Abstract
Background:
Blood blister aneurysms (BBAs) are a rare tiny subset of intracranial aneurysms, located at the nonbranching site of an artery, representing a therapeutic challenge from both surgical and endovascular approach. Flow-diverting efficacy, by preserving flow through the parent artery, was approved for its use in unruptured cerebral aneurysms, but no consensus was reached on its use for BBAs ruptured in the acute setting. We report a multicenter experience of use of flow diversion in acute setting of ruptured BBA, to analyze the safety and efficacy of these devices.
Methods:
We performed a retrospective study of 6 consecutive intracranial BBAs treated with flow diverter devices (FDD) between 2018 and 2020 at 3 italian institutions. Materials, therapy used, complications, clinical and radiographic outcomes were reviewed.
Results:
We used different FDD, in all cases immediate change in contrast opacification at the end of the procedure was reported. Intraprocedural IIb/IIIa inhibitor agent was the major antiplatelet protocol administered. Any complications occurred. All patients showed complete BBA obliteration at 3 months follow-up. 5/6 patients achieved good clinical outcome (0–2 mRS) at 3 months, all of which were presented with low grade SAH (Hunt Hess I–III) and a lower Fisher grade.
Conclusion:
Our data support this endovascular technique as a safe and effective therapeutic modality for this pathology in the acute setting. (www.actabiomedica.it).
Keywords: flow-diverter, SAH, blister aneurysm (BBA), endovascular treatment (EVT), double antiplatelet therapy (DAPT), IIb/IIIa inhibitors.
Background
Blood blister aneurysms (BBAs) are a rare tiny subset of intracranial aneurysms, located at non branching site of an artery usually settled in the medial wall of the supraclinoid segment of the internal carotid artery (ICA) (1,2). BBAs are life treating, they typically occurred with acute subarachnoid hemorrhage (SAH), since they are characterized by morphological instability and high tendency to grow and rupture, due to their structural frailty (3), consisting in internal elastic lamina and media focal arterial wall defect, covered by a thin layer of fibrous tissue and adventitia (4), representing a therapeutic challenge from both surgical and endovascular approach.
Endovascular treatment (EVT) has been representing a promising alternative, showing lower morbidity and mortality compared with open surgical treatment (1,5), represented by clip reconstruction with or without aneurysm wrapping, reserving arterial bypass as a last resort option in selected cases, with high mortality and morbidity rates (6). In particular flow-diverting efficacy, by preserving flow through the parent artery, was approved for its use in unruptured BBAs, however no consensus was reached for its use in the acute setting (7,8).
We report a italian multicenter experience of use of flow diversion in acute setting of ruptured BBA, to analyze the safety and efficacy of these devices.
Methods
From January 2018 to January 2020, 8 consecutive cases of ruptured BBA, treated with flow-diverter device, in the acute setting of subarachnoid hemorrhage, by three Neurointerventional Center were reviewed. Since data were retrospective reviewed, some follow-up data were missing, whereby only 6 cases were included in our three centric retrospective study. The study was approved by the institutional review board at each participating institution. Informed consent was obtained from the patient or legal representative for the EVT of BBA with use of the FDD.
The diagnosis of BBA was made by the treating physician at each contributing center based on clinical presentation and neuroradiological findings. Treatment of BBA was based on each operator preference, including timing of the procedure, vascular access, choice and number of devices, administration and dose of antiplatelet agents, and post procedure care.
All procedures were performed under general anesthesia and continuous infusion of heparin. The device was delivered in the standard fashion, through a microcatheter, having been navigated distally over a shaped microwire, often with the addition of an intermediate support catheter. The FDD was then deployed across the neck of the BBA under fluoroscopy. Angiograms ensured the right positioning. Post-embolization CT was chosen to detect periprocedural complications.
Patient demographic data (sex, age), presenting symptoms (Fisher scala, Hunt and Hess scala and Glasgow’Come scala) radiologic images (NCCT, CTA, MRI and DSA), operative reports such as flow diverter type and administration of antiplatelet agents, complications and postprocedural angiographic outcomes and follow-up were reviewed by local investigators at each participating institution and collected in an online database, without a central core laboratory. Two blinded physicians analysed the data.
Results
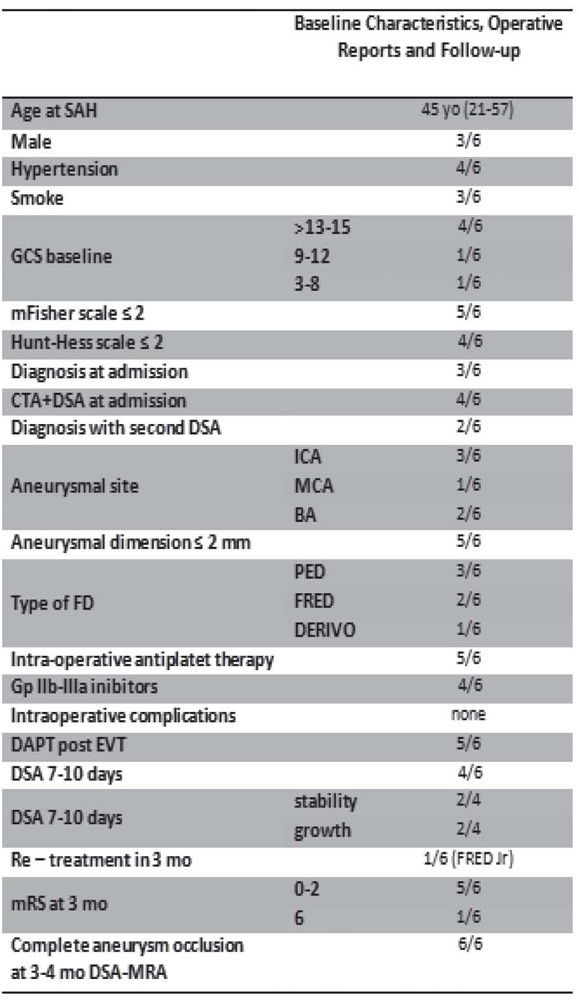
There were a total of 6 patients with BBA, treated with flow diverter devices in the acute setting of SAH, from three participating centers. Baseline clinical and imaging characteristics of these cases reviewed are shown in table 1.
Table 1.
Baseline Characteristics, Operative reports and Follow-up of patients with BBA treated with FDD in the acute setting of SAH
|
Mean age was 45 years old, and there were no differences in sex among patients treated (3/6 were men). 4/6 patients had hypertension, and were admitted with mild GCS score (>13) and a Hunt and Hess score ≤2; 5/6 patients had a Fisher scale ≤2. Only one patient had a severe GCS score (GCS 6), with Hunt and Hess score of 4 and a grade 3 of Fisher scale.
A ruptured BBA was detected at admission in the 50% of patients after DSA study, while about 33% of BBA (2/6) was detected at the second DSA control after 7-10 days from SAH; only one BBA was detected on MRA.
3/6 patients showed a BBA of supraclinoid ICA, 2/6 patients showed BBA of basilar artery, one patient showed a BBA of middle cerebral artery. Median aneurysm size was 2 mm.
In all cases was used a single FDD. 3/6 were treated with the Pipeline Embolization Device (PED; Covidien, Irvine, California); 2/6 with the Flow-Redirection Endoluminal Device (FRED; MicroVention, Tustin, California); in one patient was used the Derivo embolisation device (DED; Acandis GmbH & Co. KG, Pforzheim, Germany).
In 4/6 patients, FDD were delivered with intraprocedural IIb/IIIa inhibitor agents, among which Tirofiban was the most used. Double antiplatelet bolus (clopidogrel and aspirin) was administered before the procedure in only one, while in the other case it was administered tirofiban together with clopidogrel. No intra-periprocedural complications were registered.
Patients were maintained on dual antiplatelet therapy (DAPT) after placement of flow diversion devices. A combination of aspirin and clopidogrel was the most common DAPT regimen after EVT, which was used in 5/6 of cases.
Immediate change in contrast opacification at the end of the procedure described as no residual aneurysm filling or as reduced filling or contrast agent stasis inside the bleb, as a result of initial aneurysm flow exclusion was reported in all cases of treated blister aneurysms.
4/6 patients were submitted at 7-10 days DSA control, with 50% of angiographic results’ stability and one 50% of dimensional BBA growth. One of these was retreated with a second FDD (Flow-Redirection Endoluminal Junior Device - FRED Jr; MicroVention, Tustin, California), with a telescopic technique.
Angiographic follow-up was performed for all patients, on average, 3,5 months after the treatment. All showed complete obliteration. There was one case of subintimal in-stent thickening recognized on follow-up imaging control, even patient remained asymptomatic.
Clinical 3 months follow-up showed good clinical outcome with modified Rankin Scale (mRS) score of 0-2 at 3 months for 5/6 patients, in patients that started with low grade SAH (Hunt Hess I–III) and a lower Fisher grade.
Discussion
This study evaluated 6 cases presenting with BBA at non-branching sections of the supraclinoid ICA, of BA and of MCA in the acute setting of SAH, which represents the main patient presentation, even it accounts for 0.5-2.0% of ruptured intracranial aneurysms (9).
BBAs are often undiagnosed at admission, above all due to their small size and broad-based shape, making the diagnosis on neuroradiological imaging challenging, so that DSA after CTA is often required, as in our study where 3/6 patients needed DSA after CTA, moreover 2/6 BBA were detected at the second DSA control at 7-10 days, probably due to their wall instability and morphological changes, and tendency to rupture which contributed also making them technically difficult to treat (3).
Figure 1.
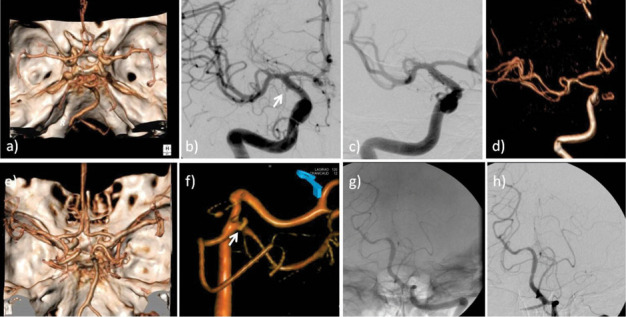
On the top Case 1: a) any aneurysm was detected on Angio-CT (VR reconstruction) at admission. b) DSA of the right ICA: blood blister aneurysm of the right ICA (1 mm), was found at ICA C6 segment (white arrow). c) DSA of the right ICA post EVT: stenting of supraclinoid tract of the right ICA, after FRED insertion under fluoroscopy d) ANGIO-RM-TOF-3D sequence (VR reconstruction): at 3 months follow-up the BBA was excluded from the circulation. On the bottom Case 2: e) any aneurysm was detected on Angio-CT (VR reconstruction) at admission. f) DSA of left vertebral artery (VR reconstruction): 2 mm blood blister aneurysm was detected at the corner between SCA - P1 segment (white arrow). g) DSA of left vertebral artery after EVT: stenting with PED of the basilar trunk. At the end of the procedure no residual aneurysm filling was detected, as a result of aneurysm flow exclusion. h) 3 months DSA of left vertebral artery: at the follow-up the exclusion of BBA from circulation was confirmed.
For all these reasons, FDD by redirecting blood flow along the normal course of the parent artery, has becoming widely accepted for the treatment of BBAs, as a safe and effective treatment for this subset of aneurysms, since induce intra-aneurysmal thrombosis with low re-rupture risk (3), indeed it has been reported to be associated with high rates of complete occlusion and good long-term neurological outcomes, even in the acute SAH setting (5).
In the last years, several evidences had been reported, supporting the safety and effectiveness of the use of FDD for ruptured BBA: in 2018 Mokin et al reported a multicenter experience with flow diversion exclusively for BBA of supraclinoid ICA, by including results of 32 BBA treatment, that supported the use of FDD with 87,5% of complete occlusion, and 68% of good clinical outcome at 3 months, with 5% of delayed complications, and only one case of fatal delayed re-rupture after the initial treatment. (10). In 2017 Ryan et al retrospectively reviewed a serie of 13 patients with ruptured BBA treated with PEDs by reporting 77% of good clinical outcomes with no episodes of procedural or delayed aneurysmal rebleeding (7). In 2016, Linfante et al treated 10 patients with ruptured BBAs of the supraclinoid ICA using a PED, describing a 90% good clinical outcome rate with no procedural complications and no aneurysmal re-ruptures (11). In 2015, Aydin et al. in a series of 11 ruptured blister aneurysms, reported good clinical outcomes in 92%, cases treated with the Silk flow diverter. No aneurysmal re-ruptures, and a procedural minor ischemic stroke in 1 case (12). Another series in 2015, reported by Rouchaud et al., of 62 ruptured blister aneurysms treated with flow diversion, 86% achieved good clinical outcomes, and 17% suffered procedural complications including an almost 8% risk of procedural ICH (13). In 2014, Yoon et al. reported a 12-patient multicenter series of PEDs in ruptured ICA blister aneurysms with 83% of good clinical outcomes rate, and no procedural or post flow diversion aneurysm rerupture (14), while Chalouhi and colleagues reported 100% of complete aneurysm occlusion and an mRS score of ≤ 2 in all 8 patients in their series on PED treatment of blister aneurysms, with no procedural complications and no aneurysmal re-ruptures (15). Hu et al. all reported similar results (16). In 2013 Çinar et al. published a 7-patient ruptured blister aneurysm series with PED treatment and reported good clinical outcome in 71% and no procedural or postprocedural aneurysm rerupture (4).
In our study all the patients treated obtained immediate change in contrast opacification at the end of the EVT, as a result of initial aneurysm flow exclusion, with complete occlusion at 3 months follow-up, and good clinical outcomes, achieving 0-2 score of modified Rankin scale at 3 months, in 5/6 patients. These results were similar to all the three different flow diverting devices chosen, with PED resulting mainly used (see table 1). It is to note that PED is the most commonly studied flow diverting stent for the treatment of BBA, reported in literature (10). Any intra-or periprocedural complications were found, even in the issue of incomplete or delayed occlusion rates, or regrowth.
Although complete occlusion of BLA immediately following FDD placement has been demonstrated (16), the persistence or growth of the BBA often occurs after the placement of flow-diverting stents probably due to mismatching of the stent or insufficient stent expansion (1,4-5, 15).
Recent studies have not shown an increased re-hemorrhage risk, even with confirmed residual aneurysm (10), such a low incidence of rebleeding in the immediate period can be explained by the reduction of jet inflow of blood and hemodynamic shearing stress on the aneurysmal wall (11,15).
Even for delayed occlusion cases or minimal increase, complete obliteration on follow-up angiography at 3.4 months was found, concurring with those previously reported where complete occlusion is usually observed in 80–90% of cases on follow-up (10,16).
Notwithstanding, it is important to note, that some intra and periprocedural complication have been reported in literature, such as side branch occlusion,stent thrombosis, vasospasm, intraoperative rebleeding, or post-procedural intracranial hemorrhage (1,3), these worsened by systemic heparinization and antiplatelet application, even if a comprehensive meta-analysis of the literature, showed how morbidity and mortality associated with BBA, treated with FDD, was lower than with surgical treatment, even in the presence of DAPT (17).
However the use of FDD in the acute setting remains a major issue since DAPT is needed to prevent thromboembolic events, however may be challenging in the acute phase, representing the major risk for the patient.
In the two patient in which BBA was detected at the second DSA, we used DAPT before procedure only in the patient clinically stable and IIb/IIIa inhibitor agent together with clopidogrel in the other one who showed worse clinical condition, while preferring intraprocedural IIb/IIIa inhibitor bolus in 4/6 cases in which BBA was detected at admission and promptly treated to avoid further hemorrhagic complications risk. None of our cases showed periprocedural intraparenchymal hemorrhage.
Some authors use and support the safety of tirofiban together with DAPT as protocol for patients with intracranial aneurysms who underwent flow diversion (18) while only a small series focused on the safety of IIb/IIIa inhibitor use in the case of ruptured aneurysm (19), so that consensus above antiplatelet therapy administration should be achieved.
Limitations of our study include the narrow number of patients, its retrospective nature, which is subject to the accuracy of case reporting, the lacking of treatment protocols at the participating institutions and the use of different FDD.
Further studies with large numbers of patients are needed to compare the effectiveness and safety of each kind of device since computational fluid dynamic analysis suggests that different flow diverters vary in their hemodynamic effects on cerebral aneurysms (20).
Conclusion
Endovascular flow diversion of intracranial blister aneurysms is a treatment that is increasingly practiced for this challenging subset of aneurysms, even if antiplatelet therapy is required and could represent a major limitation in the acute setting of subarachnoid hemorrhage presentation.
Our experience adds to previous reports other cases of ruptured BBA treated with the FDD in the acute setting of subarachnoid hemorrhage. Our data support this endovascular technique as a safe and effective therapeutic modality for BBA even in the acute setting. Larger studies are needed to compare flow diverters device efficacy.
Ethical Approval:
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent:
Written informed consent to the interventions, CT and the MR exams was obtained from all subjects in this study.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Peitz GW, Sy CA, Grandhi R. Endovascular treatment of blister aneurysms. Neurosurg Focus. 2017;42:E12. doi: 10.3171/2017.3.FOCUS1751. [DOI] [PubMed] [Google Scholar]
- 2.Jha AN, Gupta V. Blister aneurysms. Neurol India. 2009;57:2–3. doi: 10.4103/0028-3886.48789. [DOI] [PubMed] [Google Scholar]
- 3.Chinchure SD, Gupta V, Goel G, Gupta A, Jha A. Subarachnoid hemorrhage with blister aneurysms: endovascular management. Neurol India. 2014;62:393–9. doi: 10.4103/0028-3886.141262. [DOI] [PubMed] [Google Scholar]
- 4.Çinar C, Oran İ, Bozkaya H, et al. Endovascular treatment of ruptured blister-like aneurysms with special reference to the flow-diverting strategy. Neuroradiology. 2013;55:441–447. doi: 10.1007/s00234-013-1136-y. [DOI] [PubMed] [Google Scholar]
- 5.Ji T, Guo Y, Huang X, Xu B, Xu K, Yu J, et al. Current status of the treatment of blood blister-like aneurysms of the supraclinoid internal carotid artery: A review. Int J Med Sci. 2017;14:390–402. doi: 10.7150/ijms.17979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Princiotta C, Dall’olio M, Cirillo L, Leonardi M. Staged treatment of a blood blister-like aneurysm with stent-assisted coiling followed by flow diverter in-stent insertion. A case report. Interv Neuroradiol. 2011;17:365–370. doi: 10.1177/159101991101700314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan RW, Khan AS, Barco R, et al. Pipeline flow diversion of ruptured blister aneurysms of the supraclinoid carotid artery using a single-device strategy. Neurosurg Focus. 2017;42:E11. doi: 10.3171/2017.3.FOCUS1757. [DOI] [PubMed] [Google Scholar]
- 8.Ding D, Starke RM, Hope A, et al. Flow-diverting stent-assisted coil embolization of a ruptured internal carotid artery blister aneurysm with the Pipeline Flex Embolization Device. J Neurosci Rural Pract. 2017;8:664–667. doi: 10.4103/jnrp.jnrp_336_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee BH, Kim BM, Park MS, et al. Reconstructive endovascular treatment of ruptured blood blister-like aneurysms of the internal carotid artery. J Neurosurg. 2009;110:431–6. doi: 10.3171/2008.7.JNS08257. [DOI] [PubMed] [Google Scholar]
- 10.Mokin M, Chinea A, Primiani CT, et al. Treatment of blood blister aneurysms of the internal carotid artery with flow diversion. J Neurointerv Surg. 2018;10:1074–8. doi: 10.1136/neurintsurg-2017-013701. [DOI] [PubMed] [Google Scholar]
- 11.Linfante I, Mayich M, Sonig A, et al. Flow diversion with pipeline embolic device as treatment of subarachnoid hemorrhage secondary to blister aneurysms: dual-center experience and review of the literature. J Neurointerv Surg. 2017;9:29–33. doi: 10.1136/neurintsurg-2016-012287. [DOI] [PubMed] [Google Scholar]
- 12.Aydin K, Arat A, Sencer S, et al. Treatment of ruptured blood blister-like aneurysms with flow diverter SILK stents. J Neurointerv Surg. 2015;7:202–209. doi: 10.1136/neurintsurg-2013-011090. [DOI] [PubMed] [Google Scholar]
- 13.Rouchaud A, Brinjikji W, Cloft HJ, et al. Endovascular treatment of ruptured blister-like aneurysms: a systematic review and meta-analysis with focus on deconstructive versus reconstructive and flow-diverter treatments. AJNR Am J Neuroradiol. 2015;36(12):2331–2339. doi: 10.3174/ajnr.A4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon JW, Siddiqui AH, Dumont TM, et al. Feasibility and safety of pipeline embolization device in patients with ruptured carotid blister aneurysms. Neurosurgery. 2014;75:419–29. doi: 10.1227/NEU.0000000000000487. [DOI] [PubMed] [Google Scholar]
- 15.Chalouhi N, Zanaty M, Whiting A, et al. Treatment of ruptured intracranial aneurysms with the pipeline embolization device. Neurosurgery. 2015;76:165–72. doi: 10.1227/NEU.0000000000000586. [DOI] [PubMed] [Google Scholar]
- 16.Hu YC, Chugh C, Mehta H, et al. Early angiographic occlusion of ruptured blister aneurysms of the internal carotid artery using the pipeline embolization device as a primary treatment option. J Neurointerv Surg. 2014;6:740–3. doi: 10.1136/neurintsurg-2013-010937. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez AM, Narata AP, Yilmaz H, et al. Blood blister-like aneurysms: single center experience and systematic literature review. Eur J Radiol. 2014;83:197–205. doi: 10.1016/j.ejrad.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Samaniego EA, Gibson E, Nakagawa D, et al. “Safety of tirofiban and dual antiplatelet therapy in treating intracranial aneurysms.”. Stroke and vascular neurology. Feb. 2019;4(1):36–42. doi: 10.1136/svn-2018-000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aviv RI, O’Neill R, Patel MC, et al. Abciximab in patients with ruptured intracranial aneurysms. Am J Neuroradiol. 2005;26:1744–1750. [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C, Tian Z, Liu J, et al. Flow diverter effect of LVIS stent on cerebral aneurysm hemodynamics: a comparison with Enterprise stents and the Pipeline device. J Transl Med. 2016;14:199. doi: 10.1186/s12967-016-0959-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


