Abstract
Purpose:
Haemoptysis (Hp) is a potentially life-threatening medical condition. We investigated the safety, efficacy and usability of bronchial artery embolization using a new anti-reflux microcatheter in patients with haemoptysis.
Materials and methods:
The study was held as a single-center retrospective study. Four patients underwent bronchial arterial embolization, using the new microcatheter. Then, we evalueted technical success, immediate clinical success, haemoptysis recurrance rate and safety in reducing reflux complications.
Conclusion
Bronchial artery embolization for hemoptysis with the new microcatheter is a safe and effective method with high technical and clinical success rates. Short and medium-term results are excellent. (www.actabiomedica.it).
Keywords: radiology, embolization, bleeding, microcatheter, intervantional radiology, bronchial arteries
Introduction
Haemoptysis (Hp) is a potentially life-threatening medical condition (1–3).
Its aetiology and epidemiology vary a lot among studies according to geographic locations, time of publication and epidemiological design (1,2,4-8). The most common causes of life-threatening Hp include bronchiectasis from infectious and non-infectious aetiologies, bronchogenic carcinoma, and various lung infections such as tuberculosis (TB), depending on geographical location (1,9-14).
In Italy, malignancies and bronchiectasis were most common causes of moderate (20–500 mL in 24 h) and severe (> 500 mL in 24 h) Hp (3); there are also other aetiologies described in the literature include mycetomas, necrotizing pneumonia, and cryptogenic Hp (1,9-14).
No consensus has been determined about Hp severity throughout the literature; however, more and more agree to divide Hp into two categories: massive and non-massive, rather than “mild, moderate and severe”. There is no precise cut-off in relation to which it is decided whether to subject a patient to embolization.
It is estimated that 5–14% of patients presenting with Hp will have life-threatening Hp (1,15,16). Life-threatening Hp, also called massive Hp, has been variably defined based upon criteria such as the volume per hour of bleeding, the total volume of bleeding per 24h, or the presence of abnormal gas exchange or hemodynamic instability (1,9,15). Hp fatality is much more frequently associated with asphyxiation rather than exsanguination.
Although over 90% of Hp are self-limiting (17), both diagnosis and treatment of massive Hp are challenging (18).
The optimal diagnostic approach to Hp has not been determined. The most frequently used modalities for studying Hp include chest radiography (CXR), bronchoscopy (FOB) and multidetector computed tomography (MDCT) angiography, useful to determine the bleeding site and they can be used alone or in combination.
In the absence of guidelines on the treatment of Hp, there are essentially four treatment options for haemoptysis with respiratory and haemodynamic supports: drug therapy, catheter intervention, such as bronchial artery embolization (BAE), bronchoscopic intervention and surgery (lung resection) (19-24).
Mild or moderate Hp can often be managed by conservative treatment of the underlying pathology.
On the other hand, there are several modalities to treat life-threatening Hp, but there are no existing guidelines on how to best manage it.
Following initial stabilization and localization of the bleeding site, bronchial artery embolization (BAE) via the transfemoral approach has become the first-line therapy for treating massive and recurrent Hp (22,25-27,28).
BAE, which controls the bleeding by angiography-guided injection of embolic substance into pathologic bronchial arteries, has largely replaced emergent surgery for the management of life-threatening Hp, due to the availability of safe and effective endovascular embolization techniques (29).
Sopko et al. described that a surgical lung resection has a 40% of mortality when executed in emergency, instead of 18% of mortality when an election procedure is performed. Therefore, there are vary indications to surgical intervention in patients with massive Hp, such as technical failure of BAE or recurrent Hp despite multiple BAE intervention. (30)
Microcatheters are commonly used during several arterial embolization procedures. SeQure® is a new microcatheter for peripheral embolization procedures, which is a reflux-control microcatheter that uses flow dynamics to create a fluid barrier designed to deliver more treatment to the target vessel and reduce the risk of non-target embolization for less potential damage to surrounding tissue. The device has side slits through which contrast media can’t exit, putting up a barrier that prevents embolic agents from going where they shouldn’t. It means that SeQure® microcatheter helps interventional radiologists to deliver a high quality of care during image-guided BAEs.
Non-target embolization is a complication of embolization procedures, which can also be very serious, arising upon unintentional reflux of embolic beads to adjacent vessels, above all after occlusion of the target vessel. In particular one of the worst complications of BAE is the inadvertent embolization of anterior spinal arteries, with possible severe neurological consequent (e.g. paraplegia).
The aim of our study was to evaluate the effectiveness, the safety and the short and medium-term results of BAE performed using the new SeQure® microcatheter (Guerbet, France).
Material and methods
Patients
The study was held as a single-center retrospective study. Written informed consent was taken from all patients before the procedure, and the local ethical committee approved the study.
This case series includes 4 patients who underwent BAE using SeQure® microcatheter (Guerbet, France) for Hp. From March 2019 to February 2020, a total of 4 patients (3 men and 1 woman) with a mean age of 38 years were treated.
All patients had massive Hp with a bleeding of 100-500 ml in the 24h (9) causing desaturation, so an interventional radiology (IR) procedure was indicated.
The patients’ characteristics are shown in Table 1.
Table 1.
The patients’ characteristics
| Patients | Age | Sex | Cause | Grade | Pre-procedure Diagnostic Workup (CTA/FOB) | Time since embolization (months) |
| 1 | 40 | M | Bronchiectasis (Kartagener Syndrome) | Massive | CTA | 3 |
| 2 | 30 | M | Bronchiectasis | Massive | CTA | 4 |
| 3 | 37 | F | Bronchiectasis | Massive | CTA | 5 |
| 4 | 46 | M | Bronchiectasis | Massive | CTA | 6 |
CTA=Computed-Tomography Angiography
Imaging
All patients were evaluated with a MDCT angiography before the procedure (Fig. 1)
Figure 1.
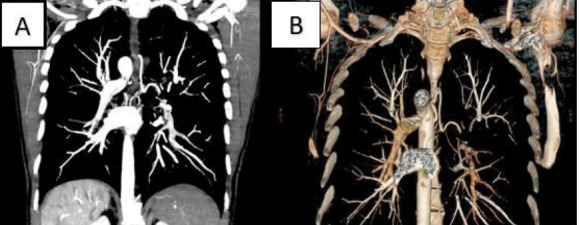
A) coronal section of a MDCT angiography. B) 3D reconstruction of a MDCT angiography.
MDCT angiography was carried out to:
- Localize the site of the bleeding and establish if there was a mono or bilateral bleeding
- Find the origin of the bronchial artery “incriminated” and measure its calibre
- Establish if there was an “active bleeding”
- Decide the type of the treatment and the choice of the catheter.
After that, we performed the procedure using a SeQure® microcatheter, that let us perform a super-selective and target embolization.
Technique
After local anaesthesia (lidocaine 2%), a 5French sheath is inserted into the right common femoral artery. Using a 5F diagnostic catheter (Cobra or Simmons) on a Terumo (0.035 inch) guidewire, catheterization of the bronchial artery is performed. Once catheterized the right artery, angiography is performed in PA and oblique projections (Fig. 2). That is to study the pathological vessels, collateral arteries (intercostal a., costo-cervical a.) and the potential presence of AV fistula or malformation. Moreover, it is important to exclude the presence of anteriors spinal arteries performing a lateral projection too.
Figure 2.
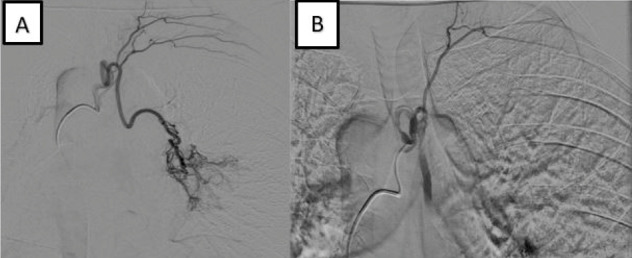
A) PA projection of an intraprocedural angiography. B) oblique projection of the angiography.
In fact, the accidental embolization of this artery can lead to paraplegia which is one of the worst complications related to this intervention.
Once studied the angiographies, the 2.8Fr SeQure® microcatheter is advanced on a 0.016 guidewire into the bronchial artery, beyond the collateral arteries to avoid non-target embolization (eg costal arteries). Another angiography is performed from the microcatheter to better study the vessel. Then the embolization is performed using microparticles Embozene (Boston Scientific Corporation) 500 or 700 micrometers, prepared with contrast media and saline solution (1:1). The injection is performed slowly. Only the contrast media exits from the lateral holes at the tip of the SeQure®, creating a turbulence that avoids reflux and non-target embolization. The injection is performed avoiding reflux of contrast beyond the proximal marker of the SeQure®, preventing non-target embolization. Injection is stopped when the blood flow in the vessel is nearly to stop.
Final angiography demonstrates good result of the embolization.
Outcomes
After performing the BAE, for each patient we evaluated the parameters below:
- Technical success (TS): was defined as the possibility to arise the bleeding vessel and carry out a target embolization.
- Immediate clinical success (ICS): was defined as the cessation of bleeding within 24 h of BAE or within the same admission.
- Hp recurrence rate (HRR): was defined as a significant Hp occurring after discharge, requiring either hospital admission, medical management, or repeat intervention of BAE in the time of two month.
- Safety in reducing reflux complications
All complications were recorded and classified as minor and major; they were assessed according to SIR Criteria (31).
Results
Patient’s results are showed in Table 2.
Table 2.
Patient’s results
| Patients | Age | Sex | TS | ICS | RR | Major complication |
| 1 | 40 | M | Yes | Yes | No | 0 |
| 2 | 30 | M | Yes | Yes | No | 0 |
| 3 | 37 | F | Yes | Yes | No | 0 |
| 4 | 46 | M | Yes | Yes | No | 0 |
In this study, 4 consecutive patients who underwent BAE were retrospectively analysed. The median patient age was 38 years and 75% were men.
All BAEs were carried out with SeQure® microcatheter using transfemoral access.
Technical success (TS) rate was 100% since every embolization was carried out targetly and a super selective injection of embolization material was performed. The immediate clinical success (ICS) rate of preventing massive Hp was 100%. There was no procedure-related mortality or morbidities. No major complication occurred during or after the procedure. Minor complications such as chest pain were observed in 1 patient (25%).
These patients showed good response after embolization with SeQure® microcatheter: recurrent Hp rate was observed in none of four patients (0%) within 2 months, and no repeat BAE is required.
In none patients (0%) a reflux complication was observed.
All the underlying pathologies for recurrent Hp were bronchiectasis (100%), in a case related to Kartagener Syndrome.
Discussion
The results of this study demonstrated that BAE with SeQure® microcatheter is a safe, effective treatment method for massive Hp with excellent short and medium-term outcomes. Hp is a potentially life-threatening occurrence, which requires prompt intervention.
Earlier, before the advent of BAE, the best method of controlling bleeding was surgery. It required patients many preparations such as chest CT, pulmonary function, even bronchoscopy to evaluate the patient’s physical condition and determine the range of surgery; however, some preparations were impossible for the patients in emergency conditions (32).
BAE is a well-established procedure used to control Hp since it first performed in 1974 (33-35). Nowadays this procedure is widely used as a first-line management of Hp, because non-operable patients could be treated and other patients could be stabilized prior to surgery (36).
The use of certain complementary examinations is indispensable in patients with Hp.
The diagnostic workup for massive Hp should be undertaken as soon as possible and after the patient has been stabilized. Sometimes, the association of FOB and MDCT angiography, may be more effective than either alone.
However, the role of FOB is currently highly debated, in particular since the continuous evolution of CT. Several studies have compared MDCT with FOB (36-39). Revel and colleagues reported the comparison between MDCT and FOB in determining the site and the cause of life-threatening Hp. MDCT may be comparable to FOB for identifying the site of bleeding (70% vs. 73% respectively), however MDCT has been found to be more efficient than FOB in identifying the cause of bleeding (77% vs. 8%, respectively, P<0.001) (37).
The MDCT angiography not only allows the study of pulmonary vascularization, but, at the same time, it allows an exhaustive study of the mediastinum and the parenchyma during the same acquisition (40). The MDCT identifies all of the catheterizable bronchial arteries in angiography, that are the source of the bleeding; as well as detects pulmonary arterial anomalies, avoiding useless bronchial angiographies and correctly indicating the therapeutic attitude (41,42).
Evertheless, the MDCT does not identify certain collateral bronchial arteries, in particular the anterior median spinal artery. Only the anterior lumbar spinal artery can be detected at this time (42).
While MDCT appears to have the highest diagnostic yield for life-threatening Hp, FOB remains invaluable for patients needing airway control.
A small parenthesis concerns Chest X-Ray (CXR), the latter represents one of the imaging modalities pertinent to the evaluation of massive Hp. Depending on the hospital, CXR could be still considered the initial imaging modality for evaluating a patient with Hp. It can assist in lateralizing bleeding and reveal a focal or diffuse lung involvement. Nevertheless, the sensitivity of CXR in this context is not high; CXR identified the bleeding site in 46% of cases, and the underlying bleeding cause in 35% (37).
In our series, all the patients underwent pre-procedural MDCT, which localized the likely site of bleeding, so FOB was not performed. After performing MDCT, our patients were ready to begin the procedure. BAE should be performed as soon as possible after contrast-enhanced MDCT (and/or bronchoscopy).
The goal of BAE is reduction of the systemic arterial perfusion pressure in the bronchial arteries of the affected area in order to stop bleeding (43).
There are no absolute contraindications to BAE for the treatment of massive Hp. Coagulopathy, contrast allergy and renal failure are only relative contraindications.
A variety of agents have been used for bronchial artery embolization. We performed 4 procedures of BAE using the relatively new SeQure® microcatheter.
BAE must be carried out by an experienced interventional radiologist. The diameter of the bronchial arteries increases to several millimeters in patients with chronic inflammatory lung disease, especially cystic fibrosis (44).
Active bleeding is demonstrated in only 3.6 to 10.8% of cases (45,46).
Bronchial artery diameter >2 mm, tortuosity of the bronchial arteries, shunts, aneurysms, extravasation of contrast medium, and hypervascularized zones of lung parenchyma represent some features of pathological bronchial arteries that could be source of bleeding (27, 45, 47). Pathological artery identification is typically followed by embolization with the material of choice, how it happens in all IR procedures.
The choice of the embolization material is important to the success of the intervention and is dependent upon the size and site of the bleeding vessel, the ease of access and deployment of the occlusive material to the vessel, the size of the catheter being used, the durability of occlusion as well as the tendency for recanalization (30, 48, 49).
Before the beginning of the procedure, we need to evaluate the existence of branches supplying the spine or the existence of shunts between the bronchial arteries and the pulmonary arteries or pulmonary veins in order, to avoid a systemic embolism.
According to this, our results are very confident compared to literature which report an ICS of 70-99% (50).
The results of this study demonstrated that BAE performed using SeQure® microcatheter is a safe, effective treatment method for moderate or massive Hp with excellent short and medium-term outcomes.
In our study bronchial arteriography and embolization were well-tolerated by all patients.
We obtained a 100% of TS, carrying out 4/4 interventional procedures.
Of the 4 cases, an immediate control of bleeding was achieved with embolization in all patients (100%), with a full ICS. The procedural efficacy in controlling Hp was comparable to other studies.
Our short-term success rates evaluated through HRR was comparable to other studies, since HRR was 0%.
The study showed the procedure to be very safe.
In summary, Hp represents a significant clinical entity with high morbidity and potential mortality. Medical management (in terms of resuscitation and bronchoscopic interventions) and surgery have severe limitations in these patient populations. BAE procedures represent the first-line treatment for Hp arising from bronchial arterial source.
The most frequent side effects of BAE are transient chest pain (24 to 91%) and dysphagia (0.7 to 18.2%) (46). Nevertheless, one of the most severe complications is transverse myelitis. Unintended spinal cord ischemia, due to the embolization of spinal arteries (1.4 to 6.5%) (10, 46), leads to transient or persistent paraparesis or paraplegia (50). The spinal artery originates from a bronchial artery in 5% of patients. During the procedure of BAE, when spinal artery is founding, it’s usually advised to use super-selective BAE to prevent the complication.
Another quite common complication is the reflux of embolic material into the artery. In this study, this type of complication has proved in some way to be avoidable thanks the use of SeQure® microcatheter.
The SeQure® microcatheter reduced the risk of non-target embolization to help maximize selective embolization, and above all, it seems to reduce significantly the reflux of embolic material. This microcatheter has a novel distal tip filter with side slits, allowing outflow of the embolic material, without passage of embolization beads. These features reduce non-target embolization and also reduce the risk of beads reflux at higher injection rates, enabling the injection of more embolic material until full stasis is achieved.
Our study has important practical implications. SeQure® microcatheter could represent a viable alternative to usually used microcatheter. It could reduce the risk of non-target embolization and it flow rate at reflux and bead accumulation in a flow model, compared to a standard microcatheter. All these features allow to reduce the risk of complication such as spinal cord ischemia or other unintended artery embolization.
The limitations of this study were its small patient population, availability of only short-term follow-up, its retrospective and observational nature, and it’s conducted at single site.
A larger patient sample should be considered to ascertain a better efficacy and safety of this microcatheter.
A more prolonged follow-up may also establish long term prognosis in these patients.
Conclusion
This study demonstrates the safety and short/medium-term efficacy of BAE using SeQure® microcatheter. SeQure® ensured safe target-vessel embolization: it can reduce the risks of bleads reflux and, accordingly, the risk of non-target embolization events. BAE using SeQure® microcatheter can be a valuable therapeutic option for treating patients with haemoptysis.
Ethical approval:
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent:
Written informed consent to the CT and the MR exams was obtained from all subjects in this study.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Hirshberg B, Biran I, Glazer M, Kramer MR. Hemoptysis: etiology, evaluation, and outcome in a tertiary referral hospital. Chest. 1997;112:440–4. doi: 10.1378/chest.112.2.440. http://www.ncbi.nlm.nih.gov/pubmed/9266882 . Accessed 13 June 2018. [DOI] [PubMed] [Google Scholar]
- 2.Tsoumakidou M, Chrysofakis G, Tsiligianni I, Maltezakis G, Siafakas NM, Tzanakis N. A prospective analysis of 184 hemoptysis cases: diagnostic impact of chest X-ray, computed tomography, bronchoscopy. Respiration. 2006;73:808–14. doi: 10.1159/000091189. https://doi.org/10.1159/000091189 . [DOI] [PubMed] [Google Scholar]
- 3.Mondoni M, Carlucci P, Job S, Parazzini EM, Cipolla G, Pagani M, et al. Observational, multicentre study on the epidemiology of haemoptysis. Eur Respir J. 2018;51:1701813. doi: 10.1183/13993003.01813-2017. https://doi.org/10.1183/13993003.01813-2017 . [DOI] [PubMed] [Google Scholar]
- 4.Soares Pires F, Teixeira N, Coelho F, et al. Hemoptysis etiology, evaluation and treatment in a university hospital. Rev Port Pneumol. 2011;17:7–14. doi: 10.1016/s2173-5115(11)70004-5. [DOI] [PubMed] [Google Scholar]
- 5.Abdulmalak C, Cottenet J, Beltramo G, et al. Haemoptysis in adults: a 5-year study using the French nationwide hospital administrative database. Eur Respir J. 2015;46:503–511. doi: 10.1183/09031936.00218214. [DOI] [PubMed] [Google Scholar]
- 6.Vanni S, Bianchi S, Bigiarini S, et al. Management of patients presenting with haemoptysis to a Tertiary Care Italian Emergency Department: the Florence Haemoptysis Score (FLHASc) Intern Emerg Med. 2017. https://doi.org/10.1007/s11739-017-1618-8 . [DOI] [PubMed]
- 7.Nielsen K, Gottlieb M, Colella S, et al. Bronchoscopy as a supplement to computed tomography in patients with haemoptysis may be unnecessary. Eur Clin Respir J. 2016;3:31802. doi: 10.3402/ecrj.v3.31802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mondoni M, Sferrazza Papa GF, Sotgiu G, et al. Haemoptysis: a frequent diagnostic challenge. Eur Respir J. 2016;47:348–350. doi: 10.1183/13993003.01344-2015. [DOI] [PubMed] [Google Scholar]
- 9.Knott-Craig CJ, Oostuizen JG, Rossouw G, Joubert JR, Barnard PM. Management and prognosis of massive hemoptysis. Recent experience with 120 patients. J Thorac Cardiovasc Surg. 1993;105(3):394–7. [PubMed] [Google Scholar]
- 10.Mal H, Rullon I, Mellot F, Brugière O, Sleiman C, Menu Y, et al. Immediate and long-term results of bronchial artery embolization for life-threatening hemoptysis. Chest. 1999;115(4):996–1001. doi: 10.1378/chest.115.4.996. [DOI] [PubMed] [Google Scholar]
- 11.Porter DK, Van Every MJ, Anthracite RF, Mack JW. Massive hemoptysis in cystic fibrosis. Arch Intern Med. 1983;143(2):287–90. [PubMed] [Google Scholar]
- 12.Cahill BC, Ingbar DH. Massive hemoptysis. Assessment and management. Clin Chest Med. 1994;15(1):147–67. [PubMed] [Google Scholar]
- 13.Santiago S, Tobias J, Williams AJ. A reappraisal of the causes of hemoptysis. Arch Intern Med. 1991;151(12):2449–51. [PubMed] [Google Scholar]
- 14.Johnston H, Reisz G. Changing spectrum of hemoptysis. Underlying causes in 148 patients undergoing diagnostic flexible fiberoptic bronchoscopy. Arch Intern Med. 1989;149(7):1666–8. doi: 10.1001/archinte.149.7.1666. [DOI] [PubMed] [Google Scholar]
- 15.Corey R, Hla KM. Major and massive hemoptysis: reassessment of conservative management. Am J Med Sci. 1987;294(5):301–9. doi: 10.1097/00000441-198711000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Garzon AA, Gourin A. Surgical management of massive hemoptysis. A ten-year experience. Ann Surg. 1978;187(3):267–71. doi: 10.1097/00000658-197803000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lordan JL, Gascoigne A, Corris PA. The pulmonary physician in critical care * Illustrative case 7: Assessment and management of massive haemoptysis. Thorax. 2003;58:814–9. doi: 10.1136/thorax.58.9.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haponik EF, Fein A, Chin R. Managing life-threatening hemoptysis: has anything really changed? Chest. 2000;118:1431–5. doi: 10.1378/chest.118.5.1431. [DOI] [PubMed] [Google Scholar]
- 19.Swanson KL, Johnson CM, Prakash UB, et al. Bronchial artery embolization: experience with 54 patients. Chest. 2002;121:789–95. doi: 10.1378/chest.121.3.789. [DOI] [PubMed] [Google Scholar]
- 20.Govind M, Maharajh J. The impact of coinfection with human immunodeficiency virus and pulmonary tuberculosis on the success of bronchial artery embolization. Br J Radiol. 2013;86:20120256. doi: 10.1259/bjr.20120256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee MK, Kim SH, Yong SJ, et al. Moderate hemoptysis: recurrent hemoptysis and mortality according to bronchial artery embolization. Clin Respir J. 2015;9:53–64. doi: 10.1111/crj.12104. [DOI] [PubMed] [Google Scholar]
- 22.Poyanli A, Acunas B, Rozanes I, et al. Endovascular therapy in the management of moderate and massive haemoptysis. Br J Radiol. 2007;80:331–6. doi: 10.1259/bjr/34204483. [DOI] [PubMed] [Google Scholar]
- 23.Yoon W, Kim JK, Kim YH, et al. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics. 2002;22:1395–409. doi: 10.1148/rg.226015180. [DOI] [PubMed] [Google Scholar]
- 24.Ittrich H, Klose H, Adam G. Radiologic management of haemoptysis: diagnostic and interventional bronchial arterial embolisation. Rofo. 2015;187:248–59. doi: 10.1055/s-0034-1385457. [DOI] [PubMed] [Google Scholar]
- 25.Fernando HC, Stein M, Benfield JR, Link DP. Role of bronchial artery embolization in the management of hemoptysis. Arch Surg. 1998;133:862–6. doi: 10.1001/archsurg.133.8.862. [DOI] [PubMed] [Google Scholar]
- 26.Remy J, Arnaud A, Fardou H, Giraud R, Voisin C. Treatment of hemoptysis by embolization of bronchial arteries. Radiology. 1977;122:33–7. doi: 10.1148/122.1.33. [DOI] [PubMed] [Google Scholar]
- 27.Uflacker R, Kaemmerer A, Picon PD, et al. Bronchial artery embolization in the management of hemoptysis: technical aspects and long-term results. Radiology. 1985;157:637–44. doi: 10.1148/radiology.157.3.4059552. [DOI] [PubMed] [Google Scholar]
- 28.Expert Panel on Thoracic Imaging. Olsen KM, Manouchehr-Pour S, et al. ACR Appropriateness Criteria® Hemoptysis. J Am Coll Radiol. 2020;17(5S):S148–S159. doi: 10.1016/j.jacr.2020.01.043. doi: 10.1016/j.jacr.2020.01.043. [DOI] [PubMed] [Google Scholar]
- 29.Ierardi AM, Floridi C, Pellegrino C, et al. Role of percutaneous transcatheter embolization (PTE) in the treatment of spontaneous bleeding associated with anticoagulant therapy. Radiol Med. 2015;120(1):149–157. doi: 10.1007/s11547-014-0470-4. doi: 10.1007/s11547-014-0470-4. [DOI] [PubMed] [Google Scholar]
- 30.Sopko DR, Smith TP. Bronchial artery embolization for hemoptysis. Semin Intervent Radiol. 2011;28(1):48–62. doi: 10.1055/s-0031-1273940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khalilzadeh Omid, Baerlocher Mark O, Paul Shyn B, et al. Proposal of a New Adverse Event Classification by the Society of Interventional Radiology Standards of Practice Committee. J Vasc Interv Radiol. 2018 Jan;29(1):146. doi: 10.1016/j.jvir.2017.06.019. doi: 10.1016/j.jvir.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 32.Sehitogullari A, Bilici S, Sayir F, Cobanoglu U, Kahraman A. A long-term study assessing the factors infuencing survival and morbidity in the surgical management of bronchiectasis. J Cardiothorac Surg. 2011;6:161. doi: 10.1186/1749-8090-6-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burke CT, Mauro MA. Bronchial artery embolization. Semin Intervent Radiol. 2004;21:43–8. doi: 10.1055/s-2004-831404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lorenz J, Sheth D, Patel J. Bronchial artery embolization. Semin Intervent Radiol. 2012;29:155–60. doi: 10.1055/s-0032-1326923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sidhu M, Wieseler K, Burdick TR, Shaw DW. Bronchial artery embolization for hemoptysis. Semin Intervent Radiol. 2008;25:310–8. doi: 10.1055/s-0028-1085931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwon W, Kim YJ, Lee YH, Lee WY, Kim MS. The effectiveness of embolotherapy for treatment of hemoptysis in patients with varying severity of tuberculosis by assessment of chest radiography. Yonsei Med J. 2006;47:377–83. doi: 10.3349/ymj.2006.47.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Revel MP, Fournier LS, Hennebicque AS, Cuenod CA, Meyer G, Reynaud P, et al. Can CT replace bronchoscopy in the detection of the site and cause of bleeding in patients with large or massive hemoptysis? AJR Am J Roentgenol. 2002;179(5):1217–24. doi: 10.2214/ajr.179.5.1791217. [DOI] [PubMed] [Google Scholar]
- 38.Millar AB, Boothroyd AE, Edwards D, Hetzel MR. The role of computed tomography (CT) in the investigation of unexplained haemoptysis. Respir Med. 1992;86(1):39–44. doi: 10.1016/s0954-6111(06)80146-0. [DOI] [PubMed] [Google Scholar]
- 39.Naidich DP, Funt S, Ettenger NA, Arranda C. Hemoptysis: CT-bronchoscopic correlations in 58 cases. Radiology. 1990;177(2):357–62. doi: 10.1148/radiology.177.2.2217769. [DOI] [PubMed] [Google Scholar]
- 40.Hidenori M, Yasushi O, Yusuke T, Masanori K, Fumitaka I, Junki E, et al. Use of multidetector row CT to evaluate the need for bronchial arterial embolisation in hemoptysis patients. Respiration. 2010;80:24–31. doi: 10.1159/000253882. [DOI] [PubMed] [Google Scholar]
- 41.Rémy-Jardin M, Bouaziz N, Dumont P, Brillet PY, Bruzzi J, Rémy J. Bronchial and non-bronchial systemic arteries at multidetector row CT angiography: comparaison with conventional angiography. Radiology. 2004;233:741–9. doi: 10.1148/radiol.2333040031. [DOI] [PubMed] [Google Scholar]
- 42.Carette MF, Parrot A, Fartoukh M, Tassart M, Khalil A. Normal and abnormal systemic pulmonary circulation: CT imaging features. J Radiol. 2009;90:1789–800. doi: 10.1016/s0221-0363(09)73283-4. [DOI] [PubMed] [Google Scholar]
- 43.Marshall TJ, Jackson JE. Vascular intervention in the thorax: bronchial artery embolization for haemoptysis. Eur Radiol. 1997;7:1221–7. doi: 10.1007/s003300050279. [DOI] [PubMed] [Google Scholar]
- 44.Botenga AS. Broncho-bronchial anastomosis. A selective angiographic study. Ann Radiol. 1970;13:1–16. [PubMed] [Google Scholar]
- 45.Vujic I, Pyle R, Hungerford GD, Griffin CN. Angiography and therapeutic blockade in the control of hemoptysis. The importance of nonbronchial systemic arteries. Radiology. 1982;143:19–23. doi: 10.1148/radiology.143.1.7063726. [DOI] [PubMed] [Google Scholar]
- 46.Ramakantan R, Bandekar VG, Gandhi MS, Aulakh BG, Deshmukh HL. Massive hemoptysis due to pulmonary tuberculosis: control with bronchial artery embolization. Radiology. 1996;200:691–4. doi: 10.1148/radiology.200.3.8756916. [DOI] [PubMed] [Google Scholar]
- 47.Fellows KE, Stigol L, Shuster S, Khaw KT, Shwachman H. Selective bronchial arteriography in patients with cystic fibrosis and massive hemoptysis. Radiology. 1975;114:551–6. doi: 10.1148/114.3.551. [DOI] [PubMed] [Google Scholar]
- 48.Laganà D, Carrafiello G, Mangini M, et al. Indications for the use of the Amplatzer vascular plug in interventional radiology. Radiol Med. 2008;113(5):707–718. doi: 10.1007/s11547-008-0306-1. doi: 10.1007/s11547-008-0306-1. [DOI] [PubMed] [Google Scholar]
- 49.Mangini M, Laganà D, Fontana F, et al. Use of Amplatzer Vascular Plug (AVP) in emergency embolisation: preliminary experience and review of literature. Emerg Radiol. 2008;15(3):153–160. doi: 10.1007/s10140-007-0696-8. doi: 10.1007/s10140-007-0696-8. [DOI] [PubMed] [Google Scholar]
- 50.Panda A, Bhalla AS, Goyal A. bronchial artery embolization in hemoptysis: a systematic review. Diagn Interv Radiol. 2017;23(4):307–17. doi: 10.5152/dir.2017.16454. [DOI] [PMC free article] [PubMed] [Google Scholar]


