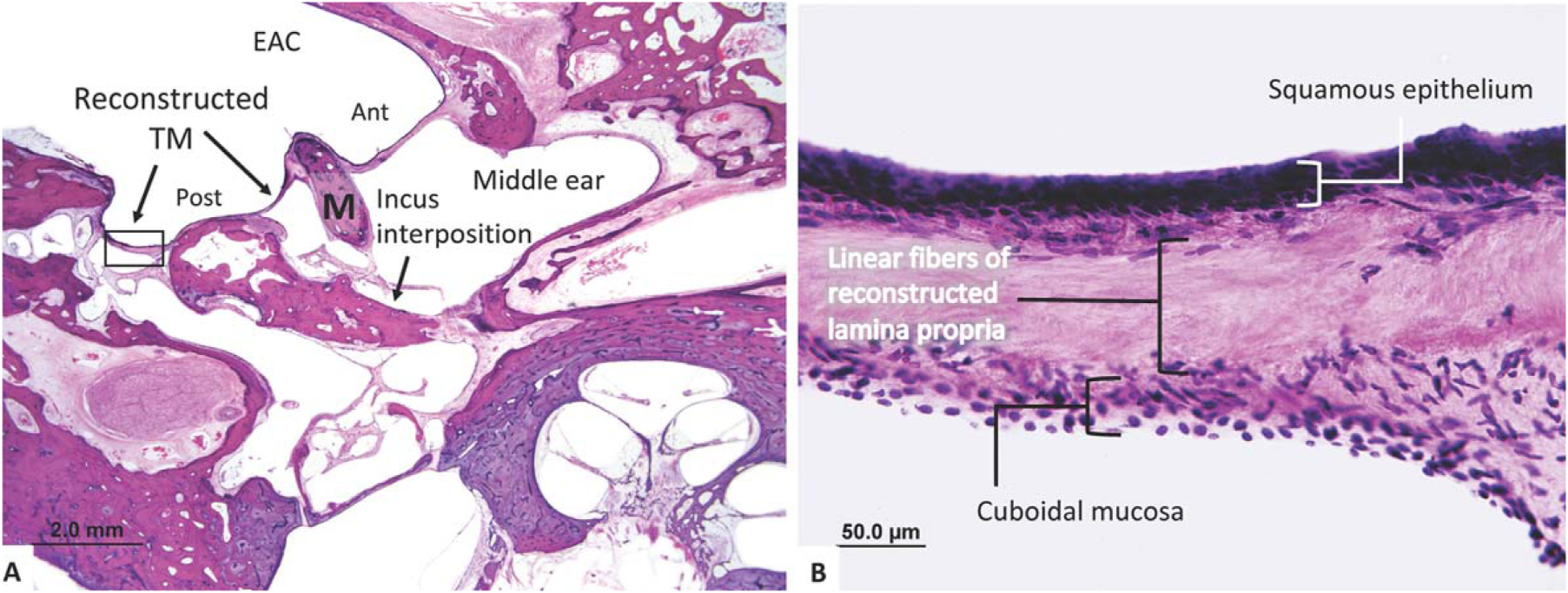
An 87-year-old patient underwent a left temporalis fascia repair of a subtotal perforation with incus interposition ossiculoplasty. (A) The reconstructed TM sits over the body of the incus posteriorly, whereas the native TM remains anteriorly. (B) A high-power view of the reconstructed TM demonstrates a thickened lamina propria with primarily a linear fiber arrangement. This was found throughout the reconstructed TM.
Ant = anterior; EAC = external auditory canal; M = malleus; Post = posterior; TM = tympanic membrane.
Fascia Reconstructed TM Case 3
The specimen is from a 62-year-old male who suffered from bilateral chronic otitis media. At age 50, the patient underwent right-sided intact canal wall tympanomastoidectomy procedure. The procedure involved the removal of an attic cholesteatoma and placement of a silastic sheet, a cartilage ossicular prosthesis, and a temporalis fascia reconstruction of the TM. Audiometric data was available just before death and showed a persistent air–bone gap of 30 to 40 dB.
Histological analysis of the right temporal bone showed an intact reconstructed TM composed of a uniformly thick temporalis fascia graft (Fig. 4A). The malleus, incus, and crural arch of the stapes are missing, but the stapes footplate is intact. Figure 4B shows a thickened drum with uniform, linear collagen fibers comprising a monolayer lamina propria of the reconstructed TM. The average thickness of this reconstructed drum was 266.0 μm, with a SD of 5.8 μm; the majority of the TM was comprised of the fascia graft (89% ± 1% of thickness of TM), with an average epithelial layer thickness of 20.0 ± 4.1 μm (8% ± 2% of thickness of TM) and an average mucosal layer thickness of 10.5 ± 2.4 μm (4% ± 1% of thickness of TM).
Fig. 4.
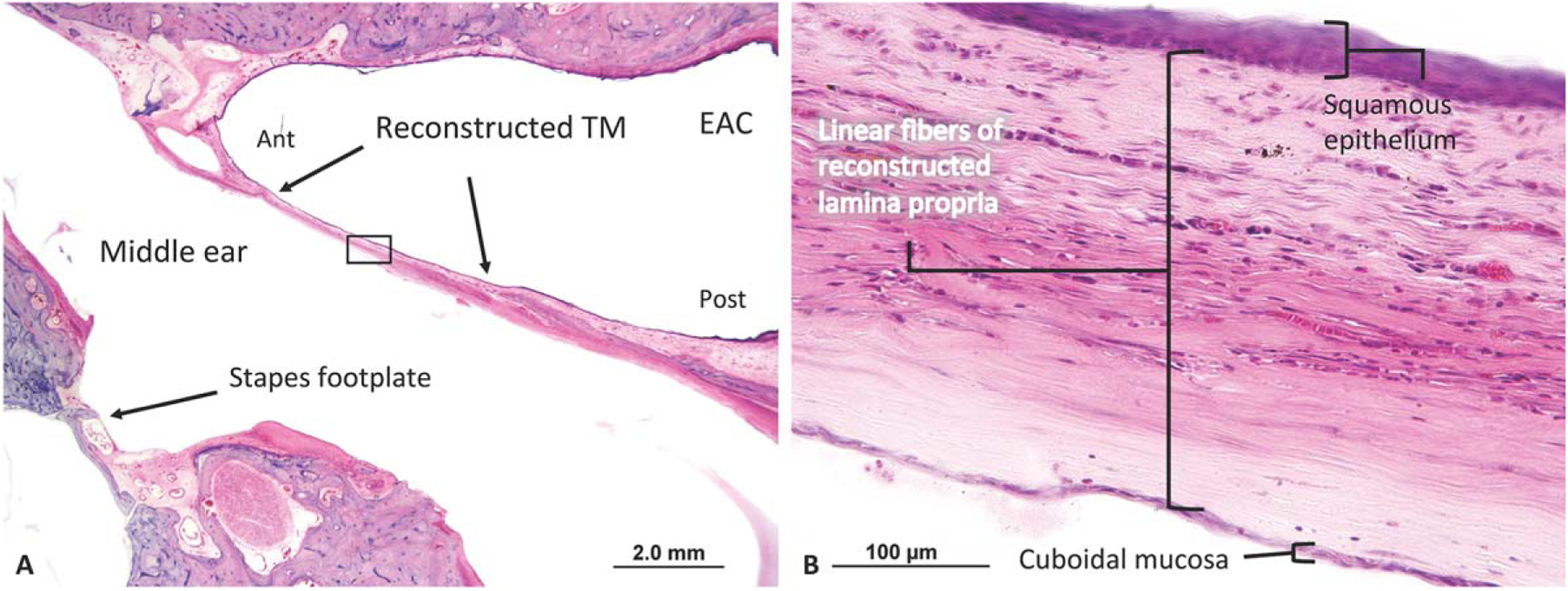
A 62-year-old patient underwent a right total drum replacement with temporalis fascia with cartilage ossiculoplasty. (A) The TM does not demonstrate blunting but is uniformly thickened anteriorly and posteriorly. (B) A high-power view of the reconstructed TM clearly demonstrates thick linear fibers.
Ant = anterior; EAC = external auditory canal; Post = posterior; TM = tympanic membrane.
Temporalis Fascia Thickness Does Not Change Significantly Following Implantation
To investigate structural change in temporalis fascia following successful tympanoplasty, we compared the average thickness of PreTF controls to implanted fascia in reconstructed TM cases. PreTF specimens had a mean thickness of 234.9 ± 144.9 μm (Table I). When compared to the lamina propria of reconstructed TM cases (162.9 ± 71.9 μm), no significant difference between PreTF and reconstructed lamina propria was identified (P = 0.1). We did not observe thickening of the outer keratinizing squamous epithelium or inner cuboidal mucosa in any of the examined cases (Figs. 2–4). In case 1, the anterior angle of the reconstructed TM was significantly thickened (Fig. 2), consistent with postoperative blunting. Measurements from this area of the TM were excluded so as not to bias the results. Finally, there was no significant association between the duration of implantation and thickness of implanted temporalis fascia grafts (r = −0.53, P = 0.64).
Temporalis Fascia Fiber Orientation Does Not Change Significantly Following Implantation
Figure 5 shows the collagen fibrous structure of harvested, temporalis fascia explants. Throughout the tissue, there is a uniform pattern of thick, linear collagen fibers. The structure of temporalis fascia in all three reconstructed TM cases exhibited a similar qualitative fiber orientation to that of native temporalis fascia. There is clear persistence of a linear monolayer collagen fiber orientation without evidence of bilayer or multidirectional fibrous architecture (Figs. 2B, 3B, 4B).
Fig. 5.
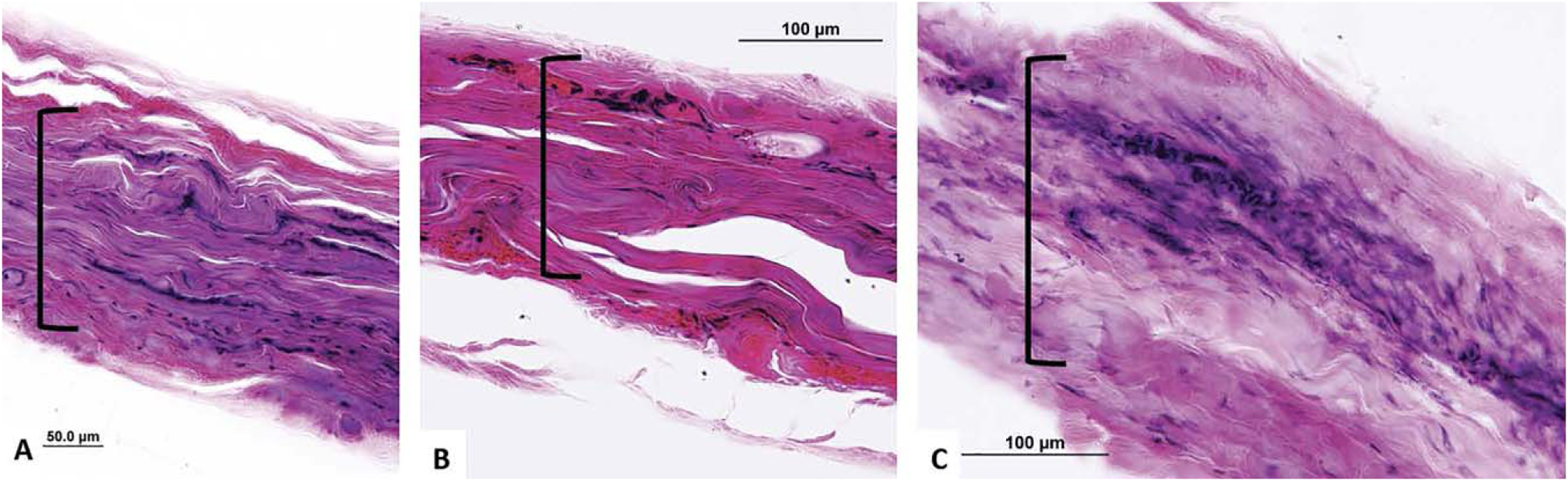
Surgically prepared temporalis fascia demonstrates typical linear fiber arrangement ([) (A–C) with thickness similar to reconstructed tympanic membranes.
Temporalis Fascia Reconstructed TM Cases Are Thicker Than Normal TM Controls
Using above-described measurement techniques, the mean thickness of native (normal) TMs (Fig. 6) was 83.7 ± 42.4 μm (Table I), with an average epithelial layer thickness of 16.0 ± 6.4 μm, lamina propria thickness of 59.8 ± 39.3 μm, and mucosal layer thickness of 9.6 ± 3.2 μm. The lamina propria of normal TMs was significantly thinner than the mean thickness of harvested PreTF specimens (P < 0.001), and the total thickness of normal TMs was significantly thinner than the mean thickness of fascia reconstructed TM cases (P < 0.001). This difference was consistent across all specimens; every native TM was thinner than each reconstructed TM and PreTF control specimen in both temporalis fascia comparison groups (Table I) (Fig. 7). Additionally, the relative ratios of tissue layers within temporalis fascia reconstructed TMs differed from those within native TMs. The middle fascia layer comprised a significantly greater portion of reconstructed TMs compared to the middle lamina propria layer of native TMs (mean 81% ± 7% vs. 67% ± 11% of TM thickness, respectively; P < 0.001). Both epithelial and mucosal layers also comprised significantly smaller portions of reconstructed TMs (mean 10% ± 4% and 8% ± 4% of TM thickness, respectively) compared to native TMs (18% ± 7% and 13% ± 6% of TM thickness, respectively) (P < 0.001, P < 0.02, respectively).
Fig. 6.
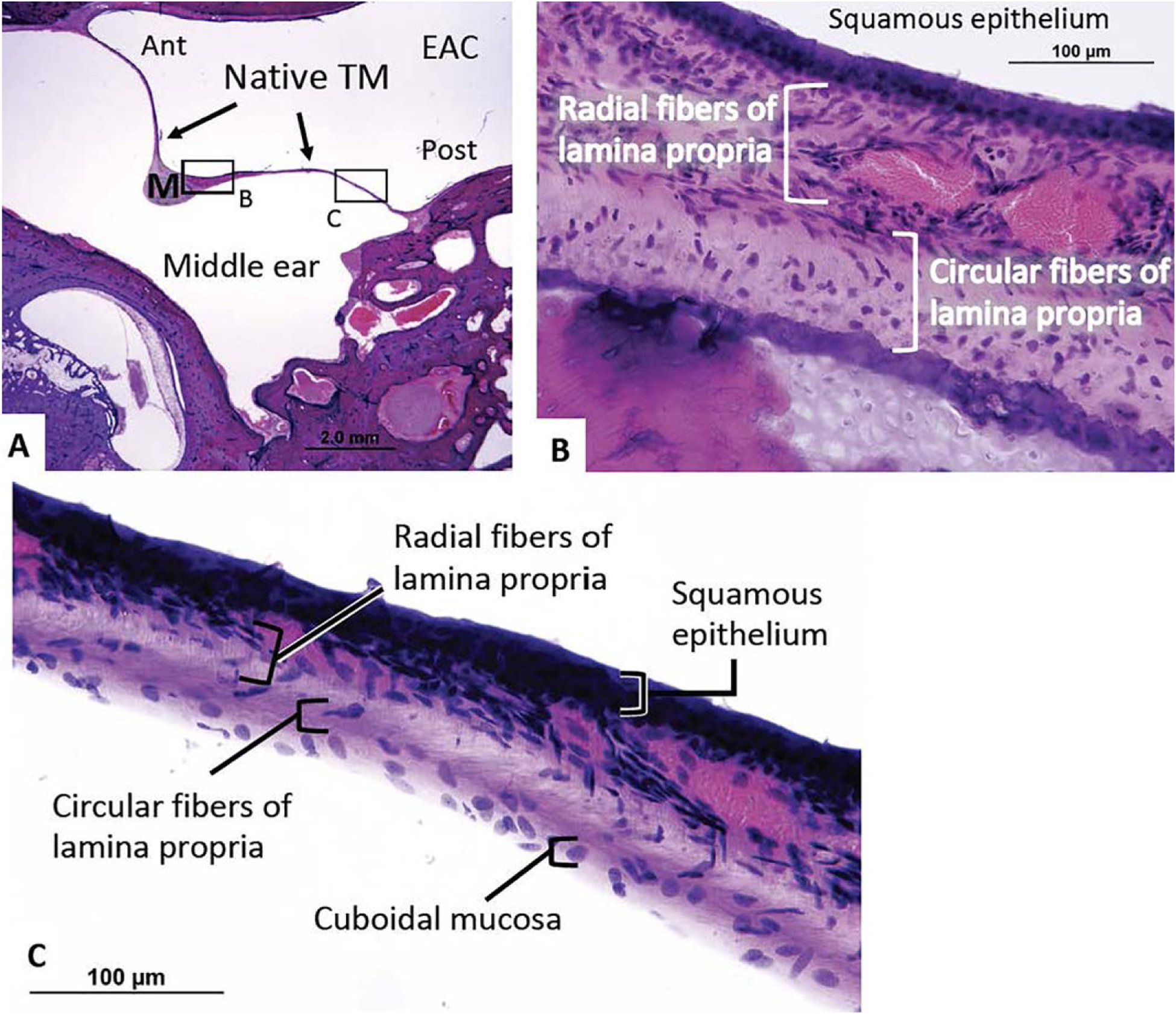
(A) A representative healthy left TM from a 77-year-old patient without history of middle ear disease or surgery. (B) There is a clear pattern of outer radial fibers and inner circular fibers within the lamina propria. (C) This thin radial and circular fiber pattern is easily seen throughout the TM.
Ant = anteriorly; EAC = external auditory canal; M = malleus; Post = posteriorly; TM = tympanic membrane.
Fig. 7.
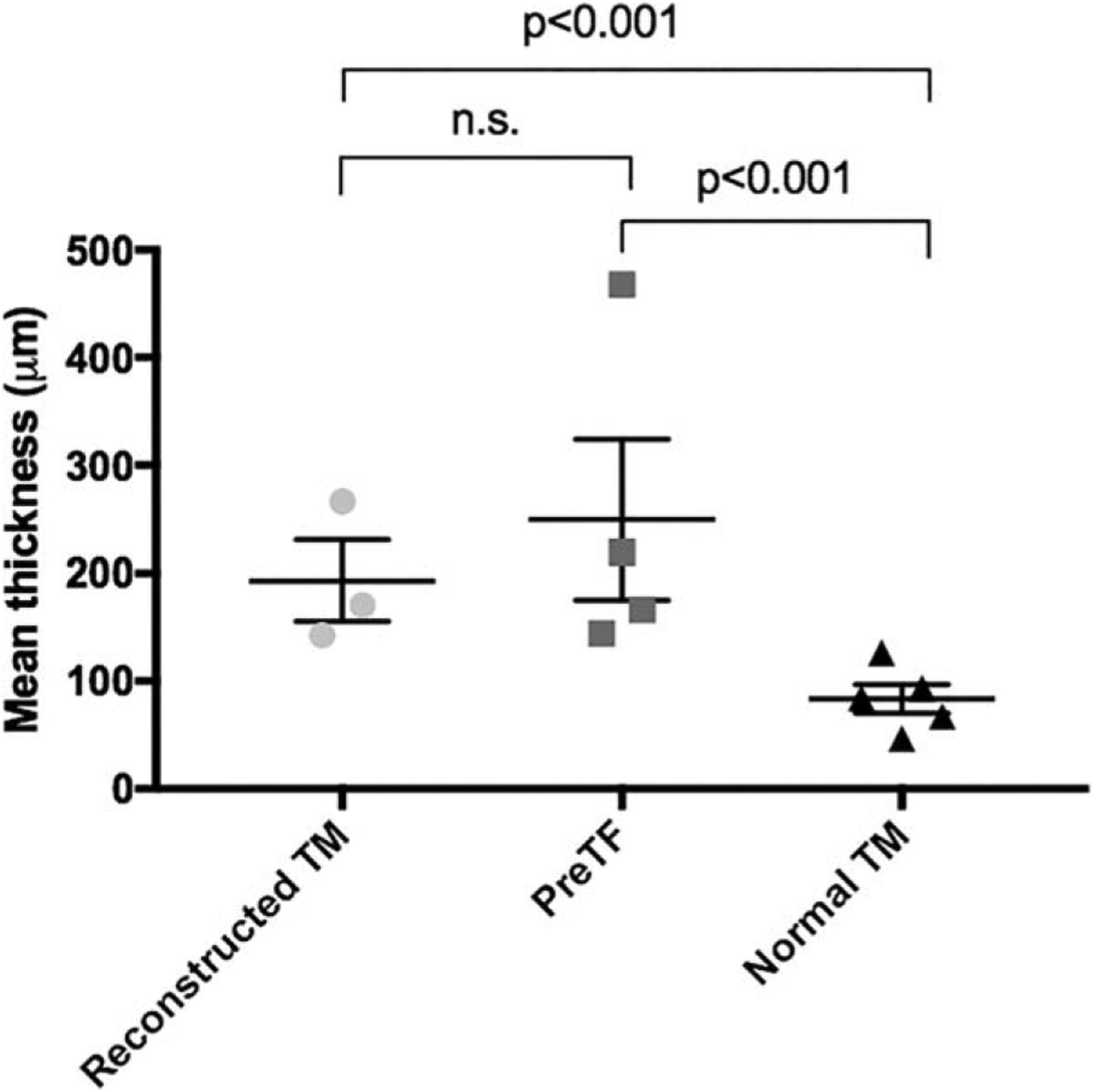
Comparison of the mean thickness of specimens from temporalis fascia reconstructed TMs (light gray circles), temporalis fascia explants (dark gray squares), and normal TMs (black triangles). Error bars represent standard errors of the mean. Whereas the mean thicknesses of reconstructed TMs and PreTF specimens are comparable, both reconstructed TMs and PreTF specimens are significantly thicker than normal TMs (P < 0.001, P < 0.001, respectively).
n.s. = not significant; PreTF = pre-implantation temporalis fascia; TM = tympanic membrane.
DISCUSSION
This study is the first to investigate the histology of temporalis fascia grafts following tympanoplasty in humans. In three cases of successful tympanoplasty, we find that fascia reconstructed TMs appear histologically similar in thickness and linear fiber structure to surgically prepared, preimplanted temporalis fascia. This would imply that fascia grafts do not significantly remodel or change in thickness following successful tympanoplasty. Follow-up duration was on average 16 years, thus indicating the long-term stability of the autograft tissue. Additionally, we find that in comparison to the normal, healthy TM, reconstructed eardrums and PreTF were significantly thicker, possessed variability in thickness, and lacked a bilayer fibrous structure.
We also find that whereas lateral epithelialization and medial mucosalization of fascia grafts occur, these changes do not appear to significantly affect the thickness of the successful graft. The reconstructed TM appears to be primarily an autograft construct, with the graft comprising > 80% of the total TM thickness and with similar thickness and unchanged fibrous structure to graft material at the time of implantation. This is relevant given the thickness of the postoperative TM can affect sound transmission, as suggested by previously published finite element models of the middle ear that predict higher stapes displacements with a thinner TM.18 Clinical studies have also observed the negative effect of thickened reconstructed TMs on postoperative hearing outcomes.19,20 Unfortunately, our small sample size and ossicular abnormalities in study cases preclude analysis of the effect of graft thickness on clinical hearing outcomes. Nevertheless, the lack of remodeling observed in successful tympanoplasty cases from this study would suggest that the native structure and sound conduction properties of preimplantation grafted materials are relevant to the long-term structure and function of the reconstructed eardrum.
Temporalis fascia has been extensively studied ex vivo. The characteristics of our PreTF specimens are consistent with previous reports, demonstrating a uniform layer of thick, linearly arranged collagen fibers.10 In addition to fiber arrangement, the collagen content of the native TM is important for its function.7 Histological studies of healthy TMs demonstrate a majority of radial fibers composed of type II collagen, which provides resistance to deformation and structural support. Within the periphery, circular fibers are composed primarily of type III collagen, with high elasticity that is essential for sound transmission.21 In comparison, temporalis fascia is composed primarily of type I collagen, which provides resistance to force but lacks the elasticity and sound conduction properties of the native TM.14 Our study shows that the structure of the temporalis fascia grafts remains constant even after incorporation between epithelial and mucosal layers, and that in addition to thickness, differences in collagen type between the native and reconstructed TM may impact graft performance.
Animal studies of temporalis fascia grafts in tympanoplasty have delineated early and long-term changes that occur following implantation.15 In a fascia tympanoplasty study in > 100 guinea pigs, Szabo observed that within days following tympanoplasty, immediate ingrowth of host vessels and fibroblasts occurs within the graft, which appear to maintain the graft’s extracellular matrix.15 Histological analysis shows these grafted TMs retain a uniform lamina propria composed of a linear monolayer of collagen fibers and intact vascular structures for up to 1 year. Additionally, these specimens showed an absence of giant cells, implying that phagocytosis and resorption of the graft does not typically occur. These results add additional evidence to claims that implanted fascia is inert and serves as a scaffold that does not significantly remodel over time. Histologic analysis from the presented human study reveals that fascia within reconstructed human TM specimens also appears inert postimplantation and can remain so over the course of multiple decades.
The findings from this study have direct relevance for clinical practice. First, temporalis fascia grafts should be harvested from the thinnest area available to better replicate the native TM thickness. This is typically the most superior portion of temporalis fascia, several centimeters above the temporal line.22 Second, the preparation of fascia grafts should focus on thinning the fascia with a press or with surgical instruments. The surgeon’s control of thickness in the harvested temporalis fascia graft was suggested as early as 1961 by Storrs but is not frequently discussed.23 Removing adherent fat and muscle from the fascia will further decrease the graft thickness. Whereas no fat or muscle was observed in fascia reconstructed TM cases in this study, prior research has shown the long-term persistence of adipose tissue in fat myringoplasty.24 The degree of variability in the thickness of surgically prepared temporalis fascia grafts observed in this study may be due to differences in preparation. Last, further research is needed to develop biocompatible grafts with fibrous or collagen architecture similar to the native TM. If successful, such a graft could provide a thin biomimetic lamina propria and potentially improve strength and sound transmission through the reconstructed TM.25
This study is primarily limited by the small sample size. We did perform a post-hoc power analysis given the available cases for review and found that our sample size was adequate to detect a clinically significant difference in reconstructed TM thickness when compared to controls. Nevertheless, additional cases would help better define a range of outcomes following successful fascia tympanoplasty. We hope that continued collection and processing of human temporal bones will permit a larger study to be performed in the future. Second, due to the retrospective nature of the histopathologic review, the clinical history of included cases may be incomplete, and pre-/postsurgical audiometry was not universally available. Furthermore, ossicular abnormalities in several cases precluded isolated assessment of reconstructed TM transfer function. Whereas we aimed to understand how temporalis fascia grafts change over time, it is impossible to know the initial structure of the fascia that was implanted in each reconstruction case. To overcome this obstacle, we used nonimplanted temporalis fascia specimens from four separate individuals that were prepared and thinned for implantation. These grafts provide an estimate of the initial state of the autologous graft. Although the thickness and morphology of fascia grafts remain constant following tympanoplasty, it is possible that the distribution of collagen types within the graft may change overtime. Future studies may investigate whether this occurs in humans using immunohistochemical techniques.
This human temporal bone study is the first to provide evidence on the structure of implanted temporalis fascia grafts following tympanoplasty in humans. We show that the structure and thickness of fascia reconstructed TMs are different than that of the normal human eardrum. Additionally, the structure of implanted fascia appears to remain constant over time, without significant changes in thickness or fiber arrangement. These results should reiterate the importance of graft preparation and thinning at the time of tympanoplasty.
CONCLUSION
Temporalis fascia grafts in successful human tympanoplasty demonstrate significantly different structure and thickness when compared to the native human TM. The structure and thickness of fascia grafts do not significantly change following implantation, remaining constant over multiple decades. These results have implications for graft selection and preparation at the time of surgery.
Acknowledgment
We would like to acknowledge Dianne Jones and Garyfallia Pagonis of the Massachusetts Eye and Ear Otopathology Laboratory for their assistance with case identification and photomicrographs, respectively.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Footnotes
Presented at the Triological Society Combined Sections Meeting, New Orleans, Louisiana, U.S.A., January 19–21, 2017.
BIBLIOGRAPHY
- 1.Doyle PJ, Schleuning AJ, Echevarria J. Tympanoplasty: should grafts be placed medial or lateral to the tympanic membrane? Laryngoscope 1972; 82:1425–1430. [DOI] [PubMed] [Google Scholar]
- 2.Wullstein H. Theory and practice of tympanoplasty. Laryngoscope 1956;66: 1076–1093. [DOI] [PubMed] [Google Scholar]
- 3.Kaylie DM, Gardner EK, Jackson CG. Revision chronic ear surgery. Otolaryngol Head Neck Surg 2006;134:443–450. [DOI] [PubMed] [Google Scholar]
- 4.Hardman J, Muzaffar J, Nankivell P, et al. Tympanoplasty for chronic tympanic membrane perforation in children: systematic review and meta-analysis. Otol Neurotol 2015;36:796–804. [DOI] [PubMed] [Google Scholar]
- 5.Tan HE, Santa Maria PL, Eikelboom RH, et al. Type I Tympanoplasty meta-analysis: a single variable analysis. Otol Neurotol 2016;37: 838–846. [DOI] [PubMed] [Google Scholar]
- 6.Nardone M, Sommerville R, Bowman J, et al. Myringoplasty in simple chronic otitis media: critical analysis of long-term results in a 1,000-adult patient series. Otol Neurotol 2011;33:48–53. [DOI] [PubMed] [Google Scholar]
- 7.O’Connor KN, Tam M, Blevins NH, et al. Tympanic membrane collagen fibers: a key to high-frequency sound conduction. Laryngoscope 2008; 118:483–490. [DOI] [PubMed] [Google Scholar]
- 8.Lim DJ. Tympanic membrane. Electron microscopic observation. I: pars tensa. Acta Otolaryngol 1968;66:181–198. [DOI] [PubMed] [Google Scholar]
- 9.Maftoon N, Funnell WR, Daniel SJ, et al. Finite-element modelling of the response of the gerbil middle ear to sound. J Assoc Res Otolaryngol 2015;16:547–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boedts D, De Cock M, Andries L, et al. A scanning electron-microscopic study of different tympanic grafts. Am J Otol 1990;11:274–277. [PubMed] [Google Scholar]
- 11.Morales-Avalos R, Soto-Dominguez A, Garcia-Juarez J, et al. Characterization and morphological comparison of human dura mater, temporalis fascia, and pericranium for the correct selection of an autograft in duraplasty procedures. Surg Radiol Anat 2017;39:29–38. [DOI] [PubMed] [Google Scholar]
- 12.Wormald PJ, Alun-Jones T. Anatomy of the temporalis fascia. J Laryngol Otol 1991;105:522–524. [DOI] [PubMed] [Google Scholar]
- 13.Tellioglu AT, Tekdemir I, Erdemli EA, et al. Temporoparietal Fascia: An anatomic and histologic reinvestigation with new potential clinical applications. Plast Reconstr Surg 2000;105:40–45. [DOI] [PubMed] [Google Scholar]
- 14.Chhapola S, Matta I. Cartilage-perichondrium: an ideal graft material? Indian J Otolaryngol Head Neck Surg 2012;64:208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szabo LZ. How can an underlaid fascia graft form the middle layer of a reconstructed tympanic membrane? Laryngoscope 2006;116:1674–1677. [DOI] [PubMed] [Google Scholar]
- 16.Merchant SN, Schuknecht HF, Rauch SD, et al. The National Temporal Bone, Hearing, and Balance Pathology Resource Registry. Arch Otolaryngol Head Neck Surg 1993;119:846–853. [DOI] [PubMed] [Google Scholar]
- 17.Schuknecht HF. Pathology of the Ear. 2nd ed. Philadelphia, PA: Lea & Febiger; 1993. [Google Scholar]
- 18.Gan RZ, Feng B, Sun Q. Three-dimensional finite element modeling of human ear for sound transmission. Ann Biomed Eng 2004;32:847–859. [DOI] [PubMed] [Google Scholar]
- 19.Vadiya S, Bhatt S. Comparison of partial thickness and full thickness tragal cartilage graft during modified cartilage shield tympanoplasty for type I procedures. Indian J Otolaryngol Head Neck Surg 2016;68:30–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mokbel KM, Thabet EM. Repair of subtotal tympanic membrane perforation by ultrathin cartilage shield: evaluation of take rate and hearing result. Eur Arch Otorhinolaryngol 2013;270:33–36. [DOI] [PubMed] [Google Scholar]
- 21.Knutsson J, Bagger-Sjoback D, von Unge M. Collagen type distribution in the healthy human tympanic membrane. Otol Neurotol 2009;30: 1225–1229. [DOI] [PubMed] [Google Scholar]
- 22.Beheiry EE, Abdel-Hamid FA. An anatomical study of the temporal fascia and related temporal pads of fat. Plast Reconstr Surg 2007;119:136–144. [DOI] [PubMed] [Google Scholar]
- 23.Storrs LA. Myringoplasty with the use of fascia grafts. Arch Otolaryngol 1961;74:45–49. [Google Scholar]
- 24.Gold SR, Chafoo RA. Fat myringoplasty in the guinea pig. Laryngoscope 1991;101:1–5. [DOI] [PubMed] [Google Scholar]
- 25.Kozin ED, Black NL, Cheng JT, et al. Design, fabrication, and in vitro testing of novel three-dimensionally printed tympanic membrane grafts. Hear Res 2016;340:191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]


