Abstract
The COVID-19 epidemic, which began in Wuhan in December 2019, quickly spread all over the world, leading in a few months to a high number of deaths also in healthcare workers. The purpose of the study is to a) describe the importance of a correct management of SARS-CoV-2 infections; b) report the number of positive healthcare workers after the epidemic phase and to describe their socio-characteristics data, the main methods of transmission and the symptoms; c) to report the seroconversion rate of healthcare workers (HCWs). The study was conducted from March 9, 2020 to June 19, 2020 in three phases:1) in a first phase, we implemented the guidelines to be followed for patient care in our hospital; 2) in a second phase, we provided the epidemiological investigation/contact tracing of HCWs; 3) we collected swabs on all healthcare workers and we also performed serological investigation. The number of healthcare workers under surveillance is of 2611 subjects and, of these, only 0.65% contracted COVID-19. In particular, 70.6% of these have been infected in the healthcare setting, 11, 8% in the family and 17.6% returning from high risk areas. Ultimately, only 0.1% of HCWs dedicated to the treatment of COVID-19 patients contracted the infection (one was asymptomatic). Only 2% of HCWS were positive for serological investigation. (www.actabiomedica.it)
Keywords: management, healthcare workers, COVID-19, SARS-CoV-2, symptoms
Introduction
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) epidemic, which began in Wuhan, China in December 2019, quickly spread all over the world, leading in a few months to a high number of deaths and 8,986,016 infected (last update June 22) (1).
Many initial cases reported outside of China were imported or were linked to travellers from China (2). However, as community transmission has become widespread, the source of cases of COronaVIrus Disease 19 (COVID-19) in several countries has not been established (3).
In Italy, the first cases have been described in Rome on January 29 involving two Chinese tourists referring to the Spallanzani Institute, representing imported cases (4). Subsequently, unfortunately, on February 21 the first autochthonous Italian case of COVID 19 was identified, in Lombardy (Codogno), the region that first struck by this invisible infection paid the highest price in terms of mortality and morbidity (5,6).
The epidemiological nature of the disease, with its long incubation period, has given time to the health systems of other regions, thanks also to the lockdown measures undertaken, to get ready by setting up proper COVID hospitals or wards (7). At the same time, the continuous updating of international and national guidelines, also allowed a further limitation of the contagions, as far as well applied.
In particular, on January 30 2020, after the second meeting of the Security Committee, the General Director of the World Health Organization (WHO) has declared the international outbreak of COVID-19 as a public health emergency of international relevance (Public Health Emergency of International Concern - PHEIC), as established in the International Health Regulations (8).
In Italy, on February 21, the Ministry of Health enforced the quarantine measure with active surveillance for fourteen days for individuals who have had close contacts with confirmed COVID-19 positive subjects, or in the last fourteen days had returned from high risk areas of China, by the means of the territorially competent health authorities which had the obligation to notify the Local Health Units (9).
The definition of close contact and the indications related to laboratory diagnosis were established by the Ministry of Health (10).
Contact tracing, in combination with the early detection of cases and in synergy with other measures such as physical distancing, are essential actions to combat the ongoing epidemic, as well as for preventive purposes. The purpose of identifying and managing the contacts of probable or confirmed cases of COVID-19 is to quickly identify and isolate the secondary cases, in order to intervene and interrupt the transmission chain. It is also worth remembering the role of asymptomatic in the dynamics of the epidemic spread of COVID-19 (11).
In healthcare workers (HCWs) “the provision referred to in article 1, paragraph 2, letter h), of the decree-law of 23 February 2020, no. 6 (quarantine) does not apply and they must instead have supervised. The same HCWs must suspend their work activity only in the case of respiratory symptoms or a positive test for SARS-CoV-2 (12). Additionally, in Sicily, the local government introduced as mandatory the search for SARS-CoV-2 on all HCWs using a nasopharyngeal swab, thus testing also asymptomatic subjects (13).
The molecular diagnostic protocols for SARS-CoV-2 drawn by WHO are based on the identification of viral RNA by Real-Time reverse transcription polymerase chain reaction (Real Time RT-PCR) (14-16). In Italy, Annex 4 of the circular of the Ministry of Health dated February 22 (17) establishes the guidelines for laboratory protocols and all details on the collection and sending of biological samples for diagnosis. From that time on SARS-CoV-2 detection could be performed only by certified laboratories and main hospitals identified by regional health departments on the basis of the afore mentioned protocols. For specimen collection in clinical settings samples were taken from the lower respiratory tract using sputum, endotracheal aspiration, or bronchoalveolar lavages. In the event that patients do not show signs of lower respiratory tract disease, or if the specimen collection was not possible, even if clinically indicated, samples taken from the upper respiratory tract such as nasopharyngeal aspirate or nasopharyngeal swabs was recommended.
In this context, serological testing can have a crucial role in identifying convalescent cases or people with milder symptoms who might have been missed by other surveillance methods. The preliminary observations, available from the initial outbreaks in China, quantified in about 85% the total number of infected people from high risk areas (18). It should be emphasized that the presence of antibodies does not necessarily translate into immunity, since not all antibodies are able to neutralize the virus. Serological tests can provide a qualitative (yes/no) or quantitative measurement of antibodies relative to a specific viral antigen. However, the ability of antibodies to prevent viral replication and clear infection is determined through neutralization assays. To date, it appears that the truly neutralizing antibodies are those specific to the spike protein (S) and nucleocapsid protein (N) of SARS-CoV-2 (19). It should be emphasized that one of the most important limits for the extension of serological test to the entire population is represented by the sensitivity and specificity values of the test, but thanks to a Bayesian method, it is possible to build a range of credibility values of the prevalence when the sensitivity and the specificities are unknown (20).
The purpose of this study is to a) describe the importance of a correct management of SARS-CoV-2 infections and of a correct organization b) report the number of positive HCWs after the epidemic phase and to describe their socio-characteristics data, the main methods of transmission and the symptoms c) report the seroconversion rate of HCWs.
Materials and methods
The University Hospital “G.Martino” of Messina includes 14 pavilions, indicated with the letters of the alphabet which have four to six raised floors with a total of about 570 beds. Following the COVID-19 epidemic, an entire pavilion was dedicated to the care of these patients (70 places) and other 90 have been added at a later stage, also creating a “surgical area” for the exclusive use of positive patients. The number of healthcare workers in the entire structure is of 2311 units plus 330 postgraduate medical doctors for a total of 2611 HCWs.
The study was conducted from March 9, 2020 to June 19, 2020 in three phases:
1) In a first phase, the guidelines to be followed in patient care, the indications on a correct and rational use of individual protection devices and sanitizers were implemented in the University Hospital by the Hospital Hygiene Unit and the Health Department;
2) In a second phase, a plan was adopted for the epidemiological investigation and contact tracing of HCWs who for clinical or epidemiological reasons (confirmed / probable case contacts or individuals coming from city at high risk or foreign countries) required the start of the protocol. The pharyngeal swab was then performed on symptomatic subjects for the detection of SARS-CoV-2.
In particular, the epidemiological investigation was carried out by adapting the model provided by the superior health institution (SHI) and implemented with the European Center of Disease Control (ECDC) guidelines, by collecting the socio-personal information (gender, age, job, hospital unit), the date of onset of symptoms and the type of symptoms (by monitoring the subjects twice a day on a daily bases), the possible return from areas at high risk, the way of contact with any suspected/ confirmed case or with subjects with flu-like symptoms and the adherence during the patient’s approach of the company guidelines on the use of personal protective equipment (PPE) and on hand sanitization measures (as indicated in the document provided by ECDC), the vaccination status for influenza, any contact with other individuals and compliance with isolation measures.
3) The third phase, in accordance with the indications of the regional decree, was represented by the performing of the swabs on all the healthcare workers and moreover, at a later time, from the start of the serological investigation.
We collected nnasopharyngeal swabs by all HCWs and they were immediately processed for molecular SARS-CoV-2 detection using Allplex™ 2019-nCoV Assay (Seegene, Korea). Briefly, viral RNA was extracted using a Nuclisens Easymag platform (BioMérieux), which can process 24 samples per run. A total of 200 µl of each sample was extracted and eluted with 100 µl of elution buffer, according to manufacturer’s recommendations with minor modifications. Briefly, to enhance the recovery of viral RNA, 10 μL of poly (A) RNA carrier (Qiagen) were added, after the lysis incubation step, to each sample before the addition of magnetic silica. Amplification and identification of 2 target genes specific for COVID-19 and an E gene specific for all of Sarbecovirus including SARS-CoV-2, were performed using the Allplex™ 2019-nCoV, a multiplex RT PCR assay, according to the manufacturer’s instructions, on a CFX96 Instrument (Bio-Rad Laboratories). Single RT-PCR was performed in a 25-µL reaction mixture for each sample. The threshold cycle (Ct) from the following fluorogenic probes: FAM (E gene), Cal Red 610 (RdRp gene), Quasar 670 (N gene) and HEX (internal control) were acquired. Samples were considered positive with a Ct value <40 for any gene. The samples must be considered negative when the internal control, but not the viral genes, are amplified. When there was no amplification of the internal control the samples must always be considered invalid.
Later, according with the Document of the Ministry of Health titled “Patient cured for Covid-19” (21), concerning the screening of the asymptomatic HCWs; and the Circular of the Ministry of Health of May 9, 2020 (22) concerning the interpretation of serology, we proceed to serological investigation as follows:
1) If the HCW is positive for IgM (with or without IgG) serum, he/she must remain in home isolation as long as it is negative for the presence of SARS-CoV-2 ribonucleic acid (RNA) on nasopharyngeal swab, repeated twice for 2 consecutive days, and only on this case the HCW will be able to return to work. However, for prudential purposes, serological test was carried out again after 7 days together with nasopharyngeal swab for SARS-CoV-2 RNA detection.
2) If the HCW is positive for serum IgG only, he/she will have to remain in close home isolation until it is negative for the presence of SARS-CoV-2 RNA on nasopharyngeal swab, repeated twice for 2 consecutive days. At that point the HCW will be able to return to work.
For the assessment of IgG and IgM antibodies against SARS-CoV-2 we used MAGLUMI 2019-nCoV IgG and IgM (two indirect CLIAs) in human serum or plasma samples, on the fully automated MAGLUMI analyser (SNIBE–Shenzhen New Industries Biomedical Engineering Co., Ltd, Shenzhen, China). According to the manufacturer’s declarations, the antibodies used in these assays are directed against both CoV-S (spike) and CoV-N (nucleocapsid). A value ≥1.10AU/mLis considered reactive, whilst the overall reproducibility declared by the manufacturer is between 6.8% and 8.7%.
Results
The number of healthcare workers under surveillance was equal to 2611 units and of these only 0.65% (17) contracted SARS-CoV-2 infection.
In particular, 43 epidemiological investigations were carried out for the presence of SARS-CoV-2 like symptoms or for the exposure to suspected/confirmed cases of COVID-19 disease with the detection of 17 positive cases among HCWs. The average age of the subjects interviewed was 47 ± 11 years, equally distributed by gender. The socio-personal and working characteristics of the positive subjects and of all the interviewees are summarized in Table 1.
Table 1.
Socio-personal and working characteristics of the positive subjects and of all the interviewees
| Characteristics | Interviewed HCWs (n=43) %(n) | Positive HCWs (n=17) %(n) |
|
Gender females |
51 (22) | 58,8 (10) |
| Mean Age ±SD | 47 ± 11 years | 46 ± 13 years |
Hospital ward^
|
18,4 (8) 44,2 (19) 23,3(10) 11,6 (5) 2,3 (1) |
17,6 (3) 52, 9 (9) 5,9 (1) 23,5 (4) 0 (0) |
HCWs type
|
52,5 (21) 40 (16) 12,5 (5) 2,5 (1) |
58,8 (10) 23,5 (4) 17,6 (3) 0 (0) |
^hospital driver = 1
The type of exposure was most represented by the workplace (67.4%; n = 29), followed by contacts with suspected/confirmed cases in the family environment, or outside the work area, or the onset of flu like symptoms (23.3%; n = 10: of these 33.3% lived in the same house of COVID-19 positive subject) and, finally, from the return from high risk areas/foreign states (11.6%; n = 4).
The most frequent type of contact in the healthcare area was represented by permanency in the same room of a confirmed case of COVID 19 at a distance of less than 2 meters and for over 15 minutes (58.6%); while the least frequent was contact with secretions (6.9%). The types of contacts are shown in Figure 1. Only in one case the subject performed aerosol-generating procedures. Only in 3 cases the patient wore the surgical mask. All healthcare workers wore PPE, although the surgical mask and gloves were the most worn. All healthcare workers have stated that they always wear all PPE as recommended and disposed of it as per hospital guidelines. This lead, except in few cases, in a reduction of the intra-hospital infection rate.
Figure 1.
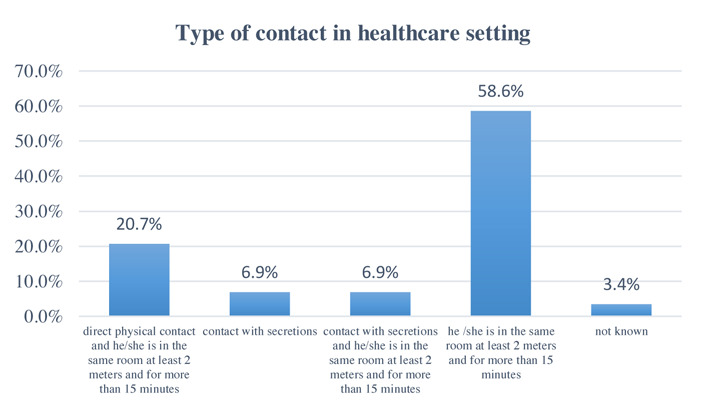
Type of contact in healthcare setting
76.5% of COVID-19 positive HCWs developed symptoms (23.5% of asymptomatic subjects) and in particular cough (29%) and fever (47%) (see Figure 2). In two cases, antibiotic or antiviral therapy had to be started.
Figure 2.
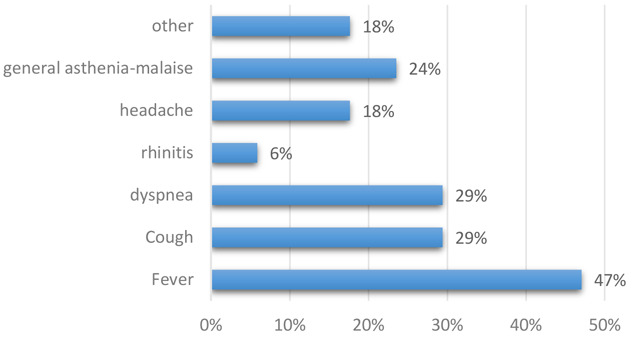
Type of symptoms in HCWs infected
In two cases the subject required hospitalization which resulted in clinical recovery without complications. None of the subjects had ever been vaccinated for flu.
The swabs gave a positive result in 0.67% (17) of healthcare workers whose socio-personal and working characteristics are described in Table 1. In particular, 70.6% of these contracted infection in the healthcare setting, 11,8% in the family environment and 17.6% returning from high risk areas before the onset of epidemic in Italy. Among those who contracted the disease in the healthcare area, 83.3% worked in the internal medicine operating unit, where the arrival of a patient then confirmed positive for COVID-19 (first two swabs were negative) seems to be related to transmission in the HCWs. The other transmission cases (17.7%) derived from close contacts with cases confirmed in two healthcare workers of the COVID Hospital. Ultimately, only 0.1% (2) of the healthcare workers dedicated to the treatment of COVID-19 patients acquired the infection (1 of which in asymptomatic form).
With regard to the serological survey, 2% (n = 53) of the healthcare workers were positive, of which 41.5% (n = 22) male and 58.5% (n = 31) female. The average age of the subjects who tested positive was 50.12 ±12.09 years. 74% of the subjects tested for Ig G/Ig M positive for Ig G, 20% for Ig M and 6% for both. The data are represented in figure 3.
Figure 3.
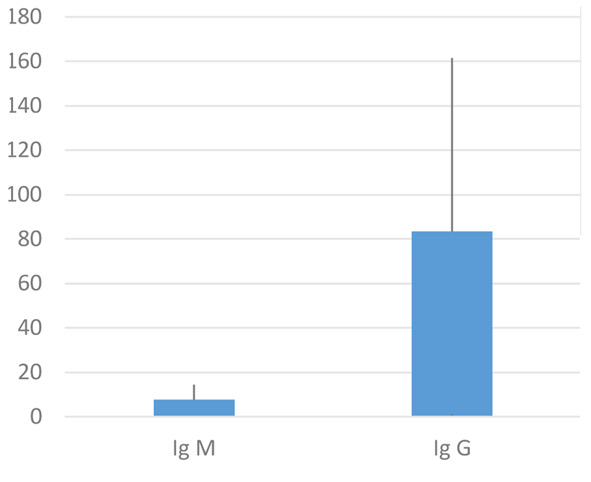
Box plot of Ig M and Ig G of HCWs
None of the serological positive subjects were positive for SARS-CoV-2 RNA (no asymptomatic HCWs were detected after the first step of nasopharyngeal swabs). The detection performed after one week of distance, as described in the materials and methods, revealed similar values to those described above.
Discussion and conclusion
The SARS-CoV-2 epidemic has led to a great impact on all social and healthcare systems all over the world, with a high number of victims even among the healthcare workers (23).
Until June 21, 2020 38,901 cases of COVID-19 were registered in Italy, of which 33,369 died; and 29,174 healthcare workers have been infected (24). This data does not refer to the number of subjects infected during healthcare assistance but only to the type of professional role.
Healthcare workers face an elevated risk of exposure to infectious diseases, including COVID-19, thus, it is imperative to ensure the safety of healthcare workers not only to safeguard continuous patient care but also to ensure they do not transmit the virus. COVID-19 can spread via cough or respiratory droplets, contact with bodily fluids, or from contaminated surfaces and so hospital environment is a potential source of infection both of HCWs and of patients (25).
Our study finds that a correct adherence to the guidelines and the correct use and disposal of PPE are fundamental measures to reduce the risk of contagion, which however cannot be completely eliminated (26).
The greatest risk of transmission occurred in addition to the nosocomial context in the family as previously described in the literature (26,27). In our study, most of the COVID-19 cases among healthcare workers were mild and were managed at home with self-isolation measures, however two infected healthcare workers (11.8%) were hospitalized, despite this no one died. The serological investigation confirmed the previous infection of the positive subjects, but the positivity for IgM and/or Ig G did not lead to new diagnoses, unlike other studies described (28-30).
The availability of serological tests for the assay of anti-SARS-CoV-2 antibodies is however fundamental both for studying the humoral response in infected subjects and for conducting seroprevalence studies in the general population. In this phase 2 that we are addressing the need to identify those who are still susceptible to infections, those who are undergoing acute infection, and those who are cured and, therefore, potentially immune to reinfection remains of paramount importance. Serological tests, suitably validated, could prove useful for acquiring information on the real extent of the pandemic, especially in relation to asymptomatic, and for contributing to the management of the population in the SARS-CoV-2 pandemic. However, it is believed that, at the moment, it is necessary to acquire data that demonstrate the real effectiveness of the immunity conferred by the antibodies. Furthermore, a factor that could complicate the strategy of testing SARS-CoV-2 antibodies on a large scale could be the difficulty of identifying all those who actually present the antibodies, as tests should be repeated at regular intervals to identify new infections through the seroconversion (positivity for IgM and/or IgG).
In fact, considering the nonnegligible percentage of false positives and false negatives, the results provided by the serological methods are less accurate than the RT-PCR performed on samples taken with nasopharyngeal swabs, which remains the most reliable and detect method for diagnosis of COVID-19 even before the onset of symptoms.
In our study, none of the subjects interviewed were vaccinated for the flu and this not only made the differential diagnosis difficult, but led to a reduction in staff (even if negligible) in the emergency phase (31). In addition, a recent study in the literature has hypothesized a protective role of influenza vaccination: the influenza virus would seem to lead to an amplification of the expression of ACE-2, used as the gateway receptor by the SARS-CoV-2 virus (32). In light of this, as with other diseases, vaccination in healthcare workers is essential both to reduce absenteeism and to prevent healthcare professionals from becoming potential greasers (33-38).
In conclusion, according to our results, the best strategy to reduce the possibility of intra-hospital and intrafamily contagion, and to immunize all HCWs, is to test, track and treat SARS-CoV-2 positive subjects. In this perspective, the correct application of guidelines, the role of public health and prevention represent are fundamental points in the health system of each country and their importance should be highlighted also into account of the recent epidemic that hit the world (39-47).
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Data on COVID. Johns Hopkins. https://coronavirus.jhu.edu/map.html . [Google Scholar]
- 2.Spiteri G, Fielding J, Diercke M, et al. First cases of coronavirus disease 2019 (COVID-19) in the WHO European Region, 24 January to 21 February 2020. Euro Surveill. 2020;25(9):2000178. doi: 10.2807/1560-7917.ES.2020.25.9.2000178. doi: 10.2807/1560-7917.ES.2020.25.9.2000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yong SEF, Anderson DE, Wei WE, et al. Connecting clusters of COVID-19: an epidemiological and serological investigation [published online ahead of print, 2020 Apr 21] Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30273-5. S1473-3099(20)30273-5. doi: 10.1016/S1473-3099(20)30273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capobianchi MR, Rueca M, Messina F, et al. Molecular characterization of SARS-CoV-2 from the first case of COVID-19 in Italy [published online ahead of print, 2020 Mar 27] Clin Microbiol Infect. 2020;26(7):954–956. doi: 10.1016/j.cmi.2020.03.025. doi: 10.1016/j.cmi.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romagnani P, Gnone G, Guzzi F, et al. The COVID-19 infection: lessons from the Italian experience [published online ahead of print, 2020 May 29] J Public Health Policy. 2020;1-7 doi: 10.1057/s41271-020-00229-y. doi: 10.1057/s41271-020-00229-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Odone A, Delmonte D, Scognamiglio T, Signorelli C. COVID-19 deaths in Lombardy, Italy: data in context [published correction appears in Lancet Public Health 2020 Jun; 5:6.e315. Lancet Public Health. 2020;5(6):e310. doi: 10.1016/S2468-2667(20)30099-2. doi: 10.1016/S2468-2667(20)30099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Signorelli C, Scognamiglio T, Odone A. COVID-19 in Italy: impact of containment measures and prevalence estimates of infection in the general population. Acta Bio Med [Internet] 2020Apr.10;91(3-S):175–9. doi: 10.23750/abm.v91i3-S.9511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.second-meeting-of-the-international-health-regulation. Available on https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov. )) [Google Scholar]
- 9.Ordinanza 21 febbraio 2020 Ulteriori misure profilattiche contro la diffusione della malattia infettiva COVID-19. (20A01220). (G.U. Serie Generale, n. 44 del 22 febbraio 2020 [Google Scholar]
- 10.ORDINANZA 20 marzo 2020 Ulteriori misure urgenti in materia di contenimento e gestione dell’emergenza epidemiologica da COVID-19, applicabili sull’intero territorio nazionale. (20A01797) (GU Serie Generale n.73 del 20-03-2020) [Google Scholar]
- 11.Documento relativo ai criteri per sottoporre soggetti clinicamente asintomatici alla ricerca d’infezione da SARS-CoV-2 attraverso tampone rino-faringeo e test diagnostico [Google Scholar]
- 12.DECRETO-LEGGE 9 marzo 2020, n. 14. Disposizioni urgenti per il potenziamento del Servizio sanitario nazionale in relazione all’emergenza COVID-19 [Google Scholar]
- 13.Ordinanza contingibile e urgente n. 7 del 20.03.2020 Ulteriori misure per la prevenzione e gestione dell’emergenza epidemiologica da Covid-2019. Ordinanza ai sensi dell’art.32, comma 3, della legge 23 dicembre 1978, n. 833 in materia di igiene e sanità pubblica. Regione Sicilia [Google Scholar]
- 14. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/laboratoryguidance . [Google Scholar]
- 15.Corman VM, Eckerle I, Bleicker T, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. pii=2000045. https://doi.org/10.2807/1560-7917.ES.2020.25.3.2000045 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reusken CBEM, Broberg EK, Haagmans B, et al. Laboratory readiness and response for novel coronavirus (2019-nCoV) in expert laboratories in 30 EU/EEA countries, January 2020. Euro Surveill. 2020;25(6):2000082. doi: 10.2807/1560-7917.ES.2020.25.6.2000082. doi: 10.2807/1560-7917.ES.2020.25.6.2000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Circolare del Ministero della salute prot. n. 5443 del 22 febbraio 2020 [Google Scholar]
- 18.Protocollo metodologico per un’indagine di siero-prevalenza sul SARS-CoV-2 condotta dal Ministero della salute e dall’ISTAT Decreto Legge 10 maggio 2020 n.30 [Google Scholar]
- 19.Park T, Sang-Yeop L, Seil K, et al. Spike protein binding prediction with neutralizing antibodies of SARS-CoV-2 bioRxiv. 2020 02.22.951178; doi: https://doi.org/10.1101/2020.02.22.951178 . [Google Scholar]
- 20.Diggle DJ Estimating Prevalence Using an Imperfect Test, Epidemiology Research International, 2011. https://www.hindawi.com/journals/eri/2011/608719/ [Google Scholar]
- 21.Document of the Ministry of Health of 28/02/2020 concerning the definition of “Patient cured of Covid-19” [Google Scholar]
- 22.Circular of the Ministry of Health of May 9, 2020 [Google Scholar]
- 23.Grech V. Unknown unknowns - COVID-19 and potential global mortality. Early Hum Dev. 2020;144:105026. doi: 10.1016/j.earlhumdev.2020.105026. doi: 10.1016/j.earlhumdev.2020.105026. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Sorveglianza Integrata COVID-19 in Italia. Available on https://www.epicentro.iss.it/coronavirus/bollettino/Info-grafica_19giugno%20ITA.pdf . [Google Scholar]
- 25.Chang D, Xu H, Rebaza A, Sharma L, Dela Cruz CS. Protecting health-care workers from subclinical coronavirus infection. The Lancet Respiratory Medicine. 8(3):e13. doi: 10.1016/S2213-2600(20)30066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chou R, Dana T, Buckley DI, Selph S, Fu R. Totten AM. Epidemiology of and Risk Factors for Coronavirus Infection in Health Care Workers [published online ahead of print, 2020 May 5] Ann Intern Med. 2020 M20-1632. doi: 10.7326/M20-1632. [Google Scholar]
- 27.Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grant R, Malik MR, Elkholy A, Van Kerkhove MD. A Review of Asymptomatic and Subclinical Middle East Respiratory Syndrome Coronavirus Infections. Epidemiol Rev. 2019;41(1):69–81. doi: 10.1093/epirev/mxz009. doi: 10.1093/epirev/mxz009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilder-Smith A, Teleman MD, Heng BH, Earnest A, Ling AE, Leo YS. Asymptomatic SARS coronavirus infection among healthcare workers, Singapore. Emerg Infect Dis. 2005;11(7):1142–1145. doi: 10.3201/eid1107.041165. doi: 10.3201/eid1107.041165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haveri A, Smura T, Kuivanen S, et al. Serological and molecular findings during SARS-CoV-2 infection: the first case study in Finland, January to February 2020. Euro Surveill. 2020;25(11):2000266. doi: 10.2807/1560-7917.ES.2020.25.11.2000266. doi: 10.2807/1560-7917.ES.2020.25.11.2000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maltezou HC, Poland GA. Vaccination policies for healthcare workers in Europe. Vaccine. 2014;32(38):4876–4880. doi: 10.1016/j.vaccine.2013.10.046. doi: 10.1016/j.vaccine.2013.10.046. [DOI] [PubMed] [Google Scholar]
- 32.Chen L, Hao G. The role of angiotensin-converting enzyme 2 in coronaviruses/influenza viruses and cardiovascular disease [published online ahead of print, 2020 Apr 8] Cardiovasc Res. 2020 doi: 10.1093/cvr/cvaa093. cvaa093. doi: 10.1093/cvr/cvaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costantino C, Ledda C, Genovese C, et al. Immunization Status against Measles of Health-Care Workers Operating at Three Sicilian University Hospitals: An Observational Study. Vaccines (Basel) 2019 Nov 3;7(4):175. doi: 10.3390/vaccines7040175. doi: 10.3390/vaccines7040175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Squeri R, Di Pietro A, La Fauci V, Genovese C. Healthcare workers’ vaccination at European and Italian level: a narrative review. Acta Biomed. 2019 Sep 13;90(9-S):45–53. doi: 10.23750/abm.v90i9-S.8703. doi: 10.23750/abm.v90i9-S.8703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Genovese C, La Fauci V, Costa GB, et al. A potential outbreak of measles and chickenpox among healthcare workers in a university hospital. EMBJ. 2019;14(10):045–048. [Google Scholar]
- 36.Genovese C, Picerno IAM, Trimarchi G, et al. Vaccination coverage in healthcare workers: a multicenter cross-sectional study in Italy. J Prev Med Hyg. 2019 Mar 29;60(1):E12–E17. doi: 10.15167/2421-4248/jpmh2019.60.1.1097. doi: 10.15167/2421-4248/jpmh2019.60.1.1097. eCollection 2019 Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Squeri R, La Fauci V, Picerno IAM, et al. Evaluation of Vaccination Coverages in the Health Care Workers of a University Hospital in Southern Italy. Ann Ig. 2019 Mar-Apr;31(2 Supple 1):13–24. doi: 10.7416/ai.2019.2273. doi: 10.7416/ai.2019.2273. [DOI] [PubMed] [Google Scholar]
- 38.Montagna MT, Mascipinto S, Pousis C, et al. Knowledge, experiences, and attitudes toward Mantoux test among medical and health professional students in Italy: a cross-sectional study. Ann Ig. 2018 Sep-Oct;30(5 Supple 2):86–98. doi: 10.7416/ai.2018.2253. doi: 10.7416/ai.2018.2253. [DOI] [PubMed] [Google Scholar]
- 39.La Fauci V, Riso R, Facciolà A, Merlina V, Squeri R. Surveillance of microbiological contamination and correct use of protective lead garments. Ann Ig. 2016 Sep-Oct;28(5):360–6. doi: 10.7416/ai.2016.2116. doi: 10.7416/ai.2016.2116. [DOI] [PubMed] [Google Scholar]
- 40.La Fauci V, Costa GB, Arena A, Ventura Spagnolo E, Genovese C, Palamara MA, Squeri R. Trend of MDR-microorganisms isolated from the biological samples of patients with HAI and from the surfaces around that patient. New Microbiol. 2018 Jan;41(1):42–46. Epub 2018 Jan 9. [PubMed] [Google Scholar]
- 41.Ventura Spagnolo, E Stassi, C Mondello, C Zerbo, S Milone, L., Argo A. Forensic microbiology applications: A systematic review. Legal Medicine. 2019;36:73–80. doi: 10.1016/j.legalmed.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Spagnolo , E.V Mondello, C Stassi, C Baldino, G D’Aleo, F. Conte M, Argo A, Zerbo S. Forensic microbiology. A case series analysis EMBJ. 2019;14(27):117–121. [Google Scholar]
- 43.Spagnolo E.V, Cannavò G, Mondello C, Cardia L, Bartoloni G, Cardia G. Unexpected death for takayasu aortitis associated with coronary ostial stenosis. American Journal of Forensic Medicine and Pathology. 2015;36(2):88–90. doi: 10.1097/PAF.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 44.Mellace L, Consonni D, Jacchetti G, DelMedico M, Colombo R, Velati M, et al. Epidemiology of Clostridiumdifficile-associated disease in internal medicine wards in northern Italy. Intern Emerg Med. 2013;8(8):717–723. doi: 10.1007/s11739-012-0752-6. [DOI] [PubMed] [Google Scholar]
- 45.Ardoino I, Zangirolami F, Iemmi D, Lanzoni M, Cargnelutti M, Biganzoli E, et al. Riskfactors and epidemiology of Acinetobacter baumannii infections in auniversity hospital in Northern Italy: A case-control study. Am J InfectControl. 2016;44(12):1600–1605. doi: 10.1016/j.ajic.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 46.Capobussi M, Sabatino G, Donadini A, Tersalvi CA, Castaldi S. Control of scabies outbreaksin an Italian hospital: An information-centered management strategy. Am JInfect Control. 2014;42(3):316–320. doi: 10.1016/j.ajic.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 47.Prigitano A, Romanò L, Auxilia F, Castaldi S, Tortorano AM. Antibiotic resistance: Italianawareness survey 2016. J Infect Public Health. 2018;11(1):30–34. doi: 10.1016/j.jiph.2017.02.010. IF 2,118Q2. [DOI] [PubMed] [Google Scholar]


