Abstract
Background and aim of the study.
COVID-19 is characterized by super spread events occurring in communities, e.g., hospitals. To limit virus diffusion among healthcare workers the use of personal protective equipment and screening tests are highly advised; also, isolation of virus positive professionals while monitoring their health condition is recommended. This study aims to assess, in a cohort of COVID-19 positive quarantined healthcare workers, the perceived source of infection and exposure risk as well as the clinical evolution of the disease through a surveillance interview.
Methods.
A retrospective observational study accounting 896 observations on 93 healthcare professionals tested positive for COVID-19. Data were collected from the Nursing and Technical Directorate of Romagna, Ravenna, Local Health Company, Italy.
Results.
99.5% of the positive workers accepted phone interviews with management staff. 2.6% of workers were positive with increasing records in the specialist medical area. Nurses and social health professionals were mostly affected. Patient exposure at a distance <1 m and a contact time > 2 hours was the first cause of positivity. In COVID-19 and territorial emergency departments, the first cause was the contact with colleagues. At the time of the infection, most of the staff wore a surgical mask. Cough, asthenia, fever, anosmia, dysgeusia, and rhinitis were common symptoms. Asymptomatic percentage was about 10%. The self-perceived physical condition was high (>7) and improved during the observation period.
Conclusions.
The diffusion rate of COVID-19 among healthcare workers is relatively low, probably due to the use of personal protective equipment. The distancing, also among colleagues, is a fundamental measure to reduce the possibility of infection. Symptoms are mild and can be controlled by surveillance measures. Constant contact with the organization is an essential strategy for promoting recovering of workers and reducing the spread of the virus within the healthcare organization. (www.actabiomedica.it)
Keywords: COVID-19 infection, healthcare professionals, risk factors, infection prevention and control measures, COVID-19 disease
Background
According to Callaway (1), the spread of Coronavirus-19 disease (COVID-19) is becoming unstoppable and has already met the epidemiological criteria necessary to be declared a pandemic, having infected more than 100,000 people in 100 countries (1, 2). At the time of writing, the countries involved in the pandemic were 214 for a total of 36,175,540 confirmed cases which led to 27,224,339 hospitalizations and 1,056,711 deaths (3).
In Italy, on February 20, 2020, a young Lombard was hospitalized with atypical pneumonia which turned out to be COVID-19. Over the next 24 hours, there were 36 more cases, none of which had contact with the first patient or with anyone known to have COVID-19. As of October 2020, the cases ascertained in Italy are more than 330,000, with a growth of about 3,600 infected per day (4). From February 21, Italian medical and health personnel began fighting one of the largest and most serious COVID-19 outbreak in the world (5).
The epidemics of COVID-19 is characterized by the so-called super spread events, which often occur in hospitals (6). For this reason, the World Health Organization (WHO) and national/international scientific societies have issued official recommendations that indicate which Personnel Protective Equipment (PPE) must be used based on the activity and contact with the patient, also giving indications on how to optimize the availability of PPE (7-9). Based on the possible ways of virus transmission (10) many studies have been performed on the risk of transmission during oxygen administration (4, 11-16), through contact/drops (4, 17) and on the precautions to minimize transmission in case of procedures that generate aerosols (8, 16, 18-20). It was highlighted that compliance with the indications for the use of PPE and the creation of “clean and dirty” paths are effective methods for preventing COVID-19 infection among healthcare personnel (21, 22). Despite these indications, a number of COVID-19 cases were reported among healthcare personnel (23, 24).
Based on the most recent pandemics, factors facilitating the virus spreading among healthcare personnel have been already identified. The lack and unproper use of PPE (21, 25, 26), heavy workload with inadequate resources, were major causes of healthcare personnel positivity in COVID-19 pandemic (27, 28). Anxiety and disaffection from work also facilitate infection. Other reported factors were: incorrect information from the organization, high mortality among hospitalized people, stigmatization of healthcare workers who were seen as possible sources of virus diffusion among population (29). Others studies focused on the virus transmission through contaminated surfaces (22), the lack of traceability of asymptomatic workers, the lack of separation between high-intensity care environments and less protected environments, such as dressing rooms and rest areas (23, 30).
The protection of personnel is crucial, especially when the human resources are limited. In addition to the supply of adequate PPE, diagnostic and/or screening tests must be guaranteed to diagnose or identify previous contacts with the virus and to proceed with the isolation of the worker in case of COVID-19 positivity (23). A recent experience in a COVID-19 outbreak in a military recruit school focused on the usefulness of surveillance to collect data from quarantined people; in this way, precious information relating to the source of the infection and to the evolution of symptoms in positive pre-symptomatic or asymptomatic subjects can be recorded. It is expected that these information may help in limiting virus spreading in communities and recovering effectively people after the isolation period (31).
Aim
The aim of the study was to evaluate among the affected by COVID-19 healthcare personnel: the perceived source of the infection, the perceived exposure risks, and the course of the disease.
Method
Study design
A retrospective observational study designed according to the STROBE guidelines on the observational clinical studies in epidemiology (32).
Setting
From March 25, 2020 to May 05, 2020, the Nursing and Technical Department of the Local Health Company of Romagna, in Ravenna (Italy), collected data on the quarantined hospital staff due to a positive swab. The surveillance was carried out for 15 days through daily phone interviews. In some cases, at the request of the worker, the surveillance extended over this period.
Sample
The sample was non-probabilistic, and included all non-medical health personnel working in hospitals in Ravenna’s district who tested positive to COVID-19.
Instrument
Data were collected using an on-line questionnaire. During the interview the following data were recorded: personnel information, personnel profile, working area, work shift characteristics, swab positivity date, perceived source of infection, distance from the COVID-19 positive presumed contact, duration of exposure (24,28), type of PPE worn during the workshift, or at the time when presumed exposure occurred (4, 33, 34, 35, 36), symptoms (24, 34, 35, 37, 38, 39, 40, 41), therapy (42) and assessment of physical condition in a scale from 1 to 10.
Data analysis
Data were analyzed with the demo version of SPSS statistical software. Descriptive statistical analysis (frequency, percentage, mean, Standard Deviation, median) were performed with a 95% confidence interval. A Chi-square test was used for analyzing the nominal variables and an ANOVA test for the cardinal variables. Multiple comparisons were analyzed using the post hoc Tukey’s HSD test. The Pearson index was used for the correlations, while the sample adequacy was calculated with the Kaiser - Meyer - Olkin test (KMO).
Results
Characteristics of the Sample
896 observations were recorded on the database, as follows: 865 (96.6%) were surveillance interviews; 14 (1.6%) were hospitalizations involving 9 employees; 17 (1.9%) were “null” records (subjects not contactable by phone or refusing the interview).
The sample, consisting of 93 (2.6%) out of 3565 hospital personnels (data source: DIT - Ravenna, 2020), had an average age of 45.96 years (SD = 10.71); 71,0% (n = 66) were female.
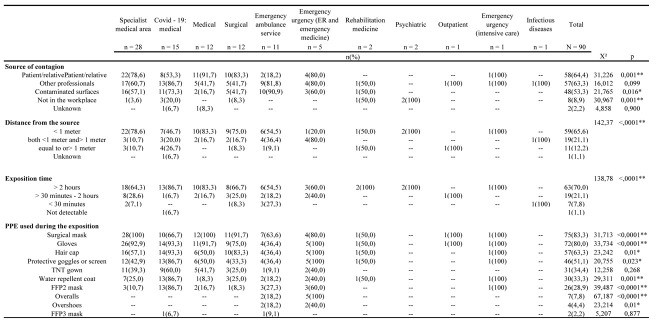
Most of the positive workers were nurses (54.8%, n = 51), and health and social care workers (31.2%, n = 29); these two categories represented 86% of the overall sample while the others were only occasionally involved. As detailed in Figure 1, areas with the greater prevalence of infection between workers were the specialist medical area (30.1%, n = 28), followed by COVID-19 wards (16.1%, n = 15), the surgical areas (14,0%, n = 13), the general medical area (12.9%, n = 12), the emergency ambulance service (11.8%, n = 11). The sample size was adequate with a KMO = .745 and with the Bartlett sphericity test with a significance < ,0001.
Figure 1.
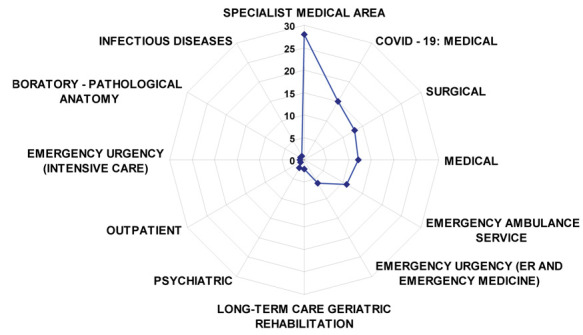
Highlights the services in which supervised professional tested positive for COVID-19
Figure 1 illustrates the department distribution of infected healthcare personnel. The curve shows a significant shift to the left (p = < ,0001), overlapping the COVID-19 free medical and surgical wards.
62.4% (n = 58) of the sample were h24 shift workers, with a high prevalence of nurses (74.1%, n = 43; X² = 39.916; p = < 0,0001). As to the perceived route of the infection, significant differences were found. Infection from the patient / relative was the first reported cause [64.4% (n = 58)], especially in the medical [91.7% (n = 11)], surgical [83.3% (n = 10)], emergency urgency [80% (n = 4)] and in specialist medicine areas [78.6% (n = 22)] (p =, 001). As for the item “a colleague as a source of infection”, the hypothesis was null (p = .099). However, this route was perceived as the most probable by 86.7% (n = 13) of personnels working in COVID-19 departments and by 81.8% (n = 9) of the crew in the emergency ambulance service. Also the few positive personnels working in outpatient services, intensive care, and infectious diseases units, had the perception of having contracted the infection from their colleague. In the psychiatry area, all respondents reported that they were infected outside the workplace (Table 1).
Table 1.
Perceived source of contagion, distance and time of exposure from the presumed source, and PPE used during the workshift or at the time when the presumed exposure occured
* p = ,05; ** p = ,01.
65.6% of the entire sample reported that the distance from the presumed source of infection was <1 meter; this percentage was even higher for personnels working in the medical (83.3%), surgical (75%), specialist (78.6%) and emergency ambulance service (54.5%) areas. Distances less and more than 1 meter were mainly reported by workers in emergency room and emergency medicine ward (80%). Data from personnels working in COVID-19 areas had a more homogeneous distribution.
There were differences also about the presumed exposure time: 70% of the entire sample reported an exposure > than 2 hours, especially the 86.7% personnels working in the COVID-19 areas, followed by the medical areas (83.3%), from the surgical ones (66.7%) and lastly from the specialist areas (64.3%).
As to the PPE worn during the workshift in wich the presumed exposure occured, the surgical mask was the most frequently used [83.3% (n = 75)]. The medical, outpatient, intensive care and surgical areas were those in which 100% of operators have worn the masks, followed by the surgical area with 91.7% (n = 12), the emergency room and the emergency medical operative unit with 80.0% (n = 4), the COVID-19 wards with 66.7% (n = 10) and the emergency ambulance service with 63.6% (n = 7) (p = <, 0001). Contrary to surgical masks, FFP2s were used by the 28.9% (n = 26) of the sample, mainly in the COVID-19 wards (86.7%, n = 13).
Gloves were used by 80.0% (n = 72) of personnels; however, crew of emergency ambulance service used this protective equipment in a significantly lower percentage (36%, n = 4). This number could justify the 90.9% of fomite infections among staff operating in the emergency - territorial urgency. The hair cap was used in 63.3% (n = 57) of the sample, especially in COVID-19 wards (93.3%) and in surgical areas (83.3%). Water-repellent and TNT gowns were used by 30.5% of the health personnels, especially by personnel working in COVID-19 areas. Operators in the psychiatric area and infectious disease wards reported that they did not wear any PPE because in the first case the infection occurred outside work, while in the second case the infection seemed to have occurred in the kitchenette with another colleague.
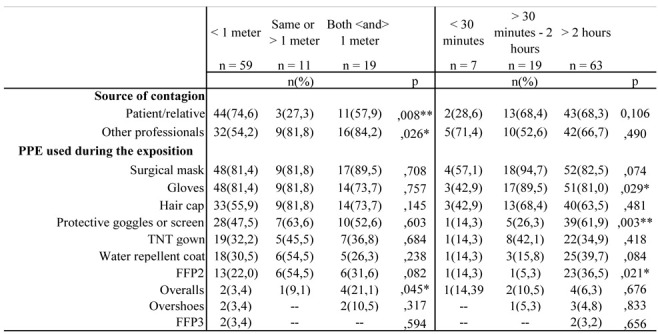
Considering the distance and time from the source of the contagion perceived by workers (Table 2), it was noted that at a distance of less than one meter, the 74.6% (n = 44) came into contact with patients and/or relatives (p =, 008), while 84.2% (n = 16) of workers suspect they have contracted the infection through colleagues with whom they have maintained a distance of less or more than 1 meter (p =. 026). As far as exposure time was concerned, there were no differences in patients and/or relatives, however, with percentages higher than 65%, workers were exposed to patients for more than 30 minutes and even for more than 2 hours. As for the PPE used during the related exposure, no significant differences were found for the distance from the source. The surgical mask appeared to be the most used device, with a percentage of more than 80% regardless of distance. Also concerning the exposure time, the workes used the mask more. Gloves were the most used devices with 81.0% in the exposure time of more than 2 hours (p = .029), followed by the glasses and the protective shield (61.9%; p = .003) and the FFP2 (36.5%; p = .021).
Table 2.
Relationship between distance and presumed exposure time
 |
* p = ,05; ** p = ,01.
The parametric calculation did not show significant differences to the number of exposures perceived by staff within the wards/services (F = .716; p = .613). In practice, the personnels reported having come into contact with an average number of infected persons equal to 9.14 ± 11.272 in the COVID-19 wards, with 8.33 (SD = ± 9.948) in the surgical areas, with 6.07 (SD = ± 5.786) in the specialized medical areas, with 5.91 (SD = ± 6.395) in emergency ambulance service, with 4.92 (SD = ± 5.107) in the medical areas and with 4.33 (SD = ± 3.327) in the other operative units.
During the data processing, non-parametric calculations were made by relating the personnel profiles with the use of PPE, the source and the duration of the exhibition. No significant differences were observed.
Surveillance
Regarding the clinical conditions of the quarantined personnels, 863 observations were performed (2 missing). 828 observations were completed within 15 days in accordance with the protocol, while the remaining 35 observations continued, upon worker request, until the operators recovered.
On average, the Nursing and Technical Department performed 9.58 interviews each worker. Considering that 9.59 (SD = ± 5.435) is the mean and (μe = 9.00 days) is the median of the days in which the personnels entered the surveillance protocol, it can be extrapolated that every worker has used the service for the entire time he/she was on surveillance.
As regards the duration of the symptoms, each worker reported a clinical course lasting on average of 12.44 days (SD = ± 5.920 days; μe = 12.00).
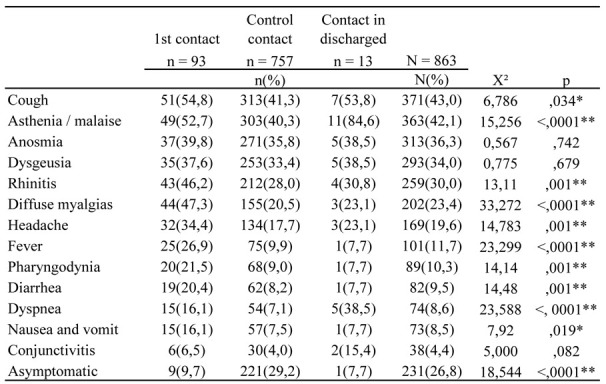
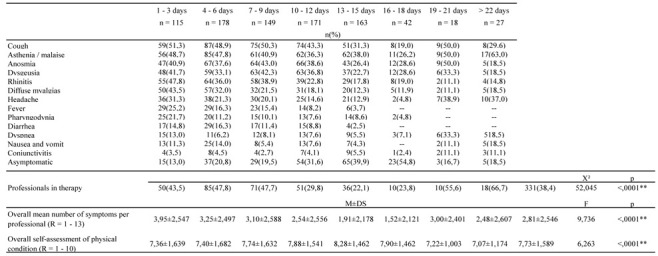
A total of 13 symptoms were reported. Significant differences in symptomatology have been detected by dividing the surveillance into three types of contact: 1st contact (after the positive swab), control contact, and contact in workers discharged from the COVID-19 wards. Cough was the most common symptom (higher than 50% both in the first contact and in the post-discharge one). Other common symptoms were asthenia and malaise (84.6% of discharged workers and 52.0% of the first contact). Anosmia and dysgeusia were reported in a percentage of more than 30%. Rhinitis was reported in 46.2% at the 1st contact, and reduced during the control contacts. The same course was also found for diffuse myalgia, headache, fever, and pharyngodynia. Diarrhea, nausea/vomiting, and conjunctivitis were associated with a very favorable course. In fact, compared to the 1st contact, the percentage of workers affected reduced to below 10% in subsequent control observations. Dyspnea was significant in the cases discharged from the COVID-19 wards (<.0001) and persisted in the 38.5% of cases after discharge. The asymptomatic positive workers increased from 9.7% (first contact) to 29.2% (control contact).
Results are schematized in Table 3.
Table 3.
Symptoms reported by workers
 |
* p = ,05; ** p = ,01.
The data collection made it possible to assess the quality of the cough and the progress of the fever in the periods between the 1st contact, the control contacts, and discharge. For both symptoms, no significant differences emerged. Workers mainly reported a dry cough (56.2%, n=208); a productive cough was recorded in 24.3% (N =90; X² =, 711; p =.950); as regards the fever, apyrexia was more common in the morning (M =36.74; SD = ±, 758) and low-grade fever in the evening (M =37.46; SD = ±, 628; F = 1.352; p = .264).
There was a substantial alignment between the symptoms present at the 1st contact and the subsequent control contacts. Unlike the otolaryngological symptoms, the clinical course showed a significant symptom reduction and increase (p = <, 0001) of the asymptomatic subjects (Fig. 2).
Figure 2.
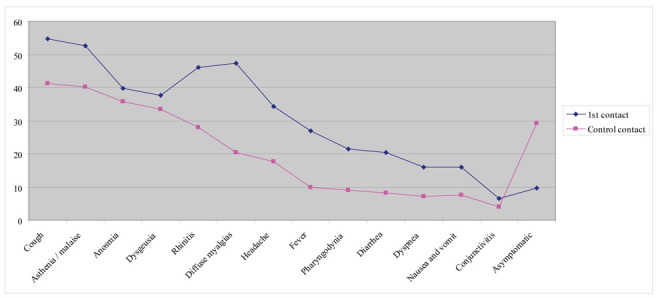
The figure describes the course of the symptoms during surveillance
Also considering the time elapsed since the first swab, and grouping the entire surveillance period into three-day classes, symptoms constantly reduced. However, after 20 days 63,0% of workers still reported malaise and asthenia. Statistically significant differences were found (p = <, 0001) on the average number of symptoms reported by workers in the period considered. From the date of the swab until the third day of surveillance, on average each worker reported about 4 symptoms (M =3.95; SD = ± 2.547), from the 4th to the 9th day about three symptoms, with a progressive reduction up to the 18th day (M =1.52; SD = ± 2.121). A symptom recurrence was recorded from day 19 on a limited number of works that also included the 9 discharged from COVID facilities (Fig. 2). The perception of health was generally good in all the surveillance period. Despite the significant differences (p = <, 0001) on a scale of 1 - 10, the values remained above 7.00 with a progressive improvement up to 8.00 on the 18th day. The value decreased slightly from the 19th day, in relation to the workers discharged from the COVID-19 wards (Table 4).
Table 4.
Symptoms and personnels - perception of their physical conditions
 |
** p = ,01.
By relating the types of contact with the perception that the workers had of their health condition, the univariate analysis showed significant differences (F = 9.794; p = <, 0001) attributing to the first contact an average of well-being of 7.10 (SD= ± 1.649), in the control contact a value of 7.82 (SD= ± 1.567) and in the discharged an average of 7.08 (SD= ± 1.382).
The multiple comparisons confirmed a null hypothesis between the first contact and the contacts in the discharged workes (p =, 999), while they proved a significant improvement in the physical condition between the first contact and the control contacts (MD = -, 720; p = <, 0001). In summary, there was no difference between the first contact group and the workers discharged from the COVID-19 wards.
The Pearson correlation coefficient showed a positive correlation between the health perception and the days elapsed from the first swab (ρ = 0.98; Sig. =. 004), while a negative correlation was recorded between the health perception and the ongoing therapy (ρ = -, 360; Sig. = <, 0001).
From the swab positivity, the course of the symptoms was distributed within a wide range (1-27 days). During this interval, there were significant differences in the clinical course. Fever significantly anticipated all the other symptoms (μe = 6.00; M = 6.69; SD = ± 3.938;p = .001), followed by diarrhea (μe = 6.00; M = 7.00; SD= ± 3.969;p = .029) and diffuse myalgias (μe = 6.00; M = 7.70; SD= ± 5.175; p = .0001). Rhinitis had a median distribution on day 7, (M = 7.91;SD = ± 4.877;p = <, 0001), headache (μe = 7.00, M = 9.00 ± 6.252;p = .005), cough (μe = 8.00; M = 8.71; SD= ± 5.168;p = .006), dyspnea (μe = 9.00; M = 10.07; SD= ± 6.697;p = .0001) and conjunctivitis (μe = 10.00, M = 10.22; SD= ± 5.966;p = .0001) (Fig. 3).
Figure 3.
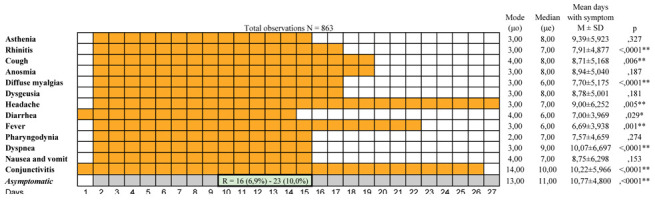
Shows the summary of the symptomatology course
* p = ,05; ** p = ,01.
Even though asymptomatic patients were recorded at the diagnosis, they peaked from the 10th to the 15th day (M = 10.77; SD = ± 4.88; 11p and 13p = <, 0001). A non-parametric analysis did not reveal any relationship between the characteristics of the symptoms and the distance and time elapsed since the likely route of the infection.
Table 5 relates the perceived health conditions with the therapy. The self-perceived health condition was good with a μo = 8.00 and an average of 7.73 (SD =± 1.589). 243 observations were scored 8 and among the workers, 14.9% (N =37) were treated with Plaquenil®, 8.5% (N =21) with low molecular weight heparin and 6.9% (N = 17) with antibiotics. Overall, chloroquine was the most used drug (17.7%, N=153), followed by antipyretics (7.9%; N = 68) and antibiotics (6.0%; N =52). The association of antibiotics, antipyretics, and anti-inflammatories was taken by subjects with a score from 2 to 4. Other recorded drugs were mucolytics, gastro protectors, mineral salts, and folic acid (5,4%; N =47).
Table 5.
Relationship between the personnels’ perception of their physical conditions with ongoing therapy
 |
Discussion
In the present retrospective observational study, we report the characteristics of COVID-19 infected health personnels working in the hospitals of the Ravenna’s district; the survey was recorded from March 25, 2020 to May 05, 2020, corresponding to the exponential growth of the first outbreak of the disease in Italy.
The quarantined or hospitalized workers were interviewed using an on-line questionnaire and information on the perceived route of the infection, the perceived exposure risks, and the course of the disease were collected.
The prevalence of confirmed healthcare personnel subjected to surveillance (2.6%) overlaps that reported in a recent Chinese study (43) and is in the values reported in the general population in the same period (2-5%; https://ourworldindata.org). This evidences that the protection measures adopted within the healthcare facilities have been effective in containing the spread of the infection among personnel.
As anticipated in other studies (43, 44) the healthcare personnel with greater contact with patients are those at greatest risk of contracting the infection; nurses, first of all, and health and social care workers represented 86% of the overall sample.
The higher prevalence of infected wokers was in the specialist medical area followed by COVID-19 dedicated wards; also, the crew working at emergency ambulance service was particularly affected. These results, which in part overlap with those reported by Liu et al (24), can be explained by the lower awareness of the risk and a lack of preparedness of workers in specialist medicine departments at the beginning of the pandemic outbreack when many clusters occurred, the burden of the virus load in dedicated COVID-19 departments and uncomplete adherence of workers to recommendations in the emergency services.
In the dedicated COVID-19 wards as well as emergency ambulance service and emergency wards, the main perceived route of infection would appear to be the colleagues or fomites; however Lai et al, 2020 (46) analyzing the possible contaminated surfaces such as diagnostic tables, door handles, bed bars, elevator buttons, PC keyboard and mouse, thermometers, and electro-medical devices (48) did not find any virus contamination.
As has already been widely documented (24, 47, 48), PPE were found effective to limit any increase in transmission and most of the healthcare personnel reported they wore the surgical mask at the time of the exposure, especially when the distance from the perceived route of infection was less than one meter and the exposure time greater than 30 minutes. Further, in our study, a non-parametric analysis performed to assess a possible relationship between the different professional profiles, routes of infection, and PPE compliance did not show any significant difference suggesting that workers tend to adopt behaviors related more to the organizational routine than to professional skills.
Overall, worker participation in the surveillance program was very high since less than 0.5% did not accept the phone interview. This result is in agreement with other studies (46, 49) that also recorded, in the quarantined workers, a reduction in anxiety and stress in at least 35 observations.
The symptoms reported in this study are similar to those described by others (38, 50, 51) including the high frequency of more than a sign or symptom. However, we found the most reported symptom was the cough and not the fever, contrary to what is commonly reported in the literature (38, 39, 51). Dry cough was present in more than half of personnel as also reported by Li et al, 2020 (52). Of interest is the presentation of anosmia and dysgeusia in more than 30% of the cases; these unusual symptoms have recently been associated with COVID-19 (53, 54, 55), and possibly are, when present, a characteristic of the disease.
In the present study, the rate of asymptomatic healthcare workers stands about 10% and is comparable with most of the reported data in the literature. It is well known that in mild COVID-19 disease, subjects could be completely asymptomatic or present only common flu-like symptoms such as cough, nasal congestion, and hyposmia (56). Until now, little was known about the risk of transmission from asymptomatic COVID-19 carriers. Since the viral load detected in asymptomatic patients is similar to that detected in symptomatic patients, it is thought that patients with mild or asymptomatic symptoms can spread COVID-19 with great ease (57).
During the surveillance, personnel had a high self-perception of their physical conditions with a gradual score improvement at control swab. On the contrary, workers discharged from wards reported residual symptoms and a self-perceived health comparable to that reported by quarantined personnel at first diagnosis; this could be a consequence of a more important clinical picture that led to hospitalization (24).
By correlating the quality of the self-perceived physical condition with the therapeutic plan, we found that personnel took more than one drug when the perceived physical condition was bad to average; after this value the correlation between the two variables became negative since the perceived health improved while the subject adopted a monotherapy. Chloroquine was the most used drug and the only one taken by subjects in excellent self-perceived health conditions.
Conclusions
The surveillance protocol on quarantined or discharged healthcare workers provided useful information on the presumed route of the infection, the perceived exposure risks, and the course of COVID-19 disease. The rate of infection was low, evidencing the efficacy of the adopted protection measures. Nurses were the most involved professional category especially those who were working in the wards where clusters occured at the beginning of the pandemic outbreak through the Ravenna’s district and in COVID-19 dedicated wards; the most frequently reported as the main perceived route of infection were patients or colleagues and the adherence to protective measures was not related to the possession of specific skills.
Worker adhesion to the surveillance program was high and allowed to maintain a relationship with the personnel and to intensify the presence and support of the local health company on infected workers. The questionnaire used during the interviews ensured a standardization in data collection and an integration between the different personnel involved in the interviews. This allowed to have a continuous survey on the health condition of the workers, while identifying weaknesses and strengths points that can be used effectively to limit the virus transmission in the healthcare environment and to improve the health management of infected healthcare personnel at the next outbreak of the COVID-19 disease.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Callaway E. Time to use the p-word? Coronavirus enter dangerous new phase. Nature. 2020;579:12. doi: 10.1038/d41586-020-00551-1. [DOI] [PubMed] [Google Scholar]
- 2.Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395:1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Worldometer COVID-19 Coronavirus Pandemic. 2020 https://www.worldometers.info/coronavirus/ . Date last updated: 02 July 2020. [Google Scholar]
- 4.Civil protection. COVID-19 Situation Italy. 2020 http://opendatadpc.maps.arcgis.com/apps/opsdashboard/index.html#/b0c68bce2cce478eaac82fe38d4138b1 . (Accessed on september 23, 2020) [Google Scholar]
- 5.Ferioli M, Cisternino C, Leo V, et al. Protecting healthcare workers from SARS-CoV-2 infection: practical indications. Eur Respir Rev. 2020;29:200068. doi: 10.1183/16000617.0068-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu IT, Xie ZH, Tsoi KK, et al. Why did outbreaks of severe acute respiratory syndrome occur in some hospital wards but not in others? Clin Infect Dis. 2007;44:1017–1025. doi: 10.1086/512819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Disposition of the Italian Minister of Health n. 5443. New indications and clarifications. http://www.trovanorme.salute.gov.it/norme/home . Date last updated: 22 February 2020; date last accessed: 20 April 2020. [Google Scholar]
- 8.World Health Organization. Rational use of personnel protective equipment for coronavirus disease 2019 (COVID-19) Interim guidance. https://apps.who.int/iris/bitstream/handle/10665/331215/WHO-2019-nCov-IPCPPE_use-2020.1-eng.pdf . Date last updated: 27 February; date last accessed: 20 April 2020. 2020 [Google Scholar]
- 9.World Health Organization. Advice on the use of masks in the community, during home care and in health care settings in the context of the novel coronavirus (2019-nCoV) outbreak. Interim guidance. Date last updated: 19 March; date last accessed: 20 April 2020. 2020 [Google Scholar]
- 10.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hui DS, Ip M, Tang JW, et al. Airflows around oxygen masks: a potential source of infection? Chest. 2006;130:822–826. doi: 10.1378/chest.130.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hui DS, Chow BK, Chu L. Exhaled air dispersion and removal is influenced by isolation room size and ventilation settings during oxygen delivery via nasal cannula. Respirology. 2011;16:1005–1013. doi: 10.1111/j.1440-1843.2011.01995.x. [DOI] [PubMed] [Google Scholar]
- 13.Hui DS, Chow BK, Chu L, et al. Exhaled air dispersion during coughing with and without wearing a surgical or N95 mask. PloS One. 2012;7:e50845. doi: 10.1371/journal.pone.0050845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hui DS, Chan MT, Chow B. Aerosol dispersion during various respiratory therapies: a risk assessment model of nosocomial infection to health care workers. Hong Kong Med J. 2014;20(Suppl. 4):9–13. [PubMed] [Google Scholar]
- 15.Hui DS, Chow BK, Lo T, et al. Exhaled air dispersion during noninvasive ventilation via helmets and a total facemask. Chest. 2015;147:1336–1343. doi: 10.1378/chest.14-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tran K, Cimon K, Severn M, et al. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PloS. 2012;7:e35797. doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Infection prevention and control of epidemic and pandemic-prone acute respiratory infections in health care. Geneva: WHO. 2014 [PubMed] [Google Scholar]
- 18.European Centre for Disease Prevention and Control. Personnel protective equipment (PPE) needs in healthcare settings for the care of patients with suspected or confirmed 2019-nCoV. Stockholm: ECDC. 2020 [Google Scholar]
- 19.Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67:568–576. doi: 10.1007/s12630-020-01591-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. Clinical management of severe acute respiratory infection (SARI) when Covid-19 disease is suspected. Interim guidance. 2020 https://apps.who.int/iris/handle/10665/331446?show=full. Date last updated: 13 March; date last accessed: 20 April 2020. [Google Scholar]
- 21.Seto WH, Tsang D, Yung RW, et al. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS) Lancet. 2003;361:1519–1520. doi: 10.1016/S0140-6736(03)13168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz J, King CC, Yen MY. Protecting Health Care Workers during the COVID-19 Coronavirus Outbreak-Lessons from Taiwan’s SARS response. Clin Infect Dis. 2020;71:858–860. doi: 10.1093/cid/ciaa255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Black JRM, Bailey C, Swanton C. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lanced. 2020;395:1418–1420. doi: 10.1016/S0140-6736(20)30917-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu M, He P, Liu HG, et al. Clinical characteristics of 30 medical workers infected with new coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:209–214. doi: 10.3760/cma.j.issn.1001-0939.2020.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Wilson NM, Norton A, Young FP, Collins DW. Airborne transmission of severe acute respiratory syndrome coronavirus-2 to healthcare workers: a narrative review. Anaesthesia. 2020;75:1086–1095. doi: 10.1111/anae.15093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rowan NJ, Laffey JG. Challenges and solutions for addressing critical shortage of supply chain for personnel and protective equipment (PPE) arising from Coronavirus disease (COVID19) pandemic - Case study from the Republic of Ireland. Sci Total Environ. 2020;725:138532. doi: 10.1016/j.scitotenv.2020.138532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yen MY, Lin YE, Lee CH, et al. Taiwan’s traffic control bundle and the elimination of nosocomial severe acute respiratory syndrome among health care workers. J Hosp Infect. 2011;77:332–337. doi: 10.1016/j.jhin.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yen MY, Schwartz J, Wu JSJ, Hsueh PR. Controlling MERS: Lesson Learned from SARS. Clin Infect Dis. 2015;61:1761–1762. doi: 10.1093/cid/civ648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su TP, Lien TC, Yang CY, et al. Prevalence of psychiatric morbidity and psychological adaptation of the nurses in a structured SARS caring unit during outbreak: A prospective and periodic assessment study in Taiwan. J Psychiatr Res. 2007;41:119–130. doi: 10.1016/j.jpsychires.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCOV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baetting SJ, Parini A, Cardona I, Morand GB. Case series of coronavirus (SARS-CoV-2) in a military recruit school: clinical, sanitary and logistical implications. BMJ Mil Health. 2020; april 16 doi: 10.1136/bmjmilitary-2020-001482. [DOI] [PubMed] [Google Scholar]
- 32.Vandenbrouckel JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4:1628–1655. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chou R, Dana T, Buckley DI, Selph S, Fu R. Totten AM. Epidemiology of and Risk Factors for Coronavirus Infection in Health Care Workers: A living Rapid Revew. Ann Intern Med. 2020;173:120–136. doi: 10.7326/M20-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization. Protocol for assessment of potential risk factors for 2019-novel coronavirus (2019-nCoV) infection among health care workers in a health care setting. 2020d https://www.who.int/publications-detail/protocol-for-assessment-of-potential-risk-factors-for-2019-novel-coronavirus-(2019-ncov)-infection-among-health-care-workers-in-a-health-care-setting. Date last updated: 25 January; date last accessed: 24 April 2020. [Google Scholar]
- 35.Kim AY, Hirsch MS, Bloom A. Coronavirus disease 2019 (COVID-19): Management in adults. In: Post TW, editor. UpTo Date. Waltham, MA: UpToDate; 2020. (Accessed on April 21, 2020.) [Google Scholar]
- 36.Tara N, Sexton DJ, Bloom A, Mitty J. Coronavirus disease 2019 (COVID-19): Infection control in health care and home settings. In: Post TW, editor. UpTo Date. Waltham, MA: UpToDate; 2020. (Accessed on April 21, 2020) [Google Scholar]
- 37.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo YR, Cao QD, Hong ZS. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Mil Med Res. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hormati A, Shahhamzeh A, Afifian M, et al. Can COVID-19 present unusual GI symptoms? Journal of Microbiology, Immunology and Infection. 2020;53:384–385. doi: 10.1016/j.jmii.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mclntosh K, Hirsch S, Bloom A. Coronavirus disease 2019 (COVID-19): Epidemiology, virology, clinical features, diagnosis, and prevention. In: Post TW, editor. UpToDate. Waltham, MA: UpToDate; 2020. (Accessed on April 21, 2020) [Google Scholar]
- 41.Scarpa N, Sghedoni D, Rosa V. COVID-19, la malattia da nuovo coronavirus (SARS-CoV-2) Quesiti clinici. 2020;11:1–50. [Google Scholar]
- 42.Horowitz RI, Freeman PR. Three novel prevention, diagnostic, and treatment options for COVID-19 urgently necessitating controlled randomized trials. Med Hypotheses. 2020;143:109851. doi: 10.1016/j.mehy.2020.109851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, Zhou M, Liu F. Reasons for healthcare workers becoming infected with novel coronavirus disease 2019 (COVID-19) in China. J Hosp Infect. 2020;205:100–101. doi: 10.1016/j.jhin.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chew NWS, Lee GKH, Benjamin YQT, Jing M, et al. A multinational, multicentre study on the psychological outcomes and associated physical symptoms amongst healthcare workers during COVID-19 outbreak. Brain Behav Immun. 2020;88:559–565. doi: 10.1016/j.bbi.2020.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Health Organization. Clinical management of severe acute respiratory infection (SARI) when Covid-19 disease is suspected. Interim guidance. 2020 https://apps.who.int/iris/handle/10665/331446?show=full. Date last accessed: 30 March 2020; date last accessed: 24 april 2020. [Google Scholar]
- 46.Lai X, Wang M, Qin C, Tan L, Ran L, Chen D, Zhang H, Shang K, Xia C, Wang S, Xu S, Wang W. Coronavirus disease 2019 (COVID-2019) infection among health care workers and implications for prevention measures in a tertiary hospital in Wuhan, China. JAMA Netw Open. 2020;3:e209666. doi: 10.1001/jamanetworkopen.2020.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin YH, Huang Q, Wang YY, Zeng XT, Luo LS, et al. Perceived infection transmission routes, infection control practices, psychosocial changes, and management of COVID-19 infected healthcare workers in a tertiary acute care hospital in Wuhan: a crosssectional survey. Mil Med Res. 2020;7:24. doi: 10.1186/s40779-020-00254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang J, Nie S, Wei S. Reflections on the present response to the pneumonia associated with a novel coronavirus (2019-nCov) New Med. 2020;30:10–3. [Google Scholar]
- 49.Röhr S, Müller F, Jung F, Apfelbacher C, Seidler A, Riedel-Heller SG. Psychosoziale Folgen von Quarantänemaßnahmen bei schwerwiegenden Coronavirus-Ausbrüchen: ein Rapid Review. Psychiatr Prax. 2020;47:179–189. doi: 10.1055/a-1159-5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shahid Z, Kalayanamitra R, McClafferty B, Kepko D, Ramgobin D, Patel R, Aggarwal CS, Vunnam R, Sahu N, Bhatt D, Jones K, Golamari R, Jain R. COVID-19 and Older Adults: What we know. J Am Geriatr Soc. 2020;68:926–929. doi: 10.1111/jgs.16472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nanshan C, Zhou M, Dong X, Qu J, Gong F, et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, Zhang HY, Sun W, Wang Y. COVID-19 Patients’ Clinical Characteristics Discharge Rate, and Fatality Rate of Meta-Analysis. J Med Virol. 2020;92:577–583. doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klopfenstein T, Kadiane-Oussou NJ, Toko L, Royer PY, Lepiller Q, Gendrin V, et al. Features of anosmia in COVID-19. Med Mal Infect. 2020;50:436–439. doi: 10.1016/j.medmal.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zayet S, N’dri Juliette KO, Royer PY, Toko L, Gendrin V, Klopfenstein T. Coronavirus disease 2019: new things to know! J Med Virol. 2020; april 13 doi: 10.1002/jmv.25874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu Z, Song C, Xu C, Jin G, Chen Y, Xu X, Ma H, Chen W, Lin Y, Zheng Y, Wang J, Hu Z, Yi Y, Shen H. Clinical Characteristics of 24 Asymptomatic Infections With COVID-19 Screened Among Close Contacts in Nanjing, China. Sci China Life Sci. 2020;63:706–711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheng X, Liu J, Li N, Nisenbaum E, Sun Q, Chen B, Casiano R, Weed D, Telischi F, Denneny JC, Liu X, Shu Y. Otolaryngology Providers Must Be Alert for Patients With Mild and Asymptomatic COVID-19. Otolaryngol Head Neck Surg. 2020;162:809–810. doi: 10.1177/0194599820920649. [DOI] [PubMed] [Google Scholar]