Abstract
Background and aim:
Infertility affects ~20% of the couples in the world. Assisted reproductive technologies (ARTs) are currently the most common treatment option for infertility. Nevertheless, ARTs may be associated with complications for mothers and/or offspring. Natural procreative technology (NaProTechnology) is a natural treatment which minimizes these risks by seeking to identify the causes of infertility to enable better treatments. This narrative review summarizes the complications related to ARTs and clarifies how the NaProTechnology approach can help ARTs to achieve better results or be used in alternative to ARTs.
Methods:
Data in the literature indicate that NaProTechnology is a natural approach for treating infertility.
Results:
The percentage of live births obtained by NaProTechnology is similar to that of ARTs.
Conclusions:
An extensive search for the genetic defects causing infertility or subfertility through genetic testing can help both ARTs and NaProTechnology to achieve successful pregnancies. By discovering the underlying causes of infertility, genetic tests enable better family counseling, like the implications of transmitting risk- and disease-alleles to future generations. (www.actabiomedica.it)
Keywords: assisted reproductive technology, genetic infertility, NaProTechnology
Table S1.
Genes associated with male and female infertility (https://www.omim.org/)
| Female infertility | |||||
| Gene | Inheritance | OMIM gene ID | OMIM phenotype | OMIM phenotype ID | Clinical Features |
| HFM1 | AR | 615684 | POF9 | 615724 | Amenorrhea |
| FIGLA | AD | 608697 | POF6 | 612310 | Small/absent ovaries, follicles absent, atrophic endometrium |
| FOXL2 | AD | 605597 | POF3 | 608996 | Hypoplastic uterus and ovaries, follicles absent, secondary amenorrhea |
| MSH5 | AR | 603382 | POF13 | 617442 | Oligomenorrhea, atrophic ovaries, follicles absent |
| STAG3 | AR | 608489 | POF8 | 615723 | Primary amenorrhea, ovarian dysgenesis |
| NOBOX | AD | 610934 | POF5 | 611548 | Secondary amenorrhea, follicles absent |
| NR5A1 | AD | 184757 | POF7 | 612964 | Irregular or anovulatory menstrual cycles, secondary amenorrhea, dysgenetic gonads, no germ cells |
| ERCC6 | AD | 609413 | POF11 | 616946 | Secondary amenorrhea |
| SYCE1 | AR | 611486 | POF12 | 616947 | Primary amenorrhea, small prepubertal uterus and ovaries, no ovarian follicles |
| MCM8 | AR | 608187 | POF10 | 612885 | Absent thelarche, primary amenorrhea, no ovaries, hypergonadotropic ovarian failure |
| BMP15 | XLD | 300247 | POF4, OD2 | 300510 | Delayed puberty, primary/secondary amenorrhea, small ovaries, follicles absent, hypoplastic uterus, hirsutism, absent pubic/axillary hair |
| FLJ22792 | XLR | 300603 | POF2B | 300604 | Weak teeth, delayed puberty, primary amenorrhea, osteoporosis |
| DIAPH2 | XLD | 300108 | POF2A | 300511 | Secondary amenorrhea |
| FSHR | AR | 136435 | OD1 | 233300 | Osteoporosis, primary amenorrhea |
| MCM9 | AR | 610098 | OD4 | 616185 | Short stature, low weight, underdeveloped breasts, no ovaries, retarded bone age and development of pubic/axillary hair, primary amenorrhea |
| SOHLH1 | AR | 610224 | OD5 | 617690 | Short stature, absent thelarche, primary amenorrhea, hypoplastic/no ovaries, small uterus, retarded bone age |
| PSMC3IP | AR | 608665 | OD3 | 614324 | Underdeveloped breasts and absent pubic hair, hypoplastic uterus, primary amenorrhea |
| AMH | AD | 600957 | POF | / | Primary/secondary amenorrhea |
| AMHR2 | AD | 600956 | POF | / | Primary ovarian insufficiency |
| DAZL | AR | 601486 | POF | / | Low ovarian reserves |
| GDF9 | AR | 601918 | POF14 | 618014 | Primary amenorrhea, no breast development, delayed pubic hair development |
| LHCGR | AR | 152790 | POF | / | Primary amenorrhea |
| INHA | AD, AR | 147380 | POF | / | Primary amenorrhea |
| PGRMC1 | AD | 300435 | POF | / | Hypergonadotropic hypogonadism, amenorrhea |
| POU5F1 | AD | 164177 | POF | / | Small ovaries without follicles |
| TGFBR3 | AD | 600742 | POF | / | Premature ovarian failure |
| WT1 | AD | 607102 | POF | / | Secondary amenorrhea |
| SGO2 | AR | 612425 | POF | / | Ovarian insufficiency |
| SPIDR | AR | 615384 | POF | / | Hypoplastic/no ovaries |
| EIF4ENIF1 | AD | 607445 | POF | / | Secondary amenorrhea |
| NUP107 | AR | 607617 | OD6 | 618078 | No ovaries, small uterus, no spontaneous puberty |
| NANOS3 | AD | 608229 | POF | / | Primary amenorrhea |
| ZP3 | AD | 182889 | OOMD3 | 617712 | Oocyte degeneration, absence of zona pellucida |
| TUBB8 | AD, AR | 616768 | OOMD2 | 616780 | Oocyte arrest at metaphase I or II; abnormal spindle |
| ZP1 | AR | 195000 | OOMD1 | 615774 | Absence of zona pellucida |
| PATL2 | AR | 614661 | OOMD4 | 617743 | Oocyte maturation arrest in germinal vesicle stage, metaphase I or polar body 1 stage; abnormal polar body 1; early embryonic arrest |
| ZP2 | AR | 182888 | OOMD6 | 618353 | Abnormal of zona pellucida |
| TLE6 | AR | 612399 | PREMBL1 | 616814 | Failure of zygote formation |
| PADI6 | AR | 610363 | PREMBL2 | 617234 | Recurrent early embryonic arrest |
| SYCP3 | AD | 604759 | RPRGL4 | 270960 | Fetal loss after 6-10 weeks of gestation |
| F2 | AD | 176930 | RPRGL2 | 614390 | Recurrent miscarriage |
| ANXA5 | AD | 131230 | RPRGL3 | 614391 | |
| NLRP7 | AR | 609661 | HYDM1 | 231090 | Gestational trophoblastic disease |
| KHDC3L | AR | 611687 | HYDM2 | 614293 | |
| Male infertility | |||||
| Gene | Inheritance | OMIM gene | OMIM phenotype | OMIM phenotype ID | Sperm defect |
| NR5A1 | AR | 184757 | SPGF8 | 613957 | AZS/OZS |
| SYCP3 | AD | 604759 | SPGF4 | 270960 | AZS/OZS |
| ZMYND15 | AR | 614312 | SPGF14 | 615842 | AZS/OZS |
| TAF4B | AR | 601689 | SPGF13 | 615841 | AZS/OZS |
| TEX11 | XLR | 300311 | SPGFX2 | 309120 | AZS |
| NANOS1 | AD | 608226 | SPGF12 | 615413 | AZS/OZS/OZS+ASTHZ+TZS |
| PLK4 | AD | 605031 | / | / | AZS |
| MEIOB | AR | 617670 | SPGF22 | 617706 | AZS |
| SYCE1 | AR | 611486 | SPGF15 | 616950 | AZS |
| USP9Y | YL | 400005 | SPGFY2 | 400042 | AZS |
| SOHLH1 | AD | 610224 | SPGF32 | 618115 | AZS |
| TEX15 | AR | 605795 | SPGF25 | 617960 | AZS/OZS |
| HSF2 | AD | 140581 | / | / | AZS |
| KLHL10 | AD | 608778 | SPGF11 | 615081 | OZS; TZS; AZS |
| AURKC | AR | 603495 | SPGF5 | 243060 | TZS (macrozoospermia) |
| DPY19L2 | AR | 613893 | SPGF9 | 613958 | TZS (globozoospermia) |
| SPATA16 | AR | 609856 | SPGF6 | 102530 | TZS (globozoospermia) |
| PICK1 | AR | 605926 | / | / | TZS (globozoospermia) |
| BRDT | AR | 602144 | SPGF21 | 617644 | ASS |
| SUN5 | AR | 613942 | SPGF16 | 617187 | ASS |
| SLC26A8 | AD | 608480 | SPGF3 | 606766 | AZS |
| CATSPER1 | AR | 606389 | SPGF7 | 612997 | AZS |
| SEPT12 | AD | 611562 | SPGF10 | 614822 | AZS; OZS+ASTHZ+TZS |
| CFAP43 | AR | 617558 | SPGF19 | 617592 | MMAF |
| CFAP44 | AR | 617559 | SPGF20 | 617593 | MMAF |
| DNAH1 | AR | 603332 | SPGF18 | 617576 | MMAF |
| PLCZ1 | AR | 608075 | SPGF17 | 617214 | OAF |
SPGF = spermatogenic failure; OZS = oligozoospermia; AZS = azoospermia; ASTHZ = asthenozoospermia; TZS = teratozoospermia; OZS+ASTHZ+TZS = oligoasthenoteratozoospermia; ASS = acephalic spermatozoa syndrome; MMAF = multiple morphological abnormalities of the flagellum; OAF = oocyte activation failure; AR = autosomal recessive; AD = autosomal dominant; XLR = X-linked recessive; YL = Y-linked; OD=ovarian dysgenesis; POF = primary ovarian failure; OOMD=oocyte maturation defect; PREMBL=preimplantation embryonic lethality; RPRGL=recurrent pregnancy loss; PREMBL=preimplantation embryonic lethality.
Introduction
Human fertilization involves the fusion of two functionally and morphologically different haploid cells (spermatozoon and oocyte) to generate a new diploid organism. In the case of women of fertile age, infertility is defined as failure to become pregnant after 12 months of regular unprotected intercourse.
A systematic analysis, published in 2012, of 277 surveys revealed that among women aged 20–44 years, exposed to unprotected intercourse, 1.9% were unable to achieve a live birth, and among women with at least one live birth, 10.5% were unable to have another child (1). Assisted reproductive technology (ART) treats infertility and obtains a high pregnancy rate (2). The most commonly used ART techniques are in vitro fertilization, intra-cytoplasmic sperm injection, controlled ovarian hyperstimulation and embryo transfer (3). Around the world, more than 500000 newborns are conceived through ART every year (4). Data in the literature indicates that ARTs may be associated, for example, with an increased rate of ovarian hyperstimulation syndrome and multiple pregnancies in mothers, and preterm birth, low birth weight, tumors and genetic/epigenetic alterations in offspring. The routine ART approach includes a set of basic clinical investigations aimed at identifying broad causes of infertility, although, recently, it is starting to focus on the increasing number of genetic factors known to impact human fertility (5).
Unlike ART, restorative reproductive medicine, such as natural procreative technology (NaProTechnology), focuses on improving gynecological health and restoring optimal reproductive function through medical and surgical reproductive procedures (6). This approach implies that if the cause of infertility is identified and treated, normal reproductive function can be restored and pregnancy can be achieved by normal intercourse without running the risk of ART-related complications (6). In addition, identification of the genetic cause of infertility in a couple gives adult offspring the opportunity to know key genetic information regarding their reproductive risk, and perhaps prevention and treatment options.
This narrative review summarizes current known ART-related risks for mothers and offspring, and illustrates the principles and treatment options of NaProTechnology.
Methods
Review of the literature
For this narrative review, PubMed was searched using the following search string: “infertility” AND “assisted reproductive technology” OR “NaProTechnology”. We evaluated articles published until August 2019 written in English. We then only selected articles related to complications associated with ART and to the NaProTechnology approach.
Results
ART-related complications for mothers
A study performed in the Netherlands showed that the mortality rate in ART pregnancies is greater than the mortality rate in normal pregnancies: 42 deaths per 100000 against 6 deaths per 100000, respectively (7).
ART can increase the risk of ovarian hyperstimulation syndrome (OHSS) and multiple pregnancies (8,9). To retrieve more oocytes, ART frequently resorts to controlled ovarian stimulation, which improves outcome in terms of likelihood of getting pregnant, but at the same time may increase the risk of OHSS (10). This risk may range from 3% to 10% in ART cycles, and can reach 20% in high risk women (11). OHSS can cause serious issues and complications for pregnant women, and if not treated promptly, can lead to miscarriage or loss of ovarian function (12).
Another major complication associated with ART is increased risk of extra uterine/ectopic pregnancies. The rate of ectopic pregnancies after ART ranges from 1% to 8.6%, whereas with normal conception it ranges from 1% to 2% (13).
According to the “Million Women Study” performed in the United Kingdom, the current practice of hormone replacement therapy is linked to a high risk of fatal breast cancer (14). Several population studies have demonstrated that infertile women undergoing hormonal stimulation for multiple oocyte production have a higher risk of breast cancer, especially when stimulation is with clomiphene or in the case of young women undergoing ART (15).
Another complication that may affect the health of women is hypertension, which is the cause of about 14% of maternal deaths (16). Specifically, women undergoing ART have double the risk of developing hypertension compared to pregnant women who conceived naturally (17).
ART-related complications for fetus and newborn
ART is associated with increased risk of low birth weight, preterm delivery, miscarriage and perinatal mortality (18). The higher risk of miscarriages embryos in the early phases of ART pregnancies may be due to chromosomal abnormalities or other genomic and epigenomic alterations (19). According to a meta-analysis that compared 12283 ART-conceived singleton infants with 1.9 million normally conceived singleton infants, the former showed a significantly higher rate of perinatal mortality, preterm births, small-for-gestational-age status and low/very low birth weight (20). In another recent analysis, researchers were unable to establish a significant association between ART and preterm births, although they found a higher risk of placenta previa, abruptio placentae, preeclampsia and caesarean delivery (21). The frequency of stillbirths is also higher in ART pregnancies (16.2/1000) than natural pregnancies (2.3/1000) (22).
Long-term potential complications of ART
A tripled risk of neural tube defects, gastrointestinal atresia, omphalocele and hypospadias was found in a cohort of Scandinavian newborns conceived by ICSI. It has been surmised that the increased risk of gastrointestinal atresia and monozygotic twinning after ART is a direct consequence of the procedure. Others have suggested that the higher risk of hypospadias after intracytoplasmic sperm injection could be related to paternal subfertility determined by a specific genetic background (23).
It was recently also established that ART may cause epigenetic defects resulting in various human disorders (24). In a Japanese study, researchers found that Beckwith-Wiedemann, Angelman, Prader-Willi and Silver-Russell syndromes are more frequent in babies conceived by ICSI and IVF than in spontaneously conceived babies (25).
Administration of exogenous hormones may affect fetal growth and organ differentiation, leading to increased risk of endocrine-sensitive cancer in later life (26). Some studies suggest a possible increased risk of cancer, including neuroectodermal tumors, malignant lymphoma and hepatoblastoma, in children conceived by ART (27-29).
Discussion
NaProTechnology and ART
The main treatment option for infertility is currently ART. It is available worldwide, but is expensive and associated with some risks for the mother and child (Table 1) (30).
Table 1.
Characteristics of ART and NaProTechnology compared to normal pregnancies
| Parameter | ART | NaProTechnology | Reference |
| Cost | ↑↑↑ | ↑ | 31 |
| Perinatal death rate | ↑ | ≈ | 30,32 |
| Extra-uterine pregnancy risk | ↑ | ≈ | 13,30 |
| Ovarian hyperstimulation syndrome risk | ↑ | ≈ | 9,30 |
| Genetic mutations risk | ↑ | ≈ | 33,34 |
| Epigenetic alterations risk | ↑ | ≈ | 35-37 |
| Chromosomal anomalies risk | ↑ | ≈ | 33,34,37 |
| Breast/ovarian cancer risk | ↑ | ≈ | 15,30,38 |
| Maternal mortality rate | ↑ | ≈ | 7,30 |
| Invasive procedures frequency | ↑ | ≈ | 39,40 |
| Low birth-weight risk | ↑ | ≈ | 6,41 |
| Long-term side effects risk | ↑ | ≈ | 42-44 |
| Genetic screening | Variable | Extensive | 19,45 |
| Genetic counseling | Variable | Extensive | 19,45 |
| Birth defects rate | ↑ | ≈ | 30,44 |
An American surgeon and gynecologist, Dr. Thomas Hilger, proposed a method for natural procreation called NaProTechnology, which takes a natural approach to regulating fertility. NaProTechnology seeks to treat infertility with surgical, endocrinological or pharmacological personalized and targeted therapies (46). NaProTechnology also focuses on locating the fertility peak to optimize the chances of conception and offers couples an opportunity to conceive by a natural intercourse (40).
The approach follows the rules of the Creighton Model Fertility Care System (CrMS) that evaluates biochemical and hormonal parameters and organ dysfunction. The parameters include short/variable luteal phases, uterine bleeding, decreased levels of progesterone and estrogen, and reduced production and release of cervical mucus (30).
In 1972, Billings and collaborators successfully tested a NaProTechnology approach by getting women themselves to notice the signs and symptoms, like cervical mucus, that indicate the ovulatory period and fertility peak (47).
Another study, published in 2008, showed that 1239 infertile couples, treated with NaProTechnology, had a live birth rate similar to that of the ART-treated group (30). In the first step, couples were educated to identify fertile days according to the CrMS; medical treatment, including clomiphene administration, was given to 75% of couples. The results showed that 52.8% of couples treated with NaProTechnology had a live birth within 24 months (30).
Another method developed to predict the probability of conception is based on the Bayesian statistical method. This method evaluates the menstrual cycle, and the mucus level and composition in order to increase the chances of conception by minimizing the frequency of intercourses (48). This simple method is based on mucus parameters and conventional markers of ovulation, such as serum hormone values and body temperature increase (49). It was estimated that outside the mid-cycle interval (day 7 to 20) the chance of conception is close to zero (49), and is directly linked to the type of mucus, classified from the most to the least fertile type in the mid-cycle interval (49). These natural fertility regulation methods may help couples recognize the most fertile period and clinicians to identify any abnormality that could be linked with infertility (50).
NaProTechnology and genetics
Infertility appears to be genetically determined in about 50% of cases (51). The burden of deleterious genetic variants in human reproduction is also documented by the fact that genetic diseases account for 20% of neonatal mortality and 10% of neonatal hospitalization (52).
NaProTechnology and ART have the same goal, namely to improve the chance of achieving pregnancies that produces healthy offspring. However, there is evidence to suggest that ART can amplify genome instability and therefore affect the chances of conceptions carrying potentially deleterious de novo mutations (53). Accordingly, several follow-up studies of children conceived by ART have proposed that ART is associated with an increased frequency of genetic and epigenetic abnormalities, as previously stated (see Long-term potential complications of ARTs).
Importantly, since genetic sequencing is now less costly and advances have been made in the interpretation of bioinformatic output, extensive genetic screening of couples for genetic factors predisposing to serious and/or neonatal/children’s diseases will soon be plausible by next generation sequencing (NGS). This approach could offer couples the opportunity to discover whether they risk transmitting serious or unexpected Mendelian pathologies not indicated by their family history. Couples with fertility problems could be the first to take advantage of NGS screening. Another important point to highlight is that if a couple does not know it carries a genetic mutation that causes infertility and ART enables them to conceive, they are postponing the problem until the next generation. In such cases, NaProTechnology is facilitated by diagnostic methods that offer a couple a more complete picture of their reproductive risks and therefore a more conscious choice between natural reproduction, ART or adoption.
In conclusion, it used to be prohibitively expensive for couples to undergo a detailed diagnostic phase including extensive genetic study, but it is now relatively accessible with NGS. Here, we propose a list of genes known to cause Mendelian infertility that could be included in a diagnostic panel for couples with idiopathic infertility (Figure 1, Table S1) (45,52,54-59).
Figure 1.
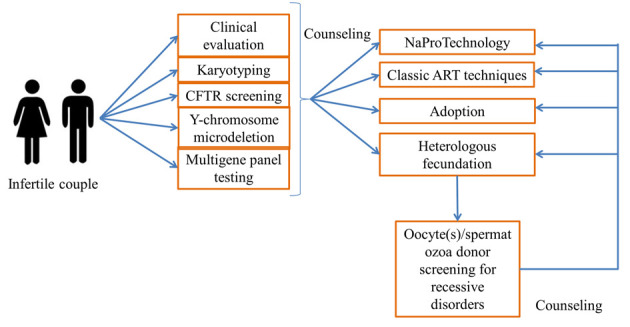
Flowchart for the counseling, diagnosis and treatment in couples with infertility
Conclusions
NaProTechnology is an approach that optimizes natural reproduction in cases of infertility with the aim of minimizing risks for mothers and offspring. NaProTechnology aims to improve the natural reproductive cycle of the couple, thereby avoiding risks related to embryo handling and hormone therapies. Knowing the underlying causes of infertility can help couples to achieve better outcomes. In this scenario, the use of NGS to assess couples with reduced fertility is making diagnosis easier, as in other areas of medicine with a significant genetic burden. Finally, NGS makes it possible to consider the pros of extensive pre-conceptive genetic screening of couples to identify alleles associated with risk of early severe/lethal disorders, and to use this information for better prevention and monitoring of reproductive risk, also in the long term.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: A systematic analysis of 277 health surveys. PLoS Med. 2012;9:e1001356. doi: 10.1371/journal.pmed.1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quaas A, Dokras A. Diagnosis and treatment of unexplained infertility. Rev Obstet Gynecol. 2008;1:69–76. [PMC free article] [PubMed] [Google Scholar]
- 3.Scaravelli G, Spoletini R. Handbook of Fertility. Tucson: Academic Press; 2015. The application of reproductive techniques (ART): worldwide epidemiology phenomenon and treatment outcomes. [Google Scholar]
- 4.Adamson GD, de Mouzon J, Chambers GM, et al. International Committee for Monitoring Assisted Reproductive Technology: World report on assisted reproductive technology, 2011. Fertil Steril. 2018;110:1067–80. doi: 10.1016/j.fertnstert.2018.06.039. [DOI] [PubMed] [Google Scholar]
- 5.Bhandari H, Choudhary M, Stewart J. Complications of assisted reproductive technology treatment and the factors influencing reproductive outcome. Obstet Gynaecol. 2018;20:177–86. [Google Scholar]
- 6.Boyle PC, de Groot T, Andralojc KM, Parnell TA. Healthy singleton pregnancies from restorative reproductive medicine (RRM) after failed IVF. Front Med. 2018;5:210. doi: 10.3389/fmed.2018.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bewley S, Foo L, Braude P. Adverse outcomes from IVF. BMJ. 2011;342:d436. doi: 10.1136/bmj.d436. [DOI] [PubMed] [Google Scholar]
- 8.Devroey P, Fauser BCJM, Diedrich K. Approaches to improve the diagnosis and management of infertility. Hum Reprod Update. 2009;15:391–408. doi: 10.1093/humupd/dmp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vrooman LA, Bartolomei MS. Can assisted reproductive technologies cause adult-onset disease? Evidence from human and mouse. Reprod Toxicol. 2017;68:72–84. doi: 10.1016/j.reprotox.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith V, Osianlis T, Vollenhoven B. Prevention of ovarian hyperstimulation syndrome: A review. Obstet Gynecol Int 2015. 2015:514159. doi: 10.1155/2015/514159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nastri CO, Teixeira DM, Moroni RM, Leitão VMS, Martins WP. Ovarian hyperstimulation syndrome: Pathophysiology, staging, prediction and prevention. Ultrasound Obstet Gynecol. 2015;45:377–93. doi: 10.1002/uog.14684. [DOI] [PubMed] [Google Scholar]
- 12.Kanayama S, Kaniwa H, Tomimoto M, Zhang B, Nishioka K, Oi H. Laparoscopic detorsion of the ovary in ovarian hyperstimulation syndrome during the sixth week of gestation: A case report and review. Int J Surg Case Rep. 2019;59:50–3. doi: 10.1016/j.ijscr.2019.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perkins KM, Boulet SL, Kissin DM, Jamieson DJ. Risk of ectopic pregnancy associated with assisted reproductive technology in the United States, 2001-2011. Obstet Gynecol. 2015;125:70–8. doi: 10.1097/AOG.0000000000000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crosignani PG. Breast cancer and hormone-replacement therapy in the Million Women Study. Maturitas. 2003;46:91–2. doi: 10.1016/j.maturitas.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Schneider J, Lahl J, Kramer W. Long-term breast cancer risk following ovarian stimulation in young egg donors: A call for follow-up, research and informed consent. Reprod Biomed Online. 2017;34:480–5. doi: 10.1016/j.rbmo.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Say L, Chou D, Gemmill A, et al. Global causes of maternal death: A WHO systematic analysis. Lancet Glob Heal. 2014;2:e323–33. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 17.Allen VM, Wilson RD, Cheung A. Genetics Committee, Reproductive Endocrinology and Infertility Committee. Pregnancy outcomes after assisted reproductive technology. J Obstet Gynaecol Canada. 2016;28:220–33. doi: 10.1016/S1701-2163(16)32112-0. [DOI] [PubMed] [Google Scholar]
- 18.Vergouw CG, Hanna Kostelijk E, Doejaaren E, Hompes PGA, Lambalk CB, Schats R. The influence of the type of embryo culture medium on neonatal birthweight after single embryo transfer in IVF. Hum Reprod. 2012;27:2619–26. doi: 10.1093/humrep/des252. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Z, Wang Y, Lin J, Xu J, Ding G, Huang H. Genetic and epigenetic risks of assisted reproduction. Best Pract Res Clin Obstet Gynaecol. 2017;44:90–104. doi: 10.1016/j.bpobgyn.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Jackson RA, Gibson KA, Wu YW, Croughan MS. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis. Obstet Gynecol. 2004;103:551–63. doi: 10.1097/01.AOG.0000114989.84822.51. [DOI] [PubMed] [Google Scholar]
- 21.Shevell T, Malone FD, Vidaver J, et al. Assisted reproductive technology and pregnancy outcome. Obstet Gynecol. 2005;106:1039–45. doi: 10.1097/01.AOG.0000183593.24583.7c. [DOI] [PubMed] [Google Scholar]
- 22.Wisborg K, Ingerslev HJ, Henriksen TB. IVF and stillbirth: A prospective follow-up study. Hum Reprod. 2010;25:1312–6. doi: 10.1093/humrep/deq023. [DOI] [PubMed] [Google Scholar]
- 23.Ericson A, Källén B. Congenital malformations in infants born after IVF: A population-based study. Hum Reprod. 2001;16:504–9. doi: 10.1093/humrep/16.3.504. [DOI] [PubMed] [Google Scholar]
- 24.Niemitz EL, Feinberg AP. Epigenetics and assisted reproductive technology: A call for investigation. Am J Hum Genet. 2004;74:599–609. doi: 10.1086/382897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hattori H, Hiura H, Kitamura A, et al. Association of four imprinting disorders and ART. Clin Epigenetics. 2019;11:21. doi: 10.1186/s13148-019-0623-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tournaire M, Devouche E, Espié M, et al. Cancer risk in women exposed to diethylstilbestrol in utero. Therapie. 2015;70:433–41. doi: 10.2515/therapie/2015030. [DOI] [PubMed] [Google Scholar]
- 27.Melamed I, Bujanover Y, Hammer J, Spirer Z. Hepatoblastoma in an infant born to a mother after hormonal treatment for sterility. N Engl J Med. 1982;307:820. doi: 10.1056/NEJM198209233071313. [DOI] [PubMed] [Google Scholar]
- 28.White L, Giri N, Vowels MR, Lancaster PAL. Neuroectodermal tumours in children born after assisted conception. Lancet. 1990;336:1577. doi: 10.1016/0140-6736(90)93350-x. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi N, Matsui I, Tanimura M, et al. Childhood neuroectodermal tumours and malignant lymphoma after maternal ovulation induction. Lancet. 1991;336:1577. doi: 10.1016/0140-6736(91)91829-j. [DOI] [PubMed] [Google Scholar]
- 30.Stanford J, Parnell T, Boyle P. Outcomes from treatment of infertility with natural procreative technology in an Irish general practice. J Am Board Fam Med. 2008;21:375–84. doi: 10.3122/jabfm.2008.05.070239. [DOI] [PubMed] [Google Scholar]
- 31.Malizia BA, Hacker MR, Penzias AS. Cumulative live-birth rates after in vitro fertilization. N Engl J Med. 2009;360:236–43. doi: 10.1056/NEJMoa0803072. [DOI] [PubMed] [Google Scholar]
- 32.Maheshwari A, Kalampokas T, Davidson J, Bhattacharya S. Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of blastocyst-stage versus cleavage-stage embryos generated through in vitro fertilization treatment: a systematic review and meta-analysis. Fertil Steril. 2013;100:1615–21. doi: 10.1016/j.fertnstert.2013.08.044. [DOI] [PubMed] [Google Scholar]
- 33.Chan PTK. Genetic risks associated with advanced assisted reproductive technology. J Sex Reprod Med. 2002;2:161–4. [Google Scholar]
- 34.Vogt PH. Genetic aspects of artificial fertilization. Hum Reprod. 1995;10:128–37. doi: 10.1093/humrep/10.suppl_1.128. [DOI] [PubMed] [Google Scholar]
- 35.Clayton-Smith J, Laan L. Angelman syndrome: a review of the clinical and genetic aspects. J Med Genet. 2003;40:87–95. doi: 10.1136/jmg.40.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeBaun MR, Niemitz EL, Feinberg AP. Association of in vitro fertilization with Beckwith-Wiedemann syndrome and epigenetic alterations of LIT1 and H19. Am J Hum Genet. 2003;72:156–60. doi: 10.1086/346031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simpson JL, Lamb DJ. Genetic effects of intracytoplasmic sperm injection. Semin Reprod Med. 2001;19:239–49. doi: 10.1055/s-2001-18043. [DOI] [PubMed] [Google Scholar]
- 38.Hemminki E, Gissler M, Toukomaa H. Exposure to female hormone drugs during pregnancy: effect on malformations and cancer. Br J Cancer. 1999;80:1092–7. doi: 10.1038/sj.bjc.6690469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vélez JR. An ethical comparison between in-vitro fertilization and NaProTechnology. Linacre Q. 2012;79:57–72. doi: 10.1179/002436312803571465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hilgers T. The Medical & Surgical Practice of NaProTechnology. Pope Paul VI Institute Press: Omaha. 2004 [Google Scholar]
- 41.Sunderam S, Kissin DM, Zhang Y, et al. Assisted reproductive technology surveillance - United States, 2016. MMWR Surveill Summ. 2019;68:1–23. doi: 10.15585/mmwr.ss6804a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiao WL, Zhang DZ, Hou JW, Sun J, Jia MY. Multiple orofacial malformations in a boy who was conceived by intracytoplasmic sperm injection. J Plast Reconstr Aesthetic Surg. 2009;62:e298–300. doi: 10.1016/j.bjps.2007.10.085. [DOI] [PubMed] [Google Scholar]
- 43.Anthony S, Buitendijk S, Dorrepaal C, Lindner K, Braat D, den Ouden A. Congenital malformations in 4224 children conceived after IVF. Hum Reprod. 2002;17:2089–95. doi: 10.1093/humrep/17.8.2089. [DOI] [PubMed] [Google Scholar]
- 44.Wen J, Jiang J, Ding C, et al. Birth defects in children conceived by in vitro fertilization and intracytoplasmic sperm injection: A meta-analysis. Fertil Steril. 2012;97:1331–7e1-4. doi: 10.1016/j.fertnstert.2012.02.053. [DOI] [PubMed] [Google Scholar]
- 45.Harper JC, Aittomäki K, Borry P, et al. Recent developments in genetics and medically assisted reproduction: From research to clinical applications. Eur J Hum Genet. 2018;26:12–33. doi: 10.1038/s41431-017-0016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.NICE Clinical Guidelines. London: Royal College of Obstetricians & Gynaecologists; 2013. Fertility: assessment and treatment for people with fertility problems. [PubMed] [Google Scholar]
- 47.Billings EL, Brown JB, Billings JJ, Burger HG. Symptoms and hormonal changes accompanying ovulation. Lancet. 1972;1:282–4. doi: 10.1016/s0140-6736(72)90291-7. [DOI] [PubMed] [Google Scholar]
- 48.Dunson DB, Stanford JB. Bayesian inferences on predictors of conception probabilities. Biometrics. 2005;61:126–33. doi: 10.1111/j.0006-341X.2005.031231.x. [DOI] [PubMed] [Google Scholar]
- 49.Scarpa B, Dunson DB, Giacchi E. Bayesian selection of optimal rules for timing intercourse to conceive by using calendar and mucus. Fertil Steril. 2007;88:915–24. doi: 10.1016/j.fertnstert.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 50.Arévalo M, Sinai I, Jennings V. A fixed formula to define the fertile window of the menstrual cycle as the basis of a simple method of natural family planning. Contraception. 1999;60:357–60. doi: 10.1016/s0010-7824(99)00106-7. [DOI] [PubMed] [Google Scholar]
- 51.Zorrilla M, Yatsenko AN. The genetics of infertility: Current status of the field. Curr Genet Med Rep. 2013:1. doi: 10.1007/s40142-013-0027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gil-Arribas E, Herrer R, Serna J. Pros and cons of implementing a carrier genetic test in an infertility practice. Curr Opin Obstet Gynecol. 2016;28:172–7. doi: 10.1097/GCO.0000000000000272. [DOI] [PubMed] [Google Scholar]
- 53.Horne SD, Abdallah BY, Stevens JB, et al. Genome constraint through sexual reproduction: application of 4D-Genomics in reproductive biology. Syst Biol Reprod Med. 2013;59:124–30. doi: 10.3109/19396368.2012.754969. [DOI] [PubMed] [Google Scholar]
- 54.Le Bouc Y, Rossignol S, Azzi S, Steunou V, Netchine I, Gicquel C. Epigenetics, genomic imprinting and assisted reproductive technology. Ann Endocrinol (Paris) 2010;71:237–8. doi: 10.1016/j.ando.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 55.Vendrell X, Escribà MJ. The model of “genetic compartments”: A new insight into reproductive genetics. J Assist Reprod Genet. 2019;36:363–9. doi: 10.1007/s10815-018-1366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin J, Asan Yi Y, Alberola T, et al. Comprehensive carrier genetic test using next-generation deoxyribonucleic acid sequencing in infertile couples wishing to conceive through assisted reproductive technology. Fertil Steril. 2015;104:1286–93. doi: 10.1016/j.fertnstert.2015.07.1166. [DOI] [PubMed] [Google Scholar]
- 57.Strauss JF, Romero R, Gomez-Lopez N, et al. Spontaneous preterm birth: Advances toward the discovery of genetic predisposition. Am J Obstet Gynecol. 2018;218:294–314. doi: 10.1016/j.ajog.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sallevelt SCEH, De Koning B, Szklarczyk R, Paulussen ADC, De Die-Smulders CEM, Smeets HJM. A comprehensive strategy for exome-based preconception carrier screening. Genet Med. 2017;19:583–92. doi: 10.1038/gim.2016.153. [DOI] [PubMed] [Google Scholar]
- 59.Normand EA, Alaimo JT, Van den Veyver IB. Exome and genome sequencing in reproductive medicine. Fertil Steril. 2018;109:213–20. doi: 10.1016/j.fertnstert.2017.12.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Genes associated with male and female infertility (https://www.omim.org/)
| Female infertility | |||||
| Gene | Inheritance | OMIM gene ID | OMIM phenotype | OMIM phenotype ID | Clinical Features |
| HFM1 | AR | 615684 | POF9 | 615724 | Amenorrhea |
| FIGLA | AD | 608697 | POF6 | 612310 | Small/absent ovaries, follicles absent, atrophic endometrium |
| FOXL2 | AD | 605597 | POF3 | 608996 | Hypoplastic uterus and ovaries, follicles absent, secondary amenorrhea |
| MSH5 | AR | 603382 | POF13 | 617442 | Oligomenorrhea, atrophic ovaries, follicles absent |
| STAG3 | AR | 608489 | POF8 | 615723 | Primary amenorrhea, ovarian dysgenesis |
| NOBOX | AD | 610934 | POF5 | 611548 | Secondary amenorrhea, follicles absent |
| NR5A1 | AD | 184757 | POF7 | 612964 | Irregular or anovulatory menstrual cycles, secondary amenorrhea, dysgenetic gonads, no germ cells |
| ERCC6 | AD | 609413 | POF11 | 616946 | Secondary amenorrhea |
| SYCE1 | AR | 611486 | POF12 | 616947 | Primary amenorrhea, small prepubertal uterus and ovaries, no ovarian follicles |
| MCM8 | AR | 608187 | POF10 | 612885 | Absent thelarche, primary amenorrhea, no ovaries, hypergonadotropic ovarian failure |
| BMP15 | XLD | 300247 | POF4, OD2 | 300510 | Delayed puberty, primary/secondary amenorrhea, small ovaries, follicles absent, hypoplastic uterus, hirsutism, absent pubic/axillary hair |
| FLJ22792 | XLR | 300603 | POF2B | 300604 | Weak teeth, delayed puberty, primary amenorrhea, osteoporosis |
| DIAPH2 | XLD | 300108 | POF2A | 300511 | Secondary amenorrhea |
| FSHR | AR | 136435 | OD1 | 233300 | Osteoporosis, primary amenorrhea |
| MCM9 | AR | 610098 | OD4 | 616185 | Short stature, low weight, underdeveloped breasts, no ovaries, retarded bone age and development of pubic/axillary hair, primary amenorrhea |
| SOHLH1 | AR | 610224 | OD5 | 617690 | Short stature, absent thelarche, primary amenorrhea, hypoplastic/no ovaries, small uterus, retarded bone age |
| PSMC3IP | AR | 608665 | OD3 | 614324 | Underdeveloped breasts and absent pubic hair, hypoplastic uterus, primary amenorrhea |
| AMH | AD | 600957 | POF | / | Primary/secondary amenorrhea |
| AMHR2 | AD | 600956 | POF | / | Primary ovarian insufficiency |
| DAZL | AR | 601486 | POF | / | Low ovarian reserves |
| GDF9 | AR | 601918 | POF14 | 618014 | Primary amenorrhea, no breast development, delayed pubic hair development |
| LHCGR | AR | 152790 | POF | / | Primary amenorrhea |
| INHA | AD, AR | 147380 | POF | / | Primary amenorrhea |
| PGRMC1 | AD | 300435 | POF | / | Hypergonadotropic hypogonadism, amenorrhea |
| POU5F1 | AD | 164177 | POF | / | Small ovaries without follicles |
| TGFBR3 | AD | 600742 | POF | / | Premature ovarian failure |
| WT1 | AD | 607102 | POF | / | Secondary amenorrhea |
| SGO2 | AR | 612425 | POF | / | Ovarian insufficiency |
| SPIDR | AR | 615384 | POF | / | Hypoplastic/no ovaries |
| EIF4ENIF1 | AD | 607445 | POF | / | Secondary amenorrhea |
| NUP107 | AR | 607617 | OD6 | 618078 | No ovaries, small uterus, no spontaneous puberty |
| NANOS3 | AD | 608229 | POF | / | Primary amenorrhea |
| ZP3 | AD | 182889 | OOMD3 | 617712 | Oocyte degeneration, absence of zona pellucida |
| TUBB8 | AD, AR | 616768 | OOMD2 | 616780 | Oocyte arrest at metaphase I or II; abnormal spindle |
| ZP1 | AR | 195000 | OOMD1 | 615774 | Absence of zona pellucida |
| PATL2 | AR | 614661 | OOMD4 | 617743 | Oocyte maturation arrest in germinal vesicle stage, metaphase I or polar body 1 stage; abnormal polar body 1; early embryonic arrest |
| ZP2 | AR | 182888 | OOMD6 | 618353 | Abnormal of zona pellucida |
| TLE6 | AR | 612399 | PREMBL1 | 616814 | Failure of zygote formation |
| PADI6 | AR | 610363 | PREMBL2 | 617234 | Recurrent early embryonic arrest |
| SYCP3 | AD | 604759 | RPRGL4 | 270960 | Fetal loss after 6-10 weeks of gestation |
| F2 | AD | 176930 | RPRGL2 | 614390 | Recurrent miscarriage |
| ANXA5 | AD | 131230 | RPRGL3 | 614391 | |
| NLRP7 | AR | 609661 | HYDM1 | 231090 | Gestational trophoblastic disease |
| KHDC3L | AR | 611687 | HYDM2 | 614293 | |
| Male infertility | |||||
| Gene | Inheritance | OMIM gene | OMIM phenotype | OMIM phenotype ID | Sperm defect |
| NR5A1 | AR | 184757 | SPGF8 | 613957 | AZS/OZS |
| SYCP3 | AD | 604759 | SPGF4 | 270960 | AZS/OZS |
| ZMYND15 | AR | 614312 | SPGF14 | 615842 | AZS/OZS |
| TAF4B | AR | 601689 | SPGF13 | 615841 | AZS/OZS |
| TEX11 | XLR | 300311 | SPGFX2 | 309120 | AZS |
| NANOS1 | AD | 608226 | SPGF12 | 615413 | AZS/OZS/OZS+ASTHZ+TZS |
| PLK4 | AD | 605031 | / | / | AZS |
| MEIOB | AR | 617670 | SPGF22 | 617706 | AZS |
| SYCE1 | AR | 611486 | SPGF15 | 616950 | AZS |
| USP9Y | YL | 400005 | SPGFY2 | 400042 | AZS |
| SOHLH1 | AD | 610224 | SPGF32 | 618115 | AZS |
| TEX15 | AR | 605795 | SPGF25 | 617960 | AZS/OZS |
| HSF2 | AD | 140581 | / | / | AZS |
| KLHL10 | AD | 608778 | SPGF11 | 615081 | OZS; TZS; AZS |
| AURKC | AR | 603495 | SPGF5 | 243060 | TZS (macrozoospermia) |
| DPY19L2 | AR | 613893 | SPGF9 | 613958 | TZS (globozoospermia) |
| SPATA16 | AR | 609856 | SPGF6 | 102530 | TZS (globozoospermia) |
| PICK1 | AR | 605926 | / | / | TZS (globozoospermia) |
| BRDT | AR | 602144 | SPGF21 | 617644 | ASS |
| SUN5 | AR | 613942 | SPGF16 | 617187 | ASS |
| SLC26A8 | AD | 608480 | SPGF3 | 606766 | AZS |
| CATSPER1 | AR | 606389 | SPGF7 | 612997 | AZS |
| SEPT12 | AD | 611562 | SPGF10 | 614822 | AZS; OZS+ASTHZ+TZS |
| CFAP43 | AR | 617558 | SPGF19 | 617592 | MMAF |
| CFAP44 | AR | 617559 | SPGF20 | 617593 | MMAF |
| DNAH1 | AR | 603332 | SPGF18 | 617576 | MMAF |
| PLCZ1 | AR | 608075 | SPGF17 | 617214 | OAF |
SPGF = spermatogenic failure; OZS = oligozoospermia; AZS = azoospermia; ASTHZ = asthenozoospermia; TZS = teratozoospermia; OZS+ASTHZ+TZS = oligoasthenoteratozoospermia; ASS = acephalic spermatozoa syndrome; MMAF = multiple morphological abnormalities of the flagellum; OAF = oocyte activation failure; AR = autosomal recessive; AD = autosomal dominant; XLR = X-linked recessive; YL = Y-linked; OD=ovarian dysgenesis; POF = primary ovarian failure; OOMD=oocyte maturation defect; PREMBL=preimplantation embryonic lethality; RPRGL=recurrent pregnancy loss; PREMBL=preimplantation embryonic lethality.


