Abstract
Introduction:
High-flow priapism is a persistent partial penile tumescence, related to high flow arterial blood into the corpora. It is much less common than the low-flow priapism, and history of trauma is the more common aetiology. In the treatment of high flow priapism, super-selective embolization is considered treatment of choice when conservative treatment fails as reported in the “European Association of Urology Guidelines on Priapism”, but there are only few series reporting the outcome, the efficacy of different embolic materials and these studies are uncontrolled and relatively small.
Objectives:
The aim of this study is to review the literature to outline the state of the art of this interventional treatment and to analyse the outcome of the different embolic agents.
Methods:
Through Medline database (via PubMed) we searched all the English-language published articles related to priapism. Keywords were chosen according to MeSH terms. We selected case-series from 1990 to 2020 including at least five cases of high-flow priapism. The variables extracted from the selected articles were: number of patients, mean age, diagnostic imaging modality, mono or bilateral involvement of the arteries, embolization material, technical success, clinical success, complications, recurrence rate and type of reintervention, mean follow up, onset of erectile dysfunction.
Results:
We analyzed 11 papers. A total of 117 patients, mean age of 30 years, were studied during a period of 8 to 72 months. Technical success average was 99%, varying from 93 to 100%. Clinical success average was 88%, varying from 56 to 100%. After two or more treatments, resolution of priapism was obtained in all patients. No major adverse events registered. Recurrence rate of 21% (25/117) was observed, and only 4 patients underwent surgery. A total of 17 patients (15%) developed erectile dysfunction (ED). We also created a subgroup analysis focusing on specific outcome with different types of materials. Technical success was very high, 100% for all materials except for PVA particles Clinical Success was at least 70% with all kind of material. Best result was obtained with gel-foam (89%) and the worse with PVA (70%).
Conclusion:
Our data suggested comparable outcomes using different types of materials. In line with the last evidences we suggest that the choice of the embolic material should be selected basing on the expertise of the operator, the characteristic of the fistula and characteristic of the patients. (www.actabiomedica.it)
Keywords: priapism, High flow priapism, endovascular treatment of priapism, penile fistula, embolization of priapism, interventional radiology, cavernous fistula
Introduction
Priapism is defined as a penile erection (partial or complete) that persists for 4h or more and is unrelated to sexual activity (1). It is a complex medical disorder described for the first time in the English medical literature in 1845 on Lancet (2). Priapism can affect all ages but its incidence is very low (0.5–0.9 cases per 100 000 person-years) (3).
For clinical management, priapism can be stratified into three groups: High-flow priapism (arterial or non ischaemic); Low-flow priapism (veno-occlusive or ischemic); Stuttering priapism (recurrent or intermittent) (4).
The most common is low-flow priapism (95% of all priapic episodes). It can be considered a compartment syndrome and it can be caused by many factors; is often idiopathic or associated with haematological dyscrasias (eg sickle cell disease, SCD) (4). It presents as a persistent and painful erection and is a medical emergency because a delay in the treatment can hesitate in penile erectile dysfunctions. The cavernosal blood has low pO2 levels (5). Management of these cases is the competence of urologists. First line treatment is penile blood aspiration and drug injection. When blood aspiration and intracavernous drug injection doesn’t work, as a second line treatment surgery should be considered (6).
Stuttering priapism is characterized by recurrent episodes of painful priapism lasting hours. The aetiology of this pathology is similar to ischaemic priapism. Often it affects patients with SCD. Episodes of stuttering priapism usually increase in frequency and duration hesitating in full episodes of ischemic priapism.
High-flow priapism is a persistent partial penile tumescence, related to high flow arterial blood into the corpora. It is much less common than the low-flow priapism, and history of trauma is the common aetiology. The trauma results in a laceration of a penile artery leading to a high-flow fistula between the artery and the corpora (7).
The clinical presentation can be delayed (hours or days) from the trauma. Clinically is classically not painful and the penis is usually non-rigid. The cavernosal blood is arteriosus, and has high pO2 level. High flow priapism does not require emergency management (4,6). First line treatment consists in conservative measures (e.g. ice compression). Often it is not enough and super-selective embolization performed by interventional radiologists is the treatment of choice. Today surgical treatment is rarely performed given its significant risks. Is performed only in selected cases (e.g. contraindications for selective embolization, or embolization failure) (6).
In the treatment of high flow priapism, super-selective embolization is considered treatment of choice when conservative treatment fails as reported in the “European Association of Urology Guidelines on Priapism”, but there are only few series reporting the outcome, the efficacy of different embolic materials and these studies are uncontrolled and relatively small (6). The aim of this study is to review the literature to outline the state of the art of this interventional treatment and to analyse the outcome of the different embolic agents.
Etiopathogenesis
Trauma is the primary cause of high-flow priapism in boys and men younger than age 55: blunt penile or perineal trauma may cause a laceration of branches of the internal pudendal artery. In older men the primary causes are malignant tumors that, in an advanced stage, can erode arteries. High-flow priapism has been described also in children with inherited diseases (e.g. SCD, Fabry’s disease). Internal pudendal artery or its branches are the site of 99% of all fistulas (8).
From a hemodynamic point of view, the laceration of a cavernous artery results in direct, persistent, and irregular flow of arterial blood inside the vascular lacuna of the erectile tissue (9). The lacunar endothelium adjacent to the fistula is exposed to oxygenated blood with high velocity and turbulent flow, which creates a shear stress and stimulates the release of nitric oxide. Shear stress and high oxygen tension stimulate the synthesis and release of endothelium-derived nitric oxide, resulting in arterial and trabecular dilatation through the corpora cavernosa. The incomplete rigidity of the penis most likely depends on incomplete corporeal smooth muscle relaxation. Arterial priapism can develop early after the trauma or with a delay of several days. The delay may possibly be explained by spasm of the injured vessel (10). As reported in literature, only two thirds of patients exhibit penile erection immediately following trauma; in the others, priapism develops over 1 to 15 hours, which suggests that hemodynamically relevant fistulas may develop from initially very small vascular defects. The bulbourethral arteries are responsible for the fistulas in about one third of patients (11).
Diagnosis of high flow priapism
To make diagnosis of priapism and classify it, it is essential to collect an accurate medical history, make a scrupulous physical examination, perform laboratory tests and complete with the most appropriate imaging.
History
High-flow priapism should be considered when the patient presents an erection not associated with pain, the erection generally is partial and the duration has not been accompanied by progressive discomfort. The patient has a history of trauma that led to the formation of an arteriovenous fistula and the appearance of priapism may be delayed for several hours or days after the initial injury (4).
Physical Exam
Examination of the genitalia, the perineum, and the abdomen is mandatory for the diagnostic evaluation of priapism. In low-flow priapism, the corpora cavernosa are rigid and very tender to palpation, but the glans penis is soft (12). In patients with high-flow priapism on exam the corpora are tumescent but not fully rigid and the penis is only partially erect (13). There may be residual bruising and some tenderness on palpation, depending on the location of the trauma and the elapsed time since the trauma.
Laboratory Testing
Laboratory evaluation should include a complete blood count, white blood cell with blood cell differential, platelet count and coagulation profile, to rule out infection and to detect hematologic abnormalities. If drugs are suspected from the psychosocial history, should be performed also urine and plasma toxicology. A corporal blood gas analysis is recommended to differentiate between low-flow and high-flow priapism: in low-flow priapism the blood is hypoxic (pO2 < 30 mm Hg, pCO2 > 60 mm Hg, pH <7.25), in high-flow priapism the blood is red in colour and oxygenated (pO2 > 90 mm Hg, pCO2 < 40 mm Hg, pH 7.40) (4).
Imaging
Colour Doppler Ultrasonography (CDUS), increasingly widespread in the setting of urologic vascular disease (14), can help in differentiation between low-flow and high-flow priapism (fig. 1). In low-flow priapism, cavernousal arterial flow typically demonstrates a “high-resistance, low velocity” waveform and arterial flow is usually absent. In high-flow priapism, CDUS demonstrates a “low-resistance, high-velocity” arterial waveform (15,16). Is important to underline that the sensitivity of CDUS in localizing an arterio-cavernosal fistula is nearly 100% (17).
Figure 1.
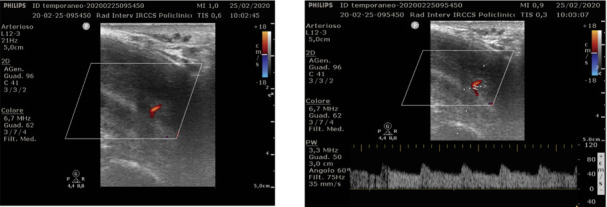
Detection of fistula on Colour Doppler examination: it is a hypoechoic area surrounded by echogenic tissue. Subsequently the Power Doppler shows a venous base flow with arterial peaks
The arteriocavernosal fistula is a hypoechoic area surrounded by echogenic tissue with mixed signal at the power doppler evaluation. CDUS is also preferred in follow-up of these patients.
The use of magnetic resonance imaging (MRI) in the diagnosis of priapism has not been already well established, and MRI has a limited role in the initial diagnosis because priapism represents an emergency situation and MRI takes too much time. There has been only few studies that reported MRI findings in cases of high-flow priapism (fig. 2-3). In the study of White et al, MRI showed the arteriocavernosal fistula in all patients (18). The authors of that article underlined that while CDUS demonstrates characteristic flow patterns of priapism, MRI can better characterize tissue and demonstrate the presence of arteriocavernosal fistula or thrombus (18). Additional benefits of MRI include its ability to predict nonviable smooth muscle within the corpora after episodes of priapism and detecting unusual conditions such as malignant infiltration (12).
Figure 2.
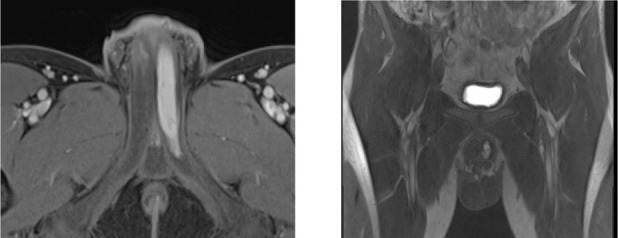
Contrast-enhanced T1 images on axial and coronal plane that show arterial enhancement of the left cavernous body because of the presence of arteriocavernosal fistula.
Figure 3.
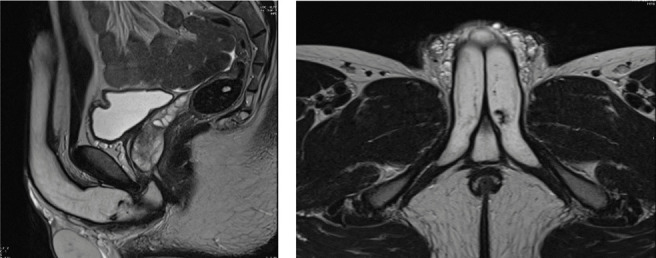
T2 images on sagittal and axial plane that show flow void in the left cavernous body.
When high-flow priapism is related to a pelvic trauma, contrast-enhanced CT is recommended to assess associated diseases (19) but is not useful in diagnosis of priapism (20).
Materials and Methods
We searched electronic information sources through Medline database (via PubMed) and bibliographic lists of relevant articles to identify studies reporting outcome of endovascular treatment of post traumatic priapism.
All the articles related to human medicine among English-language published literature were selected. Keywords were chosen according to Medical Subject Heading (MeSH) terms: “High flow priapism”, “High flow priapism endovascular treatment”, “High flow priapism and interventional radiology”, “Post traumatic penile arteriovenous fistula”, “Penile arteriovenous fistula’’, “Endovascular treatment and penile arteriovenous fistula”, “Embolization and penile fistula”, “penile cavernous arteries fistula”, “Interventional treatment penile cavernous fistula”, ‘’interventional radiology and priapism’’.
Additional studies were identified through manual research of the bibliographies from primary studies, review articles, and key journals.
Articles consisting in case-series from 1990 to 2020 including at least five cases of high-flow priapism, were considered eligible for inclusion in the present review.
First, the articles were selected by the titles focusing on our area of interest. Then the abstracts were reviewed to find the best matching articles. All articles containing data reported previously were excluded. Then the second step consisted in reviewing the full text version of the selected articles. The variables extracted from the selected articles were: number of patients, mean age, diagnostic imaging modality, mono or bilateral involvement of the arteries, embolization material, technical success, clinical success, complications, recurrence rate and type of reintervention, mean follow up, onset of erectile dysfunction. When not mentioned the information was classified as not available.
Before the extraction of data from the studies, the following clear definitions of all outcomes of interest were established. Clinical success was defined as complete resolution of priapism after one or two (when fistula was bilateral) interventions. Technical success was established as the exclusion of the fistula on the final angiography. Recurrence rate was considered as priapism persistence or relapse after one or two (when fistula was bilateral) interventions. Erectile dysfunction was defined as a IIEF-5 value under 22 (when reported) or as the impossibility to have satisfactory sexual intercourse. Adverse events were considered complications related to procedure, excluding erectile dysfunction. When adverse events were not reported we considered absence of adverse events.
Results
We analyzed endovascular treatment of high flow priapism in 11 papers (8-11,21-27), published during the last 30 years (1990-2020) which met our inclusion criteria. Table 1 summarizes all the data collected by the studies focusing on interventional treatment of high flow priapism.
Table 1.
Collection of data from our selected case series
| Study | Patients | Mean Age | Imaging | Artery involved Mono/bilateral | Embolization Matherial | Tech succ | Clin succ | Adverse Events | *Recurrence rate (prev intervention) | Type of reintervention** | Mean FUP (months) | Erectile disfuction |
| Ciampalini Et al. 2002 | 9 | 36 | CDUS | NA | Absorbable syntetic Clot | 100% | 66% | No | 3(33%) | 2(clot) 1(surgical ligature) | 41 | 20% |
| Gorich Et al. 2002 | 6 | 16 | CDUS | 2 Monolateral 4 Bilateral |
3 Gelatin Sponge 3 Microcoils |
100% | 83% | No | 1(17%) (microcoil) |
1 (microcoil) | 11.8 | 0 |
| Bertolotto Et al. 2003 | 9 | 29 | CDUS | 8monlateral 1bilateral |
PVA beads (300-500) |
100% | 56% | No | 4(44%) | 3 (PVA) 1 (PVA than surgery) |
NA | 1(11%) |
| Savoca Et al.2004 | 15 | 32 | CDUS | 13monolateral 2 bilateral |
PVA beads (350-500) |
93% | 73% | 4 (27%) bruising and pain in site of needle |
4(27%) | 3 (PVA) 1 (2 PVA than surgery) |
55 | 1(7%) |
| Bartsh Et al. 2004 | 6 | NA | CDUS | 3monolat 3bilateral |
Gelatin Sponge Microcoil |
100% | 83% | NA | 1(17%) (microcoil) |
1(vasospasm) | 48 | 0 |
| Sullivan Et al. 2006 | 5 | 31 | CDUS | 5 monolateral | 2 Gelfoam 1 autol clot 1 Microcoils 1 Microcoil+ Gelfoam |
100% | 60% | NA | 2(40%) (1blood clot, 1microcoil) |
2(gelfoam+microcoil) | 12 | 1(20%) |
| Kim Et al. 2007 | 27 | 39 | CDUS | 16monolateral 11 bilateral |
12 Gelfoam 12 autol clot 1 Microcoils 1 NBCA 1 PVA beads(500-700) |
100% | 89% | No | 3 (11%) 2 autol clot 1sponge |
2 (1 autologous clot, 1 gelfoam) 1 (1 emb, than surgery) |
13 | 7(26%) |
| Liu Et al. 2008 | 8 | 33 | CDUS | 7 monolateral 1 bilateral |
2 gelfoam 6 Microcoils |
100% | 75% | NA | 2 (25%) (gelfoam) |
2 (microcoils) | 18 | 2(25%) |
| Cantasdemir Et. al 2010 |
7 | 10 | CDUS | 7 monolater | 7 autol clot | 100% | 85% | No | 2 (29%) (aut clot) |
2 (aut clot) | 72 | 0 |
| Pei Et al.2018 | 16 | 24 | CDUS | 15monolateral 1bilateral |
10 Microcoil 4 Gelfoam 2 Microcoil+gelfoam |
100% | 94% | No | 1 (6%) (Microcoil) |
1(microcoil+ spongel) |
8 | 2(13%) |
| De Magistris Et al.2019 | 9 | 33 | CDUS | 5 monolateral 6 bilateral |
6 Microcoils 2PVA 1Gelfoam |
100% | 78% | No | 2 (22%) (Microcoil monolaterl) | 2 MicroCoil | 24 | 1(11%) |
| Total/Mean | 117 | 30 | CDUS | 81monolateral 29 bilateral |
See Tab2 | 99% | 80% | No | 25(21%) | 25 (4 surgery) | 30 | 17(15%) |
*Recurrence rate and type of embolization performed **Type of reintervention and type of used material
A total of 117 patients, mean age of 30 years, were studied during a period of 8 to 72 months, with a median follow-up of 30 months. The only imaging modality used was CDUS, which is the mainstay of diagnosis. Technical success average was 99%, varying from 93 to 100%. Clinical success average was 88%, varying from 56 to 100%, but after two or more treatments, resolution of priapism was obtained in all patients. No major adverse events registered, only 4 patients developed minor complications related to the access site, brushing or pain.
A recurrence rate of 21% (25/117) was observed, in most cases after a second treatment detumescence was obtained, and only 4 patients underwent surgery. A total of 17 patients (15%) developed erectile dysfunction (ED), most of them were already mild impotent or developed ED after surgery. Only in 8 patients, ED may be related to transarterial embolization. In any case, sildenafil administration has been sufficient to have satisfactory intercourse.
We created a subgroup analysis (Table 2) focusing on specific outcome with different types of materials, excluding the only case treated with NCBA. As expected from previous studies, technical success was very high, 100% for all materials except for PVA particles which were not able to embolize sufficiently in one case.
Table 2.
Subgroup analysis of different embolization materials. AE= Adverse events. RR= Recurrence Rate
| Materials | Patients | Tech success | Clinical success | AE | RR | Material for Reintervention | Erectile Disfunction |
| PVA (300/350-500 o 500-700) | 27 | 26(96%) | 19(70%) | No | 8(29%) | 8 | 2 (7%) |
| Microcoils | 27 | 27(100%) | 21(78%) | No | 6(22%) | 5 | 5(19%) |
| Gel-foam | 23 | 23(100%) | 20(87%) | No | 3(13%) | 1 | 4(17%) |
| Autologous Clot | 29 | 29(100%) | 21(72%) | No | 8(28%) | 5 | 5(17%) |
Clinical Success was at least 70% with all kind of material. Best result was obtained with gel-foam (89%) and the worse with PVA (70%). It is important to underline that detumescence was obtained in 100% of patient after repeating embolization or surgery.
No adverse events related to the material has been reported.
Low recurrence rate was obtained in patients treated with gel-foam followed by those treated with microcoils, respectively 13% and 22%.
Patients treated with PVA particles and autologous blood clot had a recurrence of 28% and 29% respectively.
ED was less than 20% in all cases and, if we exclude patients who was mild impotent before treatments, only 10 patients on 117 developed ED.
Discussion
Non-ischemic priapism is not a medical emergency and does not require urgent medical intervention, thus conservative management, such as watchful waiting, must be considered an alternative option (17,28). In fact it has been reported that non-ischemic priapism often resolves spontaneously (17) and most patients affected by this kind of priapism are able to have normal erections and satisfactory sexual intercourse.
The rationale of the conservative approach is to obtain detumescence at an early stage with ice or compressive perineal dressing that may be expected to cause vasospasm and thrombosis of the ruptured artery in days to weeks.
However, excessive arterial inflow with high oxygen levels and chronic erection, caused by activation of guanylate cyclase enzyme, may be dangerous to the cavernosal smooth muscle and connective tissue matrix, leading to irreversible corporal fibrosis and consequently to erectile dysfunction (9).
For these reasons it is essential to perform embolization in trauma-related cases as soon as possible avoiding too long watchful waiting periods, because of the increased risk of secondary vascular alterations.
Interventional radiology has a key role in the management of high flow priapism, as reported in many guidelines as the “European Association of Urology Guidelines on Priapism”.
Superselective TAE represents a minimally invasive and efficacious approach. In literature it is reported that near 100% of patients had a satisfactory detumescence with recurrence rate of 6.3% (25).
We reported a comparable technical and clinical success but higher recurrence rate: 25% vs 6.5%. It is important to underline that, contrary to previous studies who differentiated recurrence from persistence considering recurrence only as a relapse in our study, recurrence rate is referred to persistence and/or relapse of detumescence after one embolization, so we included all potentially includable patients.
Superselective arterial embolization may be performed with a number of agents including microcoils, polyvinylalcohol (PVA), N-butylcyanoacrylate (NBCA), gel-foam and autologous blood clot (20).
Temporary embolic agents are preferred by many interventional radiologists because there is lower risk of postprocedural impotence (1). NBCA is rarely used and we have found very few case series in the literature.
For Cantasdemir et al (25) autologous blood clot is a good occlusive agent because of its numerous advantages. It carries low risk of foreign body reaction and antigenicity, does not require additional costs for use, and more importantly, it is a temporary occlusive agent that allows recanalization of the cavernosal artery and subsequently restores the normal erectile mechanism (29).
Moreover it can be successfully used in the embolization of fistula especially for treating priapism in children (22).
Gel-foam (also called Spongel or gelatin sponge) is a temporary occlusive agent with a duration of action of 5/6 weeks after being injected. Detumescence can be achieved with gel-foam without permanent vascular occlusion (30). O’sullivan et al believe that this increases the probability of preserving the patients erectile function (22).
In the experience of Kim et al, using autologous blood clot or gelatin sponge there was no significant change in the quality of patients erection during the follow-up period and also there was no difference affecting the requirement for repeat embolization (23). The disadvantages of Gel-foam is that might lead to recanalization and priapism recurrence. It is also considered difficult to control and its positioning could result in occlusion of unexpected vessels (24).
When the preliminary angiographic study shows multiple small caliper fistulas, it is preferred to use PVA microparticles (300–500μm) because of the ability to penetrate the microvascular network (27,31). Particulate embolic injection should be performed with gradual pressure to avoid the proximal reflux and the unintentional peripheral embolization that can lead, especially with PVA, to infection and mostly ischemia (27). Ethylene-vinyl alcohol copolymer is not used in high flow priapism, even though has been demonstrated to be safe and effective in arterial embolization (32).
Microcoils are permanent occlusive agents. According with the study of Liu et al., microcoils were more effective and permanent, with accurate positioning in the desired vessel. They suggested that microcoil is a more reliable agent, even for recurrent cases and longstanding priapism. Theoretically, microcoils increase the risk of permanent vascular occlusion and subsequent erectile dysfunction (24) but no major ischaemic events have been demonstrated in literature (20).
Nevertheless, several studies have shown microcoils to have a preservative effect on erectile function, even with bilateral embolization.
Superselective cavernous artery embolization with microcoils has a satisfactory detumescence effect and might help to preserve erectile function, especially for younger patients with a unilateral arterial fistula. Therefore, Liu et al. suggest that embolization with microcoils should not be indicated for older patients (24). In our study, microcoils has not the lowest recurrent rate. Nevertheless the most of patients treated with microcoils who developed recurrence were patients with multiple fistulas and after repeat embolization fistulas has been closed either spontaneously (vasospasm) or using again microcoil and/or microcoil+spongel. Moreover, patients treated with spongel who developed recurrence, received a second embolization with microcoil solving priapism. This evidences strengthen the idea of exceptional ability of microcoils to close fistulas in high flow priapism and the importance of positioning the coil correctly which depends on operator experience in the use of this specific material (fig. 4).
Figure 4.
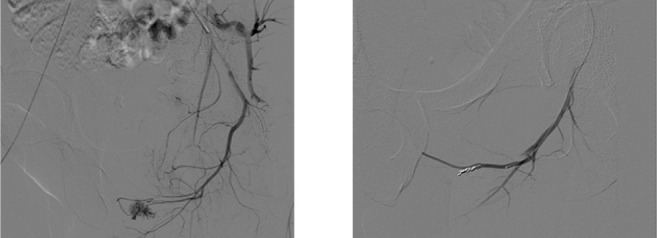
Angiography pre and post embolization with microcoils.
As expected microcoil was the material which caused more cases of ED (19%) also excluding patients with previous ED, confirming the risk of ED with the use of this type of permanent embolization material.
Conclusion
Our data suggested comparable outcomes using different types of materials.
It is clear that the type of embolo-therapy was not the major factor affecting the recurrence of priapism and this concept was underlined in more recent papers, particularly by Kim et al. (23), a multicenter study comparing treatment results among embolic agents in 27 patients, which represents the largest series reported in literature.
In line with the last evidences we suggest that the choice of the embolic material should be selected basing on the expertise of the operator, the characteristic of the fistula and characteristic of the patients.
Ethical Approval:
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent:
Written informed consent to the CT and the MR exams was obtained from all subjects in this study.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Montague DK, Jarow J, Broderick GA, et al. American Urological Association guideline on the management of priapism. J Urol. 2003 Oct;(170(4 Pt 1)):1318–24. doi: 10.1097/01.ju.0000087608.07371.ca. [DOI] [PubMed] [Google Scholar]
- 2.Tripe JW. Case of continued priapism. Lancet. 1845;2:8. [Google Scholar]
- 3.Eland IA, van der Lei J, Stricker BH, et al. Incidence of priapism in the general population. Urology. 2001 May;57(5):970–2. doi: 10.1016/s0090-4295(01)00941-4. [DOI] [PubMed] [Google Scholar]
- 4.Broderick GA, Kadioglu A, Bivalacqua TJ, et al. Priapism: pathogenesis, epidemiology, and management. J Sex Med. 2010 Jan;(7(1 Pt 2)):476–500. doi: 10.1111/j.1743-6109.2009.01625.x. [DOI] [PubMed] [Google Scholar]
- 5.El-Bahnasawy MS, Dawood A, Farouk A, et al. Low flow priapism: risk factors for erectile dysfunction. BJU int. 2002 Jan;89(3):285–290. doi: 10.1046/j.1464-4096.2001.01510.x. [DOI] [PubMed] [Google Scholar]
- 6.Salonia A, Eardley I, Giuliano F, et al. European Association of Urology guidelines on priapism. Eur Urol. 2014 Feb;65(2):480–9. doi: 10.1016/j.eururo.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Witt MA, Goldstein I, Saenz de Tejada I, et al. Traumatic laceration of intracavernosal arteries: the pathophysiology of nonischemic, high flow, arterial priapism. J Urol. 1990 Jan;143(1):129–32. doi: 10.1016/s0022-5347(17)39889-0. [DOI] [PubMed] [Google Scholar]
- 8.Bartsh G, Kuefer R, Engel O. High-flow priapism: colour-Doppler ultrasound-guided superselective embolization therapy. World J Urol. 2004;22:368–370. doi: 10.1007/s00345-004-0426-8. [DOI] [PubMed] [Google Scholar]
- 9.Ciampalini S, Savoca G, Buttazzi L, et al. High-flow priapism: treatment and long-term follow-up. Urology. 2002 Jan;59(1):110–3. doi: 10.1016/s0090-4295(01)01464-9. [DOI] [PubMed] [Google Scholar]
- 10.Bertolotto M, Quaia E, Pozzi Mucelli F, et al. Color Doppler Imaging of Posttraumatic Priapism before and after Selective Embolization. RadioGraphics. 2003;23:495–503. doi: 10.1148/rg.232025077. [DOI] [PubMed] [Google Scholar]
- 11.Gorich J, Ermis C, Kramer SC, et al. Interventional Treatment of Traumatic Priapism. J Endovasc Ther. 2002 Oct;9(5):614–7. doi: 10.1177/152660280200900511. [DOI] [PubMed] [Google Scholar]
- 12.Levey HR, Segal RL, Bivalacqua TJ. Management of priapism: an update for clinicians. Ther Adv Urol. 2014;6(6):230–244. doi: 10.1177/1756287214542096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang YC, Harraz AM, Shindel AW, et al. Evaluation and management of priapism: 2009 update. Nat Rev Urol. 2009;6(5):262–271. doi: 10.1038/nrurol.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rebonato A, Auci A, Sanguinetti F, et al. Embolization of the Periprostatic Venous Plexus for Erectile Dysfunction Resulting From Venous Leakage. J Vasc Interv Radiol. 2014 Jun;25(6):866–72. doi: 10.1016/j.jvir.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Halls JE, Patel DV, Walkden M, et al. Priapism: pathophysiology and the role of the radiologist. Br J Radiol. 2012;85(1):S79–S85. doi: 10.1259/bjr/62360925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donaldson JF, Rees RW, Steinbrecher HA. Priapism in children: a comprehensive review and clinical guideline. J Pediatr Urol. 2014;10(1):11–24. doi: 10.1016/j.jpurol.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 17.Hakim LS, Kulaksizoglu H, Mulligan R, et al. Evolving concepts in the diagnosis and treatment of arterial high flow priapism. J Urol. 1996;155(2):541–548. [PubMed] [Google Scholar]
- 18.White C, Gulati M, Gomes A, et al. Pre-embolization evaluation of high-flow priapism: magnetic resonance angiography of the penis. Abdom Imaging. 2013;38(3):588–5979. doi: 10.1007/s00261-012-9936-9. [DOI] [PubMed] [Google Scholar]
- 19.Ierardi AM, Duke E, Lucchina N, et al. The role of interventional radiology in abdominopelvic trauma. Br J Radiol. 2016;89(1061):20150866. doi: 10.1259/bjr.20150866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ierardi AM, Piacentino F, Fontana F, et al. The Role of Endovascular Treatment of Pelvic Fracture Bleeding in Emergency Settings. Eur Radiol. 2015 Jul;25(7):1854–64. doi: 10.1007/s00330-015-3589-3. [DOI] [PubMed] [Google Scholar]
- 21.Savoca G, Pietropaolo F, Scieri F, et al. Sexual function after highly selective embolization of cavernous artery in patients with high flow priapism: long-term follow-up. J Urol. 2004;172:644–647. doi: 10.1097/01.ju.0000132494.44596.33. [DOI] [PubMed] [Google Scholar]
- 22.O’Sullivan P, Browne R, McEniff N, et al. Treatment of ‘‘High-Flow’’ Priapism with Superselective Transcatheter Embolization: A Useful Alternative to Surgery. Cardiovasc Intervent Radiol. 2006;29:198–201. doi: 10.1007/s00270-005-0089-x. [DOI] [PubMed] [Google Scholar]
- 23.Kim KR, Shin JH, Song HY, et al. Treatment of High-flow Priapism with Superselective Transcatheter Embolization in 27 Patients: A Multicenter Study. J Vasc Interv Radiol. 2007 doi: 10.1016/j.jvir.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 24.Liu B, Xin Z, Zou Y, et al. High-Flow Priapism: Superselective Cavernous Artery Embolization with Microcoils. J Urol. 2008 doi: 10.1016/j.urology.2008.01.087. [DOI] [PubMed] [Google Scholar]
- 25.Cantasdemir M, Gulsen F, Solak S, et al. Posttraumatic high-flow priapism in children treated with autologous blood clot embolization: long-term results and review of the literature. Pediatr Radiol. 2011 doi: 10.1007/s00247-010-1912-3. [DOI] [PubMed] [Google Scholar]
- 26.Pei R, Yang M, Wang C, et al. Superselective Transcatheter Artery Embolization in Patients with Non-ischemic Priapism. Cardiovasc Intervent Radiol. 2018 doi: 10.1007/s00270-018-1895-2. [DOI] [PubMed] [Google Scholar]
- 27.De Magistris G, Pane F, Giurazza F, et al. Embolization of high-flow priapism: technical aspects and clinical outcome from a single-center experience. La radiologia medica. 2019 doi: 10.1007/s11547-019-01113-w. [DOI] [PubMed] [Google Scholar]
- 28.Hatzichristou D, Salpiggidis G, Hatzimouratidis K, et al. Management strategy for arterial priapism: therapeutic dilemmas. J Urol. 2002;168:2074–7. doi: 10.1016/S0022-5347(05)64299-1. [DOI] [PubMed] [Google Scholar]
- 29.Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med. 2004;1:116–120. doi: 10.1111/j.1743-6109.2004.10117.x. [DOI] [PubMed] [Google Scholar]
- 30.Cohen GS, Braunstein L, Ball DS, et al. Selective arterial embolization of idiopathic priapism. Cardiovasc Intervent Radiol. 1996;19:47–49. doi: 10.1007/BF02560148. [DOI] [PubMed] [Google Scholar]
- 31.Petrillo M, Pesapane F, Fumarola EM, et al. State of the art of prostatic arterial embolization for benign prostatic hyperplasia. Gland Surg. 2018;7(2):188–199. doi: 10.21037/gs.2018.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ierardi AM, Xhepa G, Duka E, et al. Ethylene-vinyl Alcohol Polymer Trans-Arterial Embolization in Emergency Peripheral Active Bleeding: Initial Experience. Int Angiol. 2015;34:28–35. [PubMed] [Google Scholar]


